Ultrasonic Technique for Production of Nanoemulsions for Food Packaging Purposes: A Review Study
Abstract
1. Introduction
2. Search Method
3. Results and Discussion
3.1. Role of Essential Oils in Food Packaging
3.2. An Evolutionary Era for Emerging of Nanotechnology Based Approaches in FP
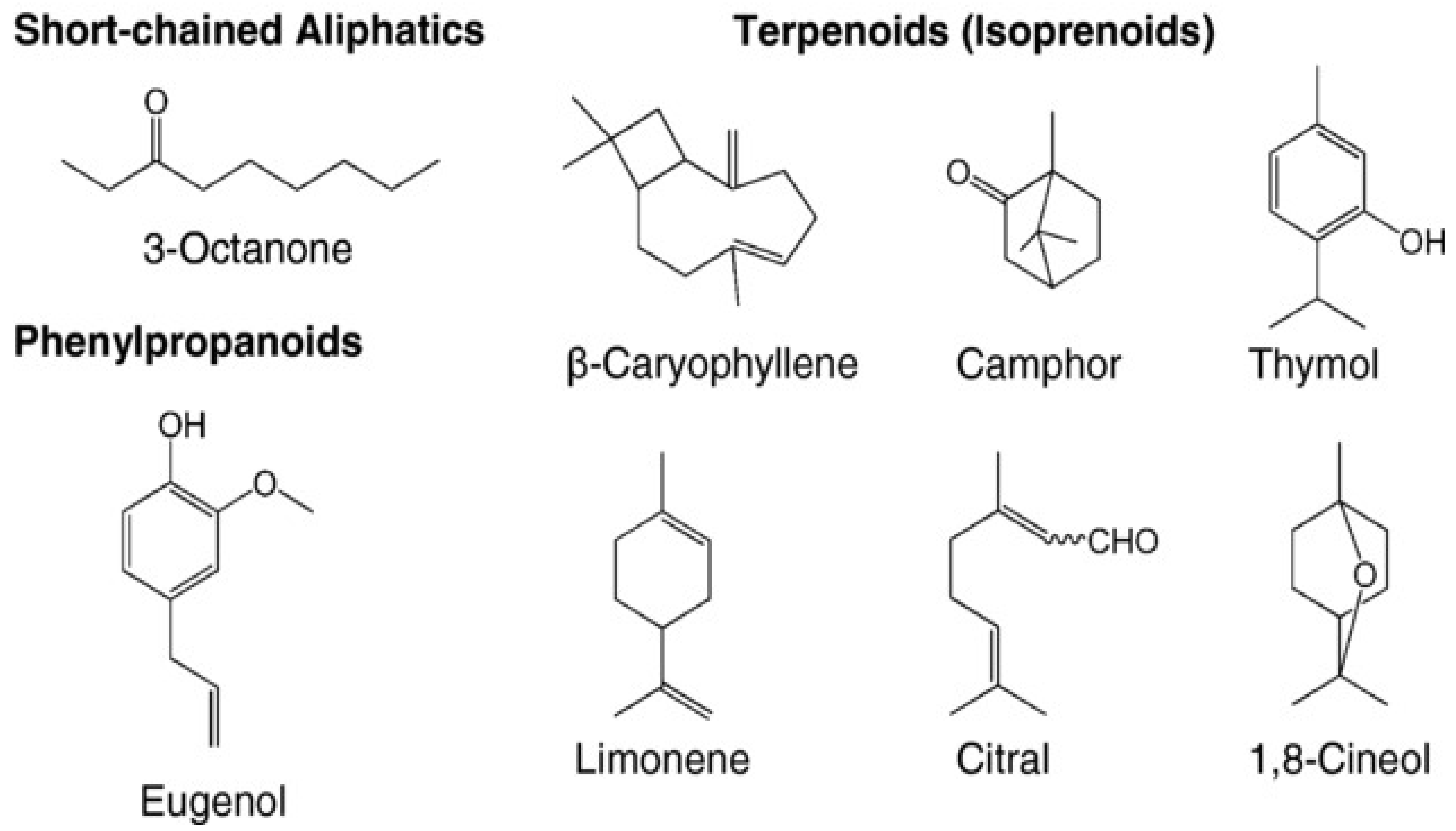
3.2.1. Employed Forms of Essential Oils in FP
3.2.2. Emergence of Nanoemulsions in Food Packaging
3.2.3. What Are Nanoemulsions?
3.3. An Introduction to Various Techniques Used for Preparation of Nanoemulsion in Food Packaging Purposes
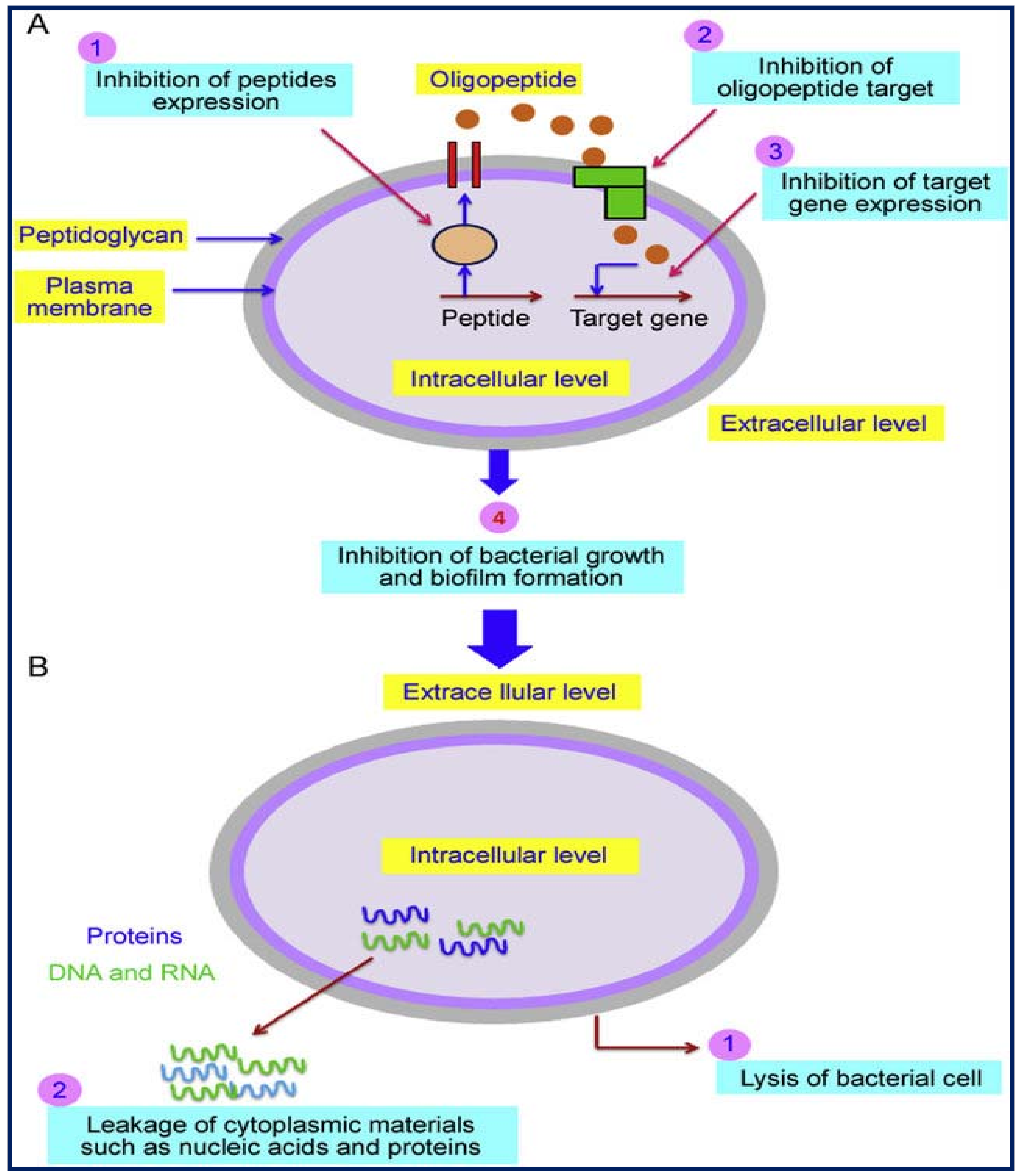
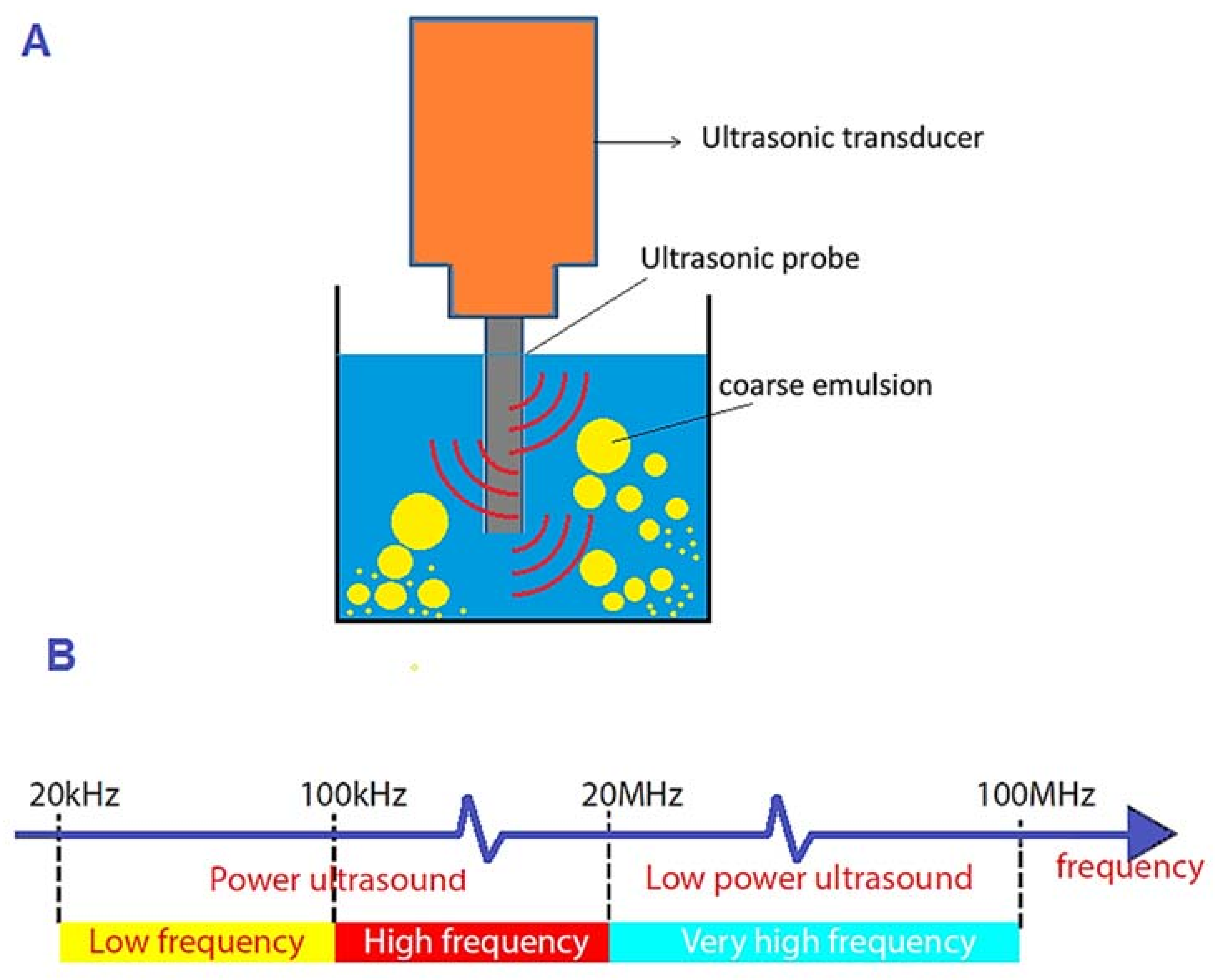
Emergence of Ultrasonic-Based Approaches as an Efficient Trend in Food Packaging
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Al-Tayyar, N.A.; Youssef, A.M.; Al-Hindi, R. Antimicrobial food packaging based on sustainable Bio-based materials for reducing foodborne Pathogens: A review. Food Chem. 2020, 310, 125915. [Google Scholar] [CrossRef] [PubMed]
- Altaf, U.; Kanojia, V.; Rouf, A. Novel packaging technology for food industry. J. Pharmacogn. Phytochem. 2018, 7, 1618–1625. [Google Scholar]
- Motelica, L.; Ficai, D.; Ficai, A.; Oprea, O.C.; Kaya, D.A.; Andronescu, E. Biodegradable Antimicrobial Food Packaging: Trends and Perspectives. Foods 2020, 9, 1438. [Google Scholar] [CrossRef] [PubMed]
- Hao, R.; Roy, K.; Pan, J.; Shah, B.R.; Mraz, J. Critical review on the use of essential oils against spoilage in chilled stored fish: A quantitative me-ta-analyses. Trends Food Sci. Technol. 2021. [Google Scholar] [CrossRef]
- Sharma, S.; Barkauskaite, S.; Jaiswal, A.K.; Jaiswal, S. Essential oils as additives in active food packaging. Food Chem. 2021, 343, 128403. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Jafari, S.M.; Sharma, S. Antimicrobial bio-nanocomposites and their potential applications in food packaging. Food Control 2020, 112, 107086. [Google Scholar] [CrossRef]
- Esmaili, F.; Sanei-Dehkordi, A.; Amoozegar, F.; Osanloo, M. A Review on the Use of Essential Oil-Based Nanoformulations in Control of Mosquitoes. Biointerface Res. Appl. Chem. 2021, 11, 12516–12529. [Google Scholar]
- Yazgan, H.; Ozogul, Y.; Durmuş, M.; Balikçi, E.; Gökdoğan, S.; Uçar, Y.; Aksun, E.T. Effects of oil-in-water nanoemulsion based on sunflower oil on the quality of farmed sea bass and gilthead sea bream stored at chilled temperature (2±2 C). J. Aquat. Food Prod. Technol. 2017, 26, 979–992. [Google Scholar] [CrossRef]
- Saifullah, M.; Ahsan, A.; Shishir, M.R.I. Production, Stability and Application of Micro-and Nanoemulsion in Food Production and the Food Processing Industry, in Emulsions; Elsevier: Amsterdam, The Netherlands, 2016; pp. 405–442. [Google Scholar]
- Lotfi, S.; Ahari, H.; Sahraeyan, R. The effect of silver nanocomposite packaging based on melt mixing and sol–gel methods on shelf life extension of fresh chicken stored at 4 °C. J. Food Saf. 2019, 39, e12625. [Google Scholar] [CrossRef]
- Majid, I.; Nayik, G.A.; Dar, S.M.; Nanda, V. Novel food packaging technologies: Innovations and future prospective. J. Saudi Soc. Agric. Sci. 2018, 17, 454–462. [Google Scholar] [CrossRef]
- Brandelli, A.; Brum, L.F.W.; dos Santos, J.H.Z. Nanobiotechnology Methods to Incorporate Bioactive Compounds in Food Packaging. In Sustainable Agriculture Reviews; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2016; pp. 27–58. [Google Scholar]
- Dávila-Rodríguez, M.; López-Malo, A.; Palou, E.; Ramírez-Corona, N.; Jiménez-Munguía, M.T. Essential oils microemulsions prepared with high-frequency ultrasound: Physical properties and antimicrobial activity. J. Food Sci. Technol. 2020, 57, 4133–4142. [Google Scholar] [CrossRef]
- Turan, D.; Gunes, G.; Kilic, A. Perspectives of Bio-nanocomposites for Food Packaging Applications. Bionanocomposites Packag. Appl. 2017, 1–32. [Google Scholar] [CrossRef]
- Hassanzad Azar, H.; Ghafari, A.; Yousefizadeh, S.; Fathollahi, M.; Aminzare, M. Antimicrobial Effects of the Nanoemulsion of Rosemary Essential Oil against Important Foodborne Pathogens. J. Hum. Environ. Health Promot. 2019, 5, 79–85. [Google Scholar]
- Ribeiro-Santos, R.; Andrade, M.; Sanches-Silva, A. Application of encapsulated essential oils as antimicrobial agents in food packaging. Curr. Opin. Food Sci. 2017, 14, 78–84. [Google Scholar] [CrossRef]
- Prakash, A.; Baskaran, R.; Paramasivam, N.; Vadivel, V. Essential oil based nanoemulsions to improve the microbial quality of minimally processed fruits and vegetables: A review. Food Res. Int. 2018, 111, 509–523. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, R.; Ahari, H.; Mahasti, P.; Paidari, S. Measuring the migration of silver from silver nanocomposite polyethylene packaging based on (TiO2) into Penaeus semisulcatus using titration comparison with migration methods. Fish. Sci. 2017, 83, 649–659. [Google Scholar] [CrossRef]
- Ahari, H.; Lahijani, L. Migration of Silver and Copper Nanoparticles from Food Coating. Coatings 2021, 11, 380. [Google Scholar] [CrossRef]
- Emamhadi, M.A.; Sarafraz, M.; Akbari, M.; Thai, V.N.; Fakhri, Y.; Linh, N.T.T.; Khaneghah, A.M. Nanomaterials for food packaging applications: A systematic review. Food Chem. Toxicol. 2020, 146, 111825. [Google Scholar] [CrossRef]
- Enayatifard, R.; Akbari, J.; Babaei, A.; Rostamkalaei, S.S.; Hashemi, S.M.H.; Habibi, E. Anti-Microbial Potential of Nano-Emulsion form of Essential Oil Obtained from Aerial Parts of Origanum Vulgare, L. as Food Additive. Adv. Pharm. Bull. 2020, 11, 327. [Google Scholar]
- Ozogul, Y.; Boğa, E.K.; Akyol, I.; Durmus, M.; Uçar, Y.; Regenstein, J.M.; Kosker, A.R. Antimicrobial activity of thyme essential oil nanoemulsions on spoilage bacteria of fish and food-borne pathogens. Food Biosci. 2020, 36, 100635. [Google Scholar] [CrossRef]
- Patra, J.K.; Baek, K.-H. Anti-Listerial Activity of Four Seaweed Essential Oils Against Listeria monocytogenes. Jundishapur J. Microbiol. 2016, 9, e31784. [Google Scholar] [CrossRef]
- Santos, M.I.; Martins, S.R.; Veríssimo, C.S.C.; Nunes, M.J.C.; Lima, A.I.G.; Ferreira, R.B.; Pedroso, L.; Sousa, I.; Ferreira, M.A.S.S. Essential oils as antibacterial agents against food-borne pathogens: Are they really as useful as they are claimed to be? J. Food Sci. Technol. 2017, 54, 4344–4352. [Google Scholar] [CrossRef]
