Application of Bone Morphogenetic Protein 7 Enhanced the Osteogenic Differentiation and Mineralization of Bone Marrow-Derived Stem Cells Cultured on Deproteinized Bovine Bone
Abstract
1. Introduction
2. Materials and Methods
2.1. Culturing of Bone Marrow-Derived Mesenchymal Stem Cells on Bone
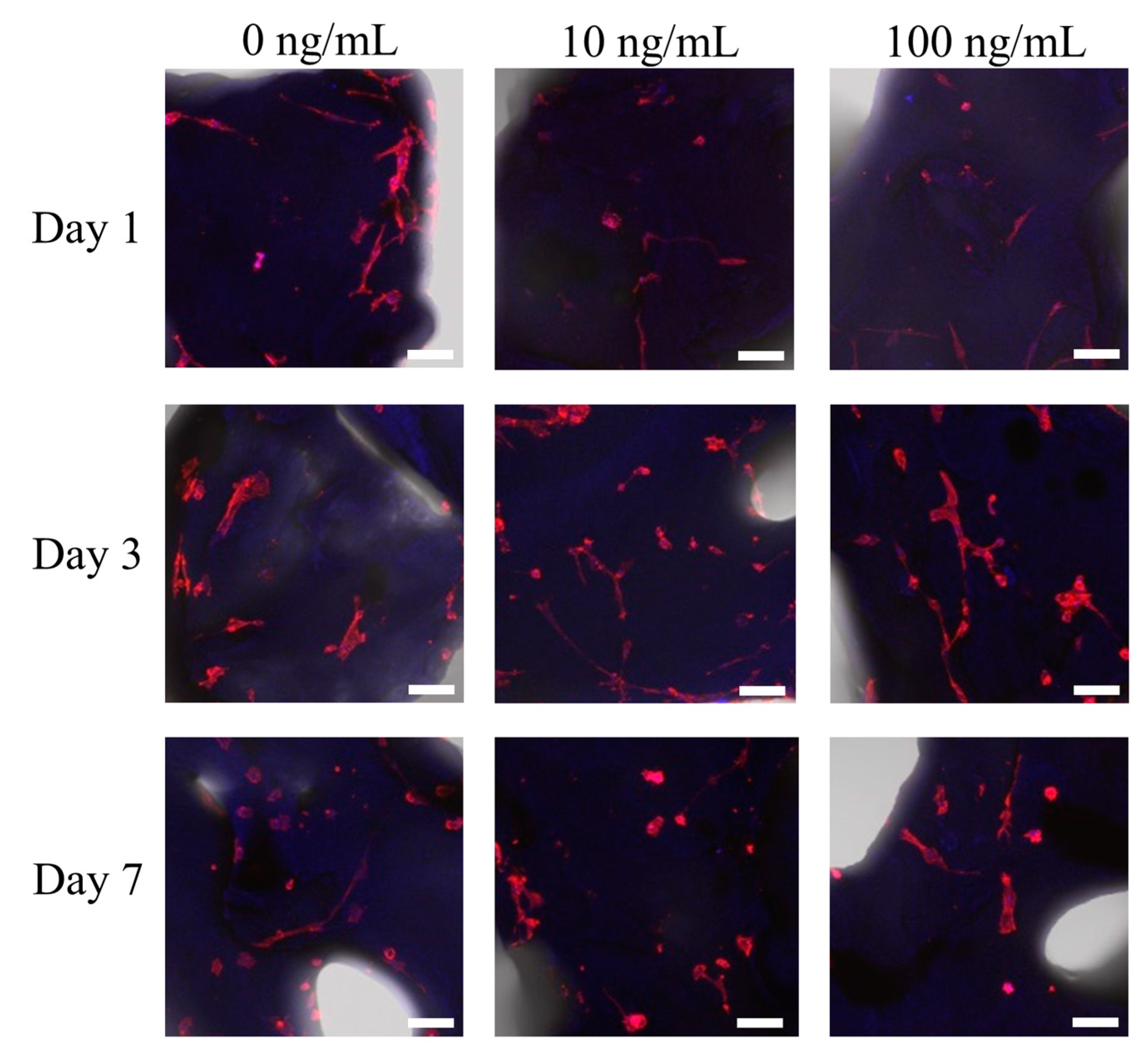
2.2. Morphologic Evaluation of Stem Cells
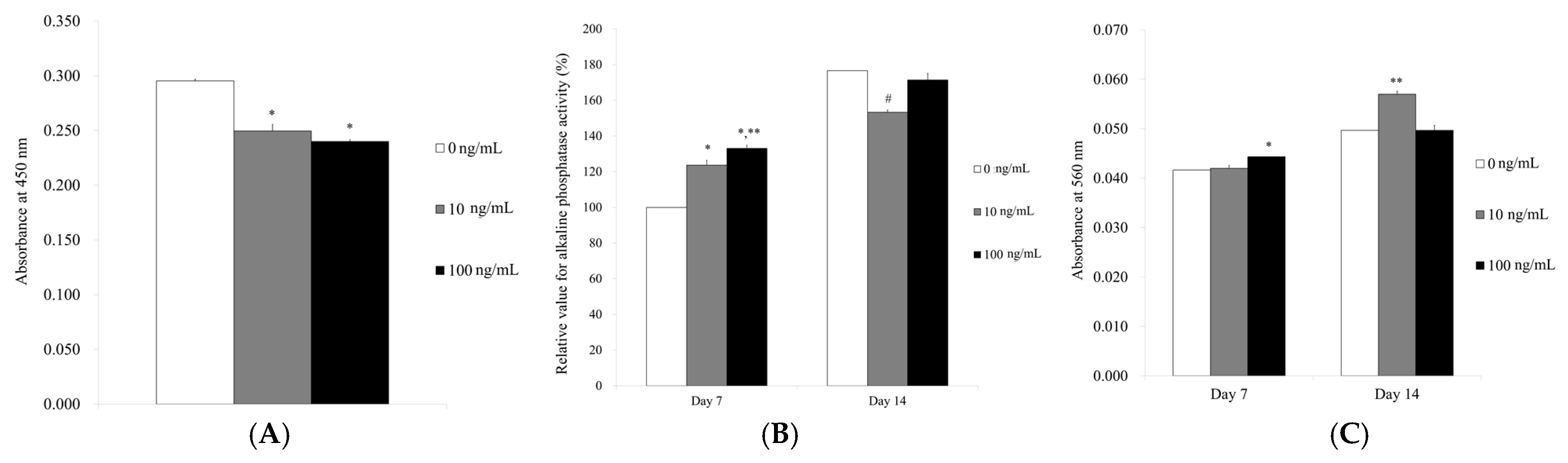
2.3. Determination of Cellular Viability
2.4. Level of Alkaline Phosphatase Activity and Calcium Deposition
2.5. Extraction of RNA and Sequencing of mRNA
2.6. Statistical Analysis
3. Results
3.1. Cell Attachment of Stem Cells to the Deproteinized Bovine Bone Mineral
3.2. Determination of Cellular Viability
3.3. Level of Alkaline Phosphatase Activity and Calcium Deposition
3.4. Evaluation of Gene Ontology and Pathway Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Benjamin, B.J. Normal aging and the multicultural population. Semin. Speech Lang. 1997, 18, 127–134. [Google Scholar] [CrossRef]
- Bouwknegt, M.; van Pelt, W.; Havelaar, A.H. Scoping the impact of changes in population age-structure on the future burden of foodborne disease in the Netherlands, 2020-2060. Int. J. Environ. Res. Public Health 2013, 10, 2888–2896. [Google Scholar] [CrossRef] [PubMed]
- Frencken, J.E.; Sharma, P.; Stenhouse, L.; Green, D.; Laverty, D.; Dietrich, T. Global epidemiology of dental caries and severe periodontitis—a comprehensive review. J. Clin. Periodontol. 2017, 44 (Suppl. S18), S94–S105. [Google Scholar] [CrossRef]
- Benjamin, R.M. Oral health: The silent epidemic. Public Health Rep. 2010, 125, 158–159. [Google Scholar] [CrossRef] [PubMed]
- Vennedey, V.; Derman, S.H.; Hiligsmann, M.; Civello, D.; Schwalm, A.; Seidl, A.; Scheibler, F.; Stock, S.; Noack, M.J.; Danner, M. Patients’ preferences in periodontal disease treatment elicited alongside an IQWiG benefit assessment: A feasibility study. Patient Prefer. Adherence 2018, 12, 2437–2447. [Google Scholar] [CrossRef]
- Krafts, K.P. Tissue repair: The hidden drama. Organogenesis 2010, 6, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Seetharaman, R.; Mahmood, A.; Kshatriya, P.; Patel, D.; Srivastava, A. An Overview on Stem Cells in Tissue Regeneration. Curr. Pharm. Des. 2019, 25, 2086–2098. [Google Scholar] [CrossRef] [PubMed]
- Mao, A.S.; Mooney, D.J. Regenerative medicine: Current therapies and future directions. Proc. Natl. Acad. Sci. USA 2015, 112, 14452–14459. [Google Scholar] [CrossRef]
