Properties of RF Magnetron-Sputtered Copper Gallium Oxide (CuGa2O4) Thin Films
Abstract
:1. Introduction
2. Experimental
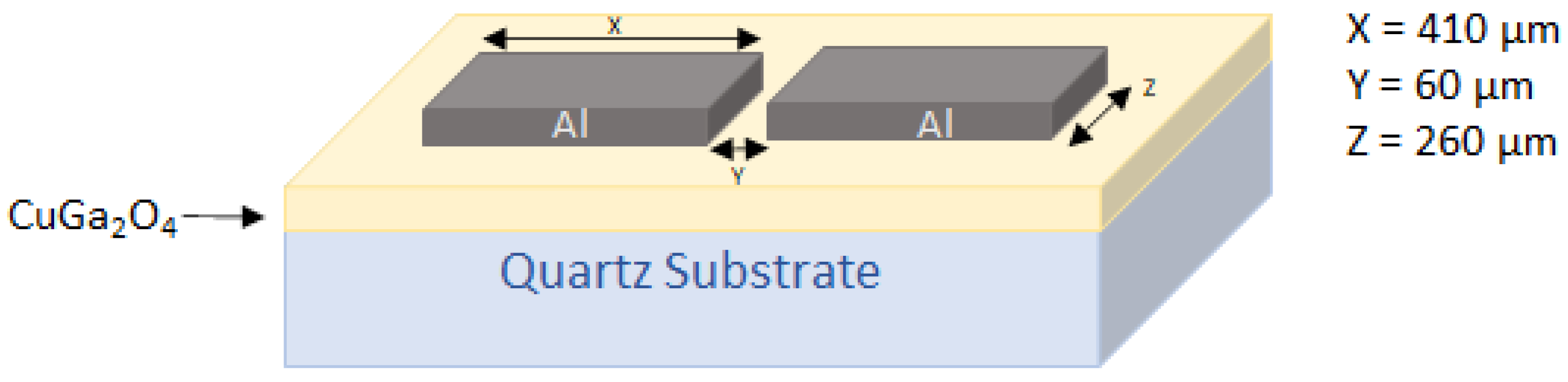
2.1. Deposition of the CuGa2O4 Thin Films
2.2. Film Characterization
3. Results and Discussions
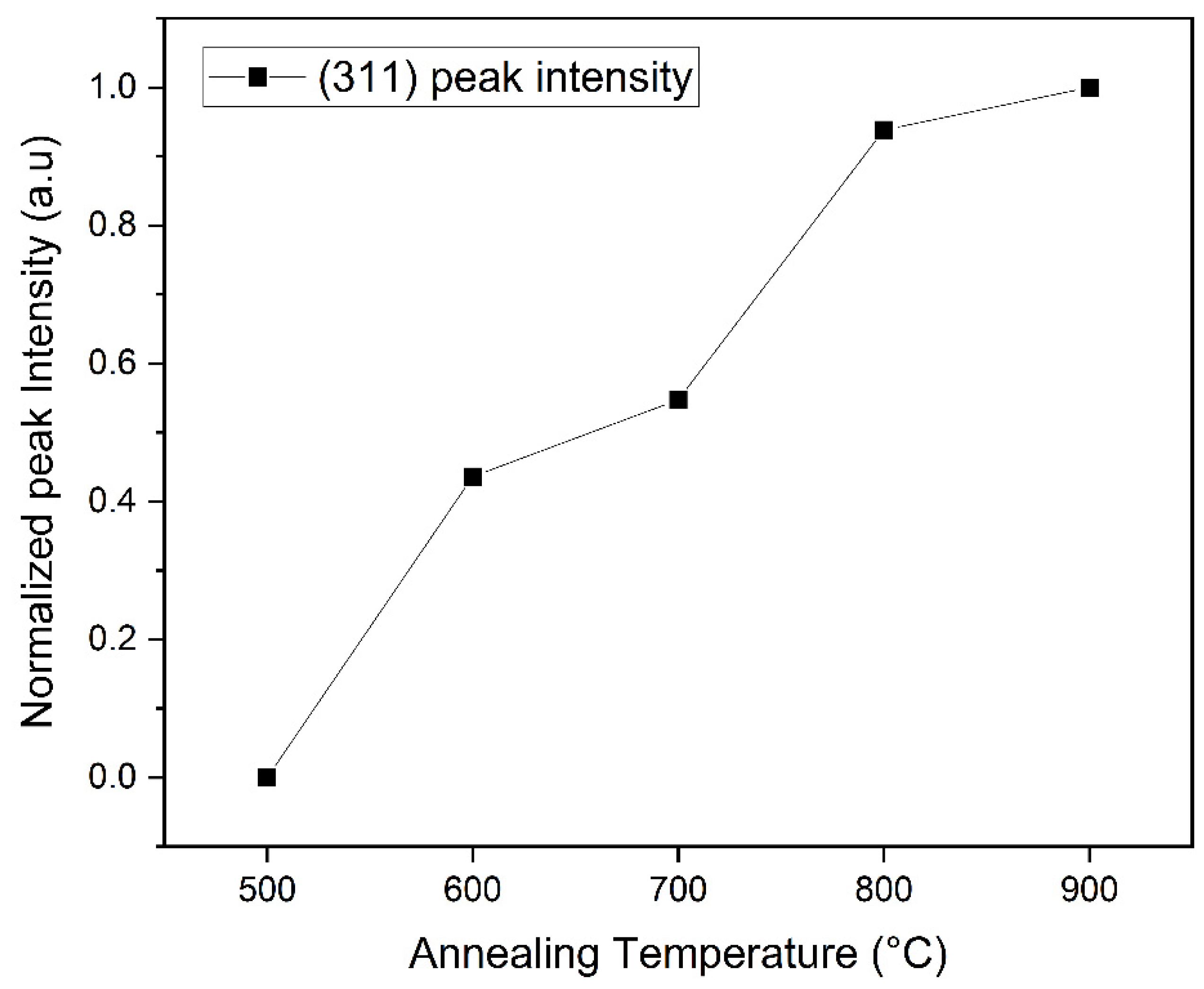
3.1. XRD Analysis
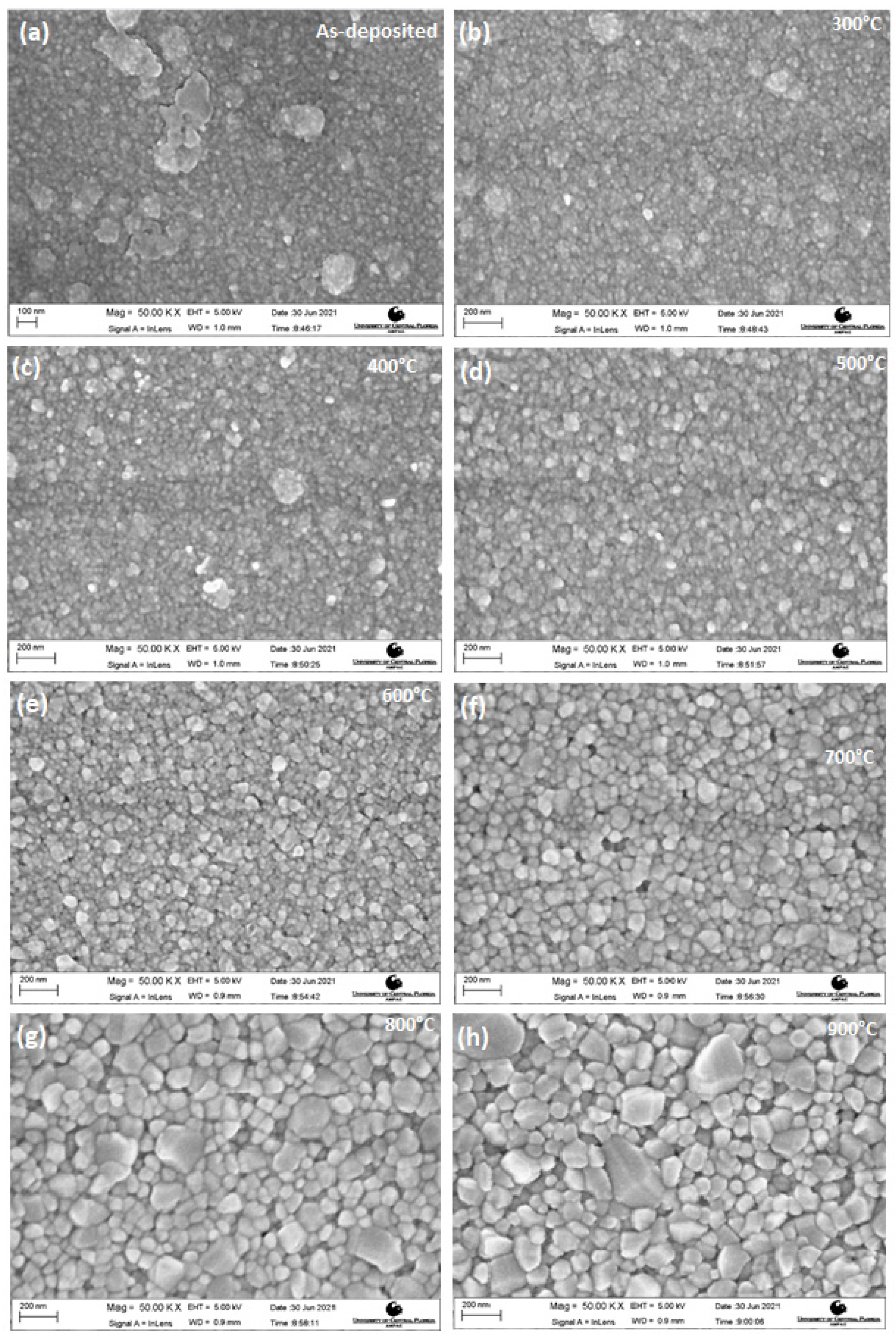
3.2. Morphology Studies
3.3. Optical Studies
3.3.1. Optical Transmission
3.3.2. Optical Bandgap
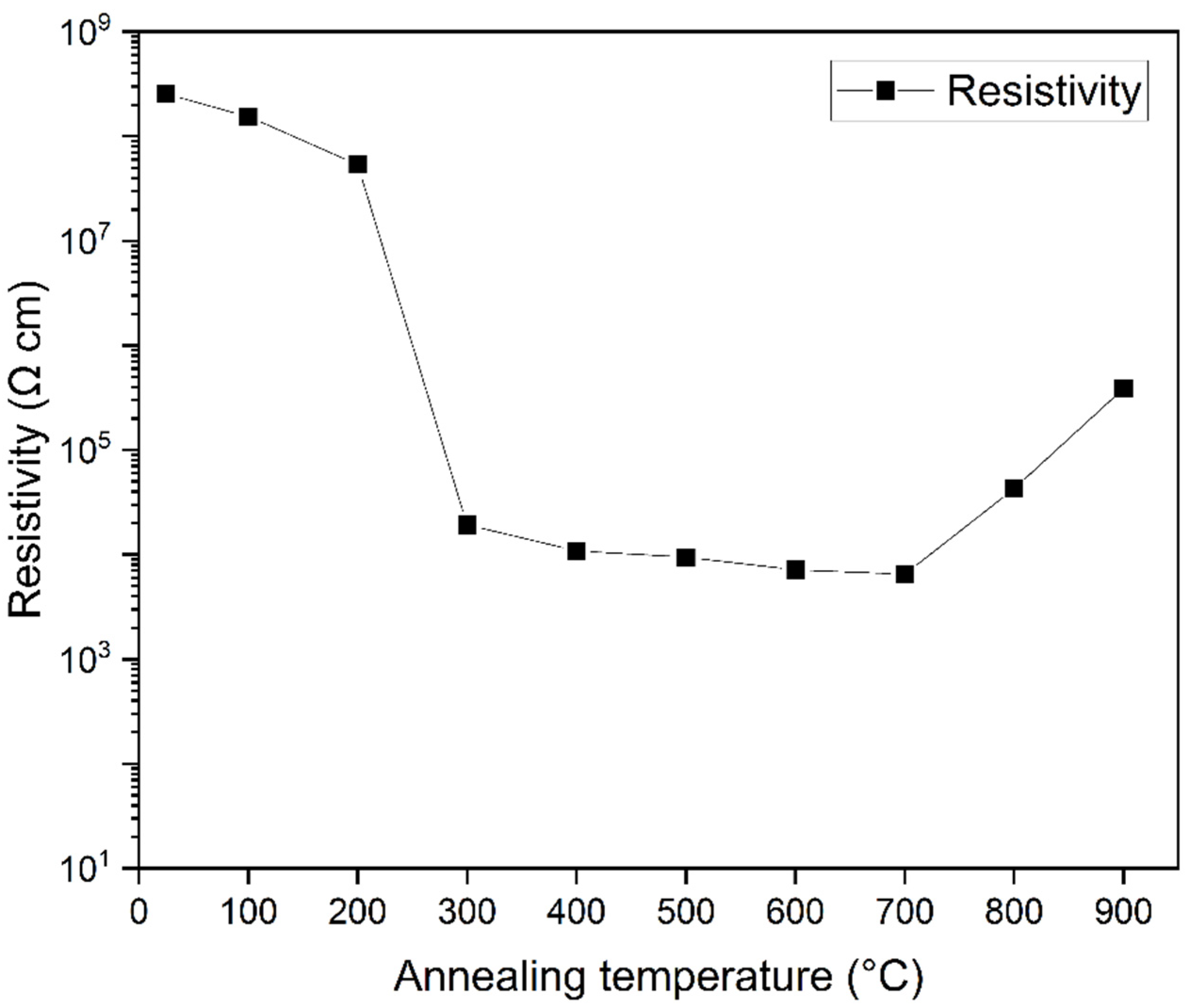
3.4. Electrical Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saikumar, A.K.; Nehate, S.D.; Sundaram, K.B. RF sputtered films of Ga2O3. ECS J. Solid State Sci. Technol. 2019, 8, Q3064. [Google Scholar] [CrossRef]
- Saikumar, A.K.; Skaria, G.; Sundaram, K.B. ZnO gate based MOSFETs for sensor applications. ECS Trans. 2014, 61, 65. [Google Scholar] [CrossRef]
- Nehate, S.D.; Prakash, A.; Mani, P.D.; Sundaram, K.B. Work Function Extraction of Indium Tin Oxide Films from MOSFET Devices. ECS J. Solid State Sci. Technol. 2018, 7, P87–P90. [Google Scholar] [CrossRef]
- Mudavakkat, V.; Atuchin, V.; Kruchinin, V.; Kayani, A.; Ramana, C. Structure, morphology and optical properties of nanocrystalline yttrium oxide (Y2O3) thin films. Opt. Mater. 2012, 34, 893–900. [Google Scholar] [CrossRef]
- Pearton, S.; Abernathy, C.; Norton, D.; Hebard, A.; Park, Y.; Boatner, L.; Budai, J. Advances in wide bandgap materials for semiconductor spintronics. Mater. Sci. Eng. R Rep. 2003, 40, 137–168. [Google Scholar] [CrossRef] [Green Version]
