Impact of Water-Repellent Products on the Moisture Transport Properties and Mould Susceptibility of External Thermal Insulation Composite Systems
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. ETICS Specimens
2.1.2. Hydrophobic Protection Products
2.2. Methods
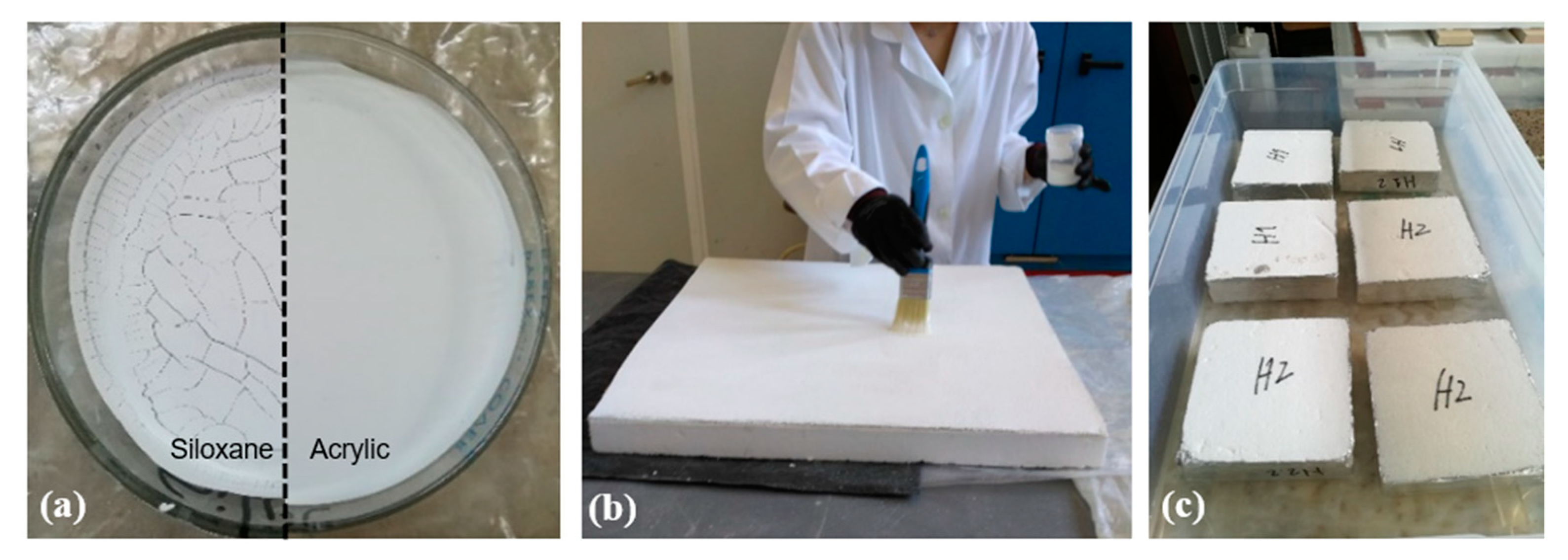
2.2.1. Application Protocol
2.2.2. Specimens
2.2.3. Moisture Transport Properties
2.2.4. Optical Microscopy
2.2.5. Susceptibility to Mould Growth
2.2.6. Accelerated Aging Test
3. Results
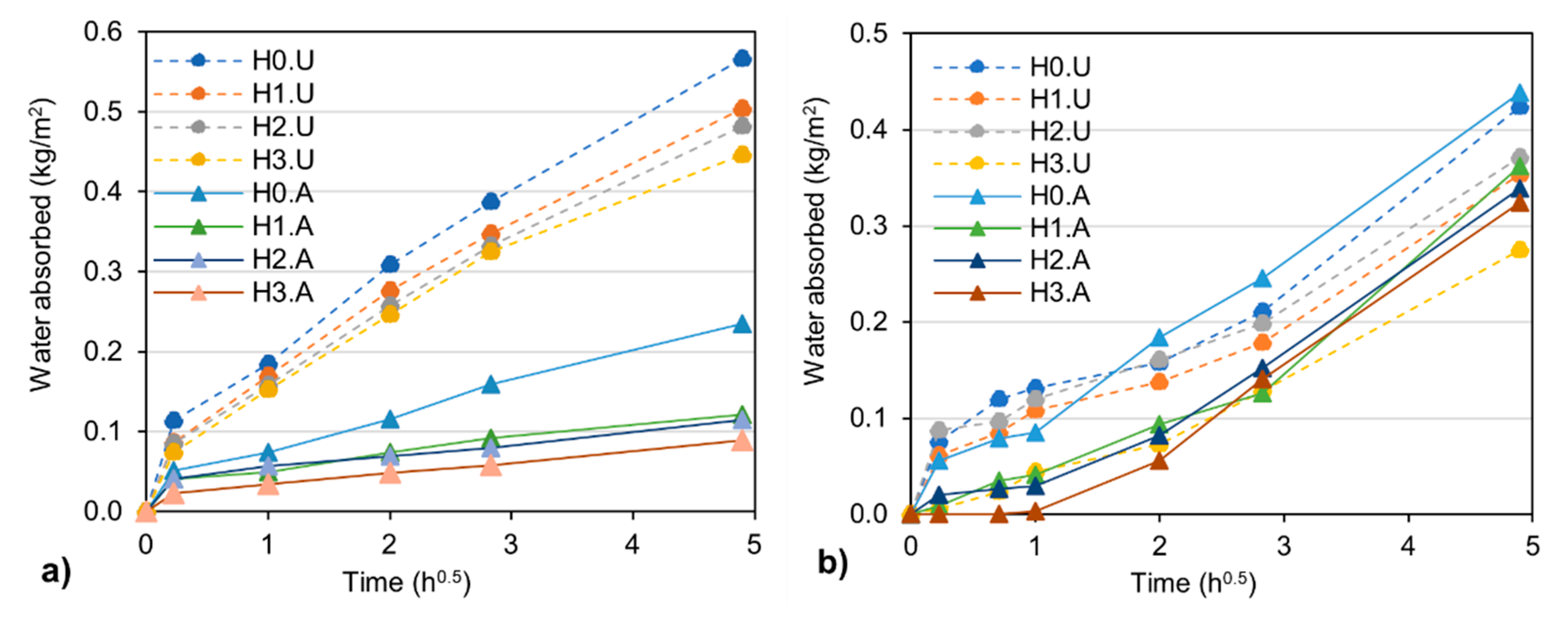
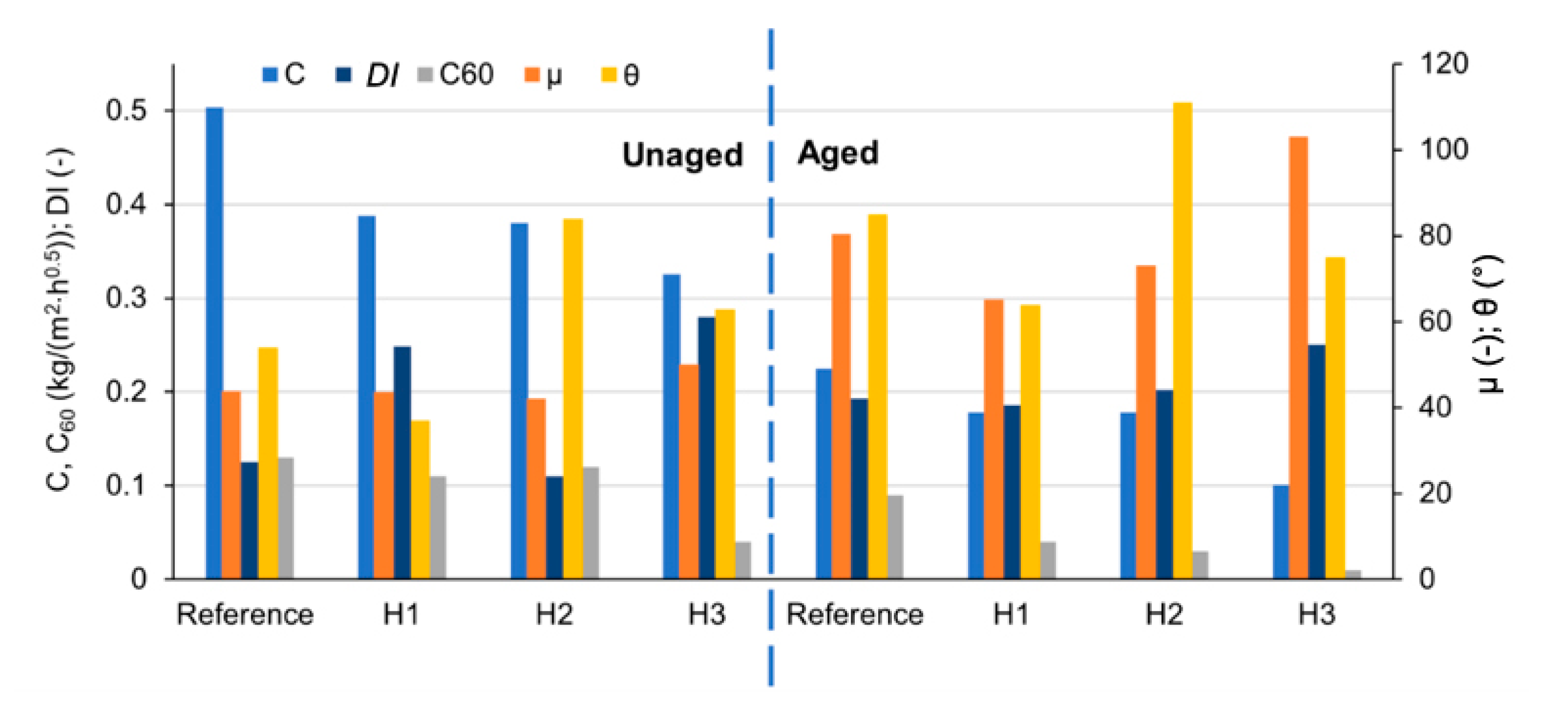
3.1. Capillary Water Absorption and Water Absorption under Low Pressure
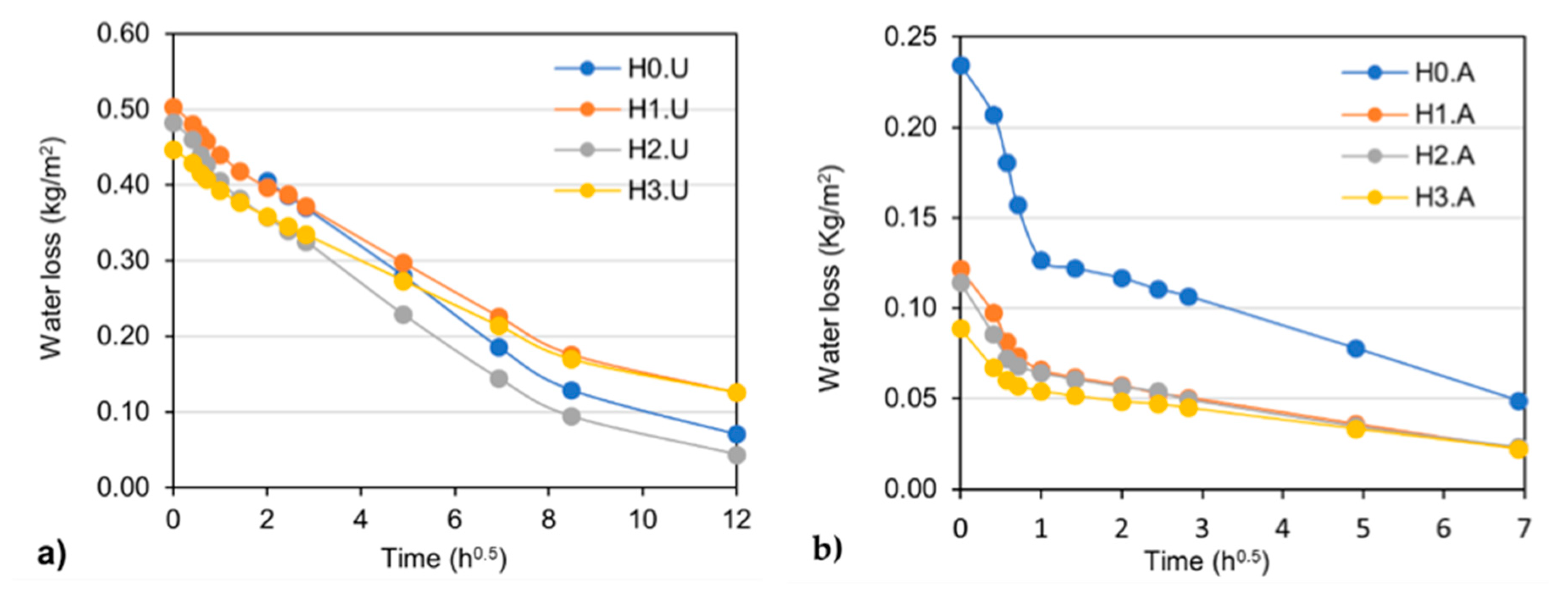
3.2. Drying
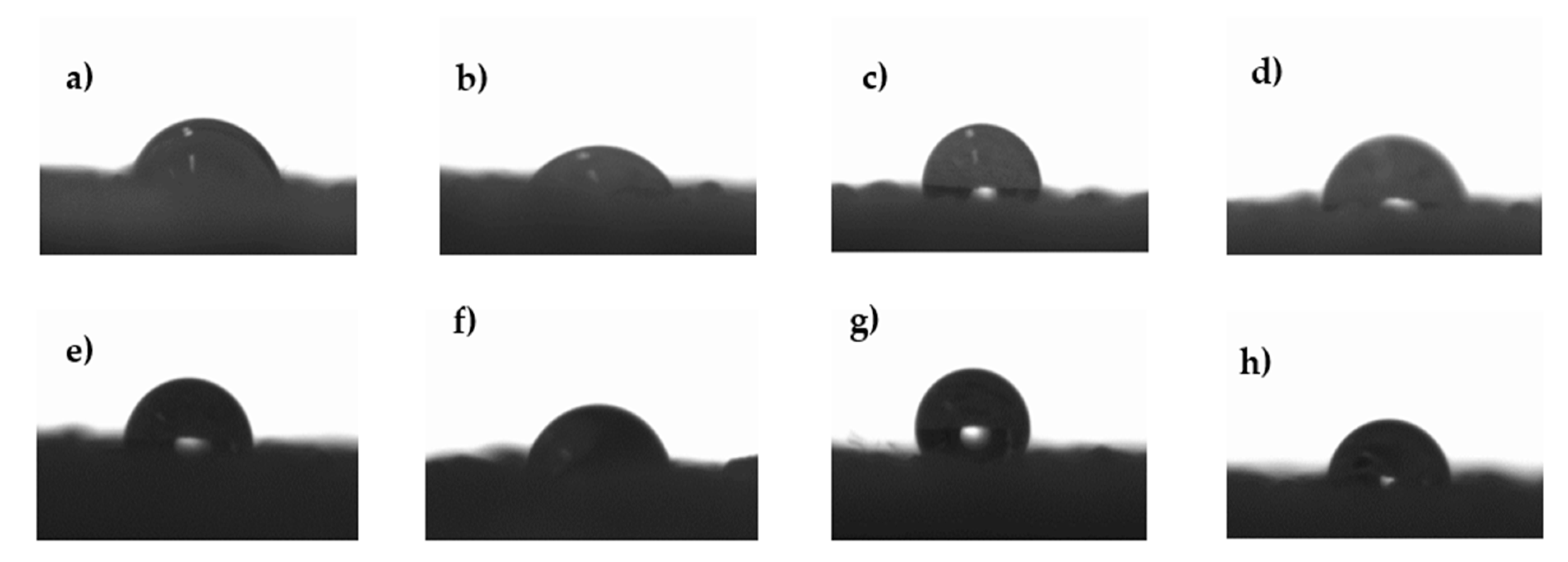
3.3. Contact Angle
3.4. Water Vapor Permeability
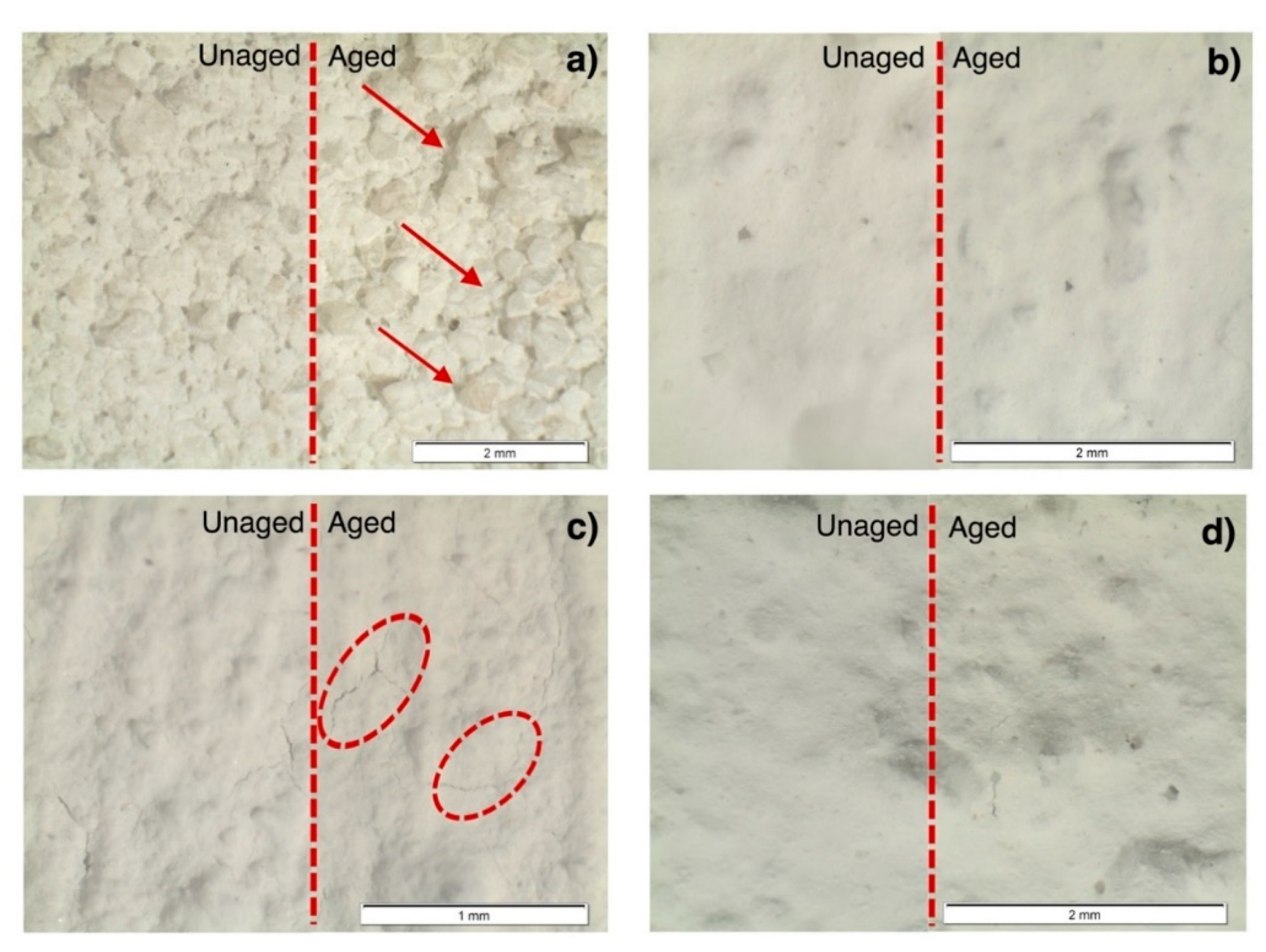
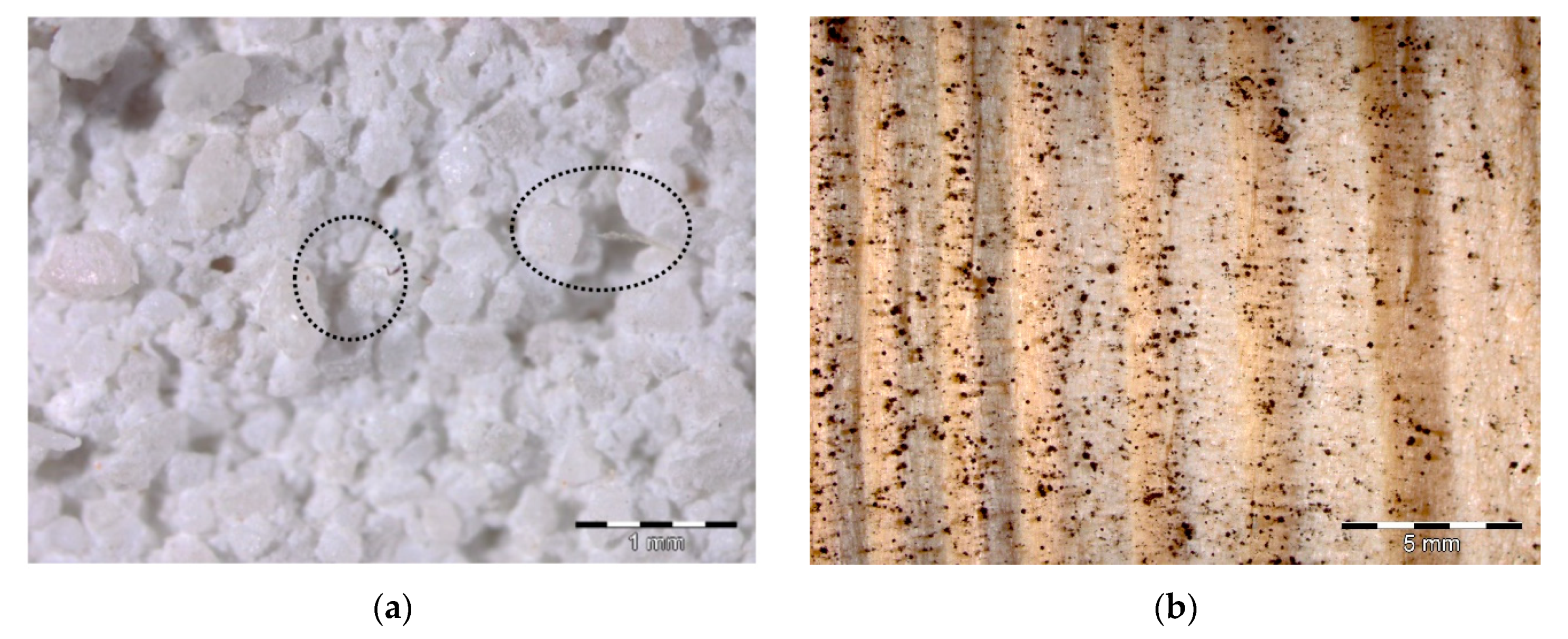
3.5. Optical Microscopy
3.6. Susceptibility to Mould Development
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Energy Performance of Building Directive (EPBD). Directive 2010-31-EU of the European Parliament and of the Council. Off. J. Eur. Union 2010, 153, 13–34. [Google Scholar]
- Barreira, E.; Freitas, V.P. Experimental study of the hygrothermal behaviour of external thermal insulation composite systems (ETICS). Build. Environ. 2013, 63, 31–39. [Google Scholar] [CrossRef]
- Borsoi, G.; Esteves, C.; Flores-Colen, I.; Veiga, R. Effect of hygrothermal aging on hydrophobic treatments applied to building exterior claddings. Coatings 2020, 10, 363. [Google Scholar] [CrossRef]