- Amini, A.R.; Laurencin, C.T.; Nukavarapu, S.P. Bone tissue engineering: Recent advances and challenges. Crit. Rev. Biomed. Eng. 2012, 40, 363–408. [Google Scholar] [CrossRef] [PubMed]
- Lo, K.W.; Ulery, B.D.; Ashe, K.M.; Laurencin, C.T. Studies of bone morphogenetic protein-based surgical repair. Adv. Drug Deliv. Rev. 2012, 64, 1277–1291. [Google Scholar] [CrossRef]
- Acil, Y.; Springer, I.N.; Broek, V.; Terheyden, H.; Jepsen, S. Effects of bone morphogenetic protein-7 stimulation on osteoblasts cultured on different biomaterials. J. Cell. Biochem. 2002, 86, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Roldan, J.C.; Jepsen, S.; Miller, J.; Freitag, S.; Rueger, D.C.; Acil, Y.; Terheyden, H. Bone formation in the presence of platelet-rich plasma vs. bone morphogenetic protein-7. Bone 2004, 34, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Terheyden, H.; Knak, C.; Jepsen, S.; Palmie, S.; Rueger, D.R. Mandibular reconstruction with a prefabricated vascularized bone graft using recombinant human osteogenic protein-1: An experimental study in miniature pigs. Part I: Prefabrication. Int. J. Oral Maxillofac. Surg. 2001, 30, 373–379. [Google Scholar] [CrossRef] [PubMed]
- McAllister, B.S.; Margolin, M.D.; Cogan, A.G.; Taylor, M.; Wollins, J. Residual lateral wall defects following sinus grafting with recombinant human osteogenic protein-1 or Bio-Oss in the chimpanzee. Int. J. Periodontics Restor. Dent. 1998, 18, 227–239. [Google Scholar]
- Min, Q.; Liu, J.; Zhang, Y.; Yang, B.; Wan, Y.; Wu, J. Dual Network Hydrogels Incorporated with Bone Morphogenic Protein-7-Loaded Hyaluronic Acid Complex Nanoparticles for Inducing Chondrogenic Differentiation of Synovium-Derived Mesenchymal Stem Cells. Pharmaceutics 2020, 12, 613. [Google Scholar] [CrossRef]
- Jeong, C.H.; Kim, S.M.; Lim, J.Y.; Ryu, C.H.; Jun, J.A.; Jeun, S.S. Mesenchymal stem cells expressing brain-derived neurotrophic factor enhance endogenous neurogenesis in an ischemic stroke model. BioMed Res. Int. 2014, 2014, 129145. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kim, M.; Park, Y.H.; Park, J.B. Dexamethasone downregulates SIRT1 and IL6 and upregulates EDN1 genes in stem cells derived from gingivae via the AGE/RAGE pathway. Biotechnol. Lett. 2018, 40, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Trapnell, C.; Pachter, L.; Salzberg, S.L. TopHat: Discovering splice junctions with RNA-Seq. Bioinformatics 2009, 25, 1105–1111. [Google Scholar] [CrossRef]