- Shadman, S.; Hosseini, S.E.; Langroudi, H.E.; Shabani, S. Evaluation of the effect of a sunflower oil-based nanoemulsion with Zataria multiflora Boiss. essential oil on the physicochemical properties of rainbow trout (Oncorhynchus mykiss) fillets during cold storage. LWT 2017, 79, 511–517. [Google Scholar] [CrossRef]
- Sahraneshin Samani, S.; Soleimanian-Zad, S.; Sheikh-Zeinoddin, M.; Fathi, M. Evaluation of Zataria multiflora Boiss. and Carum copticum L. Essential Oil Based Nanoemulsions in Inhibition of Byssochlamys fulva Growth in Apple Juice. J. Agric. Sci. Technol. 2019, 21, 357–368. [Google Scholar]
- Salem, M.A.; Ezzat, M. Nanoemulsions in food industry. Some New Asp. Colloid. Syst. Foods 2019, 2, 238–267. [Google Scholar]
- Schaefer, D.; Cheung, W. Smart Packaging: Opportunities and Challenges. Procedia CIRP 2018, 72, 1022–1027. [Google Scholar] [CrossRef]
- Brockgreitens, J.; Abbas, A. Responsive Food Packaging: Recent Progress and Technological Prospects. Compr. Rev. Food Sci. Food Saf. 2015, 15, 3–15. [Google Scholar] [CrossRef]
- Wyser, Y.; Adams, M.; Avella, M.; Carlander, D.; Garcia, L.; Pieper, G.; Rennen, M.; Schuermans, J.; Weiss, J. Outlook and Challenges of Nanotechnologies for Food Packaging. Packag. Technol. Sci. 2016, 29, 615–648. [Google Scholar] [CrossRef]
- Rehman, A.; Jafari, S.M.; Aadil, R.M.; Assadpour, E.; Randhawa, M.A.; Mahmood, S. Development of active food packaging via incorporation of biopolymeric nanocarriers containing essential oils. Trends Food Sci. Technol. 2020, 101, 106–121. [Google Scholar] [CrossRef]
- Nasiri, M.; Ahari, H.; Sharifan, A.; Anvar, A.A.; Kakolaki, S. Nanoemulsion production techniques upgrade bioactivity potential of nanoemulsified essential oils on Acipenser stellatus filet preserving. Int. J. Food Prop. 2020, 23, 2174–2188. [Google Scholar] [CrossRef]
- Dávila-Rodríguez, M.; López-Malo, A.; Palou, E.; Ramírez-Corona, N.; Jiménez-Munguía, M.T. Antimicrobial activity of nanoemulsions of cinnamon, rosemary, and oregano essential oils on fresh celery. LWT 2019, 112, 108247. [Google Scholar] [CrossRef]
- Bajpai, V.K.; Baek, K.-H.; Kang, S.C. Control of Salmonella in foods by using essential oils: A review. Food Res. Int. 2012, 45, 722–734. [Google Scholar] [CrossRef]
- Dasgupta, N.; Ranjan, S.; Gandhi, M. Nanoemulsions in food: Market demand. Environ. Chem. Lett. 2019, 17, 1003–1009. [Google Scholar] [CrossRef]
- Petkoska, A.T.; Daniloski, D.; D’Cunha, N.M.; Naumovski, N.; Broach, A.T. Edible packaging: Sustainable solutions and novel trends in food packaging. Food Res. Int. 2021, 140, 109981. [Google Scholar] [CrossRef]
- Donsì, F.; Ferrari, G. Essential oil nanoemulsions as antimicrobial agents in food. J. Biotechnol. 2016, 233, 106–120. [Google Scholar] [CrossRef] [PubMed]
- Jugreet, B.S.; Suroowan, S.; Rengasamy, R.K.; Mahomoodally, M.F. Chemistry, bioactivities, mode of action and industrial applications of essential oils. Trends Food Sci. Technol. 2020, 101, 89–105. [Google Scholar] [CrossRef]
- Bhalla, Y.; Gupta, V.K.; Jaitak, V. Anticancer activity of essential oils: A review. J. Sci. Food Agric. 2013, 93, 3643–3653. [Google Scholar] [CrossRef] [PubMed]
- Venkadesaperumal, G.; Rucha, S.; Sundar, K.; Shetty, P.H. Anti-quorum sensing activity of spice oil nanoemulsions against food borne pathogens. LWT 2016, 66, 225–231. [Google Scholar] [CrossRef]
- Pavoni, L.; Perinelli, D.R.; Bonacucina, G.; Cespi, M.; Palmieri, G.F. An overview of micro-and nanoemulsions as vehicles for essential oils: Formulation, preparation and sta-bility. Nanomaterials 2020, 10, 135. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; McLandsborough, L.; McClements, D.J. Physicochemical Properties and Antimicrobial Efficacy of Carvacrol Nanoemulsions Formed by Spontaneous Emulsification. J. Agric. Food Chem. 2013, 61, 8906–8913. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, V.; Mukherjee, A.; Chandrasekaran, N. Eugenol-loaded antimicrobial nanoemulsion preserves fruit juice against, microbi-al spoilage. Colloids Surf. B 2014, 114, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, A.; Coimbra, M.; Vicente, A. In vitro behaviour of curcumin nanoemulsions stabilized by biopolymer emulsifiers—Effect of interfacial composition. Food Hydrocoll. 2015, 52, 460–467. [Google Scholar] [CrossRef]
- Hilbig, J.; Ma, Q.; Davidson, P.M.; Weiss, J.; Zhong, Q. Physical and antimicrobial properties of cinnamon bark oil co-nanoemulsified by lauric arginate and Tween 80. Int. J. Food Microbiol. 2016, 233, 52–59. [Google Scholar] [CrossRef]
- Moraes-Lovison, M.; Marostegan, L.F.; Peres, M.S.; Menezes, I.F.; Ghiraldi, M.; Rodrigues, R.A.; Fernandes, A.; Pinho, S.C. Nanoemulsions encapsulating oregano essential oil: Production, stability, antibacterial activity and incorporation in chicken pâté. LWT 2017, 77, 233–240. [Google Scholar] [CrossRef]
- Noori, S.; Zeynali, F.; Almasi, H. Antimicrobial and antioxidant efficiency of nanoemulsion-based edible coating containing ginger (Zingiber officinale) essential oil and its effect on safety and quality attributes of chicken breast fillets. Food Control 2018, 84, 312–320. [Google Scholar] [CrossRef]
- Abdou, E.S.; Galhoum, G.F.; Mohamed, E.N. Curcumin loaded nanoemulsions/pectin coatings for refrigerated chicken fillets. Food Hydrocoll. 2018, 83, 445–453. [Google Scholar] [CrossRef]
- Prakash, A.; Vadivel, V.; Rubini, D.; Nithyanand, P. Antibacterial and antibiofilm activities of linalool nanoemulsions against Salmonella Typhimurium. Food Biosci. 2019, 28, 57–65. [Google Scholar] [CrossRef]
- Ed-Dra, A.; Nalbone, L.; Filali, F.R.; Trabelsi, N.; El Majdoub, Y.O.; Bouchrif, B.; Giarratana, F.; Giuffrida, A. Comprehensive Evaluation on the Use of Thymus vulgaris Essential Oil as Natural Additive against Different Serotypes of Salmonella enterica. Sustainability 2021, 13, 4594. [Google Scholar] [CrossRef]
- Chaudhari, A.K.; Singh, V.K.; Das, S.; Prasad, J.; Dwivedy, A.K.; Dubey, N.K. Improvement of in vitro and in situ antifungal, AFB1 inhibitory and antioxidant activity of Origanum majorana L. essential oil through nanoemulsion and recommending as novel food preservative. Food Chem. Toxicol. 2020, 143, 111536. [Google Scholar] [CrossRef]
- Chu, Y.; Gao, C.; Liu, X.; Zhang, N.; Xu, T.; Feng, X.; Yang, Y.; Shen, X.; Tang, X. Improvement of storage quality of strawberries by pullulan coatings incorporated with cinnamon essential oil nanoemulsion. LWT 2020, 122, 109054. [Google Scholar] [CrossRef]