- Silva, L.; Flores-Colen, I.; Veira, N.; Timmons, A.B.; Sequeira, P. Durability of ETICS and Premixed One-Coat Renders in Natural Exposure. In New Approaches to Building Pathology and Durability, Building Pathology and Rehabilitation; Delgado, J.M.P.Q., Ed.; Springer: Singapore, 2016; Volume 6, pp. 131–158. [Google Scholar]
- Bader, T.; Waldner, B.J.; Unterberger, S.H.; Lackner, R. On the performance of film formers versus penetrants as water-repellent treatment of high-performance concrete (HPC) surfaces. Constr. Build. Mater. 2019, 203, 481–490. [Google Scholar] [CrossRef]
- Ferreira Pinto, A.P.; Delgad Rodrigues, J. Effectiveness and Stability Over Time of Water Repellent Treatments on Carbonate and Granitic Stones. In Hydrophobe VII—7th International Conference on Water Repellent Treatment and Protective Surface Technology for Building Materials; Elena Charola, A., Delgado Rodrigues, J., Eds.; LNEC (Laboratório Nacional de Engenharia Civil): Lisbon, Portugal, 2014; pp. 151–160. [Google Scholar]
- Esteves, C.; Ahmed, H.; Flores-Colen, I.; Veiga, R. The influence of hydrophobic protection on building exterior claddings. J. Coat. Technol. Res. 2019, 16, 1379–1388. [Google Scholar] [CrossRef]
- McGettigan, E. Factors affecting the selection of water-repellent treatments. APT Bull. J. Preserv. Technol. 1995, 26, 22–26. [Google Scholar] [CrossRef]
- Levi, M.; Ferro, C.; Regazzoli, D.; Dotelli, G.; Lo presti, A. Comparative evaluation method of polymer surface treatments applied on high performance concrete. J. Mater. Sci. 2002, 37, 4881–4888. [Google Scholar] [CrossRef]