- Quinlan, A.R.; Hall, I.M. BEDTools: A flexible suite of utilities for comparing genomic features. Bioinformatics 2010, 26, 841–842. [Google Scholar] [CrossRef] [PubMed]
- Gentleman, R.C.; Carey, V.J.; Bates, D.M.; Bolstad, B.; Dettling, M.; Dudoit, S.; Ellis, B.; Gautier, L.; Ge, Y.; Gentry, J.; et al. Bioconductor: Open software development for computational biology and bioinformatics. Genome Biol. 2004, 5, R80. [Google Scholar] [CrossRef]
- Ellman, M.B.; Kim, J.; An, H.S.; Chen, D.; Kc, R.; Li, X.; Xiao, G.; Yan, D.; Suh, J.; van Wijnen, A.J.; et al. Lactoferricin enhances BMP7-stimulated anabolic pathways in intervertebral disc cells. Gene 2013, 524, 282–291. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Huang, X.; Zhong, L.; Post, J.N.; Karperien, M. Co-treatment of TGF-β3 and BMP7 is superior in stimulating chondrocyte redifferentiation in both hypoxia and normoxia compared to single treatments. Sci. Rep. 2018, 8, 10251. [Google Scholar] [CrossRef]
- Harding, A.K.; Aspenberg, P.; Kataoka, M.; Bylski, D.; Tägil, M. Manipulating the anabolic and catabolic response in bone graft remodeling: Synergism by a combination of local BMP-7 and a single systemic dosis of zoledronate. J. Orthop. Res. 2008, 26, 1245–1249. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Min, S.K.; Song, Y.; Park, Y.H.; Park, J.B. Bone morphogenetic protein-7 upregulates genes associated with osteoblast differentiation, including collagen I, Sp7 and IBSP in gingiva-derived stem cells. Exp. Ther. Med. 2019, 18, 2867–2876. [Google Scholar] [CrossRef]
- Torii, D.; Tsutsui, T.W.; Watanabe, N.; Konishi, K. Bone morphogenetic protein 7 induces cementogenic differentiation of human periodontal ligament-derived mesenchymal stem cells. Odontology 2016, 104, 1–9. [Google Scholar] [CrossRef]
- Kim, B.B.; Tae, J.Y.; Ko, Y.; Park, J.B. Lovastatin increases the proliferation and osteoblastic differentiation of human gingiva-derived stem cells in three-dimensional cultures. Exp. Ther. Med. 2019, 18, 3425–3430. [Google Scholar] [CrossRef]
