Mechanistic Study on Gold-Like Luster Development of Solution-Cast Oligo(3-methoxythiophene) Film
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Coating Solution and Film Preparation
2.3. Instrumentation and Method of Film Characterization
3. Results and Discussion
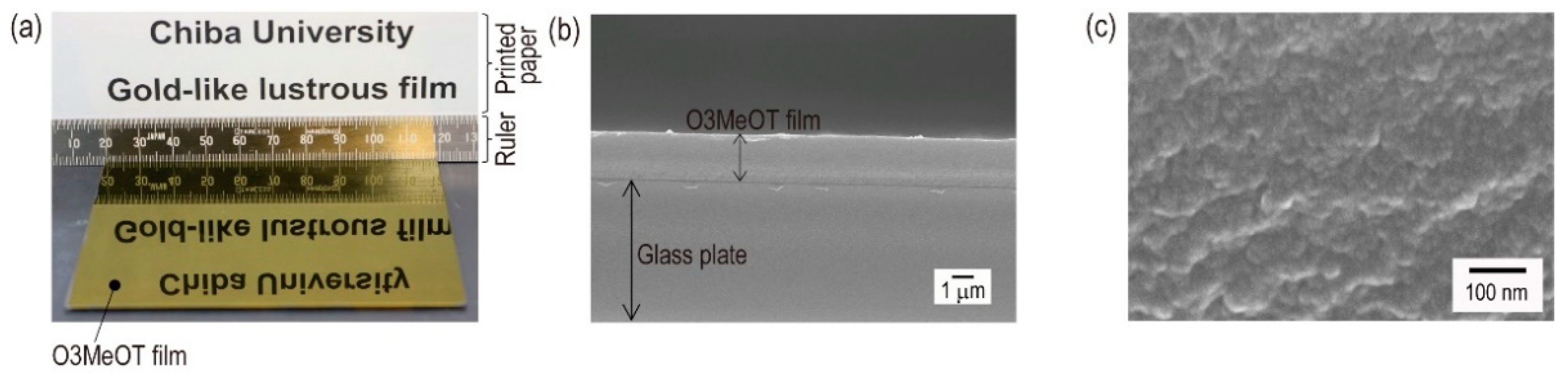
3.1. Appearance and Morphology of a Coating Film
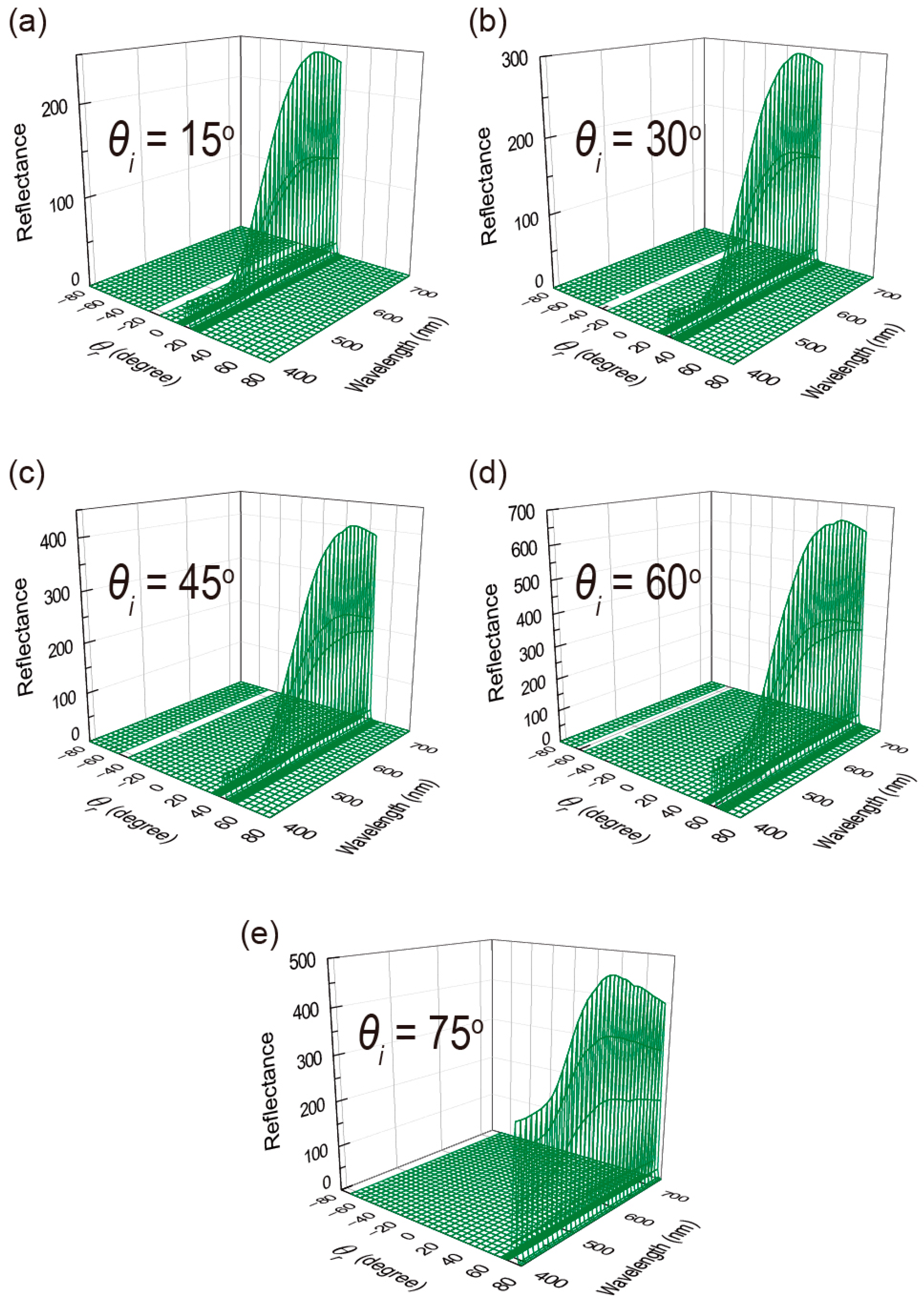
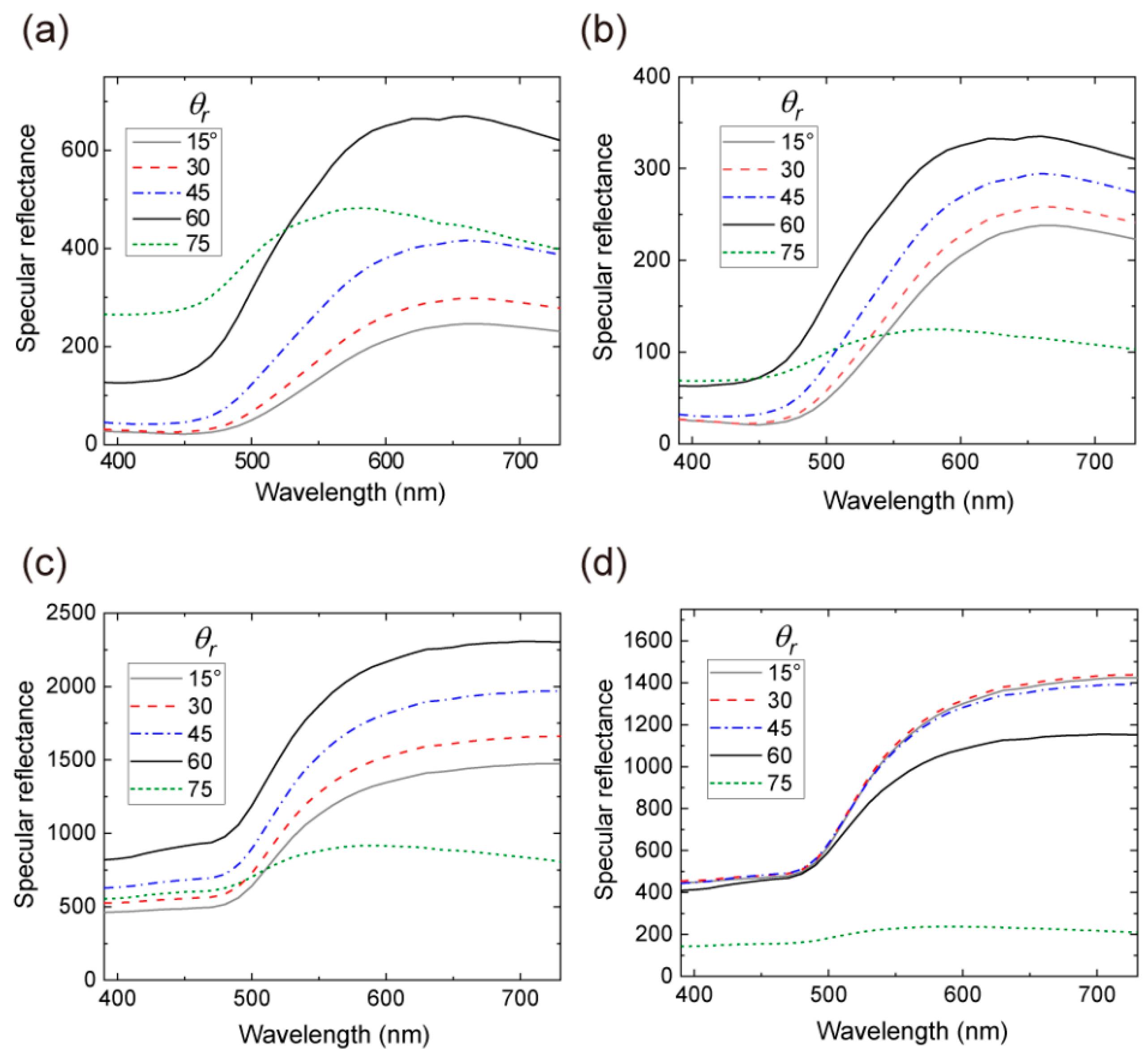
3.2. Variable-Angle Spectral Reflectance Measurements
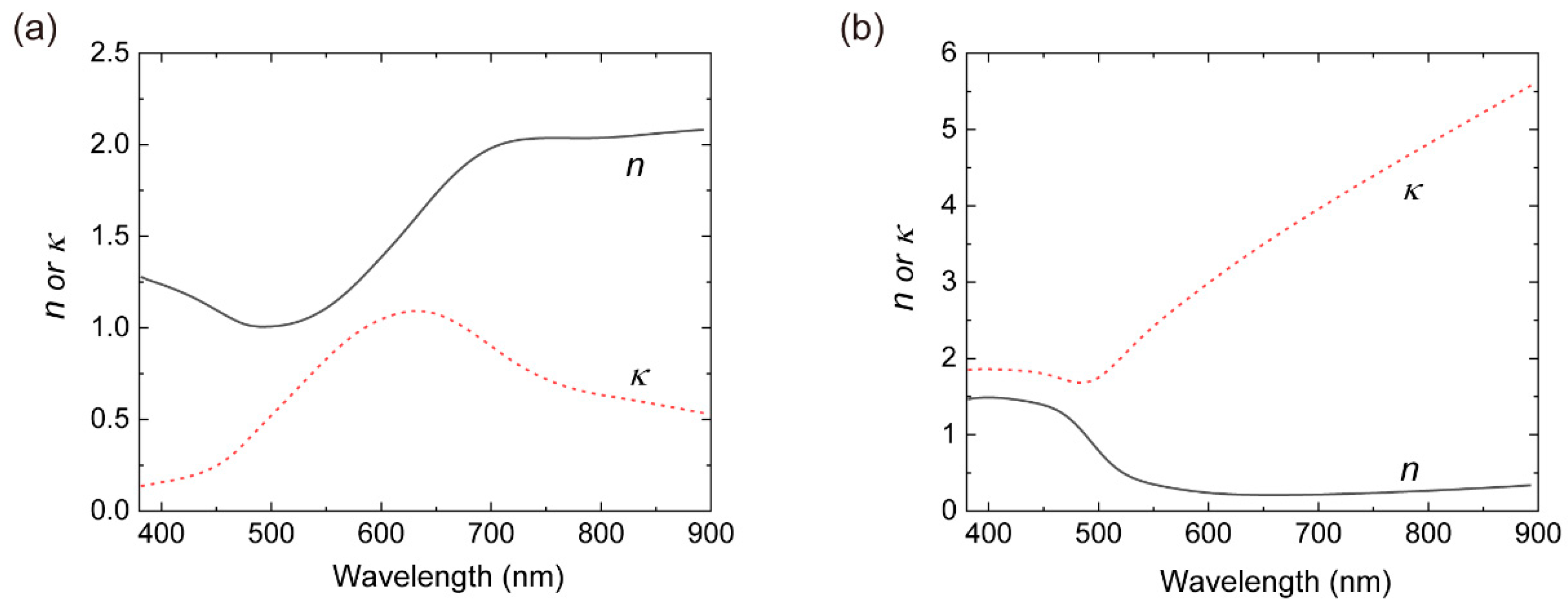
3.3. Ellipsometry Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Park, B.; Aiyar, A.; Park, M.S.; Srinivasarao, M.; Reichmanis, E. Conducting channel formation in poly (3-hexylthiophene) field effect transistors: Bulk to interface. J. Phys. Chem. C 2011, 115, 11719–11726. [Google Scholar] [CrossRef]
- Yang, Y.; Hong, Y.; Wang, X. Utilizing the diffusion of fluorinated polymers to modify the semiconductor/dielectric interface in solution-processed conjugated polymer field-effect transistors. ACS Appl. Mater. Interfaces 2021, 13, 8682–8691. [Google Scholar] [CrossRef] [PubMed]
- Milanovich, M.; Sarkar, T.; Popowski, Y.; Low, J.Z.; Campos, L.M.; Kenig, S.; Frey, G.L.; Amir, E. Enhancing P3HT/PCBM blend stability by thermal crosslinking using poly(3-hexylthiophene)-S,S-dioxide. J. Mater. Chem. C 2020, 8, 7698–7707. [Google Scholar] [CrossRef]