- Sabatini, V.; Pargoletti, E.; Longoni, M.; Farina, H.; Ortenzi, M.A.; Cappelletti, G. Stearyl methacrylate co-polymers: Towards new polymer coatings for mortars protection. Appl. Surf. Sci. 2019, 488, 213–220. [Google Scholar] [CrossRef]
- EOTA. European Assessment Document EAD 040083-00-0404–External Thermal Insulation Composite Systems (ETICS) with renderings; European Organization for Technical Approvals: Brussels, Belgium, 2019. [Google Scholar]
- CEN—European Committee for Standardization. Conservation of Cultural Heritage—Test Methods—Determination of Drying Properties; EN 16322; CEN: Brussels, Belgium, 2013. [Google Scholar]
- RILEM. Water Absorption Under Low Pressure. In Pipe Method; Test N II.4; Tentative Recommendations: Paris, France, 1980. [Google Scholar]
- CEN—European Committee for Standardization. Methods of Methods of Test for Mortar for Masonry—Part 19: Determination of Water Vapour Permeability of Hardened Rendering and Plastering Mortars; EN 1015-19; CEN: Brussels, Belgium, 2008. [Google Scholar]
- ASTM. Determining the Resistance of Paint Films and Related Coatings to Fungal Defacement by Accelerated Four-Week Agar Plate Assay; ASTM D5590-17:2017; ASTM International: Pennsylvania, PA, USA, 2017. [Google Scholar]
- ASTM. Standard Test Method for Determining Fungi Resistance of Insulation Materials and Facings; ASTM C1338-19:2019; ASTM International: Pennsylvania, PA, USA, 2019. [Google Scholar]