- Zakhary, K.; Motakis, D.; Hamdy, R.H.; Campisi, P.; Amar, Y.; Lessard, M.L. Effect of recombinant human bone morphogenetic protein 7 on bone density during distraction osteogenesis of the rabbit mandible. J. Otolaryngol. 2005, 34, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Terheyden, H.; Warnke, P.; Dunsche, A.; Jepsen, S.; Brenner, W.; Palmie, S.; Toth, C.; Rueger, D.R. Mandibular reconstruction with prefabricated vascularized bone grafts using recombinant human osteogenic protein-1: An experimental study in miniature pigs. Part II: Transplantation. Int. J. Oral Maxillofac. Surg. 2001, 30, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Terheyden, H.; Menzel, C.; Wang, H.; Springer, I.N.; Rueger, D.R.; Acil, Y. Prefabrication of vascularized bone grafts using recombinant human osteogenic protein-1-part 3: Dosage of rhOP-1, the use of external and internal scaffolds. Int. J. Oral Maxillofac. Surg. 2004, 33, 164–172. [Google Scholar] [CrossRef]
- Hakki, S.S.; Bozkurt, B.; Hakki, E.E.; Kayis, S.A.; Turac, G.; Yilmaz, I.; Karaoz, E. Bone morphogenetic protein-2, -6, and -7 differently regulate osteogenic differentiation of human periodontal ligament stem cells. J. Biomed Mater. Res. B Appl. Biomater. 2014, 102, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Loebel, C.; Burdick, J.A. Engineering Stem and Stromal Cell Therapies for Musculoskeletal Tissue Repair. Cell Stem Cell 2018, 22, 325–339. [Google Scholar] [CrossRef] [PubMed]
- Amcheslavsky, A.; Jiang, J.; Ip, Y.T. Tissue damage-induced intestinal stem cell division in Drosophila. Cell Stem Cell 2009, 4, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Iismaa, S.E.; Kaidonis, X.; Nicks, A.M.; Bogush, N.; Kikuchi, K.; Naqvi, N.; Harvey, R.P.; Husain, A.; Graham, R.M. Comparative regenerative mechanisms across different mammalian tissues. NPJ Regen. Med. 2018, 3, 6. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Chen, J.; Liu, S.; Jin, Y. Stem cell-based bone and dental regeneration: A view of microenvironmental modulation. Int. J. Oral Sci. 2019, 11, 23. [Google Scholar] [CrossRef]
- Zakrzewski, W.; Dobrzyński, M.; Szymonowicz, M.; Rybak, Z. Stem cells: Past, present, and future. Stem Cell Res. Ther. 2019, 10, 68. [Google Scholar] [CrossRef]
- Zhu, L.; Ma, J.; Mu, R.; Zhu, R.; Chen, F.; Wei, X.; Shi, X.; Zang, S.; Jin, L. Bone morphogenetic protein 7 promotes odontogenic differentiation of dental pulp stem cells in vitro. Life Sci. 2018, 202, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Schwarting, T.; Benölken, M.; Ruchholtz, S.; Frink, M.; Lechler, P. Bone morphogenetic protein-7 enhances bone-tendon integration in a murine in vitro co-culture model. Int. Orthop. 2015, 39, 799–805. [Google Scholar] [CrossRef]