- Khoshbouy Lahidjani, L.; Ahari, H.; Sharifan, A. Influence of curcumin-loaded nanoemulsion fabricated through emul-sion phase inversion on the shelf life of Oncorhynchus mykiss stored at 4 °C. J. Food Process. Preserv. 2020, 44, e14592. [Google Scholar] [CrossRef]
- Xiong, Y.; Li, S.; Warner, R.D.; Fang, Z. Effect of oregano essential oil and resveratrol nanoemulsion loaded pectin edible coating on the preserva-tion of pork loin in modified atmosphere packaging. Food Control 2020, 114, 107226. [Google Scholar] [CrossRef]
- Chu, Y.; Tang, X. Fabrication, structure and properties of pullulan-based active films incorporated with ultra-sound-assisted cinnamon essential oil nanoemulsions. Food Packag. Shelf Life 2020, 25, 100547. [Google Scholar] [CrossRef]
- Liew, S.N.; Utra, U.; Alias, A.K.; Tan, T.B.; Tan, C.P.; Yussof, N.S. Physical, morphological and antibacterial properties of lime essential oil nanoemulsions prepared via spontaneous emulsification method. LWT 2020, 128, 109388. [Google Scholar] [CrossRef]
- Garzoli, S.; Petralito, S.; Ovidi, E.; Turchetti, G.; Masci, V.L.; Tiezzi, A.; Trilli, J.; Cesa, S.; Casadei, M.A.; Giacomello, P.; et al. Lavandula x intermedia essential oil and hydrolate: Evaluation of chemical composition and antibacterial activity before and after formulation in nanoemulsion. Ind. Crop. Prod. 2020, 145, 112068. [Google Scholar] [CrossRef]
- Shokri, S.; Parastouei, K.; Taghdir, M.; Abbaszadeh, S. Application an edible active coating based on chitosan- Ferulago angulata essential oil nanoemulsion to shelf life extension of Rainbow trout fillets stored at 4 °C. Int. J. Biol. Macromol. 2020, 153, 846–854. [Google Scholar] [CrossRef] [PubMed]
- Saada, N.S.; Abdel-Maksoud, G.; El-Aziz, M.A.; Youssef, A. Evaluation and utilization of lemongrass oil nanoemulsion for disinfection of documentary heritage based on parchment. Biocatal. Agric. Biotechnol. 2020, 29, 101839. [Google Scholar] [CrossRef]
- Yazgan, H. Investigation of antimicrobial properties of sage essential oil and its nanoemulsion as antimicrobial agent. LWT 2020, 130, 109669. [Google Scholar] [CrossRef]
- Prakash, A.; Baskaran, R.; Vadivel, V. Citral nanoemulsion incorporated edible coating to extend the shelf life of fresh cut pineapples. LWT 2020, 118, 108851. [Google Scholar] [CrossRef]
- Liu, Q.; Gao, Y.; Fu, X.; Chen, W.; Yang, J.; Chen, Z.; Wang, Z.; Zhuansun, X.; Feng, J.; Chen, Y. Preparation of peppermint oil nanoemulsions: Investigation of stability, antibacterial mechanism and apoptosis effects. Colloids Surf. B 2021, 201, 111626. [Google Scholar] [CrossRef]
- Cecchini, M.; Paoloni, C.; Campra, N.; Picco, N.; Grosso, M.; Perez, M.S.; Alustiza, F.; Cariddi, N.; Bellingeri, R. Nanoemulsion of Minthostachys verticillata essential oil. In-vitro evaluation of its antibacterial activity. Heliyon 2021, 7, e05896. [Google Scholar] [CrossRef]
- Carpena, M.; Nuñez-Estevez, B.; Soria-Lopez, A.; Garcia-Oliveira, P.; Prieto, M.A. Essential Oils and Their Application on Active Packaging Systems: A Review. Resources 2021, 10, 7. [Google Scholar] [CrossRef]
- Bouyahya, A.; Abrini, J.; Dakka, N.; Bakri, Y. Essential oils of Origanum compactum increase membrane permeability, disturb cell membrane integrity, and suppress quorum-sensing phenotype in bacteria. J. Pharm. Anal. 2019, 9, 301–311. [Google Scholar] [CrossRef]
- Atarés, L.; Chiralt, A. Essential oils as additives in biodegradable films and coatings for active food packaging. Trends Food Sci. Technol. 2016, 48, 51–62. [Google Scholar] [CrossRef]
- Fahmy, H.M.; Eldin, R.E.S.; Abu Serea, E.S.; Gomaa, N.M.; Aboelmagd, G.M.; Salem, S.A.; Elsayed, Z.A.; Edrees, A.; Shams-Eldin, E.; Shalan, A.E. Advances in nanotechnology and antibacterial properties of biodegradable food packaging materials. RSC Adv. 2020, 10, 20467–20484. [Google Scholar] [CrossRef]
- Dhifi, W.; Bellili, S.; Jazi, S.; Bahloul, N.; Mnif, W. Essential Oils’ Chemical Characterization and Investigation of Some Biological Activities: A Critical Review. Medicines 2016, 3, 25. [Google Scholar] [CrossRef] [PubMed]
- Ahari, H.; Massoud, R. The Effect of Cuminum Essential Oil on Rheological Properties and Shelf Life of Probiotic Yoghurt. J. Nutr. Food Secur. 2020, 5, 296–305. [Google Scholar] [CrossRef]
- Pandey, A.K.; Kumar, P.; Singh, P.; Tripathi, N.N.; Bajpai, V.K. Essential Oils: Sources of Antimicrobials and Food Preservatives. Front. Microbiol. 2017, 7, 2161. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.; Öztürk, B. Utilization of Nanotechnology to Improve the Handling, Storage and Biocompatibility of Bioactive Lipids in Food Applications. Foods 2021, 10, 365. [Google Scholar] [CrossRef] [PubMed]
- Pathania, R.; Khan, H.; Kaushik, R.; Khan, M.A. Essential Oil Nanoemulsions and their Antimicrobial and Food Applications. Curr. Res. Nutr. Food Sci. J. 2018, 6, 626–643. [Google Scholar] [CrossRef]
- Ferreira, C.D.; Nunes, I.L. Oil nanoencapsulation: Development, application, and incorporation into the food market. Nanoscale Res. Lett. 2019, 14, 1–13. [Google Scholar] [CrossRef]
- Tripathi, A.D.; Sharma, R.; Agarwal, A.; Haleem, R. Nanoemulsions based edible coatings with potential food applications. Int. J. Biobased Plast. 2021, 3, 112–125. [Google Scholar] [CrossRef]
- Zambrano-Zaragoza, M.L.; González-Reza, R.; Mendoza-Muñoz, N.; Miranda-Linares, V.; Bernal-Couoh, T.F.; Mendoza-Elvira, S.; Quintanar-Guerrero, D. Nanosystems in Edible Coatings: A Novel Strategy for Food Preservation. Int. J. Mol. Sci. 2018, 19, 705. [Google Scholar] [CrossRef]
- Kalateh Seifari, F.; Ahari, H. Active edible films and coatings with enhanced properties using nanoemulsion and nanocrystals. Food Health 2020, 3, 15–22. [Google Scholar]
- Souza, A.G.; Ferreira, R.R.; Paula, L.C.; Mitra, S.K.; Rosa, D.S. Starch-based films enriched with nanocellulose-stabilized Pickering emulsions containing different essential oils for possible applications in food packaging. Food Packag. Shelf Life 2021, 27, 100615. [Google Scholar] [CrossRef]
- Díaz-Montes, E.; Castro-Muñoz, R. Edible Films and Coatings as Food-Quality Preservers: An Overview. Foods 2021, 10, 249. [Google Scholar] [CrossRef] [PubMed]
