Facile Preparation of Fe3O4 Nanoparticles/Reduced Graphene Oxide Composite as an Efficient Anode Material for Lithium-Ion Batteries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
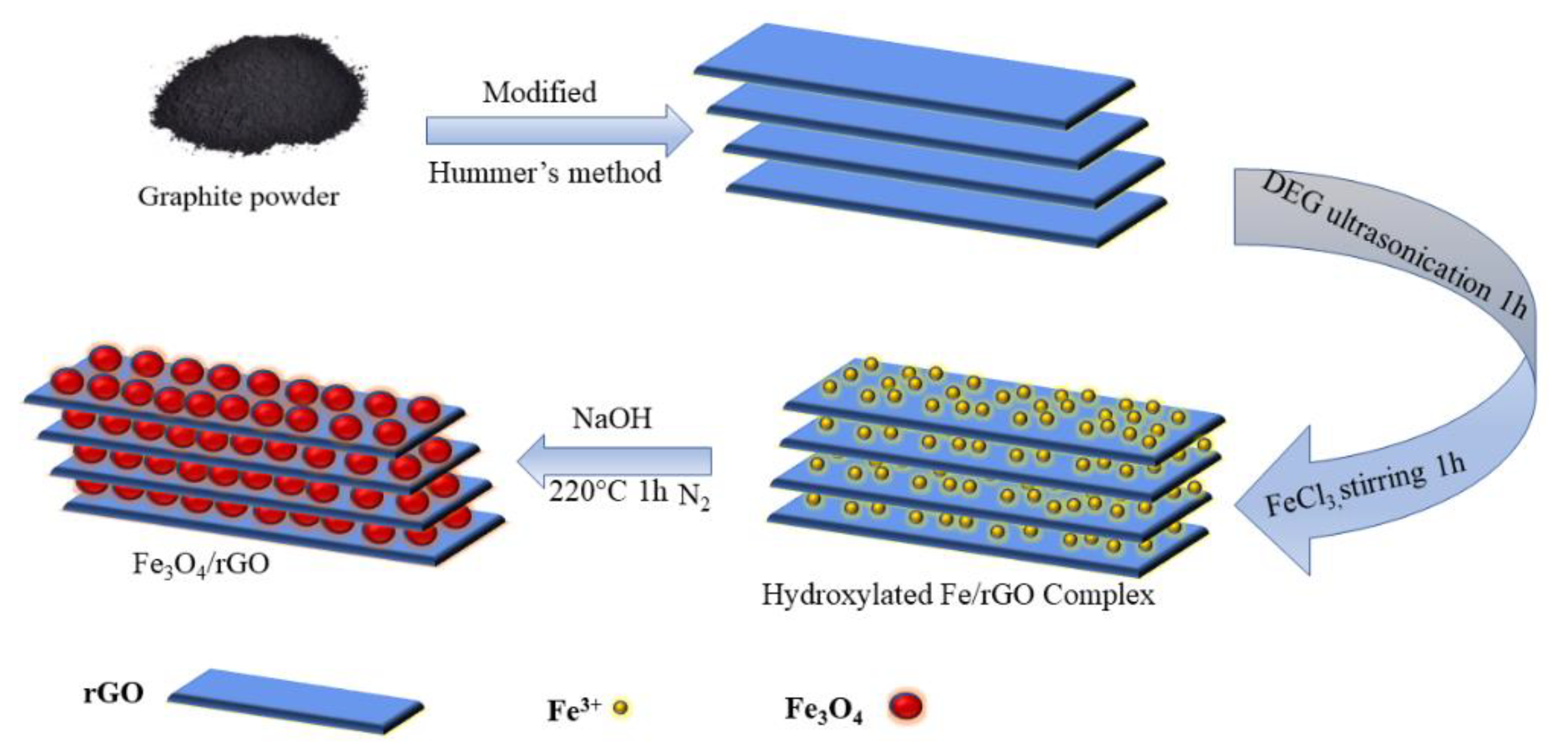
2.2. Synthesis of Fe3O4/RGO Composite
2.3. Characterization
2.4. Electrochemical Measurements
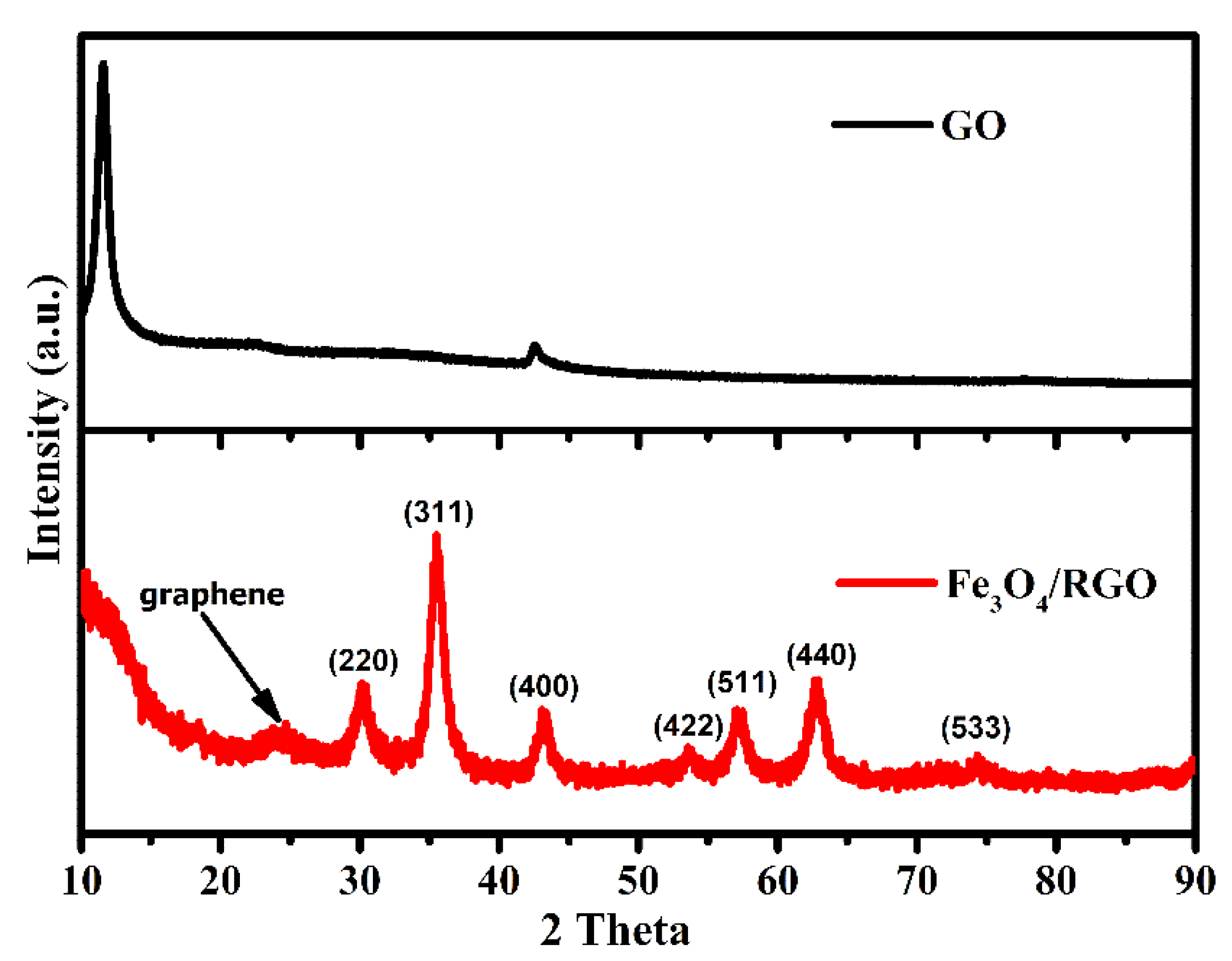
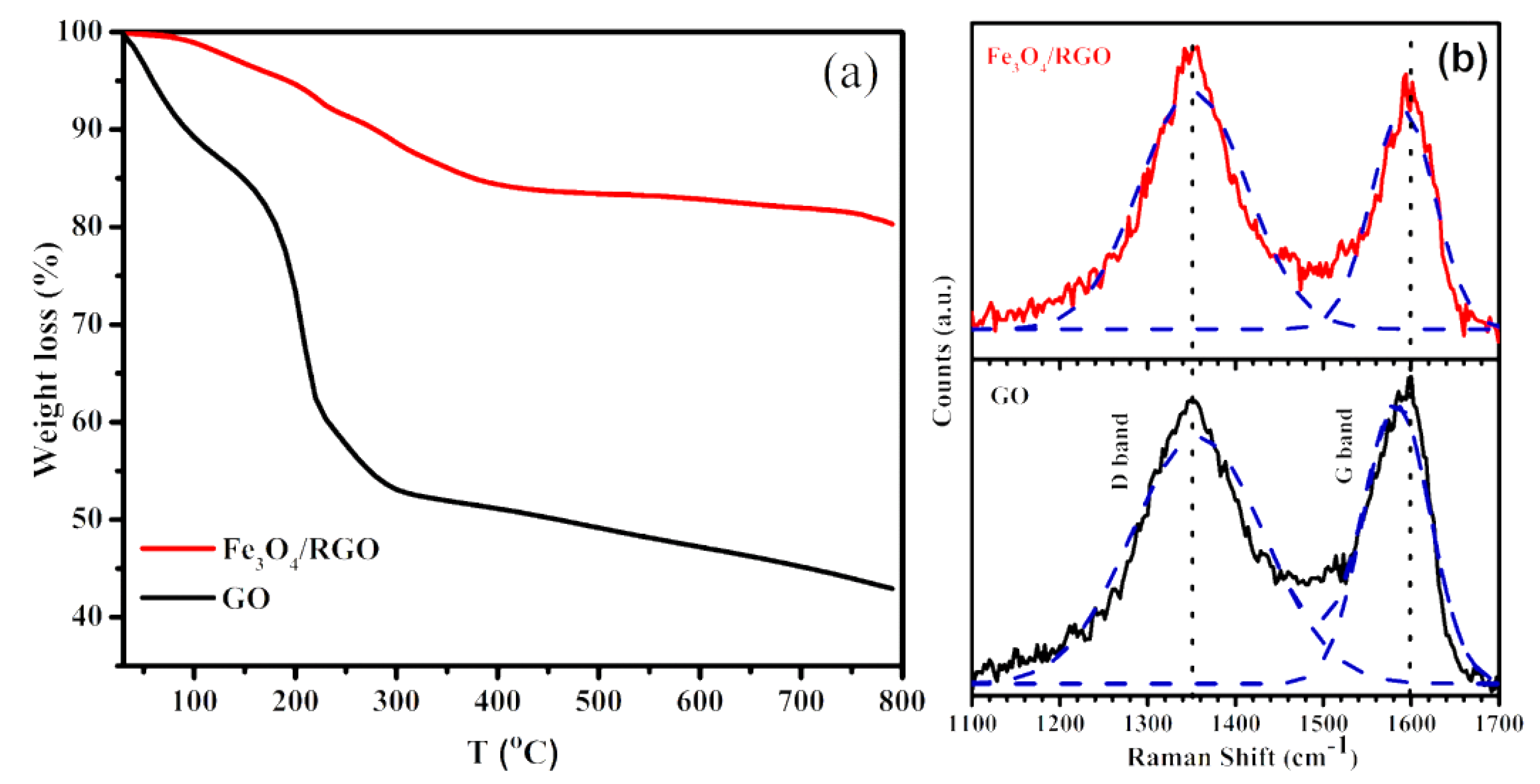
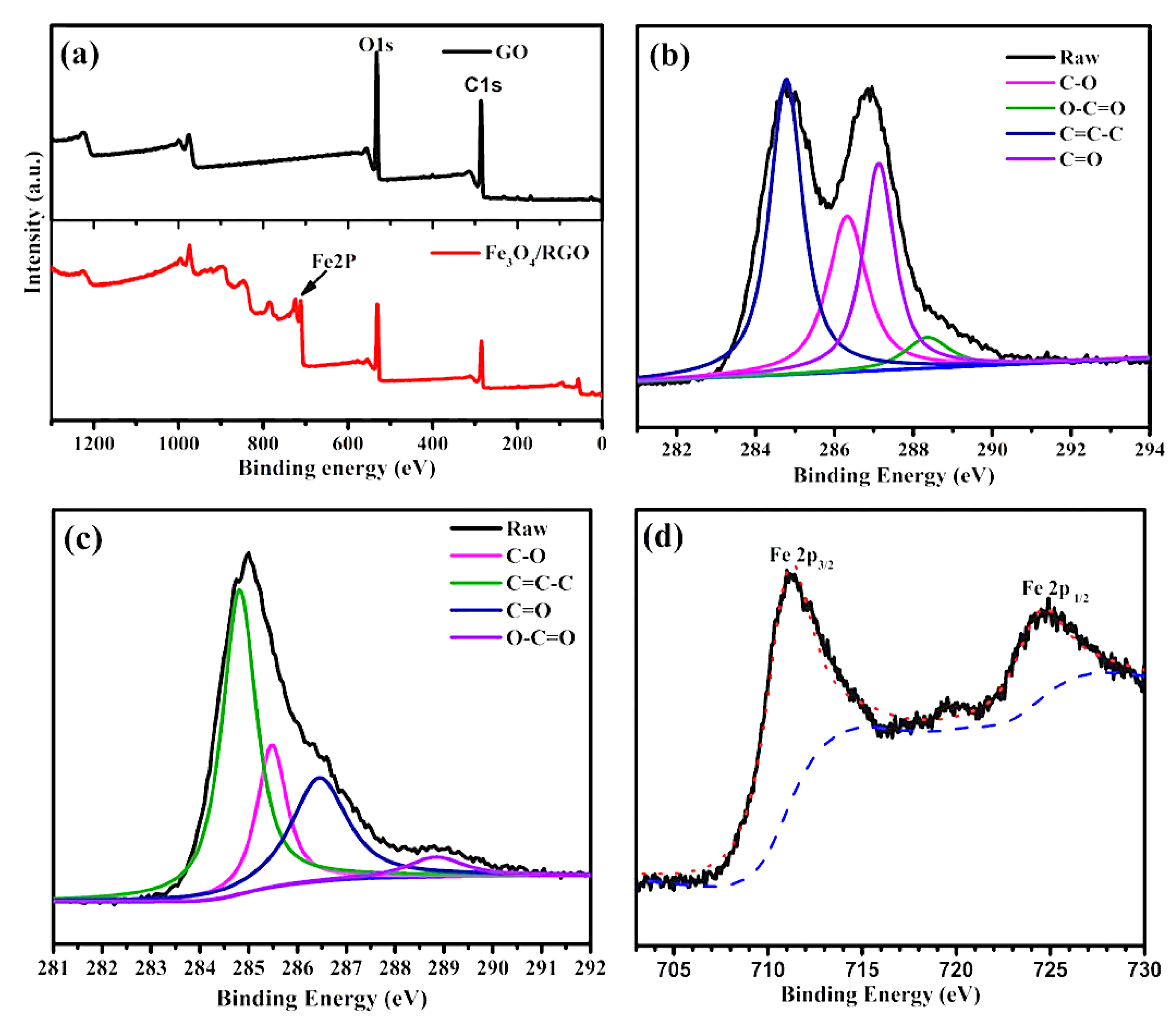
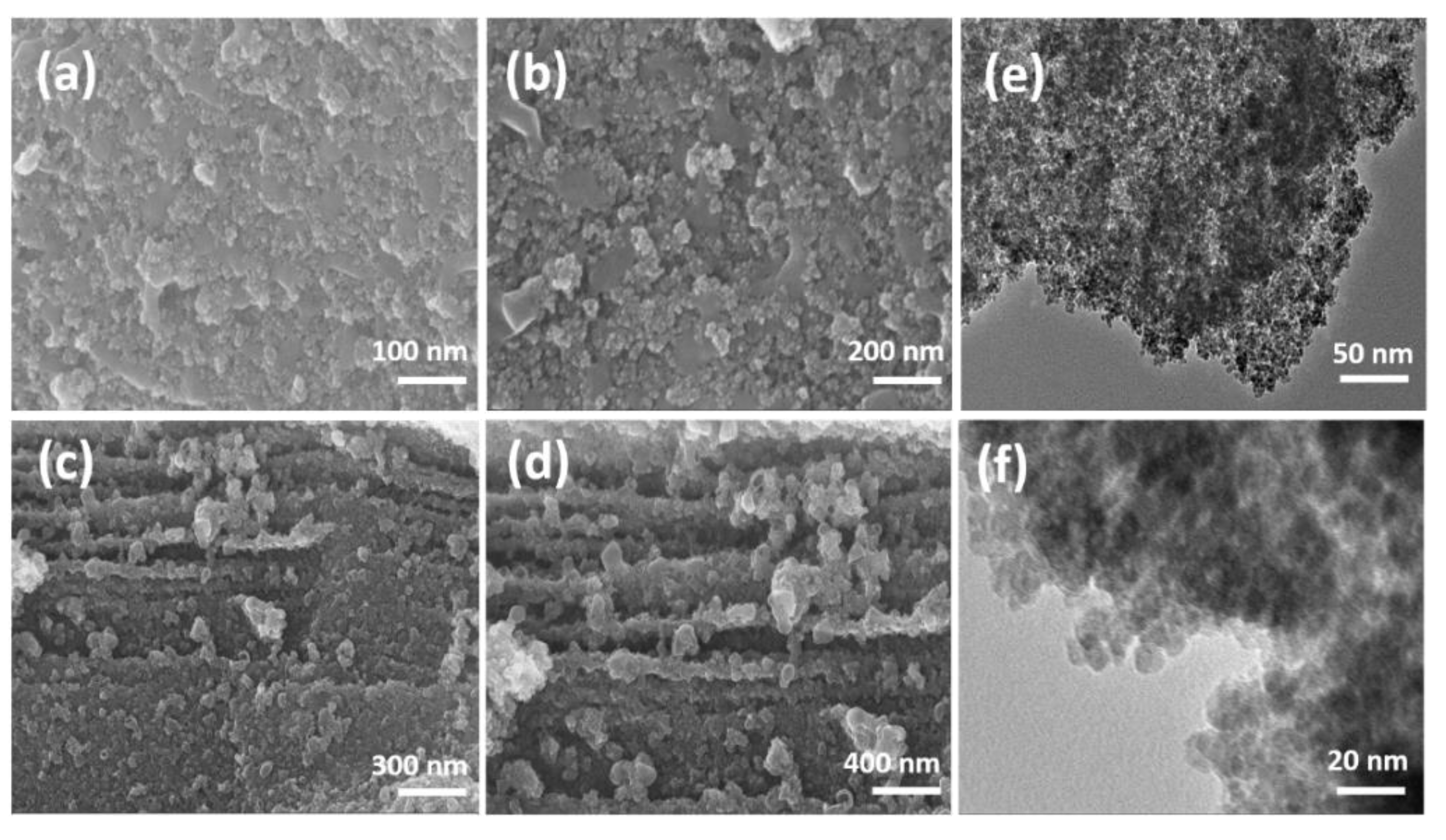
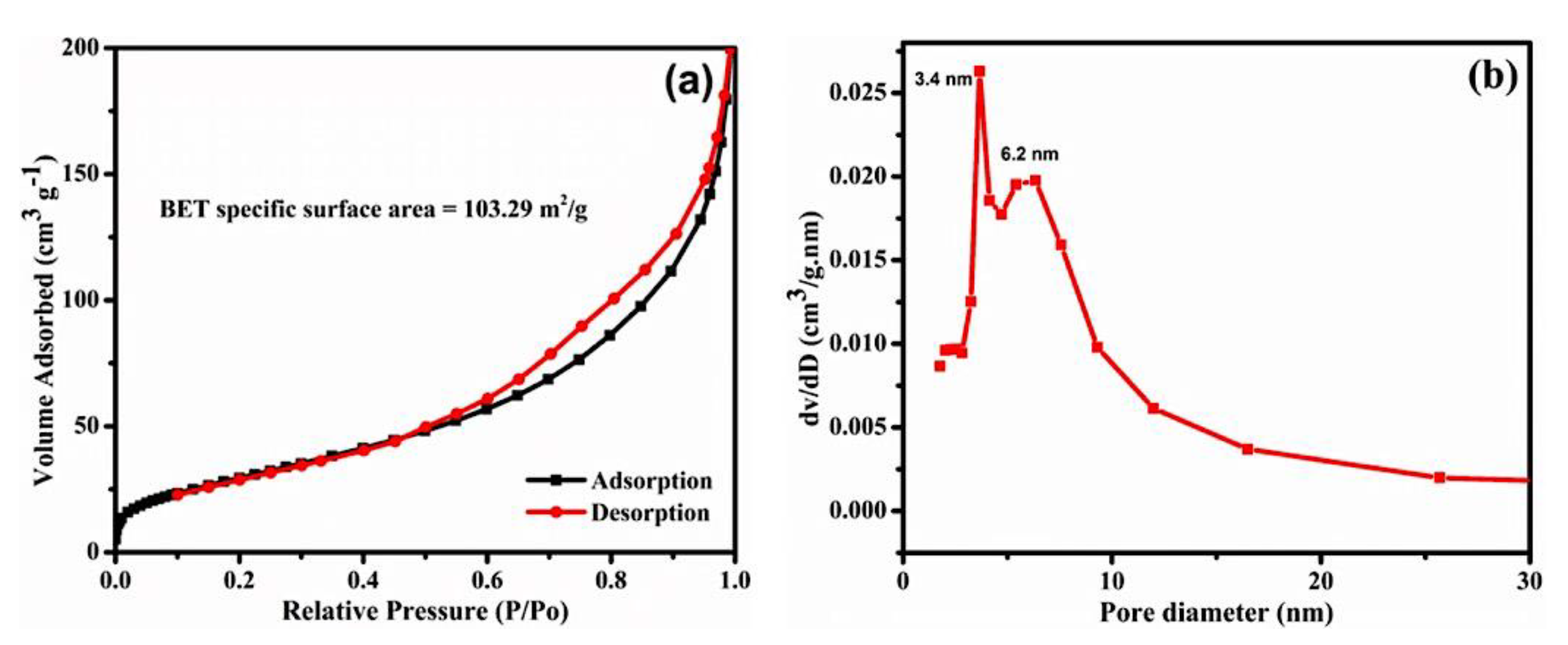
3. Results
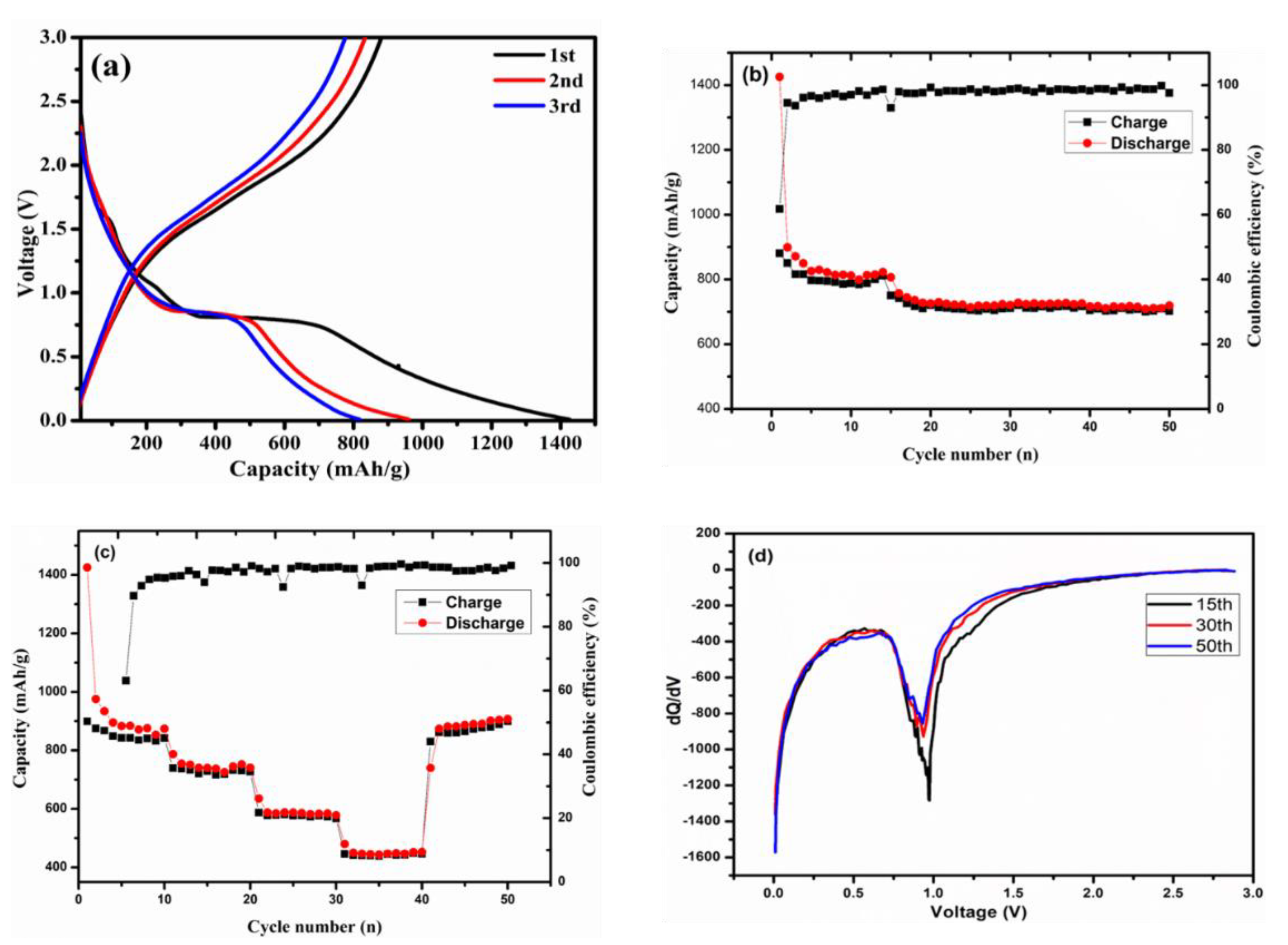
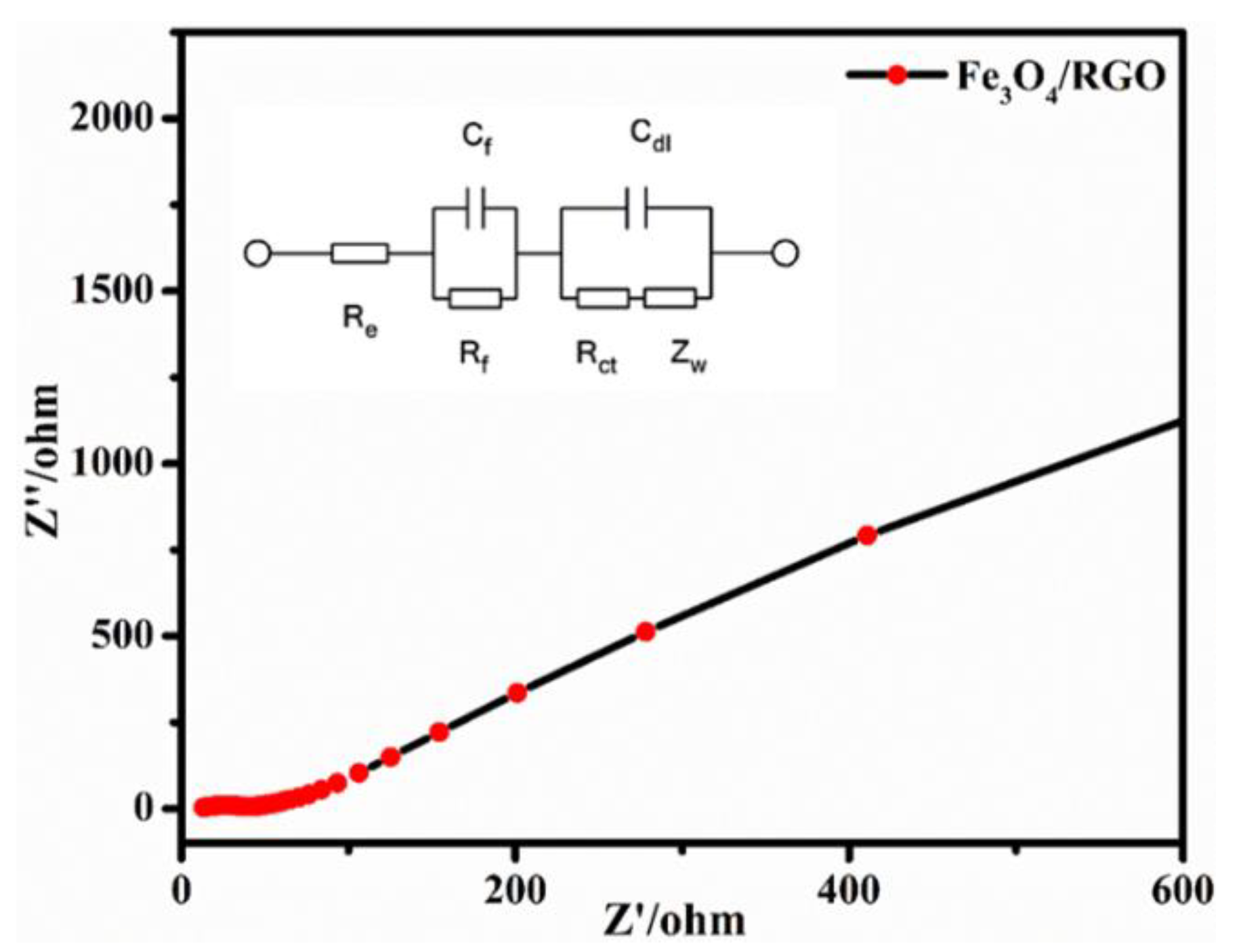
Electrochemical Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zheng, G.; Lee, S.W.; Liang, Z.; Lee, H.W.; Yan, K.; Yao, H.; Wang, H.; Li, W.; Chu, S.; Cui, Y. Interconnected Hollow Carbon Nanospheres for Stable Lithium Metal Anodes. Nat. Nanotechnol. 2014, 9, 618–623. [Google Scholar] [CrossRef]
- Lü, H.Y.; Wan, F.; Jiang, L.H.; Wang, G.; Wu, X.L. Graphene Nanosheets Suppress the Growth of Sb Nanoparticles in a Sb/C Nanocomposite to Achieve Fast Na Storage. Part. Part. Syst. Charact. 2016, 33, 204–211. [Google Scholar] [CrossRef]
- Hou, B.H.; Wu, X.L.; Wang, Y.Y.; Lü, H.Y.; Liu, D.H.; Sun, H.Z.; Zhang, J.P.; Guan, H.Y. Full Protection for Graphene- Incorporated Micro-/Nanocomposites Containing Ultra-Small Active Nanoparticles: The Best Li-Storage Properties. Part. Part. Syst. Charact. 2015, 32, 1020–1027. [Google Scholar] [CrossRef]
- Guo, J.Z.; Wu, X.L.; Wan, F.; Wang, J.; Zhang, X.; Wang, H.R.S. A Superior Na3V2(PO4)3 Based Nanocomposite Enhanced by Both N-Doped Coating Carbon and Graphene as the Cathode for Sodium-Ion Batteries. Chem. Eur. J. 2015, 21, 17371–17378. [Google Scholar] [CrossRef]
