Preparation of Surface-Modified Nano Zinc Sulfide/Polyurethane Inorganic-Organic Transparent Coating and Its Application in Resin Lens
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Reagents
2.2. Preparation of Modified Nano ZnS
2.3. Preparation of Zinc Sulfide/Polyurethane Transparent Coating
2.4. Structure Test and Characterization
2.5. Performance Characterization of ZnS/Polyurethane Transparent Coating
3. Results
3.1. Preparation of Surface Modified Nano-ZnS
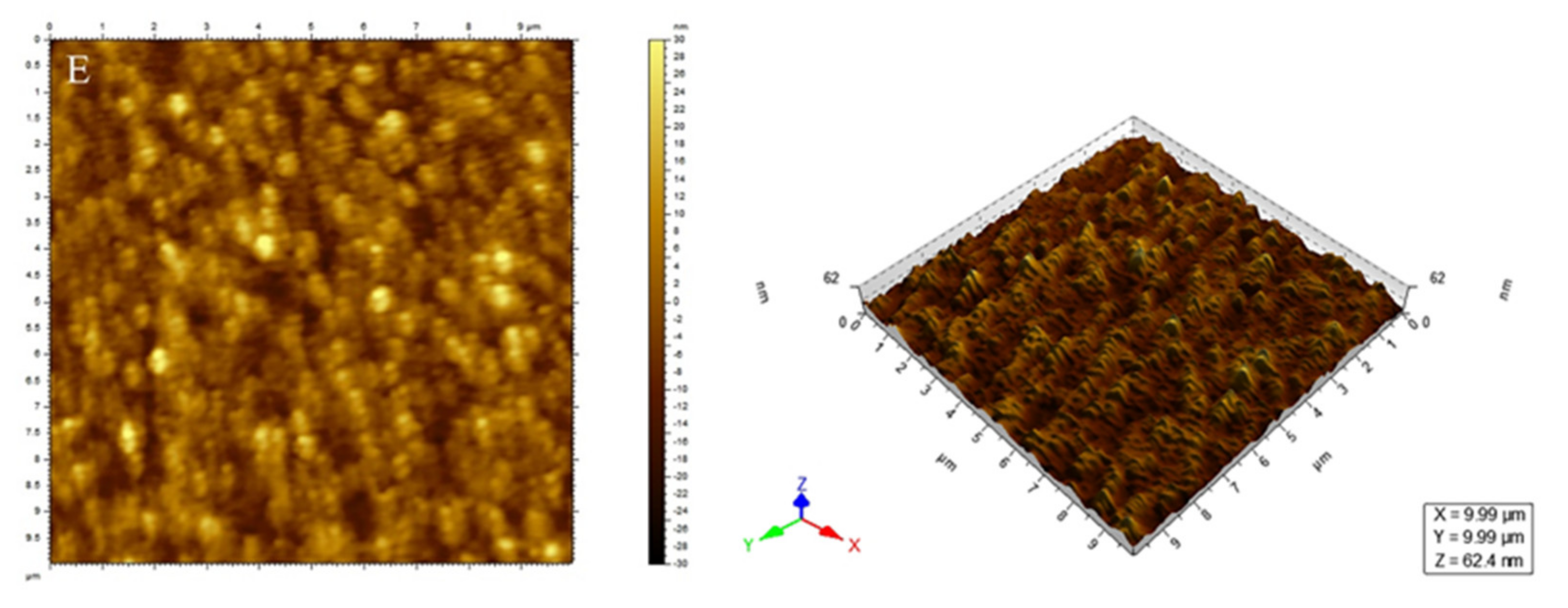
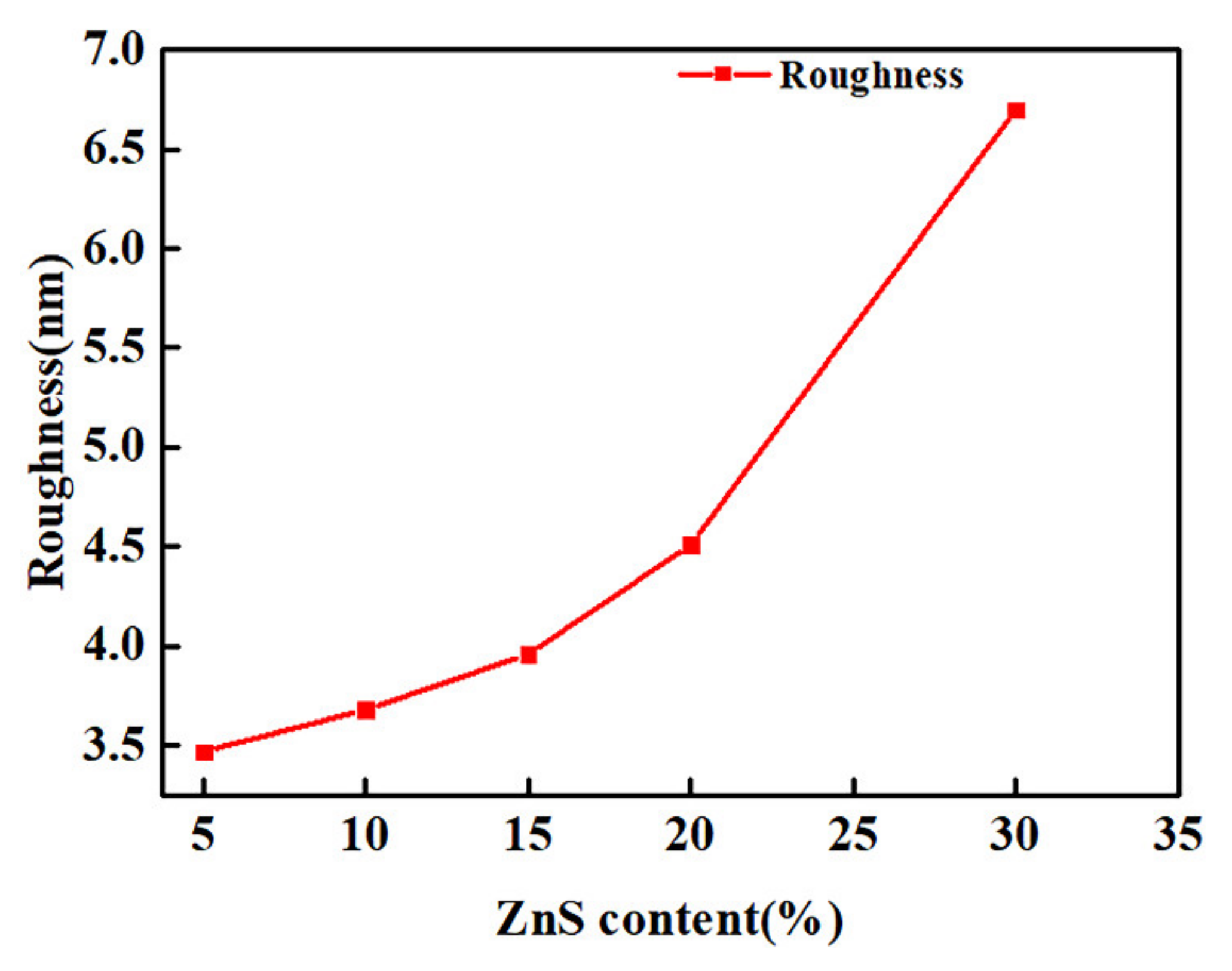
3.2. Waterborne Polyurethane Coating Modified by Nano-ZnS
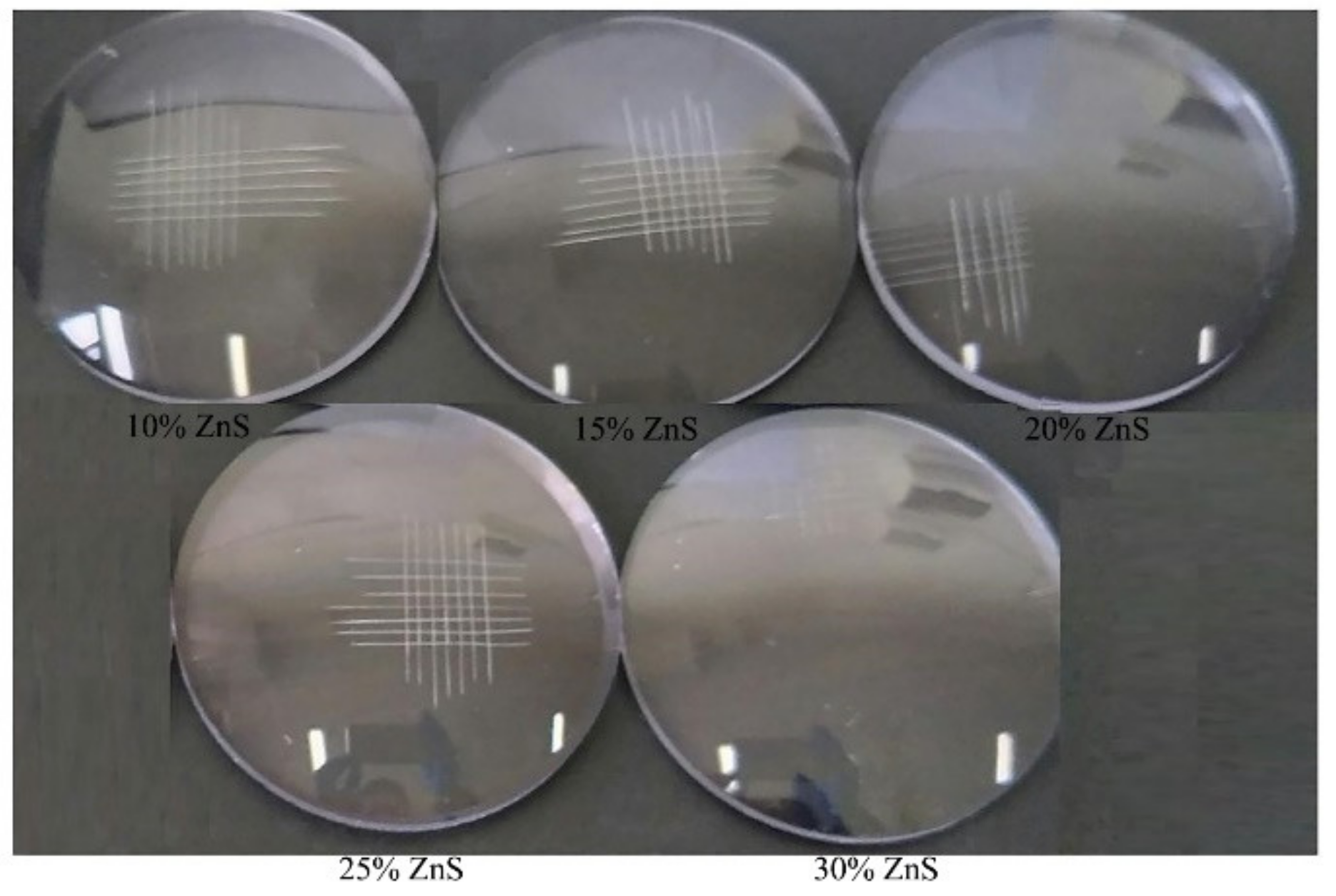
3.3. Application of ZnS/Polyurethane composite Coating on Resin Lens
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Murahara, M.; Yabe, T.; Uchida, S.; Yoshida, K.; Okamoto, Y. Anti-reflective and waterproof hard coating for high power laser optical elements. AIP Conf. Proc. 2006, 830, 457–465. [Google Scholar] [CrossRef]
- Hock, M.; Schäffer, E.; Döll, W.; Kleer, G. Composite coating materials for the moulding of diffractive and refractive optical components of inorganic glasses. Surf. Coat. Technol. 2003, 163, 689–694. [Google Scholar] [CrossRef]
- Sanchez, C.; Julian-Lopez, B.; Belleville, P.; Popall, M. Applications of hybrid organic–inorganic nanocomposites. J. Mater. Chem. 2005, 15, 3559–3592. [Google Scholar] [CrossRef]
- Xu, Q.F.; Wang, J.N.; Sanderson, K.D. Organic−inorganic composite nanocoatings with superhydrophobicity, good transparency, and thermal stability. ACS Nano 2010, 4, 2201–2209. [Google Scholar] [CrossRef]