- Tollemar, V.; Collier, Z.J.; Mohammed, M.K.; Lee, M.J.; Ameer, G.A.; Reid, R.R. Stem cells, growth factors and scaffolds in craniofacial regenerative medicine. Genes Dis. 2016, 3, 56–71. [Google Scholar] [CrossRef] [PubMed]
- Nie, W.B.; Zhang, D.; Wang, L.S. Growth Factor Gene-Modified Mesenchymal Stem Cells in Tissue Regeneration. Drug Des. Devel. Ther. 2020, 14, 1241–1256. [Google Scholar] [CrossRef]
- Nakamura, T.; Mizuno, S. The discovery of hepatocyte growth factor (HGF) and its significance for cell biology, life sciences and clinical medicine. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2010, 86, 588–610. [Google Scholar] [CrossRef]
- Biehl, J.K.; Russell, B. Introduction to stem cell therapy. J. Cardiovasc. Nurs. 2009, 24, 98–103; quiz 104–105. [Google Scholar] [CrossRef] [PubMed]
- Terheyden, H.; Jepsen, S.; Vogeler, S.; Tucker, M.; Rueger, D.C. Recombinant human osteogenic protein 1 in the rat mandibular augmentation model: Differences in morphology of the newly formed bone are dependent on the type of carrier. Mund Kiefer Gesichtschir. 1997, 1, 272–275. [Google Scholar] [CrossRef]
- Khojasteh, A.; Ghahremani, M.H.; Ostad, S.N.; Eslami, M.; Motahhary, P.; Morad, G.; Shidfar, S. The effect of deproteinized bovine bone mineral on saos-2 cell proliferation. Iran. Endod. J. 2013, 8, 118–122. [Google Scholar]
- Kim, B.B.; Kim, M.; Park, Y.H.; Ko, Y.; Park, J.B. Short-term application of dexamethasone on stem cells derived from human gingiva reduces the expression of RUNX2 and β-catenin. J. Int. Med. Res. 2017, 45, 993–1006. [Google Scholar] [CrossRef] [PubMed]
- Su, D.; Yi, L.; Guan, L.; Li, Q.; Shi, C.; Ma, X. Sequencing Analysis of mRNA Profile in Endothelial Cells in Response to ox-LDL. Biochem. Genet. 2021, 59, 767–780. [Google Scholar] [CrossRef]
- Kang, S.H.; Park, J.B.; Kim, I.; Lee, W.; Kim, H. Assessment of stem cell viability in the initial healing period in rabbits with a cranial bone defect according to the type and form of scaffold. J. Periodontal Implant Sci. 2019, 49, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Turner, P.A.; Thiele, J.S.; Stegemann, J.P. Growth factor sequestration and enzyme-mediated release from genipin-crosslinked gelatin microspheres. J. Biomater. Sci. Polym. Ed. 2017, 28, 1826–1846. [Google Scholar] [CrossRef]