- Saikumar, A.K.; Nehate, S.D.; Sundaram, K.B. A review of recent developments in aluminum gallium oxide thin films and devices. Rev. Solid State Mater. Sci. 2021, 1–32. [Google Scholar]
- Sanctis, S.; Hoffmann, R.C.; Koslowski, N.; Foro, S.; Bruns, M.; Schneider, J.J. Aqueous Solution Processing of Combustible Precursor Compounds into Amorphous Indium Gallium Zinc Oxide (IGZO) Semiconductors for Thin Film Transistor Applications. Chem. Asian J. 2018, 13, 3912–3919. [Google Scholar] [CrossRef] [PubMed]
- Petrakovskii, G.A.; Aleksandrov, K.S.; Bezmaternikh, L.N.; Aplesnin, S.S.; Roessli, B.; Semadeni, F.; Amato, A.; Baines, C.; Bartolomé, J.; Evangelisti, M. Spin-glass state in CuGa2O4. Phys. Rev. B 2001, 63, 184425. [Google Scholar] [CrossRef] [Green Version]
- Knapp, C.E.; Prassides, I.D.; Sathasivam, S.; Parkin, I.P.; Carmalt, C.J. Aerosol-Assisted Chemical Vapour Deposition of a Copper Gallium Oxide Spinel. ChemPlusChem 2013, 79, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Liang, H.; Xia, X.; Li, Z.; Long, Z.; Zhang, H.; Liu, Y. Preparation of high-quality CuGa2O4 film via annealing process of Cu/β-Ga2O3. J. Mater. Sci. 2019, 54, 11111–11116. [Google Scholar] [CrossRef]
- Zardkhoshoui, A.M.; Davarani, S.S.H. Designing a flexible all-solid-state supercapacitor based on CuGa2O4 and FeP-rGO electrodes. J. Alloy. Compd. 2019, 773, 527–536. [Google Scholar] [CrossRef]