- Salleh, N.G.N.; Alias, M.S.; Gläsel, H.-J.; Mehnert, R. High performance radiation curable hybrid coatings. Radiat. Phys. Chem. 2013, 84, 70–73. [Google Scholar] [CrossRef]
- Sangermano, M.; Voit, B.; Sordo, F.; Eichhorn, K.-J.; Rizza, G. High refractive index transparent coatings obtained via UV/thermal dual-cure process. Polymer 2008, 49, 2018–2022. [Google Scholar] [CrossRef]
- Que, W.X.; Sun, Z.; Zhou, Y.; Lam, Y.L.; Chan, Y.C.; Kam, C.H. Optical and mechanical properties of TiO2/SiO2/organically modified silane composite films prepared by sol–gel processing. Thin Solid Film 2000, 359, 177–183. [Google Scholar] [CrossRef]
- Yavas, H.; Selçuk, C.D.; Özhan, A.S.; Durucan, C. A parametric study on processing of scratch resistant hybrid sol–gel silica coatings on polycarbonate. Thin Solid Film 2014, 556, 112–119. [Google Scholar] [CrossRef]
- Pavlovska, I.; Malnieks, K.; Mezinskis, G.; Bidermanis, L.; Karpe, M. Hard TiO2–SiO2 sol–gel coatings for enamel against chemical corrosion. Surf. Coat. Technol. 2014, 258, 206–210. [Google Scholar] [CrossRef]
- Liu, B.-T.; Li, P.-S.; Chen, W.-C.; Yu, Y.-Y. Ex situ synthesis of high-refractive-index polyimide hybrid films containing TiO2 chelated by 4-aminobenzoic acid. Eur. Polym. J. 2014, 50, 54–60. [Google Scholar] [CrossRef]
- Bochev, B.; Yordanov, G. Room temperature synthesis of thioglycolate-coated zinc sulfide (ZnS) nanoparticles in aqueous medium and their physicochemical characterization. Colloids Surf. A Physicochem. Eng. Asp. 2014, 441, 84–90. [Google Scholar] [CrossRef]
- Ohno, T.; Tagawa, S.; Itoh, H.; Suzuki, H.; Matsuda, T. Size effect of TiO2–SiO2 nano-hybrid particle. Mater. Chem. Phys. 2009, 113, 119–123. [Google Scholar] [CrossRef]