- Mitchell, A.C.; Briquez, P.S.; Hubbell, J.A.; Cochran, J.R. Engineering growth factors for regenerative medicine applications. Acta Biomater. 2016, 30, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ding, L.; Zhao, Y.; Sun, W.; Chen, B.; Lin, H.; Wang, X.; Zhang, L.; Xu, B.; Dai, J. Collagen-targeting vascular endothelial growth factor improves cardiac performance after myocardial infarction. Circulation 2009, 119, 1776–1784. [Google Scholar] [CrossRef] [PubMed]
- Barrientos, S.; Brem, H.; Stojadinovic, O.; Tomic-Canic, M. Clinical application of growth factors and cytokines in wound healing. Wound Repair Regen. 2014, 22, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Kämmerer, P.W.; Pabst, A.M.; Dau, M.; Staedt, H.; Al-Nawas, B.; Heller, M. Immobilization of BMP-2, BMP-7 and alendronic acid on titanium surfaces: Adhesion, proliferation and differentiation of bone marrow-derived stem cells. J. Biomed. Mater. Res. A 2020, 108, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Zhou, Z.; Guo, L.; Zeng, Z.; Guo, Z.; Shao, Q.; Xu, W. BMP7-overexpressing bone marrow-derived mesenchymal stem cells (BMSCs) are more effective than wild-type BMSCs in healing fractures. Exp. Ther. Med. 2018, 16, 1381–1388. [Google Scholar] [CrossRef] [PubMed]
- Wada, K.; Gerbaudo, V.H.; Spector, M. Effects of PDGF-BB and OP-1 on mesenchymal stem cells in a porous mineral block. Int. J. Periodontics Restor. Dent. 2013, 33, e72–e78. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Guo, J.; Wu, G.; Zhou, Y. Effects of heterodimeric bone morphogenetic protein-2/7 on osteogenesis of human adipose-derived stem cells. Cell Prolif. 2015, 48, 650–660. [Google Scholar] [CrossRef] [PubMed]
- Gaihre, B.; Unagolla, J.M.; Liu, J.; Ebraheim, N.A.; Jayasuriya, A.C. Thermoresponsive Injectable Microparticle-Gel Composites with Recombinant BMP-9 and VEGF Enhance Bone Formation in Rats. ACS Biomater. Sci. Eng. 2019, 5, 4587–4600. [Google Scholar] [CrossRef] [PubMed]
- Hettiaratchi, M.H.; Krishnan, L.; Rouse, T.; Chou, C.; McDevitt, T.C.; Guldberg, R.E. Heparin-mediated delivery of bone morphogenetic protein-2 improves spatial localization of bone regeneration. Sci. Adv. 2020, 6, eaay1240. [Google Scholar] [CrossRef] [PubMed]
- Chiari, C.; Grgurevic, L.; Bordukalo-Niksic, T.; Oppermann, H.; Valentinitsch, A.; Nemecek, E.; Staats, K.; Schreiner, M.; Trost, C.; Kolb, A.; et al. Recombinant Human BMP6 Applied Within Autologous Blood Coagulum Accelerates Bone Healing: Randomized Controlled Trial in High Tibial Osteotomy Patients. J. Bone Miner. Res. 2020, 35, 1893–1903. [Google Scholar] [CrossRef]