- Faungnawakij, K.; Shimoda, N.; Fukunaga, T.; Kikuchi, R.; Eguchi, K. Cu-based spinel catalysts CuB2O4 (B= Fe, Mn, Cr, Ga, Al, Fe0. 75Mn0. 25) for steam reforming of dimethyl ether. Appl. Catal. A Gen. 2008, 341, 139–145. [Google Scholar] [CrossRef]
- Gurunathan, K.; Baeg, J.-O.; Lee, S.M.; Subramanian, E.; Moon, S.-J.; Kong, K.-J. Visible light active pristine and Fe3+ doped CuGa2O4 spinel photocatalysts for solar hydrogen production. Int. J. Hydrog. Energy 2008, 33, 2646–2652. [Google Scholar] [CrossRef]
- Pilliadugula, R.; Nithya, C.; Krishnan, N.G. Influence of Ga2O3, CuGa2O4 and Cu4O3 phases on the sodium-ion storage behaviour of CuO and its gallium composites. Nanoscale Adv. 2020, 2, 1269–1281. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Ibarra, V.; Barrera, D.; Xu, L.; Lee, Y.-J.; Hsu, J.W.P. Solution Synthesized p-Type Copper Gallium Oxide Nanoplates as Hole Transport Layer for Organic Photovoltaic Devices. J. Phys. Chem. Lett. 2015, 6, 1071–1075. [Google Scholar] [CrossRef] [PubMed]
- Fenner, L.A.; Wills, A.S.; Bramwell, S.T.; Dahlberg, M.; Schiffer, P. Zero-point entropy of the spinel spin glasses CuGa2O4and CuAl2O4. J. Phys. Conf. Ser. 2009, 145. [Google Scholar] [CrossRef] [Green Version]
- Biswas, S.K.; Sarkar, A.; Pathak, A.; Pramanik, P. Studies on the sensing behaviour of nanocrystalline CuGa2O4 towards hydrogen, liquefied petroleum gas and ammonia. Talanta 2010, 81, 1607–1612. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Chen, Z.; Wu, Z.; Cui, W.; Huang, Y.; Tang, W. Epitaxial growth and characterization of CuGa2O4 films by laser molecular beam epitaxy. AIP Adv. 2017, 7, 115216. [Google Scholar] [CrossRef] [Green Version]