- Hatziantoniou, S.; Deli, G.; Nikas, Y.; Demetzos, C.; Papaioannou, G. Scanning electron microscopy study on nanoemulsions and solid lipid nanoparticles containing high amounts of ceramides. Micron 2007, 38, 819–823. [Google Scholar] [CrossRef] [PubMed]
- Salvia Trujillo, L. Nanoemulsions as Delivery Systems of Food Ingredients: Improving Food Safety and Functionality. Ph.D. Thesis, Universitat de Lleida, Lleida, Spain, 2014. [Google Scholar]
- Li, W.; Chen, H.; He, Z.; Han, C.; Liu, S.; Li, Y. Influence of surfactant and oil composition on the stability and antibacterial activity of eugenol nanoemulsions. LWT-Food Sci. Technol. 2015, 62, 39–47. [Google Scholar] [CrossRef]
- Lahijani, L.K.; Ahari, H.; Sharifan, A. Enhancement of food safety using nanoemulsion with emphasize on fish food: A Review. Iran. J. Aquat. Anim. Health 2019, 5, 26–44. [Google Scholar] [CrossRef][Green Version]
- Fattahi, R.; Bahrami, A. Essential oils as a natural additive in the edible films and coatings (active packaging system): A Review. J. Babol Univ. Med. Sci. 2018, 20, 11–23. [Google Scholar]
- Iamareerat, B.; Singh, M.; Sadiq, M.B.; Anal, A.K. Reinforced cassava starch based edible film incorporated with essential oil and sodium bentonite nanoclay as food packaging material. J. Food Sci. Technol. 2018, 55, 1953–1959. [Google Scholar] [CrossRef] [PubMed]
- Chawla, R.; Sivakumar, S.; Kaur, H. Antimicrobial edible films in food packaging: Current scenario and recent nanotechnological advancements-a review. Carbohydr. Polym. Technol. Appl. 2021, 2, 100024. [Google Scholar]
- Anis, A.; Pal, K.; Al-Zahrani, S.M. Essential Oil-Containing Polysaccharide-Based Edible Films and Coatings for Food Security Applications. Polymers 2021, 13, 575. [Google Scholar] [CrossRef]
- Du, W.-X.; Avena-Bustillos, R.J.; Hua, S.S.T.; McHugh, T.H. Antimicrobial volatile essential oils in edible films for food safety. Sci. Against Microb. Pathog. 2011, 2, 1124–1134. [Google Scholar]
- Artés-Hernández, F.; Martínez-Hernández, E.A.G.B.; Aguayo, E.; Gómez, P.A.; Artés, F. Fresh-Cut Fruit and Vegetables: Emerging Eco-friendly Techniques for Sanitation and Preserving Safety. Postharvest Handl. 2017, 7–45. [Google Scholar] [CrossRef]
- Hasan, S.M.K.; Ferrentino, G.; Scampicchio, M. Nanoemulsion as advanced edible coatings to preserve the quality of fresh-cut fruits and vegetables: A review. Int. J. Food Sci. Technol. 2019, 55, 1–10. [Google Scholar] [CrossRef]
- Azadbakht, E.; Maghsoudlou, Y.; Khomiri, M.; Kashiri, M. Development and structural characterization of chitosan films containing Eucalyptus globulus essential oil: Potential as an antimicrobial carrier for packaging of sliced sausage. Food Packag. Shelf Life 2018, 17, 65–72. [Google Scholar] [CrossRef]
- Perdones, Á.; Escriche, I.; Chiralt, A.; Vargas, M. Effect of chitosan–lemon essential oil coatings on volatile profile of strawberries during storage. Food Chem. 2016, 197, 979–986. [Google Scholar] [CrossRef]
- Pandey, A.K.; Chávez-González, M.L.; Silva, A.S.; Singh, P. Essential oils from the genus Thymus as antimicrobial food preservatives: Progress in their use as nanoemulsions—A new paradigm. Trends Food Sci. Technol. 2021, 111, 426–441. [Google Scholar] [CrossRef]
- Chaudhari, A.K.; Singh, V.K.; Das, S.; Dubey, N.K. Nanoencapsulation of essential oils and their bioactive constituents: A novel strategy to control mycotoxin contamination in food system. Food Chem. Toxicol. 2021, 149, 112019. [Google Scholar] [CrossRef]
- Bedoya-Serna, C.M.; Dacanal, G.C.; Fernandes, A.; Pinho, S.C. Antifungal activity of nanoemulsions encapsulating oregano (Origanum vulgare) essential oil: In vitro study and application in Minas Padrão cheese. Braz. J. Microbiol. 2018, 49, 929–935. [Google Scholar] [CrossRef]
- Sharma, S.; Barkauskaite, S.; Duffy, B.; Jaiswal, A.K.; Jaiswal, S. Characterization and Antimicrobial Activity of Biodegradable Active Packaging Enriched with Clove and Thyme Essential Oil for Food Packaging Application. Foods 2020, 9, 1117. [Google Scholar] [CrossRef]
- Azmi, N.A.N.; Elgharbawy, A.A.M.; Motlagh, S.R.; Samsudin, N.; Salleh, H.M. Nanoemulsions: Factory for Food, Pharmaceutical and Cosmetics. Processes 2019, 7, 617. [Google Scholar] [CrossRef]
- Pradhan, N.; Singh, S.; Ojha, N.; Shrivastava, A.; Barla, A.; Rai, V.; Bose, S. Facets of Nanotechnology as Seen in Food Processing, Packaging, and Preservation Industry. BioMed Res. Int. 2015, 2015, 1–17. [Google Scholar] [CrossRef]
- Dasgupta, N.; Ranjan, S. An Introduction to Food Grade Nanoemulsions; Springer Science and Business Media LLC.: Berlin/Heidelberg, Germany, 2018; Volume 13. [Google Scholar]
- Walker, R.; Decker, E.A.; McClements, D.J. Development of food-grade nanoemulsions and emulsions for delivery of omega-3 fatty acids: Opportunities and obstacles in the food industry. Food Funct. 2014, 6, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Yalçınöz, Ş.; Erçelebi, E. Potential applications of nano-emulsions in the food systems: An update. Mater. Res. Express 2018, 5, 062001. [Google Scholar] [CrossRef]
- Mei, L.; Wang, Q. Advances in Using Nanotechnology Structuring Approaches for Improving Food Packaging. Annu. Rev. Food Sci. Technol. 2020, 11, 339–364. [Google Scholar] [CrossRef] [PubMed]
- Zambrano-Zaragoza, M.L.; Quintanar-Guerrero, D.; Mendoza-Muñoz, N.; Leyva-Gómez, G. Nanoemulsions and nanosized ingredients for food formulations. In Handbook of Food Nanotechnology; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Espitia, P.J.P.; Fuenmayor, C.A.; Otoni, C.G. Nanoemulsions: Synthesis, Characterization, and Application in Bio-Based Active Food Packaging. Compr. Rev. Food Sci. Food Saf. 2018, 18, 264–285. [Google Scholar] [CrossRef] [PubMed]
- Aswathanarayan, J.B.; Vittal, R.R. Nanoemulsions and Their Potential Applications in Food Industry. Front. Sustain. Food Syst. 2019, 3, 95. [Google Scholar] [CrossRef]
- Liu, Q.; Huang, H.; Chen, H.; Lin, J.; Wang, Q. Food-Grade Nanoemulsions: Preparation, Stability and Application in Encapsulation of Bioactive Compounds. Molecules 2019, 24, 4242. [Google Scholar] [CrossRef] [PubMed]
- Tabibiazar, M.; Hamishehkar, H. Formulation of a Food Grade Water-In-Oil Nanoemulsion: Factors Affecting on Stability. Pharm. Sci. 2015, 21, 220–224. [Google Scholar] [CrossRef]