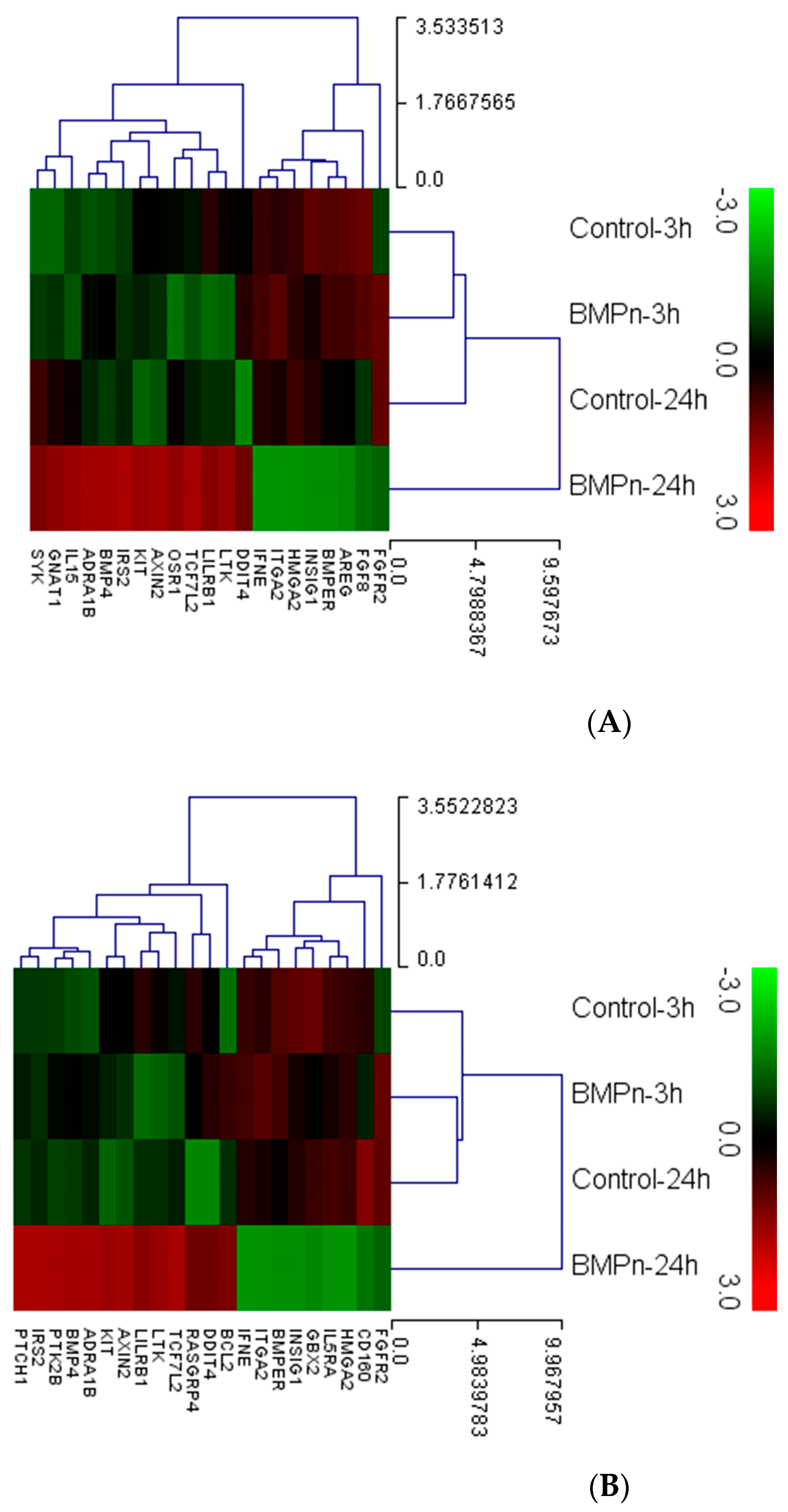
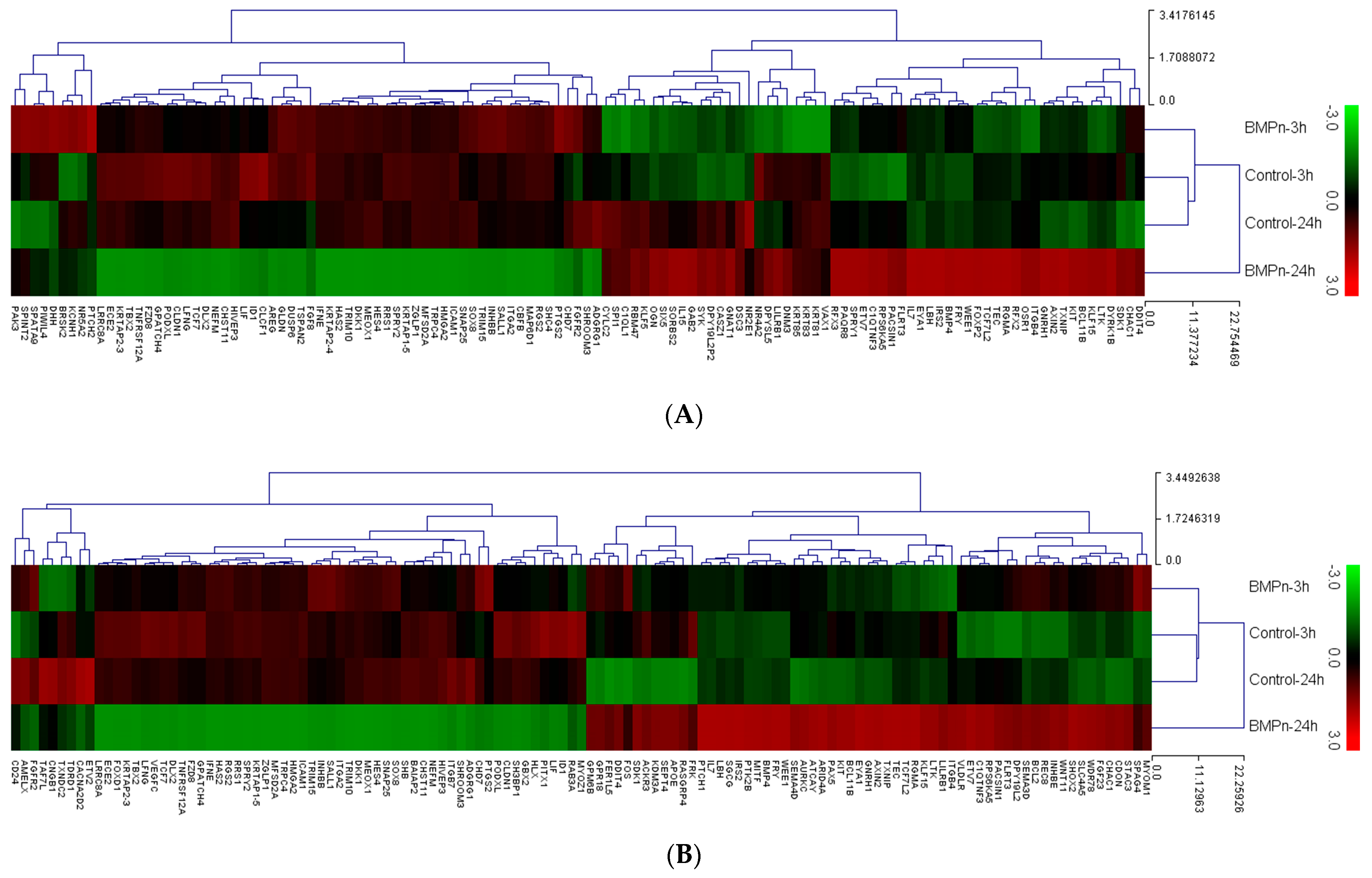



| Gene Symbol | BMP-7 24 h/Control 24 h | Gene Symbol | BMP-7 24 h/BMP-7 3 h |
|---|---|---|---|
| INSIG1 | 0.329 | ITGA2 | 0.338 |
| HMGA2 | 0.391 | INSIG1 | 0.358 |
| ITGA2 | 0.428 | BMPER | 0.401 |
| BMPER | 0.494 | HMGA2 | 0.410 |
| LTK | 2.141 | FGF8 | 0.497 |
| PTCH1 | 2.172 | BMP4 | 2.073 |
| TCF7L2 | 2.304 | OSR1 | 2.129 |
| PTK2B | 2.408 | DDIT4 | 2.157 |
| ADRA1B | 2.441 | ADRA1B | 2.174 |
| IRS2 | 2.604 | IL15 | 2.510 |
| BMP4 | 2.805 | LTK | 2.628 |
| AXIN2 | 3.781 | IRS2 | 2.696 |
| KIT | 15.821 | TCF7L2 | 2.890 |
| DDIT4 | 20.173 | AXIN2 | 3.073 |
| - | - | KIT | 7.478 |
| Gene Symbol | BMP-7 24 h/Control 24 h | Gene Symbol | BMP-7 24 h/BMP-7 3 h |
|---|---|---|---|
| PTPN22 | 0.039 | DHH | 0.034 |
| RAB3A | 0.065 | MEOX1 | 0.045 |
| NR4A2 | 0.125 | TRIM10 | 0.053 |
| PITX1 | 0.159 | TRIM15 | 0.110 |
| VAX1 | 0.184 | DKK1 | 0.117 |
| PODXL | 0.197 | FZD8 | 0.166 |
| SNAI1 | 0.209 | KRTAP2-3 | 0.222 |
| HCLS1 | 0.220 | PTGS2 | 0.238 |
| CLDN1 | 0.236 | HES4 | 0.248 |
| LFNG | 0.237 | LFNG | 0.261 |
| ANKRD1 | 0.238 | MFSD2A | 0.273 |
| MYOZ1 | 0.250 | INHBB | 0.281 |
| LIF | 0.256 | ADGRG1 | 0.294 |
| CLCF1 | 0.274 | LIF | 0.298 |
| ETV4 | 0.279 | TRPC4 | 0.298 |
| DNAH1 | 0.314 | PTCH2 | 0.320 |
| MECOM | 0.323 | CHD7 | 0.324 |
| HSF4 | 0.340 | KRTAP1-5 | 0.330 |
| SEMA3D | 0.365 | ICAM1 | 0.334 |
| CEP290 | 0.365 | ITGA2 | 0.338 |
| EGR1 | 0.370 | RRS1 | 0.343 |
| TET1 | 0.371 | KCNH1 | 0.344 |
| MAK | 0.376 | TNFRSF12A | 0.346 |
| REC8 | 0.380 | PODXL | 0.350 |
| IGSF10 | 0.391 | CLDN1 | 0.353 |
| SYNE2 | 0.404 | RGS2 | 0.354 |
| CHAC1 | 0.408 | SALL1 | 0.360 |
| JUP | 0.411 | NEFM | 0.380 |
| PTCH2 | 0.412 | HAS2 | 0.382 |
| BATF2 | 0.414 | CHST11 | 0.400 |
| HRNR | 0.418 | HMGA2 | 0.410 |
| PRDM16 | 0.419 | DLX2 | 0.410 |
| DHH | 0.420 | ECE2 | 0.421 |
| KCNH1 | 0.428 | TBX2 | 0.423 |
| - | 0.429 | CBFB | 0.427 |
| - | 0.435 | SHC4 | 0.427 |
| - | 0.443 | TCF7 | 0.429 |
| - | 0.444 | SNAP25 | 0.430 |
| - | 0.447 | HIVEP3 | 0.432 |
| - | 0.451 | SPRY2 | 0.448 |
| - | 0.457 | MAP6D1 | 0.448 |
| - | 0.457 | ID1 | 0.449 |
| - | 0.464 | PIWIL4 | 0.453 |
| - | 0.479 | CLCF1 | 0.463 |
| - | 0.488 | GPATCH4 | 0.464 |
| - | 0.489 | GLDN | 0.476 |
| - | 0.491 | DUSP6 | 0.476 |
| - | 0.494 | SHROOM3 | 0.478 |
| - | 2.063 | FGF8 | 0.497 |
