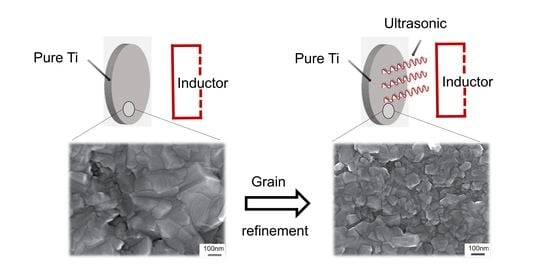
Ultrasonic Induced Refinement of Induction Heated Oxide Coating on Titanium
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
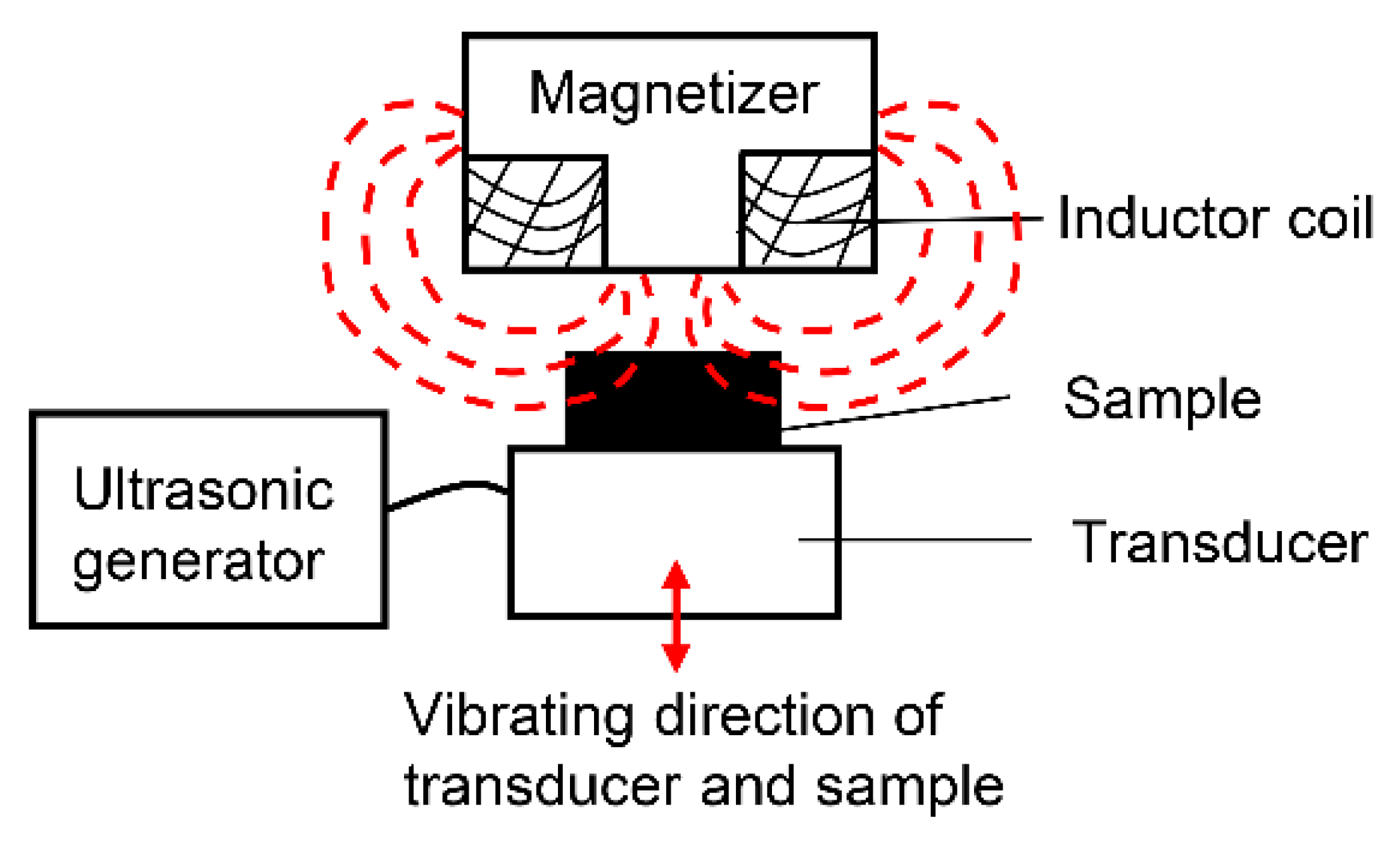
2.2. IHT Process of Ti Specimens with Ultrasonic Impact
2.3. Phase and Microstructural Characterization
2.4. Contact Angle Measurement
2.5. Micro-Hardness Test
3. Results and Discussion
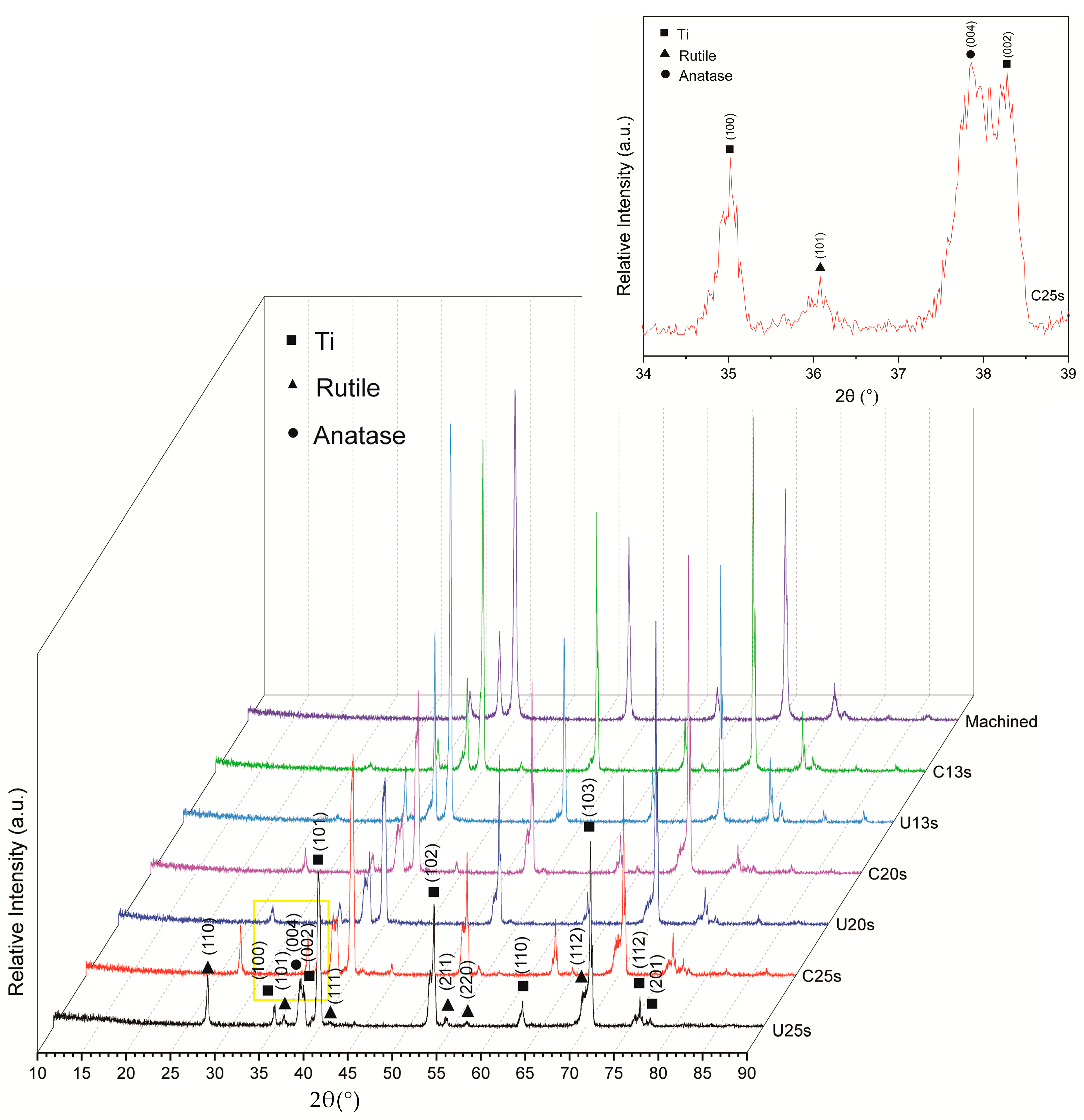
3.1. Phase Composition
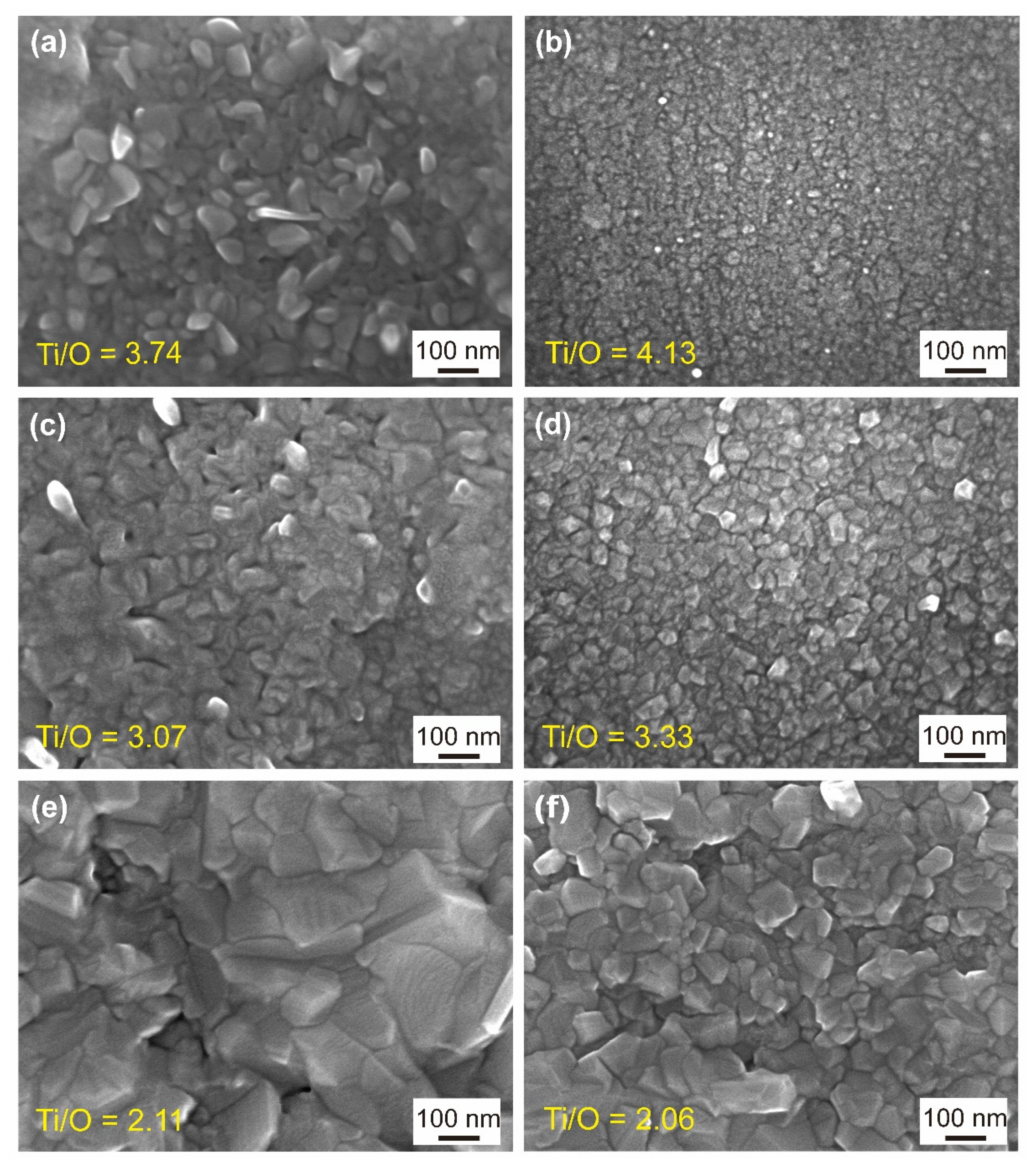
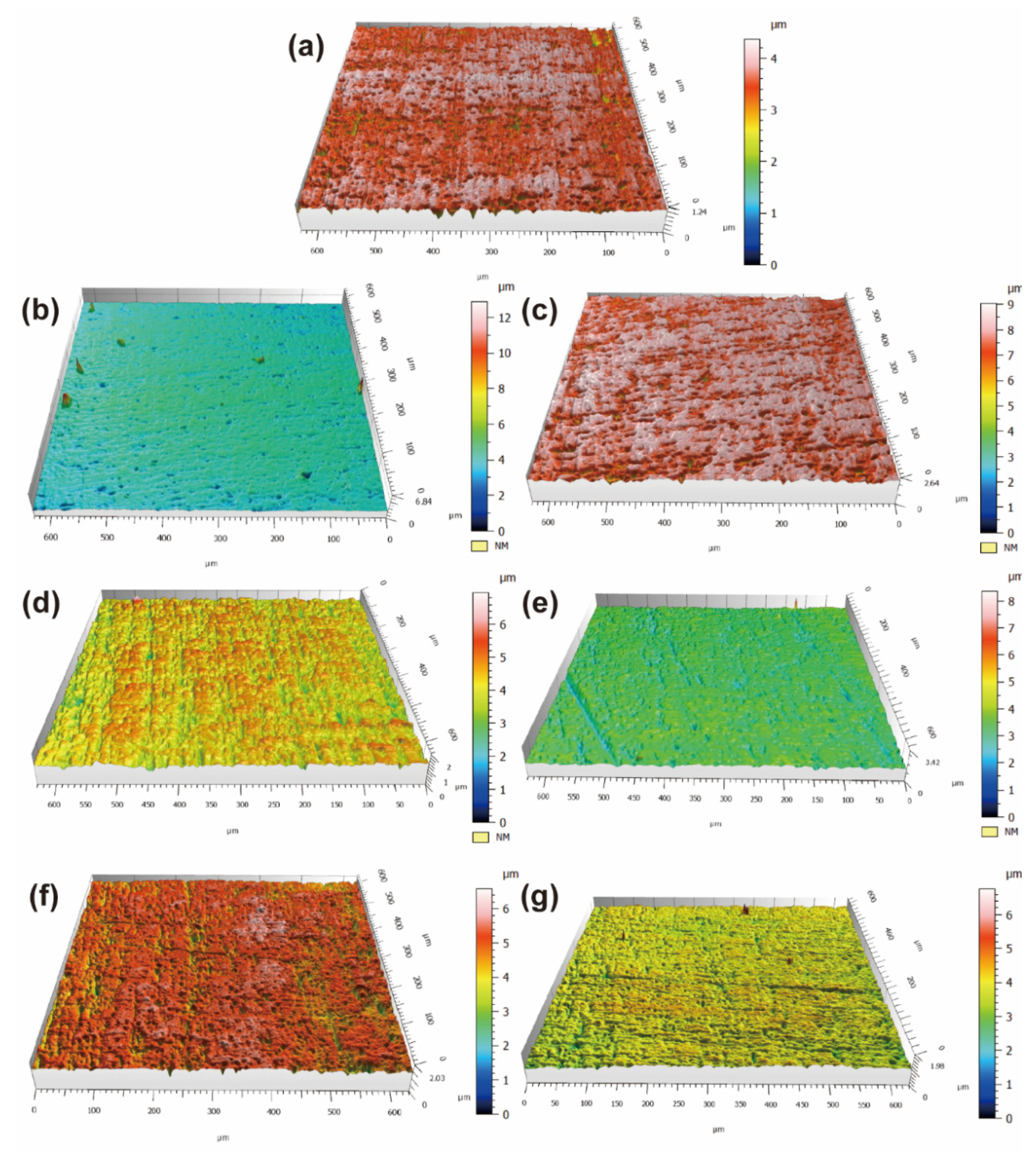
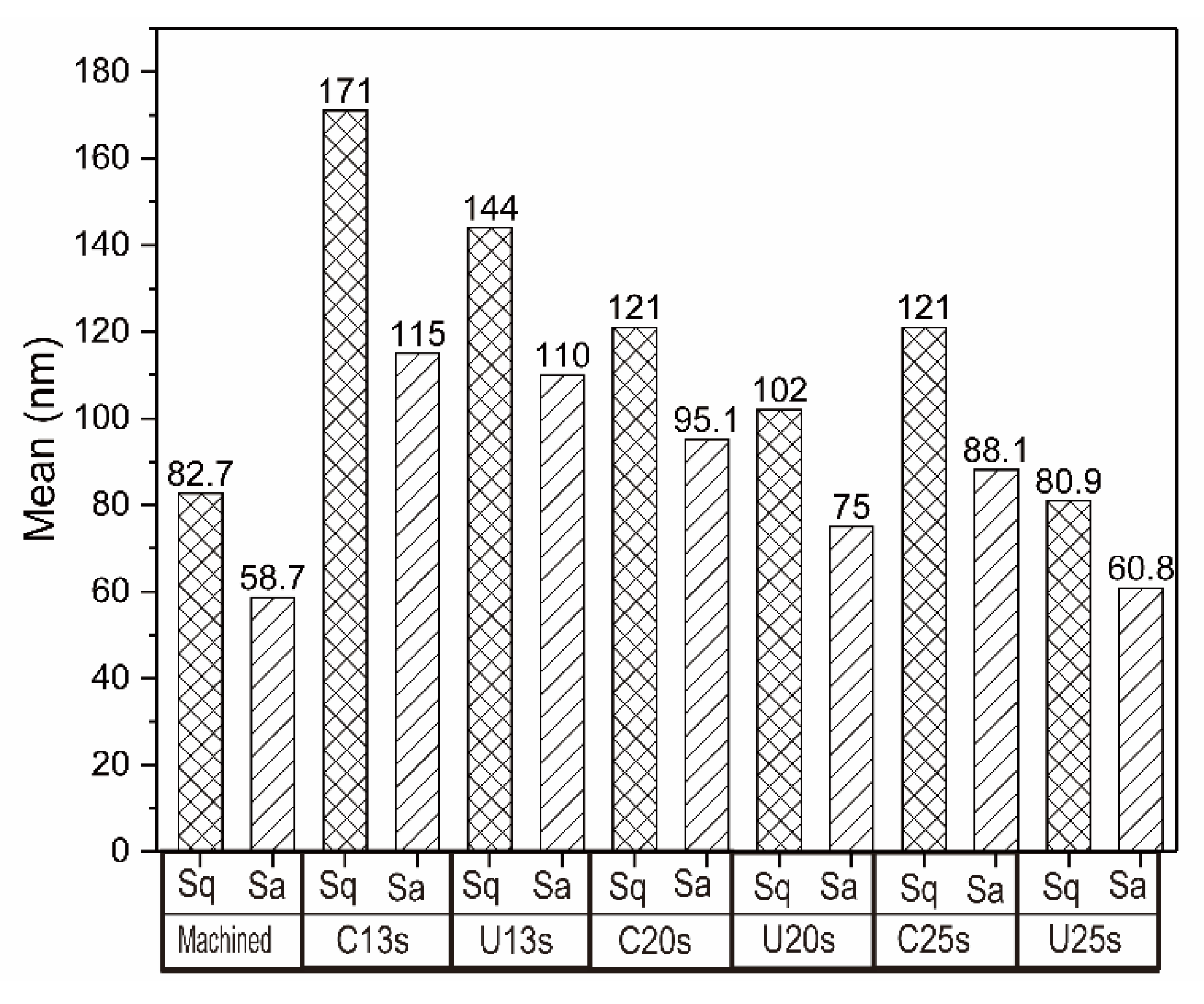
3.2. Surface Morphology and Element Distribution
3.3. Cross-Sectional Morphology and Element Distribution of the Oxide Layer
3.4. Surface Wettability
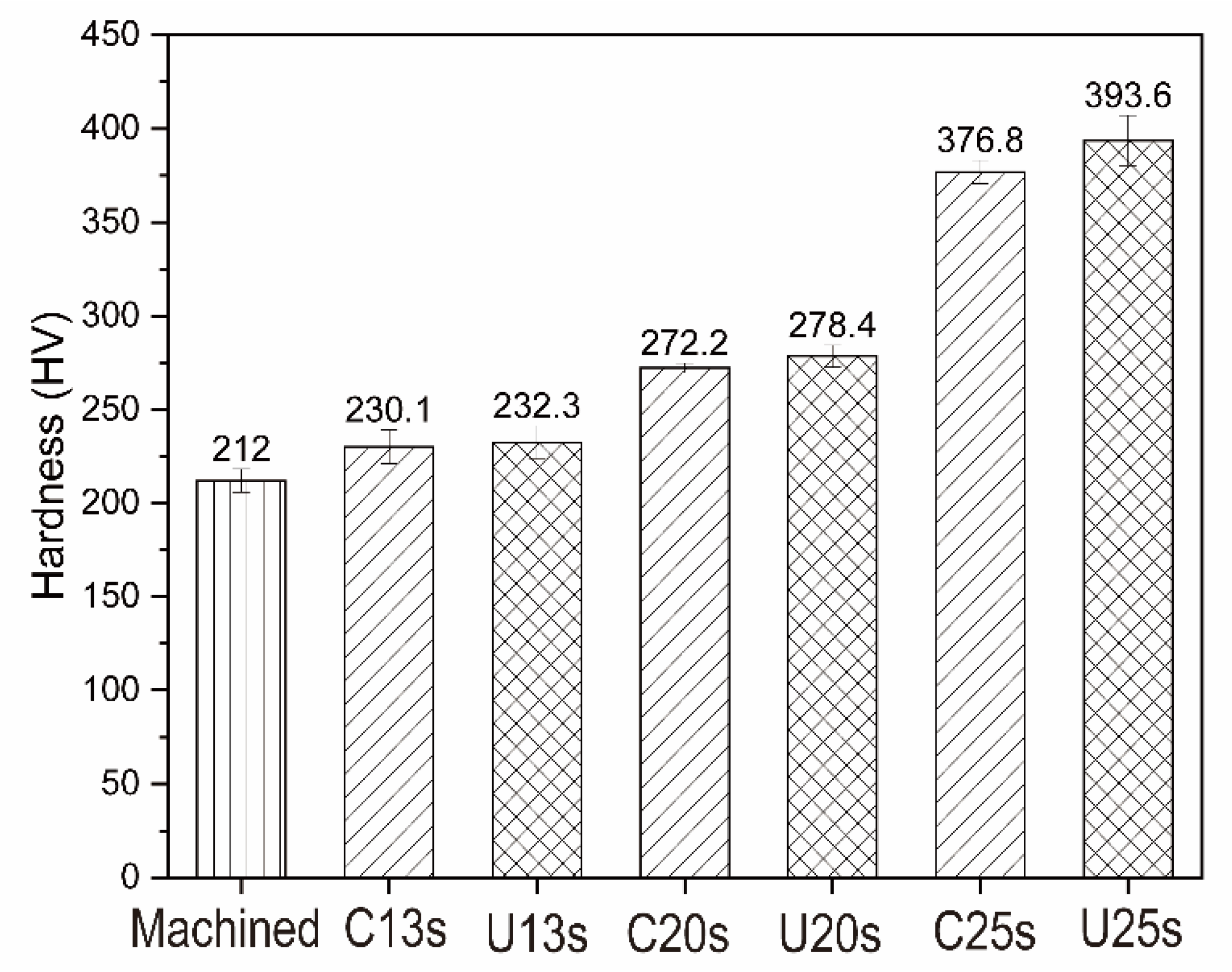
3.5. Micro-Hardness
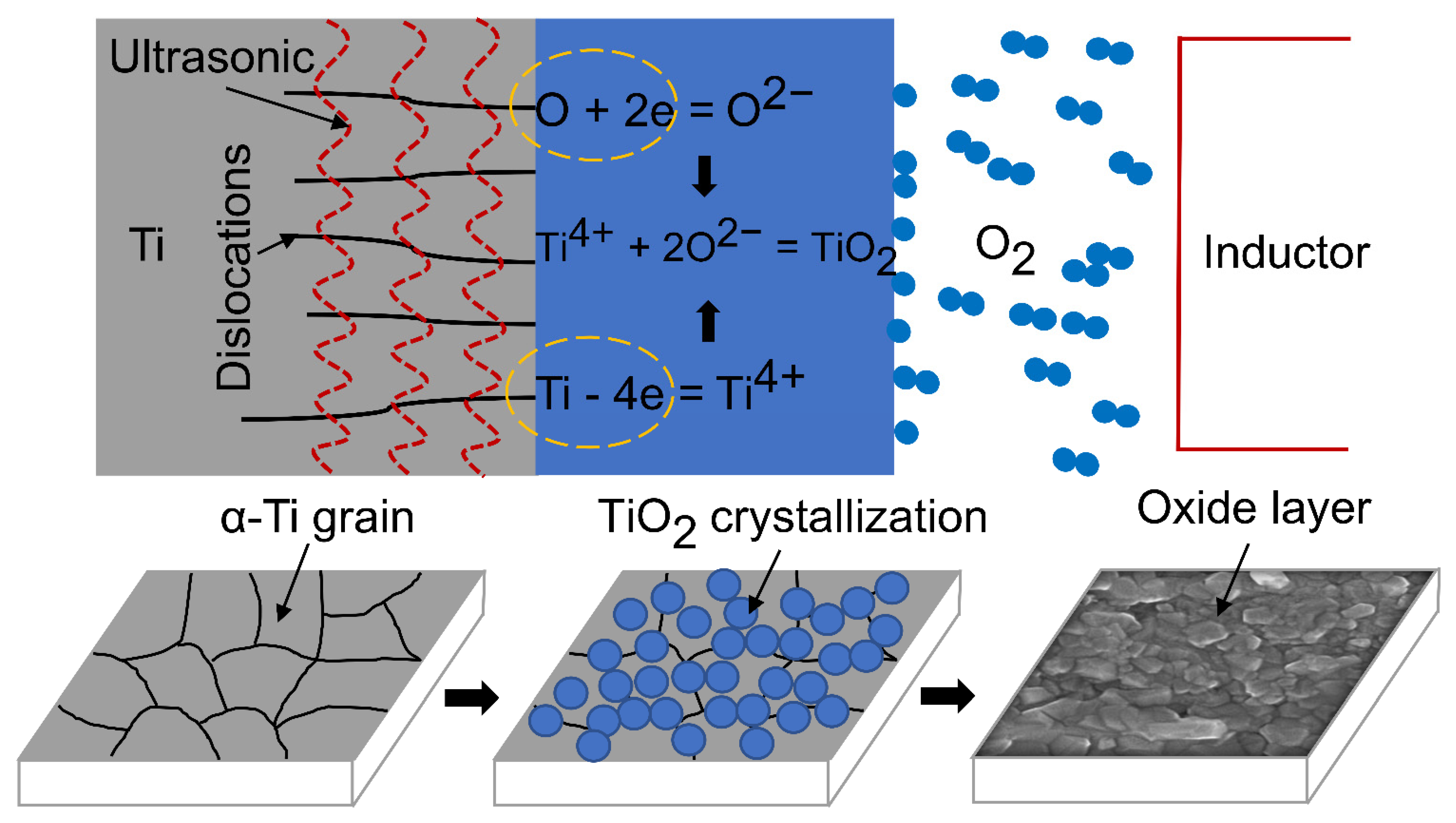
3.6. Mechanism of Ultrasonic Induced Refinement of Induction Heated Oxide Coating on Titanium
4. Conclusions
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Geetha, R.M.; Singh, A.K.; Asokamani, R.; Gogia, A.K. Ti based biomaterials, the ultimate choice for orthopaedic implants—A review. Prog. Mater. Sci. 2009, 54, 397–425. [Google Scholar] [CrossRef]
- Nuffer, J.H.; Siegel, R.W. Nanostructure–biomolecule interactions: Implications for tissue regeneration and nanomedicine. Tissue Eng. Part A 2010, 16, 423–430. [Google Scholar] [CrossRef]
- Vogel, V.; Sheetz, M.P. Local force and geometry sensing regulate cell functions. Nat. Rev. Mol. Cell Biol. 2006, 7, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Stevens, M.M.; George, J.H. Exploring and engineering the cell surface interface. Science 2005, 310, 1135–1138. [Google Scholar] [CrossRef] [PubMed]