- Soltani, N.; Saion, E.; Erfani, M.; Rezaee, K.; Bahmanrokh, G.; Drummen, G.P.C.; Bahrami, A.; Hussein, M.Z. Influence of the polyvinyl pyrrolidone concentration on particle size and dispersion of ZnS nanoparticles sythesized by microwave irradiation. Int. J. Mol. Sci. 2012, 13, 12412–12427. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Zhai, T.; Gautam, U.K.; Li, L.; Wu, L.; Bando, Y.; Golberg, D. ZnS nanostructures: From synthesis to applications. Prog. Mater. Sci. 2011, 56, 175–287. [Google Scholar] [CrossRef]
- Persits, N.; Aharoni, A.; Tur, M. Quantitative characterization of ZnS: Mn embedded polyurethane optical emission in three mechanoluminescent regimes. J. Lumin. 2017, 181, 467–476. [Google Scholar] [CrossRef]
- Chaudhuri, T.K.; Tiwari, D. Earth-abundant non-toxic Cu2ZnSnS4 thin films by direct liquid coating from metal–thiourea precursor solution. Sol. Energy Mater. Sol. Cells 2012, 101, 46–50. [Google Scholar] [CrossRef]
- Althues, H.; Palkovits, R.; Rumplecker, A.; Simon, P.; Sigle, W.; Bredol, M.; Kynast, U.; Kaskel, S. Synthesis and characterization of transparent luminescent ZnS: Mn/PMMA nanocomposites. Chem. Mater. 2006, 18, 1068–1072. [Google Scholar] [CrossRef]
- Wu, D.; Xu, H.; Qiu, F.; Yang, D. Preparation, Morphology and Properties of Waterborne-Polyurethane/Silica. Polym. Technol. Eng. 2011, 50, 498–508. [Google Scholar] [CrossRef]
- Li, G.J.; Huang, Y.G.; Zhu, Z.Z.; Luo, W.A.; Chen, X.D. Fluorescence spectra of zinc sulfide quantum dots/polyurethane nano-composite. J. South China Univ. Technol. (Nat. Sci.) 2009, 37, 1–5. [Google Scholar] [CrossRef]
