Effect of Current Density on the Wear Resistance of Ni–P Alloy Coating Prepared through Immersion-Assisted Jet-Electrodeposition
Abstract
1. Introduction
2. Experimental Materials and Method
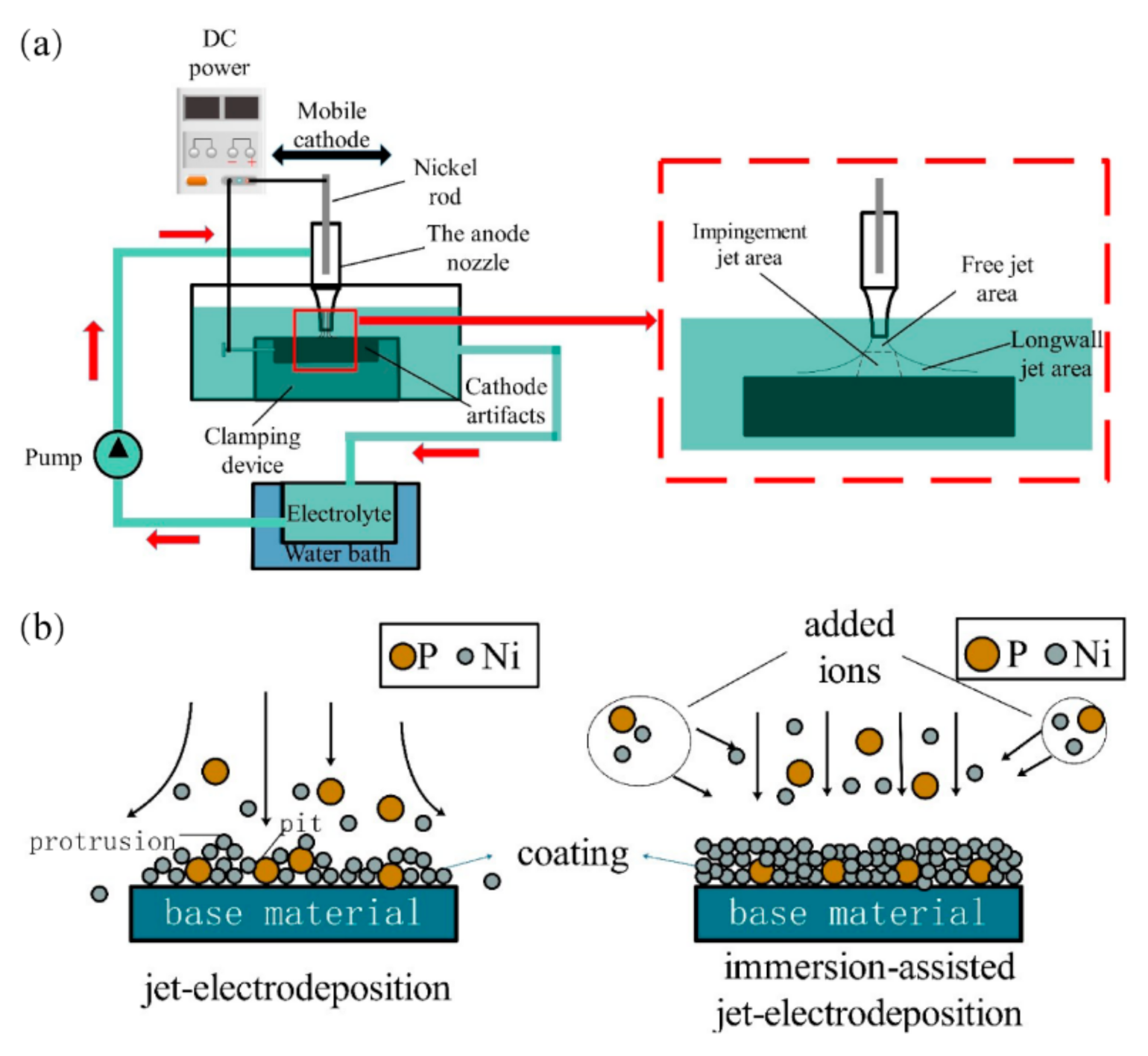