- Curtis, A.; Wilkinson, C. Nantotechniques and approaches in biotechnology. Trends Biotechnol. 2001, 19, 97–101. [Google Scholar] [CrossRef]
- Wu, K.-C.; Tseng, C.-L.; Wu, C.-C.; Kao, F.-C.; Tu, Y.-K.; So, E.C.; Wang, Y.-K. Nanotechnology in the regulation of stem cell behavior. Sci. Technol. Adv. Mater. 2013, 14, 054401. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Webster, T.J. Nanobiotechnology: Implications for the future of nanotechnology in orthopedic applications. Expert Rev. Med. Devices 2004, 1, 105–114. [Google Scholar] [CrossRef]
- Souza, J.C.; Sordi, M.B.; Kanazawa, M.; Ravindran, S.; Henriques, B.; Silva, F.; Aparicio, C.; Cooper, L.F. Nano-scale modification of titanium implant surfaces to enhance osseointegration. Acta Biomater. 2019, 94, 112–131. [Google Scholar] [CrossRef] [PubMed]
- Mendonça, G.; Mendonça, D.B.; Aragão, F.J.; Cooper, L.F. Advancing dental implant surface technology—from micron- to nanotopography. Biomaterials 2008, 29, 3822–3835. [Google Scholar] [CrossRef]
- Ueno, T.; Tsukimura, N.; Yamada, M.; Ogawa, T. Enhanced bone-integration capability of alkali- and heat-treated nanopolymorphic titanium in micro-to-nanoscale hierarchy. Biomaterials 2011, 32, 7297–7308. [Google Scholar] [CrossRef] [PubMed]
- Long, M.; Rack, H.J. Titanium alloys in total joint replacement—A materials science perspective. Biomaterials 1998, 19, 1621–1639. [Google Scholar] [CrossRef]
- Tillmann, W.; Zimpel, M.; Dias, N.F.L.; Pfeiffer, J.; Wojarski, L.; Xu, Z. Mechanical and microstructural analysis of ultrasonically assisted induction-brazed TiAl6V4 joints. Weld. World 2015, 59, 901–909. [Google Scholar] [CrossRef]