- Yin, H.; Shi, Y.; Dong, Y.; Chu, X. Synthesis of spinel-type CuGa2O4 nanoparticles as a sensitive non-enzymatic electrochemical sensor for hydrogen peroxide and glucose detection. J. Electroanal. Chem. 2021, 885, 115100. [Google Scholar] [CrossRef]
- Gingasu, D.; Mindru, I.; Patron, L.; Marinescu, G.; Tuna, F.; Preda, S.; Calderon-Moreno, J.M.; Andronescu, C. Synthesis of CuGa2O4 nanoparticles by precursor and self-propagating combustion methods. Ceram. Int. 2012, 38, 6739–6751. [Google Scholar] [CrossRef]
- Ahmadipour, M.; Ain, M.F.; Ahmad, Z.A. Effects of annealing temperature on the structural, morphology, optical properties and resistivity of sputtered CCTO thin film. J. Mater. Sci. Mater. Electron. 2017, 28, 12458–12466. [Google Scholar] [CrossRef]
- Shakti, N.; Gupta, P.S. Structural and optical properties of sol-gel prepared ZnO thin film. Appl. Phys. Res. 2010, 2, 19. [Google Scholar] [CrossRef] [Green Version]
- Ramana, C.V.; Smith, R.J.; Hussain, O.M. Grain size effects on the optical characteristics of pulsed-laser deposited vanadium oxide thin films. Phys. Status Solidi 2003, 199, R4–R6. [Google Scholar] [CrossRef]
- Tauc, J.; Grigorovici, R.; Vancu, A. Optical Properties and Electronic Structure of Amorphous Germanium. Phys. Status Solidi 1966, 15, 627–637. [Google Scholar] [CrossRef]