- Silva, H.D.; Cerqueira, M.Â.; Vicente, A.A. Nanoemulsions for Food Applications: Development and Characterization. Food Bioprocess Technol. 2012, 5, 854–867. [Google Scholar] [CrossRef]
- McClements, D.J.; Rao, J. Food-Grade Nanoemulsions: Formulation, Fabrication, Properties, Performance, Biological Fate, and Potential Toxicity. Crit. Rev. Food Sci. Nutr. 2011, 51, 285–330. [Google Scholar] [CrossRef]
- Mehmood, T.; Ahmad, A.; Ahmed, A.; Ahmed, Z. Optimization of olive oil based O/W nanoemulsions prepared through ultrasonic homogenization: A response surface methodology approach. Food Chem. 2017, 229, 790–796. [Google Scholar] [CrossRef] [PubMed]
- Singh, N. An overview of the prospective application of nanoemulsions in foodstuffs and food packaging. ASIO J. Microbiol. Food Sci. Biotechnol. Innova. 2015, 1, 20–25. [Google Scholar]
- Cardoso-Ugarte, G.A.; López-Malo, A.; Jiménez-Munguía, M.T. Application of nanoemulsion technology for encapsulation and release of lipophilic bioactive compounds in food. In Emulsions; Elsevier: Amsterdam, The Netherlands, 2016; pp. 227–255. [Google Scholar]
- Mustafa, F.; Andreescu, S. Nanotechnology-based approaches for food sensing and packaging applications. RSC Adv. 2020, 10, 19309–19336. [Google Scholar] [CrossRef]
- de Jesus Cenobio-Galindo, A.; Campos-Montiel, R.G.; Jiménez-Alvarado, R.; Almaraz-Buendía, I.; Medina-Pérez, G.; Fernández-Luqueño, F. Development and incorporation of nanoemulsions in food. Int. J. Food Stud. 2019, 8. [Google Scholar] [CrossRef]
- Bahrami, A.; Delshadi, R.; Assadpour, E.; Jafari, S.M.; Williams, L. Antimicrobial-loaded nanocarriers for food packaging applications. Adv. Colloid Interface Sci. 2020, 278, 102140. [Google Scholar] [CrossRef]
- Bernardi, D.S.; Pereira, T.A.; Maciel, N.R.; Bortoloto, J.; Viera, G.S.; Oliveira, G.C.; Rocha-Filho, P.A. Formation and stability of oil-in-water nanoemulsions containing rice bran oil: In vitro and in vivo assessments. J. Nanobiotechnol. 2011, 9, 1–9. [Google Scholar] [CrossRef]
- Ozogul, Y.; Yuvka, I.; Uçar, Y.; Durmus, M.; Kosker, A.R.; Öz, M.; Ozogul, F. Evaluation of effects of nanoemulsion based on herb essential oils (rosemary, laurel, thyme and sage) on sensory, chemical and microbiological quality of rainbow trout (Oncorhynchus mykiss) fillets during ice storage. LWT 2017, 75, 677–684. [Google Scholar] [CrossRef]
- Farshbaf-Sadigh, A.; Jafarizadeh-Malmiri, H.; Anarjan, N.; Najian, Y. Preparation of Ginger Oil in Water Nanoemulsion Using Phase Inversion Composition Technique: Effects of Stirring and Water Addition Rates on their Physico-Chemical Properties and Stability. Z. Für Phys. Chem. 2019, 1. ahead-of-print. [Google Scholar] [CrossRef]
- Nile, S.H.; Baskar, V.; Selvaraj, D.; Nile, A.; Xiao, J.; Kai, G. Nanotechnologies in Food Science: Applications, Recent Trends, and Future Perspectives. Nano-Micro Lett. 2020, 12, 1–34. [Google Scholar] [CrossRef]
- Hosseinialhashemi, M.; Tavakoli, J.; Rafati, A.; Ahmadi, F. The aplication of Pistacia khinjuk extract nanoemulsion in a biopolymeric coating to improve the shelf life extension of sunflower oil. Food Sci. Nutr. 2021, 9, 920–928. [Google Scholar] [CrossRef]
- Marchese, E.; D’Onofrio, N.; Balestrieri, M.L.; Castaldo, D.; Ferrari, G.; Donsì, F. Bergamot essential oil nanoemulsions: Antimicrobial and cytotoxic activity. Z. Für Nat. C 2020, 75, 279–290. [Google Scholar] [CrossRef]
- Pinelli, J.J.; de Abreu Martins, H.H.; Guimarães, A.S.; Isidoro, S.R.; Gonçalves, M.C.; de Moraes, T.S.J. Essential oil nanoemulsions for the control of Clostridium sporogenes in cooked meat product: An alternative? LWT 2021, 143, 111123. [Google Scholar] [CrossRef]
- Özogul, Y.; Özogul, F.; Kulawik, P. The antimicrobial effect of grapefruit peel essential oil and its nanoemulsion on fish spoilage bacteria and food-borne pathogens. LWT 2021, 136, 110362. [Google Scholar] [CrossRef]
- Kumar, M.; Bishnoi, R.S.; Shukla, A.K.; Jain, C.P. Techniques for Formulation of Nanoemulsion Drug Delivery System: A Review. Prev. Nutr. Food Sci. 2019, 24, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Nasiri, M.; Sharifan, A.; Ahari, H.; Anvar, A.A.; Kakoolaki, S. Food-grade nanoemulsions and their fabrication methods to increase shelf life. Food Health 2019, 2, 26–31. [Google Scholar]
- Klang, V.; Matsko, N.B.; Valenta, C.; Hofer, F. Electron microscopy of nanoemulsions: An essential tool for characterisation and stability assessment. Micron 2012, 43, 85–103. [Google Scholar] [CrossRef]
- Ho, T.M.; Abik, F.; Mikkonen, K.S. An overview of nanoemulsion characterization via atomic force microscopy. Crit. Rev. Food Sci. Nutr. 2021, 1–21. [Google Scholar] [CrossRef]
- Galvão, K.C.S.; Vicente, A.A.; Sobral, P.J.A. Development, Characterization, and Stability of O/W Pepper Nanoemulsions Produced by High-Pressure Homogenization. Food Bioprocess Technol. 2017, 11, 355–367. [Google Scholar] [CrossRef]
- Keykhasalar, R.; Tabrizi, M.H.; Ardalan, P. Antioxidant Property and Bactericidal Activity of Linum usitatis-simum Seed Essential Oil Nanoemulsion (LSEO-NE) on Staphylococcus aureus. Int. J. Infect. 2020, 7, e101639. [Google Scholar] [CrossRef]
- Ban, Z.; Zhang, J.; Li, L.; Luo, Z.; Wang, Y.; Yuan, Q.; Zhou, B.; Liu, H. Ginger essential oil-based microencapsulation as an efficient delivery system for the improvement of Jujube (Ziziphus jujuba Mill.) fruit quality. Food Chem. 2020, 306, 125628. [Google Scholar] [CrossRef] [PubMed]
- Gallo, M.; Ferrara, L.; Naviglio, D. Application of Ultrasound in Food Science and Technology: A Perspective. Foods 2018, 7, 164. [Google Scholar] [CrossRef]
- Abbas, S.; Hayat, K.; Karangwa, E.; Bashari, M.; Zhang, X. An Overview of Ultrasound-Assisted Food-Grade Nanoemulsions. Food Eng. Rev. 2013, 5, 139–157. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, M.; Yang, C.-H. Application of ultrasound technology in processing of ready-to-eat fresh food: A review. Ultrason. Sonochem. 2020, 63, 104953. [Google Scholar] [CrossRef] [PubMed]
- Alarcon-Rojo, A.D.; Carrillo-Lopez, L.M.; Reyes-Villagrana, R.; Huerta-Jiménez, M.; Garcia-Galicia, I.A. Ultrasound and meat quality: A review. Ultrason. Sonochem. 2019, 55, 369–382. [Google Scholar] [CrossRef] [PubMed]
- Chemat, F.; Khan, M.K. Applications of ultrasound in food technology: Processing, preservation and extraction. Ultrason. Sonochem. 2011, 18, 813–835. [Google Scholar] [CrossRef]