- Liu, D.H.; Lü, H.Y.; Wu, X.L.; Hou, B.H.; Wan, F.; Bao, S.D.; Yan, Q.Y.; Xie, H.M.; Wang, R.S. Constructing the Optimal Conductive Network in MnO-Based Nanohybrids as High-Rate and Long-Life Anode Materials for Lithium-Ion Batteries. J. Mater. Chem. A 2015, 3, 19738–19746. [Google Scholar] [CrossRef]
- Wang, J.; Liu, D.H.; Wang, Y.Y.; Hou, B.H.; Zhang, J.P.; Wang, R.S.; Wu, X.L. Dual-Carbon Enhanced Silicon-Based Composite as Superior Anode Material for Lithium Ion Batteries. J. Power Sources 2016, 307, 738–745. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, R.K.; Dubey, P.K.; Singh, D.P.; Yadav, R.M.; Tiwari, R.S. Free standing 3D Graphene-Nickel Encapsulated Nitrogen-Rich Aligned Bamboo Like Carbon Nanotubes for High- Performance Supercapacitors with Robust Cycle Stability. Adv. Mater. Interfaces 2015, 2, 1–12. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, R.K.; Dubey, P.K.; Singh, D.P.; Yadav, R.M. Self-Assembled Hierarchical Formation of Conjugated 3D Cobalt Oxide Nanobead-CNT-Graphene Nanostructure Using Microwaves for High-Performance Supercapacitor Electrode. ACS Appl. Mater. Interfaces 2015, 7, 15042–15051. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.C.; Ma, R.G.; Hu, M.J.; Cheng, H.; Tsang, C.K.; Yang, Q.D.; Li, Y.Y.; Zapien, J.A. Scalable Synthesis of Fe3O4 Nanoparticles Anchored on Graphene as a High-Performance Anode for Lithium Ion Batteries. J. Solid State Chem. 2013, 201, 330–337. [Google Scholar] [CrossRef]
- Liu, S.Y.; Xie, J.; Fang, C.C.; Cao, G.S.; Zhu, T.J.; Zhao, X.B. Self-Assembly of a CoFe2O4/Graphene Sandwich by a Controllable and General Route: Towards a High-Performance Anode for Li-Ion Batteries. J. Mater. Chem. 2012, 37, 19738–19743. [Google Scholar] [CrossRef]