- Parracha, J.L.; Cortay, A.; Borsoi, G.; Veiga, M.R.; Nunes, L. Evaluation of ETICS Characteristics That Affect Surface Mould Development. In XV International Conference on Durability of Building Materials and Components—DBMC 2020; Serrat, C., Casas, J.R., Gibert, V., Eds.; Universitat Politècnica de Catalunya: Barcelona, Spain, 2020; pp. 191–200. [Google Scholar]
- CEN—European Committee for Standardization. Methods of Test for Mortar for Masonry—Part 18: Determination of Water Absorption Coefficient due to Capillary Action of Hardened Mortar; EN 1015-18; CEN: Brussels, Belgium, 2002. [Google Scholar]
- Maia, J.; Ramos, N.M.M.; Veiga, R. Assessment of test methods for the durability of thermal mortars exposure to freezing. Mater. Struct. 2019, 52, 112. [Google Scholar] [CrossRef]
- Ergenç, D.; Sierra-Fernandez, A.; Barbero-Barrera, M.M.; Gomez-Villalba, L.S.; Fort, R. Assessment on the performances of air lime-ceramic mortars with nano-Ca(OH)2 and nano-SiO2 additions. Constr. Build. Mater. 2020, 232, 117163. [Google Scholar] [CrossRef]
- Bader, T.; Unterberger, S.H.; Lackner, R. Effect of substrate moisture on the weatherability of surface treatment for high-performance concrete (HPC). Cem. Concr. Compos. 2017, 83, 57–65. [Google Scholar] [CrossRef]
- Quagliarini, E.; Bondioli, F.; Goffredo, G.B.; Licciulli, A.; Munafo, P. Self-cleaning materials on architectural heritage: Compatibility of photo-induced hydrophilicity of TiO2 coatings on stone surfaces. J. Cult. Herit. 2013, 14, 1–7. [Google Scholar] [CrossRef]