- Luo, Y.; Chen, W.; Tian, M.; Teng, S. Thermal oxidation of Ti6Al4V alloy and its biotribological properties under serum lubrication. Tribol. Int. 2015, 89, 67–71. [Google Scholar] [CrossRef]
- Dong, H.; Bell, T. Enhanced wear resistance of titanium surfaces by a new thermal oxidation treatment. Wear 2000, 238, 131–137. [Google Scholar] [CrossRef]
- Lucia, O.; Maussion, P.; Dede, E.J.; Burdio, J.M. Induction heating technology and its applications: Past developments, current technology, and future challenges. IEEE Trans. Ind. Electron. 2014, 61, 2509–2520. [Google Scholar] [CrossRef] [Green Version]
- Li, N.-B.; Sun, S.-J.; Bai, H.-Y.; Xiao, G.-Y.; Xu, W.-H.; Zhao, J.-H.; Chen, X.; Lu, Y.-P.; Zhang, Y.-L. Preparation of well-distributed titania nanopillar arrays on Ti6Al4V surface by induction heating for enhancing osteogenic differentiation of stem cells. Nanotechnology 2017, 29, 045101. [Google Scholar] [CrossRef]
- Markovsky, P.; Semiatin, S. Tailoring of microstructure and mechanical properties of Ti–6Al–4V with local rapid (induction) heat treatment. Mater. Sci. Eng. A 2011, 528, 3079–3089. [Google Scholar] [CrossRef]
- Fomin, A.A.; Steinhauer, A.B.; Rodionov, I.; Fomina, M.A.; Zakharevich, A.M.; Skaptsov, A.A.; Gribov, A.N.; Karsakova, Y.D. Properties of titanium dioxide coatings produced by induction-thermal oxidation of VT1-00 alloy. J. Frict. Wear 2014, 35, 32–39. [Google Scholar] [CrossRef]
- Fomin, A.A.; Rodionov, I.; Fomina, M.A.; Poshivalova, E.Y.; Krasnikov, A.V.; Petrova, N.N.; Zakharevich, A.M.; Skaptsov, A.A.; Gribov, A.N.; Atkin, V.S. Induction heat treatment and technique of bioceramic coatings production on medical titanium alloys. In Proceedings of the Saratov Fall Meeting 2014, Saratov, Russia, 23–26 September 2014. [Google Scholar] [CrossRef]