- Dong, Y.C.; Ma, R.G.; Hu, M.J.; Cheng, H.; Yang, Q.D.; Li, Y.Y.; Zapien, J.A. Thermal Evaporation-Induced Anhydrous Synthesis of Fe3O4–Graphene Composite with Enhanced Rate Performance and Cyclic Stability for Lithium Ion Batteries. Phys. Chem. Chem. Phys. 2013, 15, 7174–7181. [Google Scholar] [CrossRef] [PubMed]
- Behera, S.K. Facile Synthesis and Electrochemical Properties of Fe3O4 Nanoparticles for Li Ion Battery Anode. J. Power Sources 2011, 196, 8669–8674. [Google Scholar] [CrossRef]
- Chen, Y.; Xia, H.; Lu, L.; Xue, J.M. Synthesis of Porous Hollow Fe3O4 Beads and Their Applications in Lithium Ion Batteries. J. Mater. Chem. 2012, 22, 5006–5012. [Google Scholar] [CrossRef]
- Kang, E.; Jung, Y.S.; Cavanagh, A.S.; Kim, G.H.; George, S.M.; Dillon, A.C.; Kim, J.K.; Lee, J. Fe3O4 Nanoparticles Confined in Mesocellular Carbon Foam for High Performance Anode Materials for Lithium-Ion Batteries. Adv. Funct. Mater. 2011, 21, 2430–2438. [Google Scholar] [CrossRef]
- Ban, C.M.; Wu, Z.C.; Gillaspie, D.T.; Chen, L.; Yan, Y.F.; Blackburn, J.L.; Dillon, A.C. Nanostructured Fe3O4/SWNT Electrode: Binder-Free and High-Rate Li-Ion Anode. Adv. Mater. 2010, 22, E145–E149. [Google Scholar] [CrossRef]
- Liu, H.; Wang, G.X.; Wang, J.Z.; Wexler, D. Magnetite/Carbon Core-Shell Nanorods as Anode Materials for Lithium-Ion Batteries. Electrochem. Commun. 2008, 10, 1879–1882. [Google Scholar] [CrossRef]
- Xiong, Q.Q.; Tu, J.P.; Lu, Y.; Chen, J.; Yu, Y.X.; Qiao, Y.Q.; Wang, X.L.; Gu, C.D. Synthesis of Hierarchical Hollow-Structured Single-Crystalline Magnetite (Fe3O4) Microspheres: The Highly Powerful Storage versus Lithium as an Anode for Lithium Ion Batteries. J. Phys. Chem. C 2012, 116, 6495–6502. [Google Scholar] [CrossRef]