- Féat, A.; Federle, W.; Kamperman, M.; Murray, M.; Van der Gucht, J.; Taylor, P. Slippery paints: Eco-friendly coatings that cause ants to slip. Prog. Org. Coat. 2019, 135, 331–334. [Google Scholar] [CrossRef]
- Jiang, W.; Jin, X.; Li, H.; Zhang, S.; Zhou, T.; Xie, H. Modification of nano-hybrid silicon acrylic resin with anticorrosion and hydrophobic properties. Polym. Test. 2020, 82, 106287. [Google Scholar] [CrossRef]
- La Russa, M.F.; Rovella, N.; Alvarez de Buergo, M.; Belfiore, C.M.; Pezzino, A.; Crisci, G.M.; Ruffolo, A. Nano-TiO2 coatings for cultural heritage protection: The role of the binder on hydrophobic and self-cleaning efficacy. Prog. Org. Coat. 2016, 91, 1–8. [Google Scholar] [CrossRef]
- Parracha, J.L.; Borsoi, G.; Flores-Colen, I.; Veiga, R.; Nunes, L.; Dionísio, A.; Glória Gomes, M.; Faria, P. Performance parameters of ETICS: Correlating water resistance, bio-susceptibility and surface properties. Constr. Build. Mater. 2021, 272, 121956. [Google Scholar] [CrossRef]
- Oliveira, R.; De Brito, J.; Veiga, R. Incorporation of fine glass aggregates in renderings. Constr. Build. Mater. 2013, 44, 329–341. [Google Scholar] [CrossRef]
- Farinha, C.; De Brito, J.; Veiga, R. Incorporation of fine sanitary ware aggregates in coating mortars. Constr. Build. Mater. 2015, 83, 194–206. [Google Scholar] [CrossRef]
- Xiong, H.; Yuan, K.; Wen, M.; Yu, A.; Xu, J. Influence of pore structure on the moisture transport property of external thermal insulation composite system as studied by NMR. Constr. Build. Mater. 2019, 228, 116815. [Google Scholar] [CrossRef]