- Majid, I.; Nayik, G.A.; Nanda, V. Ultrasonication and food technology: A review. Cogent Food Agric. 2015, 1, 1071022. [Google Scholar] [CrossRef]
- Ashokkumar, M. Applications of ultrasound in food and bioprocessing. Ultrason. Sonochem. 2015, 25, 17–23. [Google Scholar] [CrossRef]
- Awad, T.; Moharram, H.; Shaltout, O.; Asker, D.; Youssef, M. Applications of ultrasound in analysis, processing and quality control of food: A review. Food Res. Int. 2012, 48, 410–427. [Google Scholar] [CrossRef]
- Taha, A.; Ahmed, E.; Ismaiel, A.; Ashokkumar, M.; Xu, X.; Pan, S.; Hu, H. Ultrasonic emulsification: An overview on the preparation of different emulsifiers-stabilized emulsions. Trends Food Sci. Technol. 2020, 105, 363–377. [Google Scholar] [CrossRef]
- Paniwnyk, L. Applications of ultrasound in processing of liquid foods: A review. Ultrason. Sonochem. 2017, 38, 794–806. [Google Scholar] [CrossRef]
- Hardainiyan, S. A Review on Nanoemulsions in Food Applications. Insights Aquacult. Biotechnol. 2018, 2. [Google Scholar]
- Ashokkumar, M. The characterization of acoustic cavitation bubbles—An overview. Ultrason. Sonochem. 2011, 18, 864–872. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Zhao, W.; Yang, F.; Jiang, Q.; Xu, Y.; Xia, W. A strategy of ultrasound-assisted processing to improve the performance of bio-based coating preservation for refrigerated carp fillets (Ctenopharyngodon idellus). Food Chem. 2021, 345, 128862. [Google Scholar] [CrossRef]
- Bajerski, L.; Michels, L.R.; Colomé, L.M.; Bender, E.A.; Freddo, R.J.; Bruxel, F.; & Haas, S.E. The use of Brazilian vegetable oils in nanoemulsions: An update on preparation and biological applica-tions. Braz. J. Pharm. Sci. 2016, 52, 347–363. [Google Scholar] [CrossRef]
- Chandrapala, J.; Oliver, C.; Kentish, S.; Ashokkumar, M. Ultrasonics in food processing—Food quality assurance and food safety. Trends Food Sci. Technol. 2012, 26, 88–98. [Google Scholar] [CrossRef]
- Vilkhu, K.; Manasseh, R.; Mawson, R.; AshokKumar, M. Ultrasonic Recovery and Modification of Food Ingredients; Springer Science and Business Media LLC.: Berlin/Heidelberg, Germany, 2010; pp. 345–368. [Google Scholar]
- Teng, F.; He, M.; Xu, J.; Chen, F.; Wu, C.; Wang, Z.; Li, Y. Effect of ultrasonication on the stability and storage of a soy protein isolate-phosphatidylcholine nanoemul-sions. Sci. Rep. 2020, 10, 1–9. [Google Scholar]
- Moradi, S.; Barati, A. Essential Oils Nanoemulsions: Preparation, Characterization and Study of Antibacterial Activity against Escherichia Coli. Int. J. Nanosci. Nanotechnol. 2019, 15, 199–210. [Google Scholar]
- Aboutorab, M.; Ahari, H.; Allahyaribeik, S.; Yousefi, S.; Motalebi, A. Nano-emulsion of saffron essential oil by spontaneous emulsification and ultrasonic homogenization extend the shelf life of shrimp (Crocus sativus L.). J. Food Process. Preserv. 2021, 45, 15224. [Google Scholar] [CrossRef]
- Najaf Najafi, M.; Nemati, S.; Mohammadi Sani, A.; Kadkhodaee, R. Evaluation of physical properties and stability of water-in-oil-nanoemulsions containing saffron extract. Technol. Med. Aromatic Plants Iran 2020, 2, 12–24. [Google Scholar] [CrossRef]
- Hamed Ahari, A.A.; Beik, S.A.; Moradi, S.; Rahimian, M. Formulation of Saffron and a Method of Preparation Thereof. U.S. Patent 17/086763, 23 March 2021. [Google Scholar]
- Leong, T.S.H.; Manickam, S.; Martin, G.J.O.; Li, W.; Ashokkumar, M. Ultrasonic Production of Nano-Emulsions for Bioactive Delivery in Drug and Food Applications; Springer Science and Business Media LLC.: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Ahmadi, N.; Ahari, H. A Review of the Synthesis, Properties and Application of Nanoemulsions in the Packaging of Bioactive Foods. Sci. Q. J. Packag. Sci. Technol. Iran 2020, 11, 42–53. [Google Scholar]
- Liu, Q.; Zhang, M.; Bhandari, B.; Xu, J.; Yang, C. Effects of nanoemulsion-based active coatings with composite mixture of star anise essential oil, polylysine, and nisin on the quality and shelf life of ready-to-eat Yao meat products. Food Control. 2020, 107, 106771. [Google Scholar] [CrossRef]
- Meral, R.; Ceylan, Z.; Köse, Ş. Limitation of microbial spoilage of rainbow trout fillets using characterized thyme oil antibacterial nanoemulsions. J. Food Saf. 2019, 39, 12644. [Google Scholar] [CrossRef]
- Rashed, M.M.A.; Tong, Q.; Nagi, A.; Li, J.; Khan, N.U.; Chen, L.; Rotail, A.; Bakry, A.M. Isolation of essential oil from Lavandula angustifolia by using ultrasonic-microwave assisted method preceded by enzymolysis treatment, and assessment of its biological activities. Ind. Crop. Prod. 2017, 100, 236–245. [Google Scholar] [CrossRef]
- Salvia-Trujillo, L.; Rojas-Graü, A.; Soliva-Fortuny, R.; Martín-Belloso, O. Physicochemical Characterization of Lemongrass Essential Oil–Alginate Nanoemulsions: Effect of Ultrasound Processing Parameters. Food Bioprocess Technol. 2013, 6, 2439–2446. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, M.; Fang, Z.; Liu, Y. Preparation and characterization of blended cloves/cinnamon essential oil nanoemulsions. LWT 2017, 75, 316–322. [Google Scholar] [CrossRef]
- Ghosh, V.; Mukherjee, A.; Chandrasekaran, N. Ultrasonic emulsification of food-grade nanoemulsion formulation and evaluation of its bactericidal activity. Ultrason. Sonochem. 2013, 20, 338–344. [Google Scholar] [CrossRef]
- Gul, O.; Saricaoglu, F.T.; Besir, A.; Atalar, I.; Yazici, F. Effect of ultrasound treatment on the properties of nano-emulsion films obtained from hazelnut meal protein and clove essential oil. Ultrason. Sonochem. 2018, 41, 466–474. [Google Scholar] [CrossRef]
- Norcino, L.; Mendes, J.; Natarelli, C.; Manrich, A.; Oliveira, J.; Mattoso, L. Pectin films loaded with copaiba oil nanoemulsions for potential use as bio-based active packaging. Food Hydrocoll. 2020, 106, 105862. [Google Scholar] [CrossRef]
- Roy, A.; Guha, P. Formulation and characterization of betel leaf (Piper betleL.) essential oil based nanoemulsion and its in vitro antibacterial efficacy against selected food pathogens. J. Food Process. Preserv. 2018, 42, e13617. [Google Scholar] [CrossRef]
- Jo, Y.-J.; Chun, J.-Y.; Kwon, Y.-J.; Min, S.-G.; Hong, G.-P.; Choi, M.-J. Physical and antimicrobial properties of trans-cinnamaldehyde nanoemulsions in water melon juice. LWT 2015, 60, 444–451. [Google Scholar] [CrossRef]