- Chen, Y.J.; Xiao, G.; Wang, T.S.; Ouyang, Q.Y.; Qi, L.H.; Ma, Y.P.; Gao, C.L.; Zhu, M.S.; Cao, M.S.; Jin, H.B. Porous Fe3O4/Carbon Core/Shell Nanorods: Synthesis and Electromagnetic Properties. J. Phys. Chem. C 2011, 115, 13603–13608. [Google Scholar] [CrossRef]
- Xiong, Q.Q.; Tu, J.P.; Lu, Y.; Chen, J.; Yu, Y.X.; Wang, X.L.; Gu, C.D. Three-Dimensional Porous Nano-Ni/Fe3O4 Composite Film: Enhanced Electrochemical Performance for Lithium-Ion Batteries. J. Mater. Chem. 2012, 22, 18639–18645. [Google Scholar] [CrossRef]
- Balandin, A.A.; Ghosh, S.; Bao, W.Z.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N. Superior Thermal Conductivity of Single-Layer Graphene. Nano Lett. 2008, 8, 902–907. [Google Scholar] [CrossRef]
- Yoo, E.J.; Kim, J.; Hosono, E.; Zhou, H.S.; Kudo, T.; Honma, I. Large Reversible Li Storage of Graphene Nanosheet Families for Use in Rechargeable Lithium Ion Batteries. Nano Lett. 2008, 8, 2277–2282. [Google Scholar] [CrossRef]
- Wang, D.H.; Choi, D.W.; Li, J.; Yang, Z.G.; Nie, Z.M.; Kou, R.; Hu, D.H.; Wang, C.M.; Saraf, L.V.; Zhang, J.G.; et al. Self-Assembled TiO2-Graphene Hybrid Nanostructures for Enhanced Li-Ion Insertion. ACS Nano 2009, 3, 907–914. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.L.; Cui, L.F.; Yang, Y.; Casalongue, H.S.; Robinson, J.T.; Liang, Y.Y.; Cui, Y.; Dai, H.J. Mn3O4-Graphene Hybrid as a High-Capacity Anode Material for Lithium Ion Batteries. J. Am. Chem. Soc. 2010, 132, 13978–13980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, J.; Cao, M.H.; Ren, L.; Hu, C.W. Fe3O4-Graphene Nanocomposites with Improved Lithium Storage and Magnetism Properties. J. Phys. Chem. C 2011, 115, 14469–14477. [Google Scholar] [CrossRef]