- Pan, X.; Shi, Z.; Shi, C.; Ling, T.; Li, N. A review on concrete surface treatment Part I: Types and mechanisms. Constr. Build. Mater. 2017, 132, 578–590. [Google Scholar] [CrossRef]
- Kus, H. Long-Term Performance of Water Repellants on Rendered Autoclaved Aerated Concrete. Ph.D. Thesis, Centre For Built Environment, University of Gävle, Royal Institute of Technology, Stockholm, Sweden, 2002. [Google Scholar]
- Selley, D. Chemical considerations for making low-VOC silicon-based water repellents. J. Coat. Technol. Res. 2010, 7, 26–35. [Google Scholar]
- Topçuoğlu, Ö.; Altinkaya, S.A.; Balköse, D. Characterization of waterborne acrylic based paint films and measurement of their water vapor permeabilities. Prog. Org. Coat. 2006, 56, 269–278. [Google Scholar] [CrossRef]
| Product Identification | Color | pH | Density (g/cm3) at T = 20 °C and RH = 60% | Dry Residue (g/L) | Amount of Product per Application (L/m2) |
|---|---|---|---|---|---|
| H1 | Whitish | 8.5 | 1.34 ± 0.05 | 737 | 2 applications: 1st coat: 0.13; 2nd coat: 0.12 |
| H2 | Whitish | 9.25 | 1.58 ± 0.05 | 1051 | |
| H3 | Whitish | 8.5 | 1.30 ± 0.03 | 729 |
| Heat-Cold Cycles | Freeze-Thaw Cycles | Exposure Time |
|---|---|---|
| Heating-Infrared lamps (T = 60 ± 2 °C) | Sprinkler system (water at T = 20 ± 1 °C) | 8 h ± 15 min |
| Stabilization (T = 20 ± 2 °C, RH = 65% ± 5%) | Stabilization (T = 20 ± 2 °C, RH = 65% ± 5%) | 30 ± 2 min |
| Deep freeze cabinet (T = −15 ± 1 °C) | Deep freeze cabinet (T = −15 ± 1 °C) | 15 h ± 15 min |
| Stabilization (T = 20 ± 2 °C, RH = 65% ± 5%) | Stabilization (T = 20 ± 2 °C, RH = 65% ± 5%) | 30 ± 2 min |