- Grimsdale, A.C.; Chan, K.L.; Martin, R.E.; Jokisz, P.G.; Holmes, A.B. Synthesis of light-emitting conjugated polymers for applications in electroluminescent devices. Chem. Rev. 2009, 109, 897–1091. [Google Scholar] [CrossRef] [PubMed]
- Murad, A.R.; Iraqi, A.; Aziz, S.B.; Abdullah, S.N.; Brza, M.A. Conducting Polymers for optoelectronic devices and organic Solar Cells: A Review. Polymers 2020, 12, 2627. [Google Scholar] [CrossRef] [PubMed]
- Mazzio, K.A.; Luscombe, C.K. The future of organic photovoltaics. Chem. Soc. Rev. 2015, 44, 78–90. [Google Scholar] [CrossRef]
- Lanzi, M.; Pierini, F. Efficient and thermally stable BHJ solar cells based on a soluble hydroxy-functionalized regioregular poly-dodecylthiophene. React. Funct. Polym. 2021, 158, 104803. [Google Scholar] [CrossRef]
- Kawai, T.; Nakazono, M.; Yoshino, K. Effects of doping on the crystal structure of poly(3-alkylthiophene). J. Mater. Chem. 1992, 2, 903–906. [Google Scholar] [CrossRef]
- Guay, J.; Kasai, P.; Diaz, A.; Wu, R.; Tour, J.M.; Dao, L.H. Chain-length dependence of electrochemical and electronic properties of neutral and oxidized soluble α, α-coupled thiophene oligomers. Chem. Mater. 1992, 4, 1097–1105. [Google Scholar] [CrossRef]
- Tagawa, R.; Masu, H.; Itoh, T.; Hoshino, K. Solution-cast self-assembled films of perchlorate-doped oligo(3-methoxythiophene) showing a gold-like luster. RSC Adv. 2014, 4, 24053–24058. [Google Scholar] [CrossRef]