- Li, N.-B.; Xu, W.-H.; Xiao, G.-Y.; Zhao, J.-H.; Lu, Y.-P. A bioactive coating with submicron-sized titania crystallites fabricated by induction heating of titanium after tensile deformations. J. Mech. Behav. Biomed. Mater. 2017, 75, 105–113. [Google Scholar] [CrossRef]
- Liu, Y.; Suslov, S.; Han, Q.; Xu, C.; Hua, L. Microstructure of the pure copper produced by upsetting with ultrasonic vibration. Mater. Lett. 2012, 67, 52–55. [Google Scholar] [CrossRef]
- Khatri, M.; Ahmed, F.; Jatoi, A.W.; Mahar, R.B.; Khatri, Z.; Kim, I.S. Ultrasonic dyeing of cellulose nanofibers. Ultrason. Sonochem. 2016, 31, 350–354. [Google Scholar] [CrossRef] [PubMed]
- Langenecker, B. Effects of ultrasound on deformation characteristics of metals. IEEE Trans. Sonics Ultrason. 1966, 13, 1–8. [Google Scholar] [CrossRef]
- Yao, Z.; Kim, G.-Y.; Wang, Z.; Faidley, L.; Zou, Q.; Mei, D.; Chen, Z. Acoustic softening and residual hardening in aluminum: Modeling and experiments. Int. J. Plast. 2012, 39, 75–87. [Google Scholar] [CrossRef]
- Rusinko, A. Analytical description of ultrasonic hardening and softening. Ultrasonics 2011, 51, 709–714. [Google Scholar] [CrossRef]
- Ao, N.; Liu, D.; Zhang, X.; Liu, C.; Yang, J.; Liu, D. Surface nanocrystallization of body-centered cubic beta phase in Ti–6Al–4V alloy subjected to ultrasonic surface rolling process. Surf. Coat. Technol. 2019, 361, 35–41. [Google Scholar] [CrossRef]
- Kofstad, P. High-temperature oxidation of titanium. J. Less Common Met. 1967, 12, 449–464. [Google Scholar] [CrossRef]