| System | Unaged Specimens | Aged Specimens | ||
|---|---|---|---|---|
| Cc (kg/m2·h0.5) | C60 (kg/m2·h0.5) | Cc (kg/m2·h0.5) | C60 (kg/ m2·h0.5) | |
| H0 (reference) | 0.51 ± 0.04 | 0.13 ± 0.04 | 0.23 ± 0.05 | 0.09 ± 0.03 |
| H1 | 0.38 ± 0.04 | 0.11 ± 0.05 | 0.18 ± 0.05 | 0.04 ± 0.04 |
| H2 | 0.38 ± 0.03 | 0.12 ± 0.02 | 0.18 ± 0.02 | 0.03 ± 0.03 |
| H3 | 0.33 ± 0.03 | 0.04 ± 0.03 | 0.10 ± 0.01 | 0.01 ± 0.01 |
| System | Unaged Specimens | Aged Specimens | ||||||
|---|---|---|---|---|---|---|---|---|
| DR1 (kg/m2·h) | DR2 (kg/m2·h0.5) | DI | ΔTm (%) | DR1 (kg/m2·h) | DR2 (kg/m2·h0.5) | DI | ΔTm (%) | |
| H0 (reference) | 0.062 ± 0.002 | 0.043 ± 0.001 | 0.125 ± 0.006 | 1.26 ± 0.08 | 0.106 ± 0.009 | 0.013 ± 0.003 | 0.193 ± 0.060 | 0.78 ± 0.47 |
| H1 | 0.038 ± 0.003 | 0.035 ± 0.001 | 0.249 ± 0.025 | 2.06 ± 0.15 | 0.051 ± 0.017 | 0.008 ± 0.001 | 0.186 ± 0.035 | 0.33 ± 0.08 |
| H2 | 0.046 ± 0.004 | 0.042 ± 0.001 | 0.110 ± 0.003 | 0.80 ± 0.17 | 0.043 ± 0.004 | 0.008 ± 0.001 | 0.202 ± 0.029 | 0.37 ± 0.09 |
| H3 | 0.031 ± 0.001 | 0.030 ± 0.000 | 0.280 ± 0.026 | 2.32 ± 0.19 | 0.029 ± 0.005 | 0.005 ± 0.001 | 0.250 ± 0.056 | 0.35 ± 0.09 |
| System | Unaged Specimens θ (°) | Aged Specimens θ (°) |
|---|---|---|
| H0 (reference) | 54 ± 8 | 85 ± 6 |
| H1 | 37 ± 5 | 64 ± 5 |
| H2 | 84 ± 8 | 111 ± 3 |
| H3 | 63 ± 5 | 75 ± 5 |
| System | µ EPS | Unaged Specimens | Aged Specimens | ||
|---|---|---|---|---|---|
| µ ETICS | Sd of the Rendering System (m) | µ ETICS | Sd of the Rendering System (m) | ||