- Takashina, Y.; Mitogawa, T.; Saito, K.; Hoshino, K. Chemical events in oligo(3-methoxythiophene) coating solutions and their effect on the goldlike coating film properties. Langmuir 2018, 34, 3049–3057. [Google Scholar] [CrossRef] [PubMed]
- Takashina, Y.; Hoshino, K. Effect of π–π interaction-induced secondary doping on the gold-like luster of oligo(3-methoxythiophene) cast films. Polym. J. 2019, 51, 591–599. [Google Scholar] [CrossRef]
- Tachiki, M.; Tagawa, R.; Hoshino, K. Oligo(3-methoxythiophene)s as water-soluble dyes for highly lustrous gold- and bronze-like metal-effect coatings and printings. ACS Omega 2020, 5, 24379–24388. [Google Scholar] [CrossRef]
- Tachiki, M.; Tsukada, S.; Hoshino, K. Effect of polymerization rate on the chemical and optical properties of solution-cast metal-like lustrous films of water-soluble 3-methoxythiophene oligomer dyes. Dyes Pigment. 2021, 190, 109302. [Google Scholar] [CrossRef]
- Wooten, F. Optical Properties of Solids; Academic Press: New York, NY, USA, 1972. [Google Scholar]
- Kohri, M.; Yanagimoto, K.; Kawamura, A.; Hamada, K.; Imai, Y.; Watanabe, T.; Ono, T.; Taniguchi, T.; Kishikawa, K. Polydopamine-based 3D colloidal photonic materials: Structural color balls and fibers from melanin-lke particles with polydopamine shell layers. ACS Appl. Mater. Interface 2018, 10, 7640–7648. [Google Scholar] [CrossRef]
- Kohri, M.; Nannichi, Y.; Taniguchi, T.; Kishikawa, K. Biomimetic non-iridescent structural colormaterials from polydopamine black particles thatmimic melanin granules. J. Mater. Chem. C 2015, 3, 720–724. [Google Scholar] [CrossRef]