2.1. Equipment and Principle of Immersion-Assisted Jet-Electrodeposition
2.2. Preparation of the Workpiece and Coatings
2.3. Testing Equipment
3. Result and Discussion
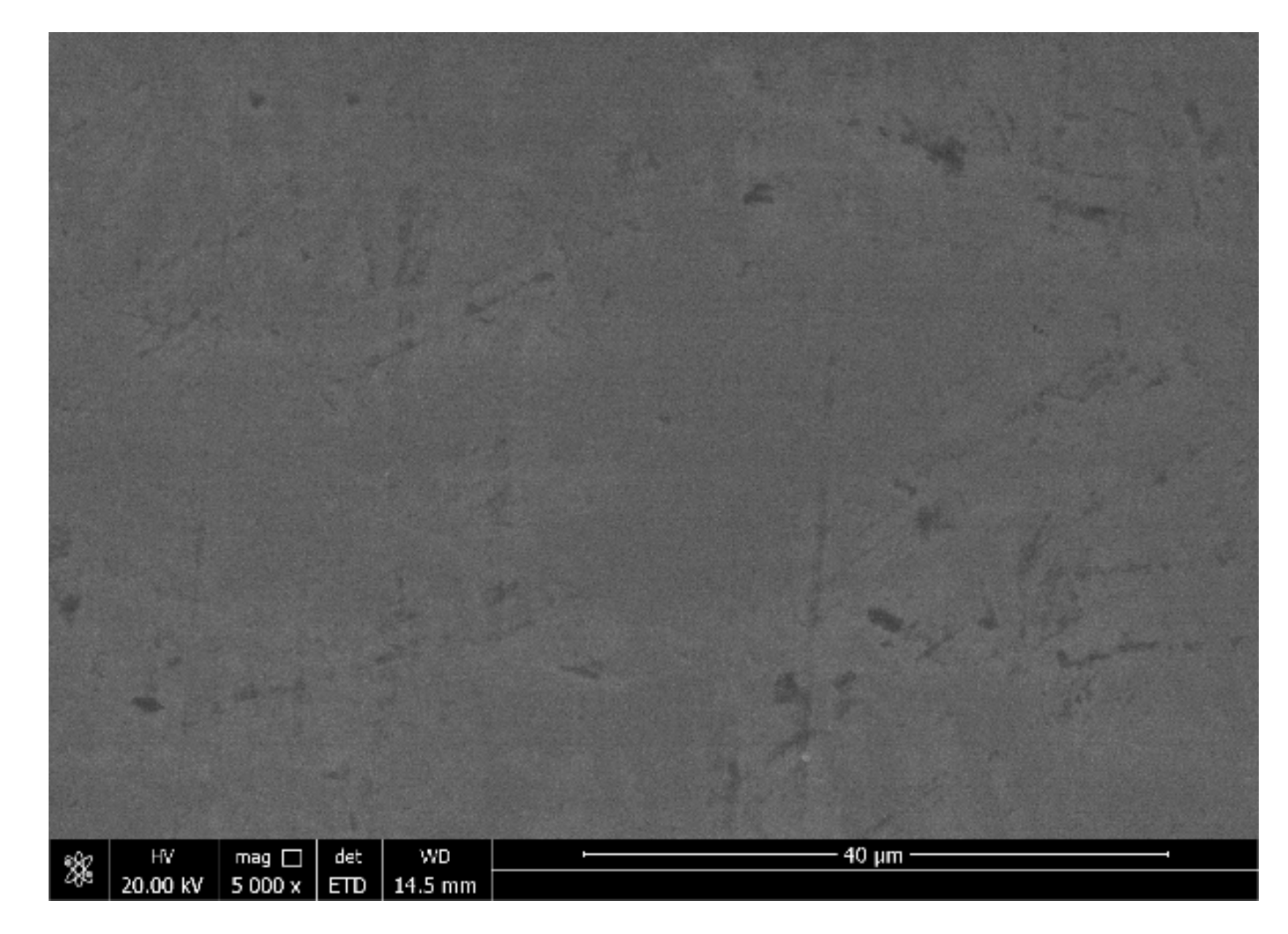
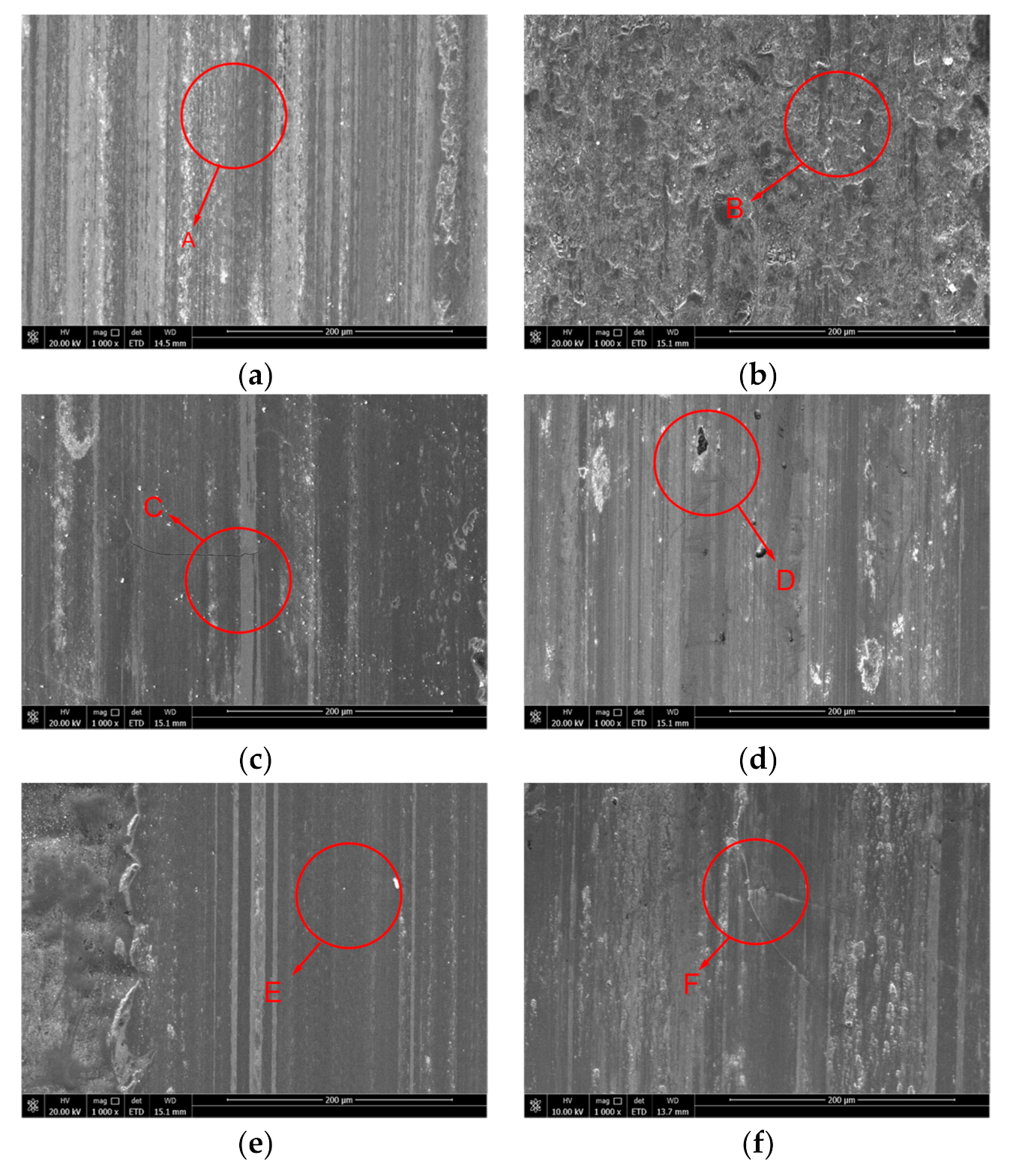
3.1. Analysis of the Surface Morphology of the Coating