- Lian, P.C.; Zhu, X.F.; Xiang, H.F.; Li, Z.; Yang, W.S.; Wang, H.H. Enhanced Cycling Performance of Fe3O4-Graphene Nanocomposite as an Anode Material for Lithium-ion Batteries. Electrochim. Acta 2010, 56, 834–840. [Google Scholar] [CrossRef]
- Zhou, G.M.; Wang, D.W.; Li, F.; Zhang, L.L.; Li, N.; Wu, Z.S.; Wen, L.; Lu, G.Q.; Cheng, H.M. Graphene-Wrapped Fe3O4 Anode Material with Improved Reversible Capacity and Cyclic Stability for Lithium Ion Batteries. Chem. Mater. 2010, 22, 5306–5313. [Google Scholar] [CrossRef]
- Li, B.J.; Cao, H.Q.; Shao, J.; Li, G.Q.; Qu, M.Z.; Yin, G. Co3O4@graphene Composites as Anode Materials for High-Performance Lithium Ion Batteries. Inorg. Chem. 2011, 50, 1628–1632. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.Q.; Wang, Y. NiO Nanosheets Grown on Graphene Nanosheets as Superior Anode Materials for Li-Ion Batteries. Nanoscale 2011, 3, 2615–2620. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wu, X.L.; Shu, C.Y.; Guo, Y.G.; Wang, C.R. Synthesis of CuO/Graphene Nanocomposite as a High-Performance Anode Material for Lithium-Ion Batteries. J. Mater. Chem. 2010, 20, 10661–10664. [Google Scholar] [CrossRef]
- Sun, Y.M.; Hu, X.H.; Luo, W.; Huang, Y.H. Self-Assembled Hierarchical MoO2/Graphene Nanoarchitectures and Their Application as a High-Performance Anode Material for Lithium-Ion Batteries. ACS Nano 2011, 5, 7100–7107. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, H.J.; Lu, L.; Stubbs, L.P.; Wong, C.C.; Lin, J.Y. Designed Functional Systems from Peapod-like Co@Carbon to Co3O4@Carbon Nanocomposites. ACS Nano 2010, 4, 4753–4761. [Google Scholar] [CrossRef]
- Guo, Y.G.; Hu, J.S.; Wan, L.J. Nanostructured Materials for Electrochemical Energy Conversion and Storage Devices. Adv. Mater. 2008, 20, 2878–2887. [Google Scholar] [CrossRef]