- Okazawa, G.; Koida, K.; Komatsu, H. Categorical properties of the color term “GOLD”. J. Vis. 2011, 11, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Daira, T.; Omodani, M. Clarification of gold color recognition mechanism: A study on the effects of lighting condition. J. Imaging Soc. Jpn. 2011, 50, 498–502. [Google Scholar]
- Tsuboi, T.; Nihira, K.; Gunji, T. A study on iridescent effect of tamamushi fabrics by hemispheric diffuse illuminator. J. Text. Mach. Soc. Jpn. 1975, 21, 46–54. [Google Scholar] [CrossRef]
- Bernad, B.; Ferrero, A.; Strothkämper, C.; Campos, J.; Pons, A.; Quast, T.; Hauer, K.-O.; Schirmacher, A. Deviation of white diffuse reflectance standards from perfect reflecting diffuser at visible and near-infrared spectral ranges. Metrologia 2019, 56, 055005. [Google Scholar] [CrossRef]
- Kim, S.; Iwakiri, T.; Yagi, R.; Ogata, T.; Kurihara, S. Fabrication of wide angle structural color with the patchy multi-bilayered films. Mol. Cryst. Liquid Cryst. 2017, 644, 36–43. [Google Scholar] [CrossRef]
- Kohri, M.; Yamazaki, S.; Kawamura, A.; Taniguchi, T.; Kishikawa, K. Bright structural color films independent of background prepared by the dip-coating of biomimetic melanin-like particles having polydopamine shell layers. Colloid Surf. A Physicochem. Eng. Asp. 2017, 532, 564–569. [Google Scholar] [CrossRef]