- Balakrishnan, A.; Saha, M.C. Effect of ultrasound and strain rate on tensile behavior of neat thermoplasticpolyurethane thin films. In Proceedings of the Asme International Mechanical Engineering Congress and Exposition, Vancouver, BC, Canada, 12–18 November 2010; pp. 123–128. [Google Scholar] [CrossRef]
- Agrawal, S.; Patidar, D.; Saxena, N. Glass transition temperature and thermal stability of ZnS/PMMA nanocomposites. Phase Transit. 2011, 84, 888–900. [Google Scholar] [CrossRef]
- Lukosz, W.; Tiefenthaler, K. Embossing technique for fabricating integrated optical components in hard inorganic waveguiding materials. Opt. Lett. 1983, 8, 537–539. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.; Kumar, P. Molecular characterization of a lipase-producing Bacillus pumilus strain (NMSN-1d) utilizing colloidal water-dispersible polyurethane. World J. Microbiol. Biotechnol. 2007, 23, 1441–1449. [Google Scholar] [CrossRef]
- Li, S.; Lin, M.M.; Toprak, M.S.; Kim, D.K.; Muhammed, M. Nanocomposites of polymer and inorganic nanoparticles for optical and magnetic applications. Nano Rev. 2010, 1. [Google Scholar] [CrossRef]
- Tiefenthaler, K.; Briguet, V.; Buser, E.; Horisberger, M.; Lukosz, W. Preparation Of planar optical SiO2-TiO2 and LiNbO3 waveguides with a dip coating method and an embossing technique for fabricating grating couplers and channel waveguides. Thin Film Technol. I 1983, 401, 165–173. [Google Scholar] [CrossRef]
- GB/T6739-2006 Paints and Varnishes-Determination of Film Hardness by Pencil Test; Standards Press of China: Beijing, China, 2006.
- QB/T2506-2017 Uncut Finished Spectacle Lenses-Optical Hard Resin Lenses; China Light Industry Press: Beijing, China, 2017.