- Li, R.; Fu, Q.; Zou, X.; Zheng, Z.; Luo, W.; Yan, L. Mn-Co-Ni-O thin films prepared by sputtering with alloy target. J. Adv. Ceram. 2020, 9, 64–71. [Google Scholar] [CrossRef] [Green Version]
- Schulze, H.; Li, J.; Dickey, E.C.; Trolier-McKinstry, S. Synthesis, Phase Characterization, and Properties of Chemical Solution-Deposited Nickel Manganite Thermistor Thin Films. J. Am. Ceram. Soc. 2009, 92, 738–744. [Google Scholar] [CrossRef]
- Kukuruznyak, D.A.; Bulkley, S.A.; Omland, K.A.; Ohuchi, F.S.; Gregg, M.C. Preparation and properties of thermistor thin-films by metal organic decomposition. Thin Solid Films 2001, 385, 89–95. [Google Scholar] [CrossRef]
- Ohyama, M.; Kozuka, H.; Yoko, T. Sol-Gel Preparation of Transparent and Conductive Aluminum-Doped Zinc Oxide Films with Highly Preferential Crystal Orientation. J. Am. Ceram. Soc. 2005, 81, 1622–1632. [Google Scholar] [CrossRef]
- Valladares, L.D.L.S.; Salinas, D.H.; Dominguez, A.B.; Najarro, D.A.; Khondaker, S.; Mitrelias, T.; Barnes, C.; Aguiar, J.A.; Majima, Y. Crystallization and electrical resistivity of Cu2O and CuO obtained by thermal oxidation of Cu thin films on SiO2/Si substrates. Thin Solid Films 2012, 520, 6368–6374. [Google Scholar] [CrossRef]




| Film | Cu atm% | Ga atm% | O atm% |
|---|---|---|---|
| As deposited film | 13.64 | 25.88 | 60.48 |
| Annealed at 300 °C | 14.92 | 24.90 | 60.18 |