- Katagiri, K.; Tanaka, Y.; Uemura, K.; Inumaru, K.; Seki, T.; Takeoka, Y. Structural color coating films composed of an amorphous array of colloidal particles via electrophoretic deposition. NPG Asia Mater. 2017, 9, e355. [Google Scholar] [CrossRef]
- Ohnuki, R.; Sakai, M.; Takeoka, Y.; Yoshioka, S. Optical Characterization of the Photonic Ball as a Structurally Colored Pigment. Langmuir 2020, 36, 5579–5587. [Google Scholar] [CrossRef]
- Wu, S.; Huang, B.; Wu, Y.; Meng, Z.; Zhang, S. Reflection and transmission two-way structural colors. Nanoscale 2020, 12, 11460–11467. [Google Scholar] [CrossRef]
- Yamada, H.; Kukino, M.; Wang, Z.A.; Miyabara, R.; Fujimoto, N.; Kuwabara, J.; Matsuishi, K.; Kanbara, T. Preparation and characterization of green reflective films of polyaniline analogs containing azobenzene units J. Appl. Polym. Sci. 2015, 132, 41275. [Google Scholar] [CrossRef]
- Kondo, Y.; Matsumoto, A.; Fukuyasu, K.; Nakajima, K.; Takahashi, Y. Gold-colored organic crystals of an azobenzene derivative. Langmuir 2014, 30, 4422–4426. [Google Scholar] [CrossRef] [PubMed]
- Takeoka, Y.; Yoshioka, S.; Takano, A.; Arai, S.; Nueangnoraj, K.; Nishihara, H.; Teshima, M.; Ohtsuka, Y.; Seki, T. Production of colored pigments with amorphous arrays of black and white colloidal particles. Angew. Chem. Int. Ed. 2013, 52, 7261–7265. [Google Scholar] [CrossRef]
- Kawamura, A.; Kohri, M.; Morimoto, G.; Nannichi, Y.; Taniguchi, T.; Kishikawa, K. Full-color biomimetic photonic materials with iridescent and non-iridescent structural colors. Sci. Rep. 2016, 6, 33984. [Google Scholar] [CrossRef] [PubMed]