- Abbas, S.; Bashari, M.; Akhtar, W.; Li, W.W.; Zhang, X. Process optimization of ultrasound-assisted curcumin nanoemulsions stabilized by OSA-modified starch. Ultrason. Sonochem. 2014, 21, 1265–1274. [Google Scholar] [CrossRef]
- Otoni, C.; Pontes, S.F.O.; Medeiros, E.A.A.; Soares, N.D.F.F. Edible Films from Methylcellulose and Nanoemulsions of Clove Bud (Syzygium aromaticum) and Oregano (Origanum vulgare) Essential Oils as Shelf Life Extenders for Sliced Bread. J. Agric. Food Chem. 2014, 62, 5214–5219. [Google Scholar] [CrossRef]
- Raji, F.; Khanzadi, S.; Hashemi, M.; Azizzadeh, M. Effect of Chitosan Coating Nano-emulsion Containing Zataria multiflora and Bunium persicum Essential Oils on Escherichia Coli O157:H7 in Vacuum-packed Rainbow Trout Fillet. J. Hum. Environ. Health Promot. 2019, 5, 21–25. [Google Scholar] [CrossRef]
- Alizadeh, Z.; Yousefi, S.; Ahari, H. Optimization of bioactive preservative coatings of starch nanocrystal and ultrasonic extract of sour lemon peel on chicken fillets. Int. J. Food Microbiol. 2019, 300, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Jesser, E.; Lorenzetti, A.; Yeguerman, C.; Murray, A.; Domini, C.; Werdin-González, J. Ultrasound assisted formation of essential oil nanoemulsions: Emerging alternative for Culex pipiens pipiens Say (Diptera: Culicidae) and Plodia interpunctella Hübner (Lepidoptera: Pyralidae) management. Ultrason. Sonochem. 2020, 61, 104832. [Google Scholar] [CrossRef]
- Abbasi, F.; Samadi, F.; Jafari, S.M.; Ramezanpour, S.; Shams-Shargh, M. Production of omega-3 fatty acid-enriched broiler chicken meat by the application of nanoencapsultsed flaxseed oil prepared via ultrasonication. J. Funct. Foods 2019, 57, 373–381. [Google Scholar] [CrossRef]
- Ceylan, Z.; Meral, R.; Kose, Y.E.; Cavidoglu, I. Wheat germ oil nanoemulsion for oil stability of the cooked fish fillets stored at 4 °C. J. Food Sci. Technol. 2019, 57, 1798–1806. [Google Scholar] [CrossRef] [PubMed]
- Rao, J.; McClements, D.J. Formation of flavor oil microemulsions, nanoemulsions and emulsions: Influence of composition and preparation method. J. Agric. Food Chem. 2011, 59, 5026–5035. [Google Scholar] [CrossRef] [PubMed]
- Sugumar, S.; Ghosh, V.; Nirmala, M.J.; Mukherjee, A.; Chandrasekaran, N. Ultrasonic emulsification of eucalyptus oil nanoemulsion: Antibacterial activity against Staphylococcus aureus and wound healing activity in Wistar rats. Ultrason. Sonochem. 2014, 21, 1044–1049. [Google Scholar] [CrossRef]
- Gahruie, H.H.; Ziaee, E.; Eskandari, M.H.; Hosseini, S.M.H. Characterization of basil seed gum-based edible films incorporated with Zataria multiflora essential oil nanoemulsion. Carbohydr. Polym. 2017, 166, 93–103. [Google Scholar] [CrossRef]
- Robledo, N.; Vera, P.; López, L.; Yazdani-Pedram, M.; Tapia, C.; Abugoch, L. Thymol nanoemulsions incorporated in quinoa protein/chitosan edible films; antifungal effect in cherry tomatoes. Food Chem. 2018, 246, 211–219. [Google Scholar] [CrossRef]
- Ghazy, O.; Fouad, M.T.; Saleh, H.H.; Kholif, A.E.; Morsy, T.A. Ultrasound-assisted preparation of anise extract nanoemulsion and its bioactivity against different pathogenic bacteria. Food Chem. 2021, 341, 128259. [Google Scholar] [CrossRef]
- He, Q.; Guo, M.; Jin, T.Z.; Arabi, S.A.; Liu, D. Ultrasound improves the decontamination effect of thyme essential oil nanoemulsions against Escherichia coli O157: H7 on cherry tomatoes. Int. J. Food Microbiol. 2021, 337, 108936. [Google Scholar] [CrossRef]
- Fan, K.; Zhang, M.; Jiang, F. Ultrasound treatment to modified atmospheric packaged fresh-cut cucumber: Influence on microbial inhibition and storage quality. Ultrason. Sonochem. 2019, 54, 162–170. [Google Scholar] [CrossRef]
- Khandpur, P.; Gogate, P.R. Evaluation of ultrasound based sterilization approaches in terms of shelf life and quality parameters of fruit and vegetable juices. Ultrason. Sonochem. 2016, 29, 337–353. [Google Scholar] [CrossRef]
- Özogul, Y.; Durmus, M.; Uçar, Y.; Ozogul, F.; Regenstein, J.M. Comparative study of nanoemulsions based on commercial oils (sunflower, canola, corn, olive, soybean, and hazelnut oils): Effect on microbial, sensory, and chemical qualities of refrigerated farmed sea bass. Innov. Food Sci. Emerg. Technol. 2016, 33, 422–430. [Google Scholar] [CrossRef]
- Marei, G.I.K.; Rabea, E.I.; Badawy, M.E.I. Ultrasonic Emulsification and Characterizations of Bio-based Nanoemulsion Formulations Containing Citral with Their Antimicrobial Activity. Egypt. Acad. J. Biol. Sci. F Toxicol. Pest Control 2017, 9, 169–182. [Google Scholar] [CrossRef]
- Ahari, H.A.; AmirAli; Rahimian, M.; Allahyaribeik, S.; Moradi, S. Water-in-Oil Nano-emulsion of Saffron and A Method of Preparing Therof. U.S. Patent 17/174340, 3 June 2021. [Google Scholar]

| Essential Oil | Technique | Emulsifier | Functions | Microorganism | Droplet Size (nm) | MIC/MBC/IZ * (ppm/ppm/mm) | Year [Ref.] |
|---|---|---|---|---|---|---|---|
| Carvacrol | Spontaneous Emulsification | Tween 20, 40, 60, 80, and 85 | Effect of interfacial composition | Zygosaccharomyces bailii, Saccharomyces cerevisiae, Brettanomyces bruxellensis, and Brettanomyces naardenensis | from >5000 to <25 nm | 625/-/- | 2013 [42] |
| Eugenol | Ultrasonic | Tween 80–Tween 20 | Anti-microbial activity | Staphylococcus aureus | 13 | - | 2014 [43] |
| Curcumin | Ultrasonic | Biopolymer emulsifiers (lactoferrin and lactoferrin/alginate multilayer structure) | Effect of interfacial composition | - | 149 | - | 2015 [44] |
| Cinnamon | Homogenizer | 3% Tween 80 | Antimicrobial activity | Salmonella enteritidis, Escherichia coli O157:H7, and Listeria monocytogenes | 100 | 400/600/- | 2016 [45] |
| Oregano | Phase Inversion Temperature method | Tween 80 | Anti-bacterial activity | Staphylococcus aureus, and Escherichia coli | 35–55 | 0.5–0.6/0.9–3.3/ | 2017 [46] |
| Ginger | Ultrasonic | Tween 80 | Anti-microbial and anti-oxidant efficiency | Salmonella Typhimurium, Listeria monocytogenes | 57–163 | -/-/12–15 | 2018 [47] |
| Curcumin-cinnamon-garlic | Homogenizer | 10% Tween 80 | Improve the structure, anti-bacterial and antioxidant properties of films | Psychrophilic bacteria, yeast and mold growth | 9–130 | - | 2018 [48] |
| Linalool | Ultrasonic | Tween 80 | Anti-bacterial and anti-biofilm activities | Salmonella Typhimurium | 11 | 60%/-/- | 2019 [49] |