| Annealed at 400 °C | 14.78 | 24.07 | 61.15 |
| Annealed at 500 °C | 13.93 | 20.21 | 65.86 |
| Annealed at 600 °C | 14.10 | 20.79 | 65.11 |
| Annealed at 700 °C | 14.56 | 20.43 | 65.01 |
| Annealed at 800 °C | 13.88 | 19.92 | 66.19 |
| Annealed at 900 °C | 13.12 | 19.71 | 67.16 |
| Annealing Temperature | Eg, Bandgap (eV) |
|---|---|
| As deposited | 3.59 |
| 100 °C | 3.72 |
| 200 °C | 3.76 |
| 300 °C | 3.8 |
| 400 °C | 3.84 |
| 500 °C | 4.12 |
| 600 °C | 4.36 |
| 700 °C | 4.4 |
| 800 °C | 4.44 |
| 900 °C | 4.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saikumar, A.K.; Sundaresh, S.; Nehate, S.D.; Sundaram, K.B. Properties of RF Magnetron-Sputtered Copper Gallium Oxide (CuGa2O4) Thin Films. Coatings 2021, 11, 921. https://doi.org/10.3390/coatings11080921
Saikumar AK, Sundaresh S, Nehate SD, Sundaram KB. Properties of RF Magnetron-Sputtered Copper Gallium Oxide (CuGa2O4) Thin Films. Coatings. 2021; 11(8):921. https://doi.org/10.3390/coatings11080921
Chicago/Turabian StyleSaikumar, Ashwin Kumar, Sreeram Sundaresh, Shraddha Dhanraj Nehate, and Kalpathy B. Sundaram. 2021. "Properties of RF Magnetron-Sputtered Copper Gallium Oxide (CuGa2O4) Thin Films" Coatings 11, no. 8: 921. https://doi.org/10.3390/coatings11080921
APA StyleSaikumar, A. K., Sundaresh, S., Nehate, S. D., & Sundaram, K. B. (2021). Properties of RF Magnetron-Sputtered Copper Gallium Oxide (CuGa2O4) Thin Films. Coatings, 11(8), 921. https://doi.org/10.3390/coatings11080921