- Wen, M.; Wen, C.; Hodgson, P.; Li, Y. Thermal oxidation behaviour of bulk titanium with nanocrystalline surface layer. Corros. Sci. 2012, 59, 352–359. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, G.-R.; Zhang, X.-C.; Li, S.; Tu, S.-T. Thermal oxidation of Ti-6Al–4V alloy and pure titanium under external bending strain: Experiment and modelling. Corros. Sci. 2017, 122, 61–73. [Google Scholar] [CrossRef]
- Osada, T.; Sonoya, K.; Abe, T.; Nakamura, M.; Goto, S.; Otagiri, S. Effect of bonding conditions on the bonding strength in an aluminum bonding using ultrasonic vibrations and high-frequency induction heating in an atmosphere. Weld. Int. 2018, 32, 535–541. [Google Scholar] [CrossRef]
- Aniołek, K. Structure and properties of titanium and the Ti-6Al-7Nb alloy after isothermal oxidation. Surf. Eng. 2020, 36, 847–858. [Google Scholar] [CrossRef]
- Biswas, A.; Manna, I.; Chatterjee, U.K.; Bhattacharyya, U.; Majumdar, D.J. Evaluation of electrochemical properties of thermally oxidised Ti–6Al–4V for bioimplant application. Surf. Eng. 2009, 25, 141–145. [Google Scholar] [CrossRef]
- Uchida, M.; Kim, H.; Kokubo, T.; Fujibayashi, S.; Nakamura, T. Structural dependence of apatite formation on titania gels in a simulated body fluid. J. Biomed. Mater. Res. A 2003, 6, 164–170. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zhou, W.; Zhou, X.; Zhong, X.; Zhang, X.; Wan, P.; Zhu, B.; Chen, W. The anatase phase of nanotopography titania plays an important role on osteoblast cell morphology and proliferation. J. Mater. Sci. Mater. Med. 2008, 19, 3465–3472. [Google Scholar] [CrossRef]