- GB/T1733-1993 Determination of Resistance to Water of Films; Standards Press of China: Beijing, China, 1993.
- GB/T9265-2009 Determination for Alkali Resistance of Film of-Architectural Paints and Coatings; Standards Press of China: Beijing, China, 2010.
- GB/T9286-1998 Paints and Varnishes-Cross Cut Test for Films; Standards Press of China: Beijing, China, 1999.
- Cheng, Z.; Meng, Y.; Zhang, B. Effect of sputtering power on the microstructure of Mg doped ZnO thin films. IOP Conf. Ser. Mater. Sci. Eng. 2020, 892. [Google Scholar] [CrossRef]
- Xiong, Y.; Zhang, J.; Huang, F.; Ren, G.; Liu, W.; Li, D.; Wang, C.; Lin, Z. Growth and phase-transformation mechanisms of nanocrystalline CdS in Na2S solution. J. Phys. Chem. C 2008, 112, 9229–9233. [Google Scholar] [CrossRef]
- Wang, X.; Hu, Y.; Song, L.; Yang, H.Y.; Xing, W.Y.; Lu, H.D. In situ polymerization of graphene nanosheets and polyurethane with enhanced mechanical and thermal properties. J. Mater. Chem. 2011, 21, 4222–4227. [Google Scholar] [CrossRef]
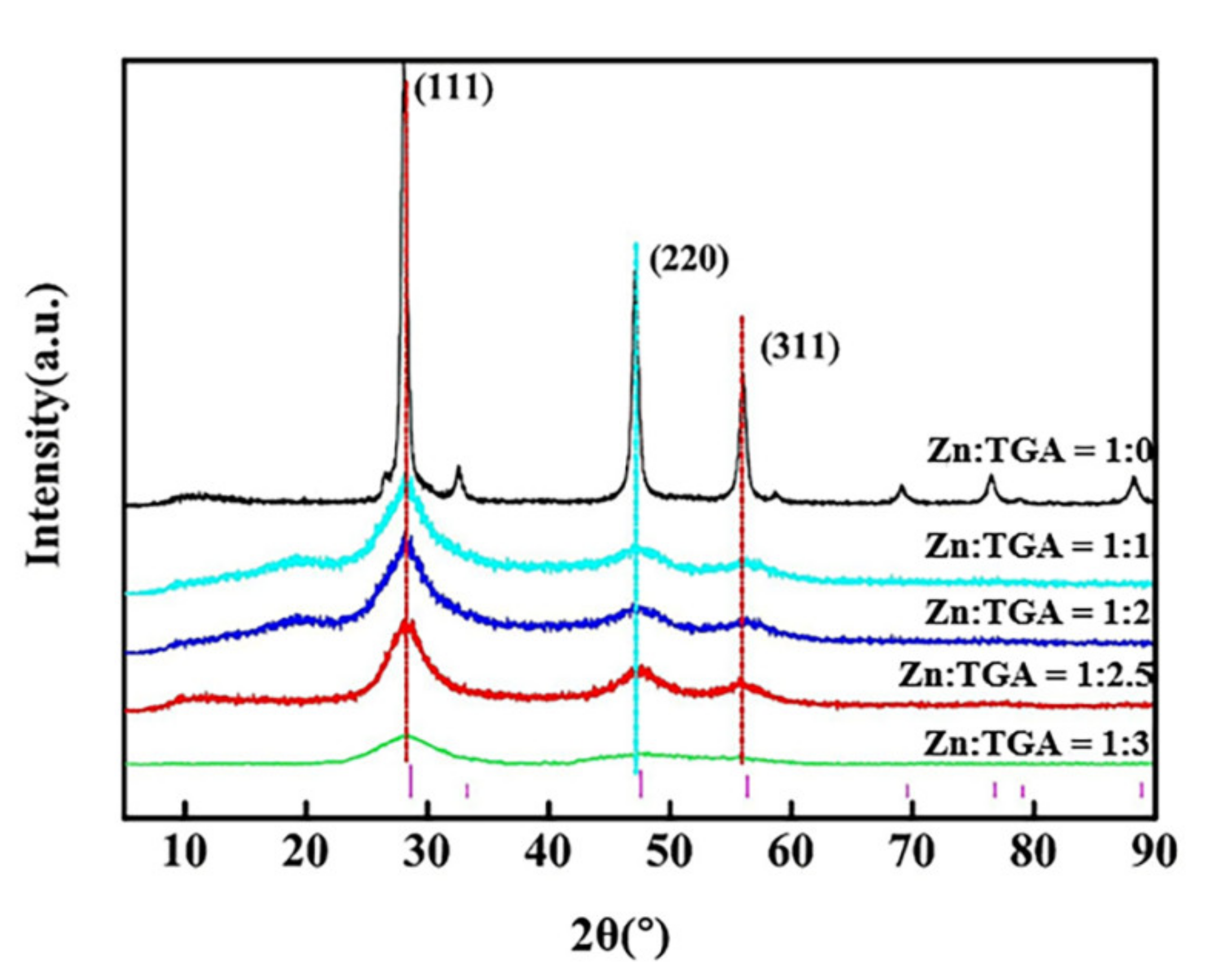
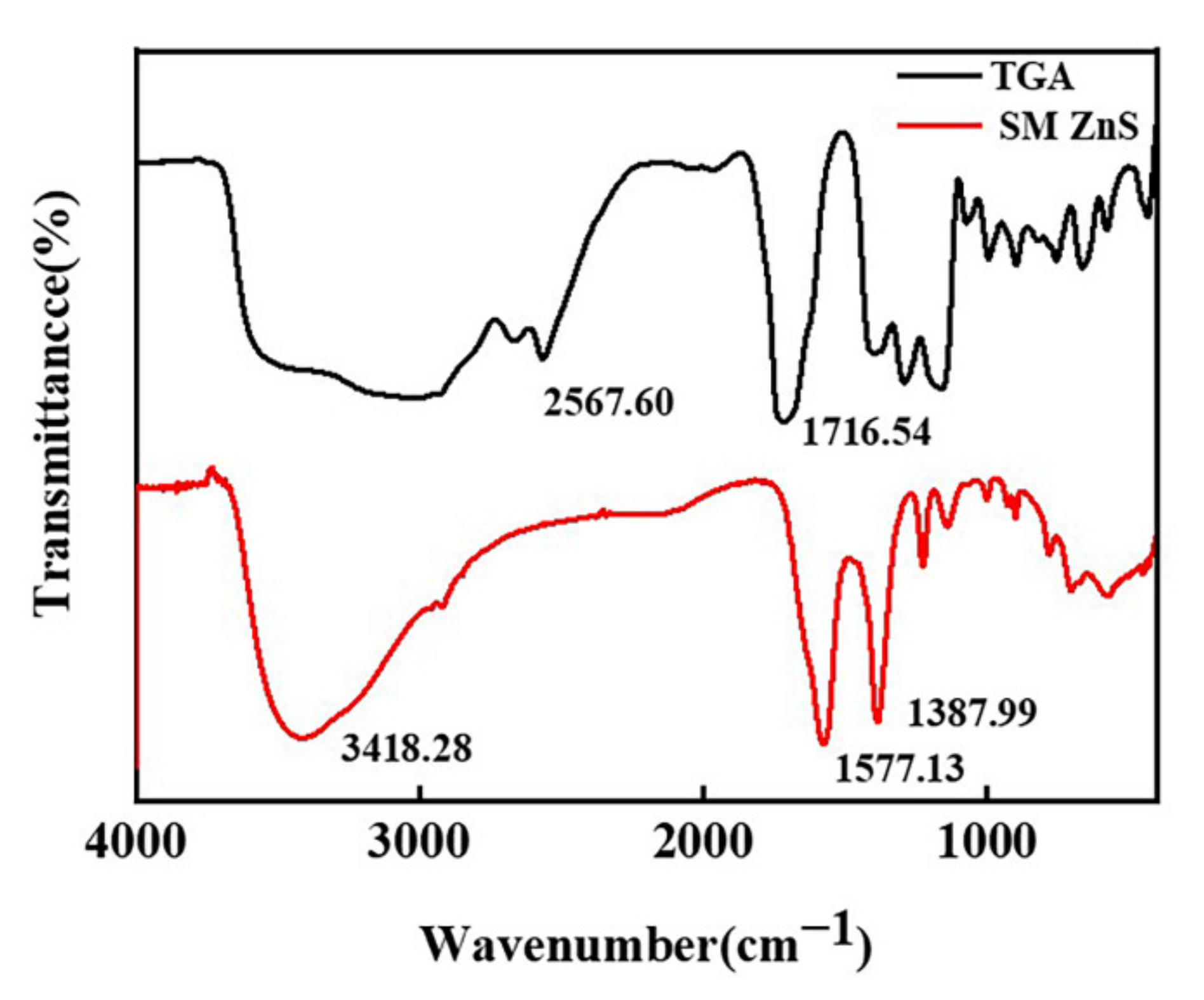

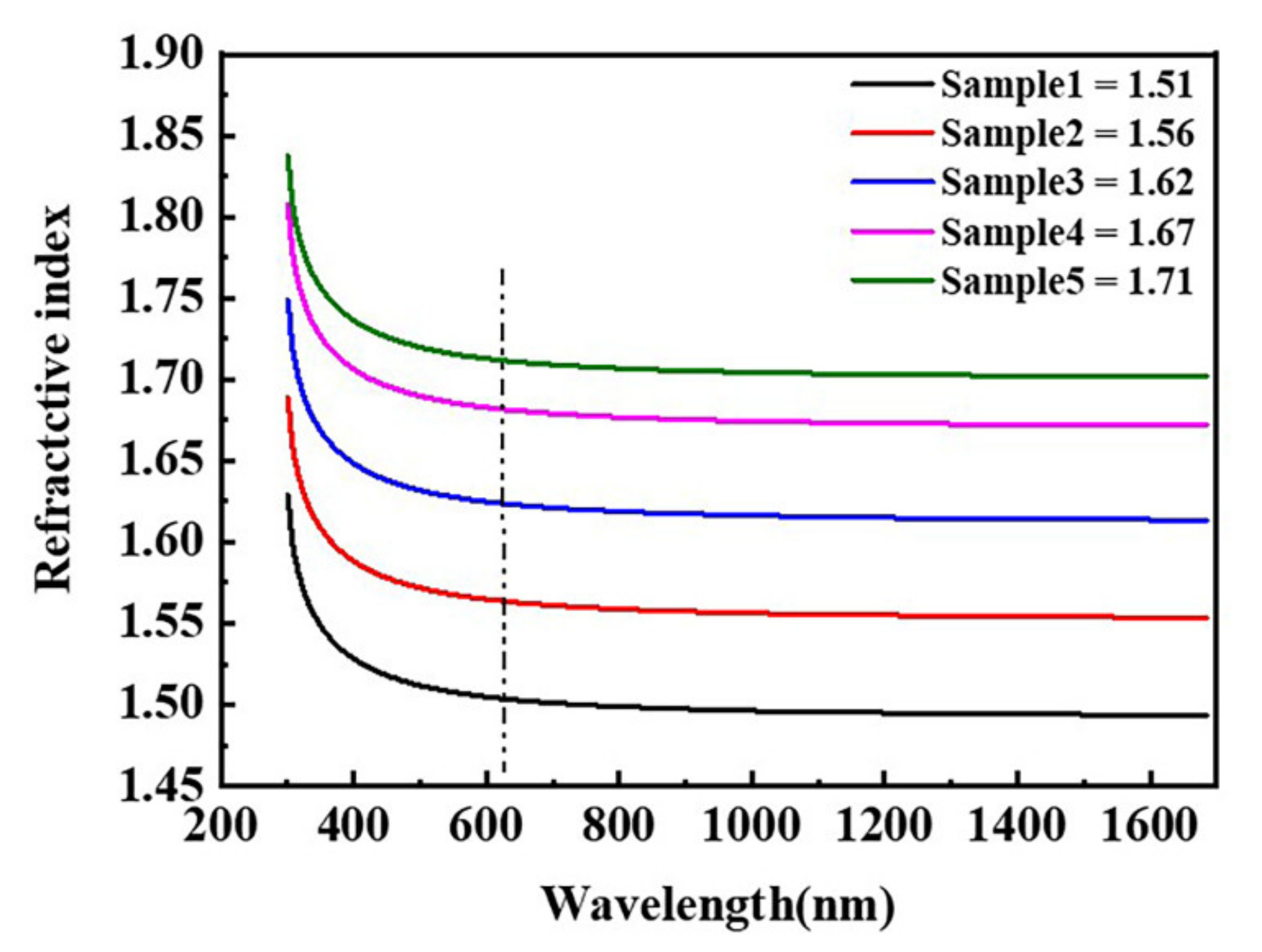

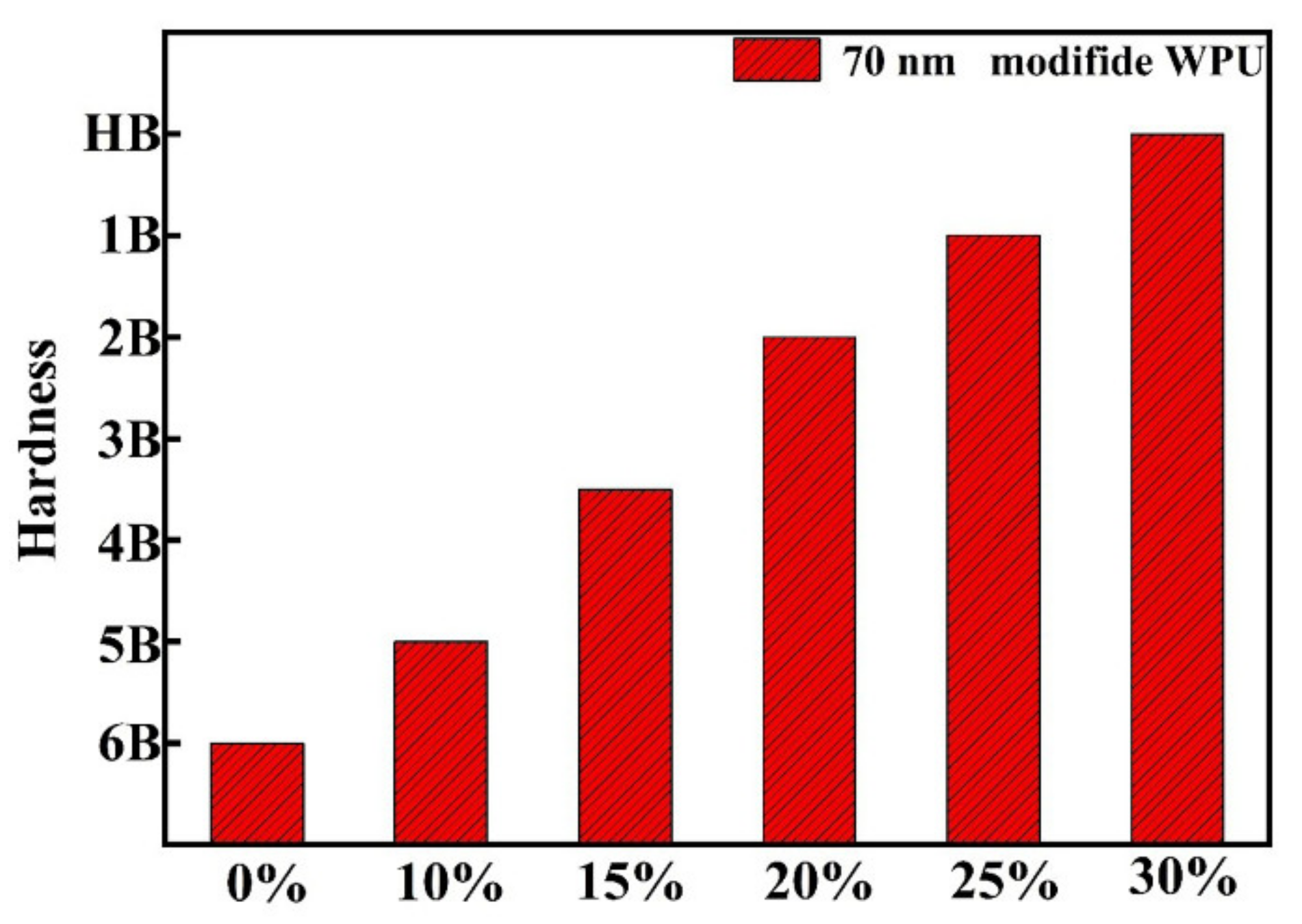





| Sample | Half Width (°) | Grain Size (nm) | Zeta Potential (mV) | Dispersity | Average Particle Size (nm) |
|---|---|---|---|---|---|