- Bruce, P.G.; Scrosati, B.; Tarascon, J.-M. Nanomaterials for Rechargeable Lithium Batteries. Angew. Chem. Int. Ed. 2008, 47, 2930–2946. [Google Scholar] [CrossRef]
- Chen, Y.; Song, B.H.; Li, M.; Lu, L.; Xue, J.M. Fe3O4 Nanoparticles Embedded in Uniform Mesoporous Carbon Spheres for Superior High-Rate Battery Applications. Adv. Funct. Mater. 2014, 24, 319–326. [Google Scholar] [CrossRef]
- Sun, Y.M.; Hu, X.L.; Luo, W.; Huang, Y.H. Ultrathin CoO/ Graphene Hybrid Nanosheets: A Highly Stable Anode Material for Lithium-Ion Batteries. J. Phys. Chem. C 2012, 116, 20794–20799. [Google Scholar] [CrossRef]
- Gong, Y.J.; Yang, S.B.; Zhan, L.; Ma, L.L.; Vajtai, R.; Ajayan, P.M. A Bottom-Up Approach to Build 3D Architectures from Nanosheets for Superior Lithium Storage. Adv. Funct. Mater. 2014, 24, 125–130. [Google Scholar] [CrossRef]
- Zhou, J.; Song, H.; Ma, L.; Chen, X. Magnetite/Graphene Nanosheet Composites: Interfacial Interaction and its Impact on the Durable High-Rate Performance in Lithium-Ion Batteries. RSC Adv. 2011, 1, 782–791. [Google Scholar] [CrossRef]
- Luo, B.; Wang, B.; Li, X.; Jia, Y.; Liang, M.; Zhi, L. Graphene-Confined Sn Nanosheets with Enhanced Lithium Storage Capability. Adv. Mater. 2012, 24, 3538–3543. [Google Scholar] [CrossRef]
- Chen, Q.W.; Zhang, L.Y.; Chen, G. Facile Preparation of Graphene-Copper Nanoparticle Composite by in situ Chemical Reduction for Electrochemical Sensing of Carbohydrates. Anal. Chem. 2012, 84, 171–178. [Google Scholar] [CrossRef]
- Zhang, F.; Li, Y.; Gu, Y.-e.; Wang, Z.; Wang, C. One-Pot Solvothermal Synthesis of a Cu2O/Graphene Nanocomposite and its Application in an Electrochemical Sensor for Dopamine. Microchim. Acta 2011, 173, 103–109. [Google Scholar] [CrossRef]
- Tuinstra, F.; Koenig, J.L. Raman Spectrum of Graphite. J. Chem. Phys. 1970, 53, 1126–1130. [Google Scholar] [CrossRef] [Green Version]