- Shokouhfar, M.; Dehghanian, C.; Montazeri, M.; Baradaran, A. Preparation of ceramic coating on Ti substrate by plasma electrolytic oxidation in different electrolytes and evaluation of its corrosion resistance: Part II. Appl. Surf. Sci. 2012, 258, 2416–2423. [Google Scholar] [CrossRef]
- Naseem, S.; Khan, W.; Khan, S.; Uddin, I.; Raza, W.; Shoeb, M.; Mobin, M.; Naqvi, A.H. Enhanced photocatalytic activity by tuning of structural and optoelectrical properties of Cr(III) incorporated TiO2 nanoparticles. J. Electron. Mater. 2019, 48, 7203–7215. [Google Scholar] [CrossRef]
- Mitchell, D.; Attard, D.; Triani, G. Transmission electron microscopy studies of atomic layer deposition TiO2 films grown on silicon. Thin Solid Films 2003, 441, 85–95. [Google Scholar] [CrossRef]
- Qian, L.; Yu, P.; Zeng, J.; Shi, Z.; Wang, Q.; Tan, G.; Ning, C. Large-scale functionalization of biomedical porous titanium scaffolds surface with TiO2 nanostructures. Sci. China Mater. 2017, 61, 557–564. [Google Scholar] [CrossRef] [Green Version]
- Omidbakhsh, F.; Ebrahimi, A.R.; Sojka, J. High temperature oxidation effects on surface roughness of Ti–4Al–2V. Surf. Eng. 2013, 29, 322–327. [Google Scholar] [CrossRef]
- Junker, R.; Dimakis, A.; Thoneick, M.; Jansen, J.A. Effects of implant surface coatings and composition on bone integration: A systematic review. Clin. Oral Implant. Res. 2009, 20, 185–206. [Google Scholar] [CrossRef]
- Huang, H.-H.; Ho, C.-T.; Lee, T.-H.; Lee, T.-L.; Liao, K.-K.; Chen, F.-L. Effect of surface roughness of ground titanium on initial cell adhesion. Biomol. Eng. 2004, 21, 93–97. [Google Scholar] [CrossRef]