| Thyme essential oil | Ultrasonic | 1% Tween 80 | Anti-microbial activity | Salmonella paratyphi, Staphylococcus aureus, Klebsiella pneumoniae, and Enterococcus. faecalis/Pseudomonas luteola, Photobacterium damselae, Vibrio vulnificus, Enterococcus faecalis, Serratia liquefaciens, and Proteus mirabilis | 448 | -/-/15–26 mm | 2020 [50] |
| Origanum majorana | Homogenizer | Tween 80 | Anti-fungal | Aflatoxin B1, Aspergillus fumigatus, Aspergillus luchuensis, Aspergillus niger, Penicillium chrysogenum, Penicillium italicum, Cladosporium cladosporioides, Fusarium poae, and Alternaria alternata | 32.65–52.38 | 2.5/-/- | 2020 [51] |
| Cinnamon | Ultrasound | Tween 80 | Anti-microbial activity | Bacteria and molds | 162 | - | 2020 [52] |
| Curcumin | Emulsion Phase Inversion | Span 80, Tween 80 | Anti-microbial activity | Escherichia coli, Staphylococcus aureus | 10–12 | - | 2020 [53] |
| Oregano | Homogenizer | Tween 80 and ethanol | Anti-microbial activity | Both Gram-positive and Gram-negative bacteria under TVC (Total Viable Count) test | 50 | TVC = 9.09 log CFU/g | 2020 [54] |
| Cinnamon | Ultrasonic | 6% Tween 80 | Improve the structure and anti-bacterial properties of films | Escherichia coli, Staphylococcus aureus under OD (Optical Density) measurement at 650 λ | 60 | OD = 0.2 | 2020 [55] |
| Lime | Magnetic stirring | 15% Tween 80 | Physical, morphological and anti-bacterial properties | Escherichia coli, Salmonella spp., and Staphylococcus aureus | 21 | -/-/8–9 | 2020 [56] |
| Lavandula x intermedia | Solvent displacement technique + magnetic stirring | - | Evaluation of chemical composition and anti-bacterial activity | Escherichia coli, Bacillus cereus | 187 | 0.01–0.37/0.02–0.37/- | 2020 [57] |
| Ferulago Angulata | Ultrasonic | Tween 80 | Anti-bacterial activities | Shewanella Putrefaciens, and Pseudomonas fluorescence | <100 | 5–15%/10–30%/- | 2020 [58] |
| Lemongrass | Low energy technique + magnetic stirring | Tween 20 | disinfection | Cladosporium xanthochromaticum, Byssochlamys spectabilis, Streptomyces albidoflavus | 275 | -/-/12–30 | 2020 [59] |
| Sage | Ultrasonic | Tween 80 | Anti-bacterial activities | Enterococcus faecalis, Klebsiella pneumonia, Salmonella paratyphi, Staphylococcus aureus, Proteus mirabilis, Photobacterium damselae, Vibrio vulnificus, Enterococcus faecalis, Pseudomonas luteola, and Serratia liquefaciens | 59 | 6.25–12.5/6.25/12–19 | 2020 [60] |
| Citral | Ultrasonic | Tween 80 | Anti-bacterial activities | Salmonella enterica, and Listeria monocytogenes | 66–131 | TVC = 4–5 log CFU/g | 2020 [61] |
| Peppermint | high speed shearing technology | 8% (Tween-60: EL-20 = 3:1) | Anti-bacterial activities | Escherichia coli, and Staphylococcus aureus | 50 | - | 2021 [62] |
| Minthostachys verticillata | homogenizer | Tween 20 | Anti-bacterial activity | Staphylococcus aureus | 10 | -/-/44.5% | 2021 [63] |
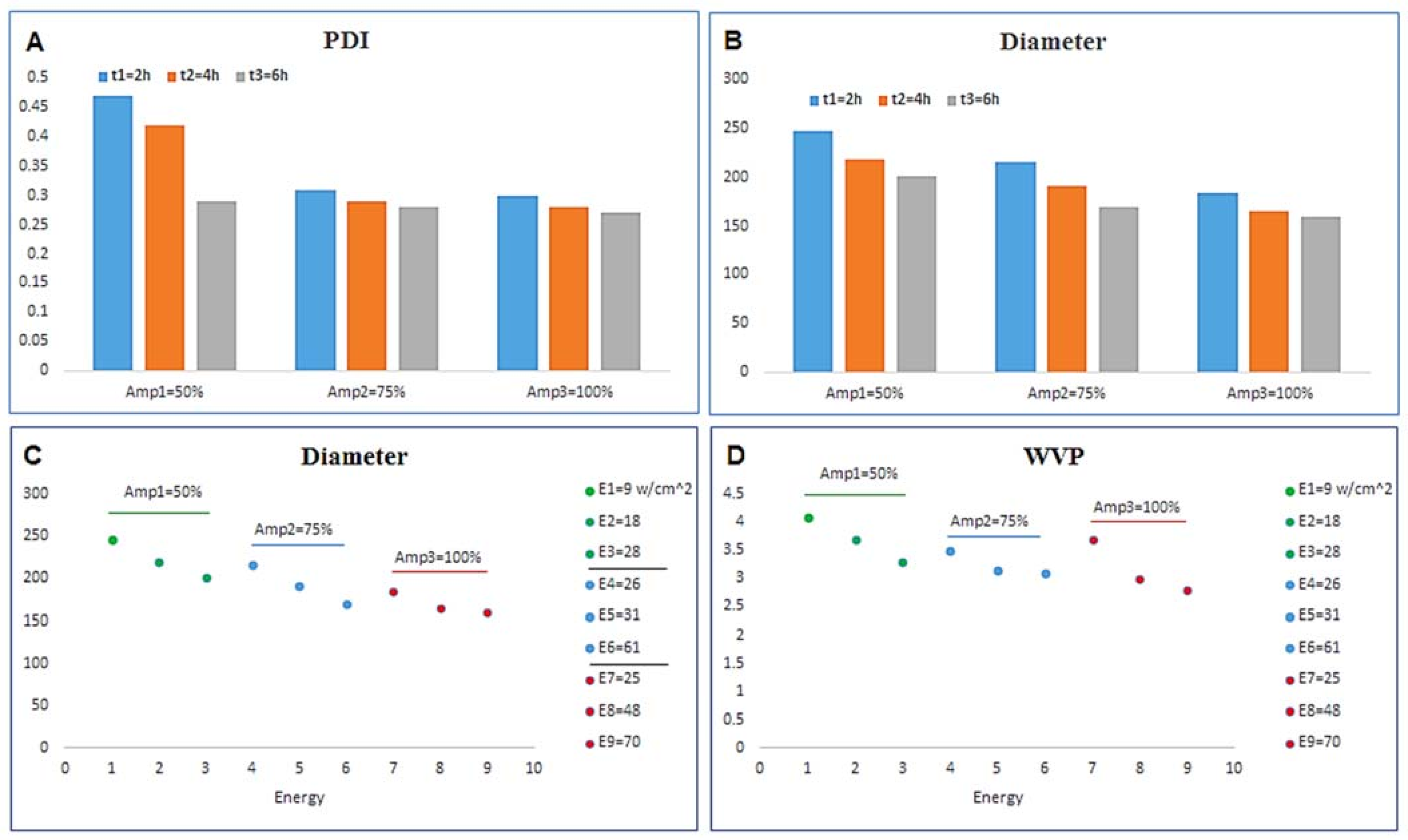
| EOs | Time | Temp. | Energy/ Frequency | Droplet Size (nm) | Zeta Potential (mV) | PDI * | Packaging Type | Consequences | [Ref] Year |
|---|---|---|---|---|---|---|---|---|---|
| Oregano (Origanum vulgare) | 10 min | 25 °C | 400 W | 180.59 ± 84.76 | - | 0.220 | Film | Increased bioavailability, less preservative content might be used and still deliver the same anti-microbial efficiency if encapsulated in smaller particles. | [163] 2014 |
| Clove Bud (Syzygium aromaticum) | 10 min | 25 °C | 400 W | 250.43 ± 100.67 | - | 0.162 | Film | Increased bioavailability, less preservative content might be used and still deliver the same anti-microbial efficiency if encapsulated in smaller particles. | [163] 2014 |
| Zataria multiflora | 6 min | 50 °C | - | - | - | - | Coating | A significant difference in mean bacterial count between the study groups during 12 days of storage. Significant reduction in initial count of E. coli O157: H7) from 6.69 ± 0.13 log CFU/g, -to 4.06 ± 0.15 log CFU/g) | [164] 2019 |
| Sour lemon peel | 10 | 25 °C | 24 kh | 150 | - | - | Coatings | Significant improvement in their physicochemical, textural and sensory characteristics compared to the control during 12-day cold-storage. | [165] 2019 |
| Hazelnuts | 2–4–6 min | 25 °C | 750 W | 201–247 | −32 ± 6 | 0.3 ± 0.2 | Film | Given the films anti-bacterial activity against L. monocytogenes, B. subtilis, S. aureus, P. aeruginosa and E. coli, and antioxidant activity | [158] 2018 |
| Geranium EO | 2 min | 25 °C | 65 W | 13–15 | - | 0.06 | Coating | Enhance the toxicity of geranium EO against larvae of Culex pipiens pipiensand Plodia interpunctella | [166] 2020 |
| Mega-3 fatty acid | 15 min | 23 °C | 24 kH | 464 | −45.5 | - | Coating | For targeted delivery | [167] 2019 |
| Ginger | 5 min | 25 °C | 160 W | 236 | - | - | Coating | Efficient delivery system for the improvement of Jujube fruit quality | [129] 2020 |
| Wheat germ oil | 10 min | 25 °C | 60% amplitude | 114 | −14.7 | 0.14 | Coating | Oil stability at 4 C | [168] 2019 |
| Lemon oil | 1 min | 25 °C | 70% amplitude | 120 | - | - | Coating | Comparing with other methods | [169] 2011 |
| Eucalyptus oil | 5–30 min | 25 °C | 750 W | 50–10 | - | - | Film | Anti-bacterial activity against Staphylococcus aureus | [170] 2014 |
| Zataria multiflora | 2.5–10 min | 25 °C | 150 W | 168–91 | - | - | Film | Extending the shelf life of food | [171] 2019 |
| Thymol | 10 min | 20 °C | 700 W | 150–250 | - | 0.2–0.15 | Film | Antifungal effect in cherry tomatoes | [172] 2018 |
| Saffron | - | - | - | - | - | - | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahari, H.; Nasiri, M. Ultrasonic Technique for Production of Nanoemulsions for Food Packaging Purposes: A Review Study. Coatings 2021, 11, 847. https://doi.org/10.3390/coatings11070847
Ahari H, Nasiri M. Ultrasonic Technique for Production of Nanoemulsions for Food Packaging Purposes: A Review Study. Coatings. 2021; 11(7):847. https://doi.org/10.3390/coatings11070847
Chicago/Turabian StyleAhari, Hamed, and Mina Nasiri. 2021. "Ultrasonic Technique for Production of Nanoemulsions for Food Packaging Purposes: A Review Study" Coatings 11, no. 7: 847. https://doi.org/10.3390/coatings11070847
APA StyleAhari, H., & Nasiri, M. (2021). Ultrasonic Technique for Production of Nanoemulsions for Food Packaging Purposes: A Review Study. Coatings, 11(7), 847. https://doi.org/10.3390/coatings11070847