| H0 | 39.07 ± 3.11 | 43.84 ± 0.10 | 0.35 ± 0.02 | 80.39 ± 10.25 | 1.93 ± 0.43 |
| H1 | 43.65 ± 0.12 | 0.34 ± 0.01 | 65.14 ± 0.73 | 1.26 ± 0.02 | |
| H2 | 42.10 ± 2.95 | 0.26 ± 0.11 | 73.08 ± 10.48 | 1.61 ± 0.46 | |
| H3 | 50.04 ± 3.90 | 0.62 ± 0.18 | 103.02 ± 20.88 | 2.86 ± 0.92 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roncon, R.; Borsoi, G.; Parracha, J.L.; Flores-Colen, I.; Veiga, R.; Nunes, L. Impact of Water-Repellent Products on the Moisture Transport Properties and Mould Susceptibility of External Thermal Insulation Composite Systems. Coatings 2021, 11, 554. https://doi.org/10.3390/coatings11050554
Roncon R, Borsoi G, Parracha JL, Flores-Colen I, Veiga R, Nunes L. Impact of Water-Repellent Products on the Moisture Transport Properties and Mould Susceptibility of External Thermal Insulation Composite Systems. Coatings. 2021; 11(5):554. https://doi.org/10.3390/coatings11050554
Chicago/Turabian StyleRoncon, Renata, Giovanni Borsoi, João L. Parracha, Inês Flores-Colen, Rosário Veiga, and Lina Nunes. 2021. "Impact of Water-Repellent Products on the Moisture Transport Properties and Mould Susceptibility of External Thermal Insulation Composite Systems" Coatings 11, no. 5: 554. https://doi.org/10.3390/coatings11050554
APA StyleRoncon, R., Borsoi, G., Parracha, J. L., Flores-Colen, I., Veiga, R., & Nunes, L. (2021). Impact of Water-Repellent Products on the Moisture Transport Properties and Mould Susceptibility of External Thermal Insulation Composite Systems. Coatings, 11(5), 554. https://doi.org/10.3390/coatings11050554