- Keller, J.C. Tissue compatibility to different surfaces of dental implants: In vitro studies. Implant. Dent. 1998, 7, 331–337. [Google Scholar] [CrossRef]
- Liu, J.; Suslov, S.; Li, S.; Qin, H.; Ren, Z.; Ma, C.; Wang, G.-X.; Doll, G.L.; Cong, H.; Dong, Y.; et al. Effects of ultrasonic nanocrystal surface modification on the thermal oxidation behavior of Ti6Al4V. Surf. Coat. Technol. 2017, 325, 289–298. [Google Scholar] [CrossRef]
- Wang, G.; Li, J.; Lv, K.; Zhang, W.; Ding, X.; Yang, G.; Liu, X.; Jiang, X. Surface thermal oxidation on titanium implants to enhance osteogenic activity and in vivo osseointegration. Sci. Rep. 2016, 6, 31769. [Google Scholar] [CrossRef] [Green Version]
- Bacakova, L.; Filova, E.; Parizek, M.; Ruml, T.; Svorcik, V. Modulation of cell adhesion, proliferation and differentiation on materials designed for body implants. Biotechnol. Adv. 2011, 29, 739–767. [Google Scholar] [CrossRef] [PubMed]
- El-Ghazali, S.; Khatri, M.; Hussain, N.; Khatri, Z.; Yamamoto, T.; Kim, S.H.; Kobayashi, S.; Kim, I.S. Characterization and biocompatibility evaluation of artificial blood vessels prepared from pristine poly (Ethylene-glycol-co-1,4-cyclohexane dimethylene-co-isosorbide terephthalate), poly (1, 4 cyclohexane di-methylene-co-isosorbide terephthalate) nanofibers and their blended composition. Mater. Today Commun. 2021, 26, 102113. [Google Scholar] [CrossRef]
- Pantaroto, H.N.; Cordeiro, J.M.; Pereira, L.T.; de Almeida, A.B.; Junior, F.H.N.; Rangel, E.C.; Neto, N.F.A.; da Silva, J.H.D.; Barão, V.A.R. Sputtered crystalline TiO2 film drives improved surface properties of titanium-based biomedical implants. Mater. Sci. Eng. C 2021, 119, 111638. [Google Scholar] [CrossRef] [PubMed]