- Shen, J.; Shi, M.; Ma, H.; Yan, B.; Li, N.; Ye, M. Hydrothermal Synthesis of Magnetic Reduced Graphene Oxide Sheets. Mater. Res. Bull. 2011, 46, 2077–2083. [Google Scholar] [CrossRef]
- Teng, X.W.; Black, D.; Watkins, N.J.; Gao, Y.L.; Yang, H. Platinum-Maghemite Core−Shell Nanoparticles Using a Sequential Synthesis. Nano Lett. 2003, 3, 261–264. [Google Scholar] [CrossRef]
- Morel, A.L.; Nikitenko, S.I.; Gionnet, K.; Wattiaux, A.; Lai-Kee-Him, J.; Labrugere, C.; Chevalier, B.; Deleris, G.; Petibois, C.; Brisson, A.; et al. Sonochemical Approach to the Synthesis of Fe3O4@SiO2 Core−Shell Nanoparticles with Tunable Properties. ACS Nano 2008, 2, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Wu, S.; Dastan, D.; Nie, S.; Liu, Y.; Li, Z.; Zhou, Y.; Li, J.; Faik, A.; Shan, K.; et al. Sensing Selectivity of SnO2-Mn3O4 Nanocomposite Sensors for the Detection of H2 and CO Gases. Surf. Interfaces 2021, 25, 101190. [Google Scholar] [CrossRef]
- He, Y.; Huang, L.; Cai, J.S.; Zheng, X.M.; Sun, S.G. Structure and Electrochemical Performance of Nanostructured Fe3O4/Carbon Nanotube Composites as Anodes for Lithium Ion Batteries. Electrochim. Acta 2010, 55, 1140–1144. [Google Scholar] [CrossRef]
- Poizot, P.; Laruelle, S.; Grugeon, S.; Dupont, L.; Tarascon, J.M. Nano-Sized Transition-Metal Oxides as Negative-Electrode Material for Lithium Ion Batteries. Nature 2000, 407, 496–499. [Google Scholar] [CrossRef]
- Shan, K.; Yi, Z.Z.; Yin, X.T.; Dastan, D.; Altaf, F.; Garmestani, H.; Alamgir, F.M. Mixed Conductivity Evaluation and Sensing Characteristics of Limiting Current Oxygen Sensors. Surf. Interfaces 2020, 21, 100762. [Google Scholar] [CrossRef]