| Zn:TGA = 1:0 | 0.676 | 12.72 | 20.8 | double-peak | 75.15 |
| Zn:TGA = 1:1 | 2.214 | 3.89 | 31.4 | single-peak | 46.7 |
| Zn:TGA = 1:2 | 2.345 | 3.67 | 46.7 | single-peak | 31.5 |
| Zn:TGA = 1:2.5 | 3.014 | 2.85 | 32.5 | double-peak | 77.6 |
| Zn:TGA = 1:3 | 4.120 | 1.70 | 25.4 | double-peak | 111.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, W.; Ding, Y.; Zhang, Y.; Chen, H. Preparation of Surface-Modified Nano Zinc Sulfide/Polyurethane Inorganic-Organic Transparent Coating and Its Application in Resin Lens. Coatings 2021, 11, 894. https://doi.org/10.3390/coatings11080894
Du W, Ding Y, Zhang Y, Chen H. Preparation of Surface-Modified Nano Zinc Sulfide/Polyurethane Inorganic-Organic Transparent Coating and Its Application in Resin Lens. Coatings. 2021; 11(8):894. https://doi.org/10.3390/coatings11080894
Chicago/Turabian StyleDu, Weiping, Yingying Ding, Yang Zhang, and Huifang Chen. 2021. "Preparation of Surface-Modified Nano Zinc Sulfide/Polyurethane Inorganic-Organic Transparent Coating and Its Application in Resin Lens" Coatings 11, no. 8: 894. https://doi.org/10.3390/coatings11080894
APA StyleDu, W., Ding, Y., Zhang, Y., & Chen, H. (2021). Preparation of Surface-Modified Nano Zinc Sulfide/Polyurethane Inorganic-Organic Transparent Coating and Its Application in Resin Lens. Coatings, 11(8), 894. https://doi.org/10.3390/coatings11080894