- Jamesh, M.; Kumar, S.; Narayanan, T.S.N.S. Effect of thermal oxidation on corrosion resistance of commercially pure titanium in acid medium. J. Mater. Eng. Perform. 2011, 21, 900–906. [Google Scholar] [CrossRef]
- Yan, W.; Wang, X.X. Surface hardening of titanium by thermal oxidation. J. Mater. Sci. 2004, 39, 5583–5585. [Google Scholar] [CrossRef]
- Roy, M.; Whiteside, L.; Xu, J.; Katerberg, B. Diamond-like carbon coatings enhance the hardness and resilience of bearing surfaces for use in joint arthroplasty. Acta Biomater. 2010, 6, 1619–1624. [Google Scholar] [CrossRef]
- Vasylyev, M.; Chenakin, S.; Yatsenko, L. Ultrasonic impact treatment induced oxidation of Ti6Al4V alloy. Acta Mater. 2016, 103, 761–774. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, H.; Yu, M.; Chen, X.; Xiao, G.; Chen, C.; Liu, B.; Lu, Y. Ultrasonic Induced Refinement of Induction Heated Oxide Coating on Titanium. Coatings 2021, 11, 812. https://doi.org/10.3390/coatings11070812
Gao H, Yu M, Chen X, Xiao G, Chen C, Liu B, Lu Y. Ultrasonic Induced Refinement of Induction Heated Oxide Coating on Titanium. Coatings. 2021; 11(7):812. https://doi.org/10.3390/coatings11070812
Chicago/Turabian StyleGao, Han, Meijie Yu, Xin Chen, Guiyong Xiao, Chuanzhong Chen, Bing Liu, and Yupeng Lu. 2021. "Ultrasonic Induced Refinement of Induction Heated Oxide Coating on Titanium" Coatings 11, no. 7: 812. https://doi.org/10.3390/coatings11070812
APA StyleGao, H., Yu, M., Chen, X., Xiao, G., Chen, C., Liu, B., & Lu, Y. (2021). Ultrasonic Induced Refinement of Induction Heated Oxide Coating on Titanium. Coatings, 11(7), 812. https://doi.org/10.3390/coatings11070812