- Morisue, M.; Hoshino, Y.; Shimizu, M.; Tomita, S.; Sasaki, S.; Sakurai, S.; Hikima, T.; Kawamura, A.; Kohri, M.; Matsui, J.; et al. A metal-lustrous porphyrin foil. Chem. Commun. 2017, 53, 10703–10706. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kubo, M.; Tachiki, M.; Mitogawa, T.; Saito, K.; Saito, R.; Tsukada, S.; Horiuchi, T.; Hoshino, K. Mechanistic Study on Gold-Like Luster Development of Solution-Cast Oligo(3-methoxythiophene) Film. Coatings 2021, 11, 861. https://doi.org/10.3390/coatings11070861
Kubo M, Tachiki M, Mitogawa T, Saito K, Saito R, Tsukada S, Horiuchi T, Hoshino K. Mechanistic Study on Gold-Like Luster Development of Solution-Cast Oligo(3-methoxythiophene) Film. Coatings. 2021; 11(7):861. https://doi.org/10.3390/coatings11070861
Chicago/Turabian StyleKubo, Minako, Minako Tachiki, Terumasa Mitogawa, Kota Saito, Ryota Saito, Satoru Tsukada, Takahiko Horiuchi, and Katsuyoshi Hoshino. 2021. "Mechanistic Study on Gold-Like Luster Development of Solution-Cast Oligo(3-methoxythiophene) Film" Coatings 11, no. 7: 861. https://doi.org/10.3390/coatings11070861
APA StyleKubo, M., Tachiki, M., Mitogawa, T., Saito, K., Saito, R., Tsukada, S., Horiuchi, T., & Hoshino, K. (2021). Mechanistic Study on Gold-Like Luster Development of Solution-Cast Oligo(3-methoxythiophene) Film. Coatings, 11(7), 861. https://doi.org/10.3390/coatings11070861