- Yin, X.T.; Li, J.; Dastan, D.; Zhou, W.D.; Garmestani, H.; Alamgir, F.M. Ultra-High Selectivity of H2 over CO with a p-n Nanojunction Based Gas Sensors and its Mechanism. Sens. Actuators B Chem. 2020, 319, 128330. [Google Scholar] [CrossRef]
- Xiang, J.Y.; Tu, J.P.; Qiao, Y.Q.; Wang, X.L.; Zhong, J.; Zhang, D.; Gu, C.D. Electrochemical Impedance Analysis of a Hierarchical CuO Electrode Composed of self-Assembled Nanoplates. J. Phys. Chem. C 2011, 115, 2505–2513. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hameed, M.U.; Akram, M.Y.; Ali, G.; Hafeez, M.; Altaf, F.; Ahmed, A.; Shahida, S.; Bocchetta, P. Facile Preparation of Fe3O4 Nanoparticles/Reduced Graphene Oxide Composite as an Efficient Anode Material for Lithium-Ion Batteries. Coatings 2021, 11, 836. https://doi.org/10.3390/coatings11070836
Hameed MU, Akram MY, Ali G, Hafeez M, Altaf F, Ahmed A, Shahida S, Bocchetta P. Facile Preparation of Fe3O4 Nanoparticles/Reduced Graphene Oxide Composite as an Efficient Anode Material for Lithium-Ion Batteries. Coatings. 2021; 11(7):836. https://doi.org/10.3390/coatings11070836
Chicago/Turabian StyleHameed, Muhammad Usman, Muhammad Yasir Akram, Ghulam Ali, Muhammad Hafeez, Faizah Altaf, Ashfaq Ahmed, Shabnam Shahida, and Patrizia Bocchetta. 2021. "Facile Preparation of Fe3O4 Nanoparticles/Reduced Graphene Oxide Composite as an Efficient Anode Material for Lithium-Ion Batteries" Coatings 11, no. 7: 836. https://doi.org/10.3390/coatings11070836
APA StyleHameed, M. U., Akram, M. Y., Ali, G., Hafeez, M., Altaf, F., Ahmed, A., Shahida, S., & Bocchetta, P. (2021). Facile Preparation of Fe3O4 Nanoparticles/Reduced Graphene Oxide Composite as an Efficient Anode Material for Lithium-Ion Batteries. Coatings, 11(7), 836. https://doi.org/10.3390/coatings11070836