| - | 2.141 | LRRC8A | 0.497 |
| - | 2.165 | RBM47 | 2.004 |
| - | 2.169 | ETV7 | 2.023 |
| - | 2.172 | DYRK1B | 2.047 |
| - | 2.174 | DPY19L2P2 | 2.060 |
| - | 2.184 | BMP4 | 2.073 |
| - | 2.206 | CYLC2 | 2.095 |
| - | 2.304 | WEE1 | 2.122 |
| - | 2.319 | KLF5 | 2.123 |
| - | 2.346 | OSR1 | 2.129 |
| - | 2.350 | PAQR8 | 2.135 |
| - | 2.368 | KRT81 | 2.138 |
| - | 2.408 | DDIT4 | 2.157 |
| - | 2.417 | SIX5 | 2.190 |
| - | 2.449 | DNM3 | 2.193 |
| - | 2.554 | CHAC1 | 2.294 |
| - | 2.604 | RFX3 | 2.303 |
| - | 2.609 | FOXP2 | 2.421 |
| - | 2.625 | EYA1 | 2.464 |
| - | 2.661 | IL15 | 2.510 |
| - | 2.682 | LBH | 2.558 |
| - | 2.684 | GAB2 | 2.560 |
| - | 2.744 | SPRY1 | 2.594 |
| - | 2.788 | LTK | 2.628 |
| - | 2.805 | TXNIP | 2.662 |
| - | 2.830 | RFX2 | 2.675 |
| - | 2.893 | NR4A2 | 2.683 |
| - | 2.973 | IRS2 | 2.696 |
| - | 3.227 | RPS6KA5 | 2.745 |
| - | 3.297 | GNRH1 | 2.798 |
| - | 3.428 | TCF7L2 | 2.890 |
| - | 3.455 | DPYSL5 | 2.985 |
| - | 3.547 | FRY | 3.013 |
| - | 3.781 | AXIN2 | 3.073 |
| - | 3.846 | TEC | 3.268 |
| - | 4.084 | SORBS2 | 3.564 |
| - | 5.012 | OGN | 3.994 |
| - | 11.467 | KLF15 | 6.356 |
| - | 15.821 | KIT | 7.478 |
| - | 20.173 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.-J.; Min, S.-K.; Park, Y.-H.; Park, J.-B. Application of Bone Morphogenetic Protein 7 Enhanced the Osteogenic Differentiation and Mineralization of Bone Marrow-Derived Stem Cells Cultured on Deproteinized Bovine Bone. Coatings 2021, 11, 642. https://doi.org/10.3390/coatings11060642
Lee H-J, Min S-K, Park Y-H, Park J-B. Application of Bone Morphogenetic Protein 7 Enhanced the Osteogenic Differentiation and Mineralization of Bone Marrow-Derived Stem Cells Cultured on Deproteinized Bovine Bone. Coatings. 2021; 11(6):642. https://doi.org/10.3390/coatings11060642
Chicago/Turabian StyleLee, Hyun-Jin, Sae-Kyung Min, Yoon-Hee Park, and Jun-Beom Park. 2021. "Application of Bone Morphogenetic Protein 7 Enhanced the Osteogenic Differentiation and Mineralization of Bone Marrow-Derived Stem Cells Cultured on Deproteinized Bovine Bone" Coatings 11, no. 6: 642. https://doi.org/10.3390/coatings11060642
APA StyleLee, H.-J., Min, S.-K., Park, Y.-H., & Park, J.-B. (2021). Application of Bone Morphogenetic Protein 7 Enhanced the Osteogenic Differentiation and Mineralization of Bone Marrow-Derived Stem Cells Cultured on Deproteinized Bovine Bone. Coatings, 11(6), 642. https://doi.org/10.3390/coatings11060642