3.2. Analysis of Elemental Composition and Microstructure of the Coating
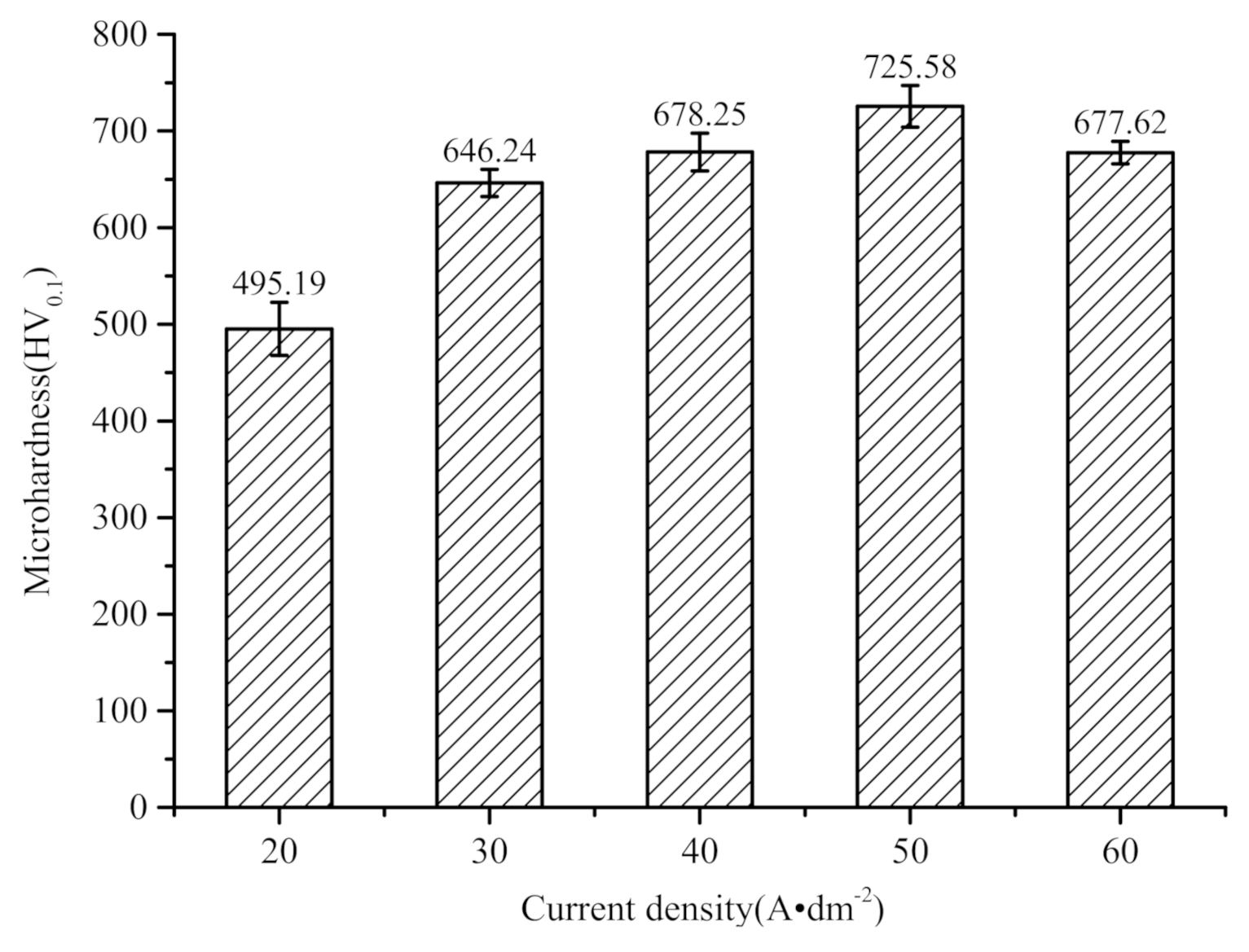
3.3. Analysis of Microhardness of Coating
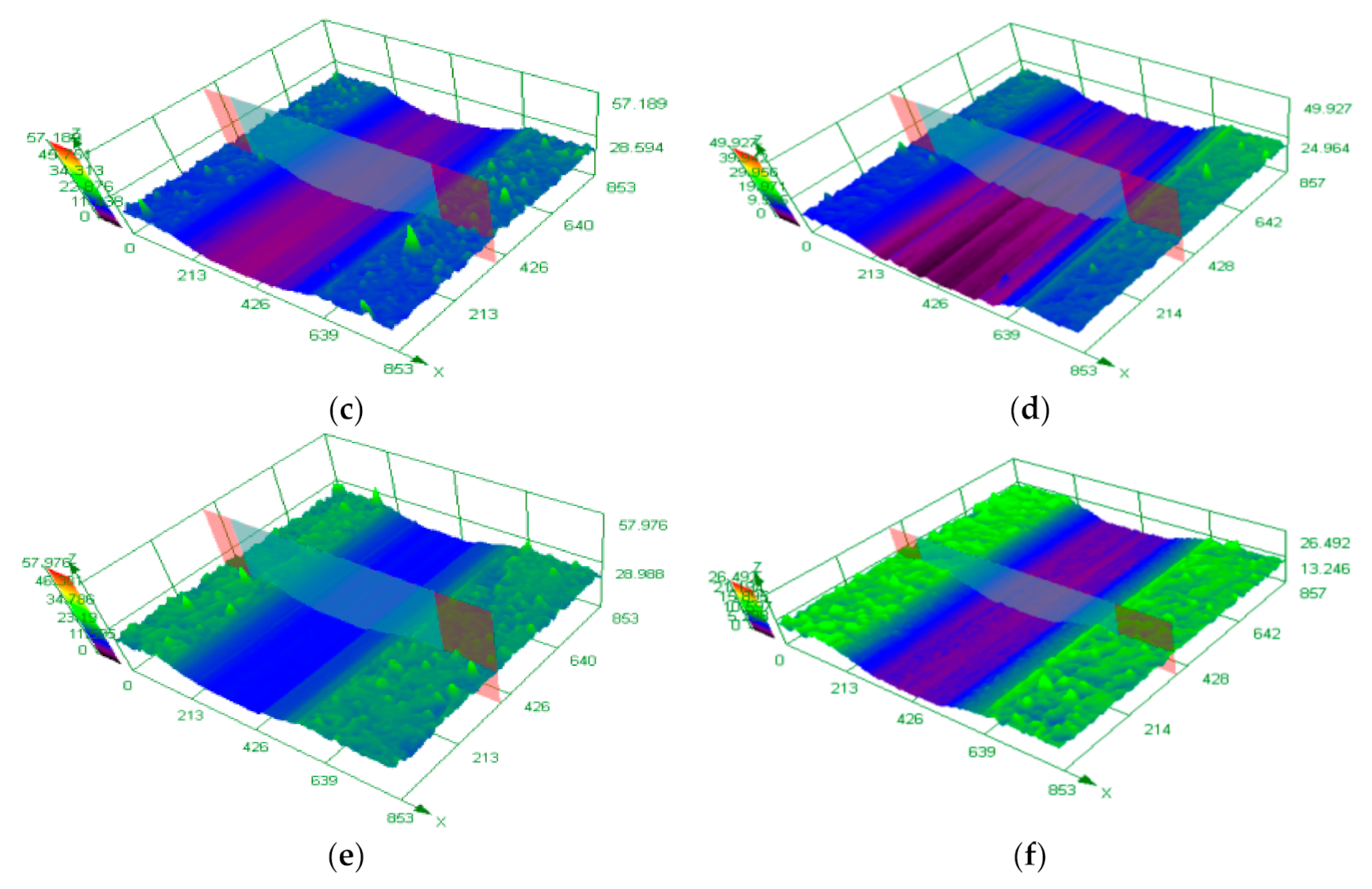
3.4. Analysis of the Wear Resistance of the Coating
4. Conclusions
- In immersion-assisted jet-electrodeposition, the workpiece is in a stable plating bath environment, the temperature is stable, and the cathode can be effectively replenished. Moreover, the limiting current density is improved, and thus the Ni−P alloy coating prepared through this process is better than that prepared through jet-electrodeposition. The surface of the coating is smoother, defects such as pits and protrusions are reduced, and hardness and wear resistance are improved;
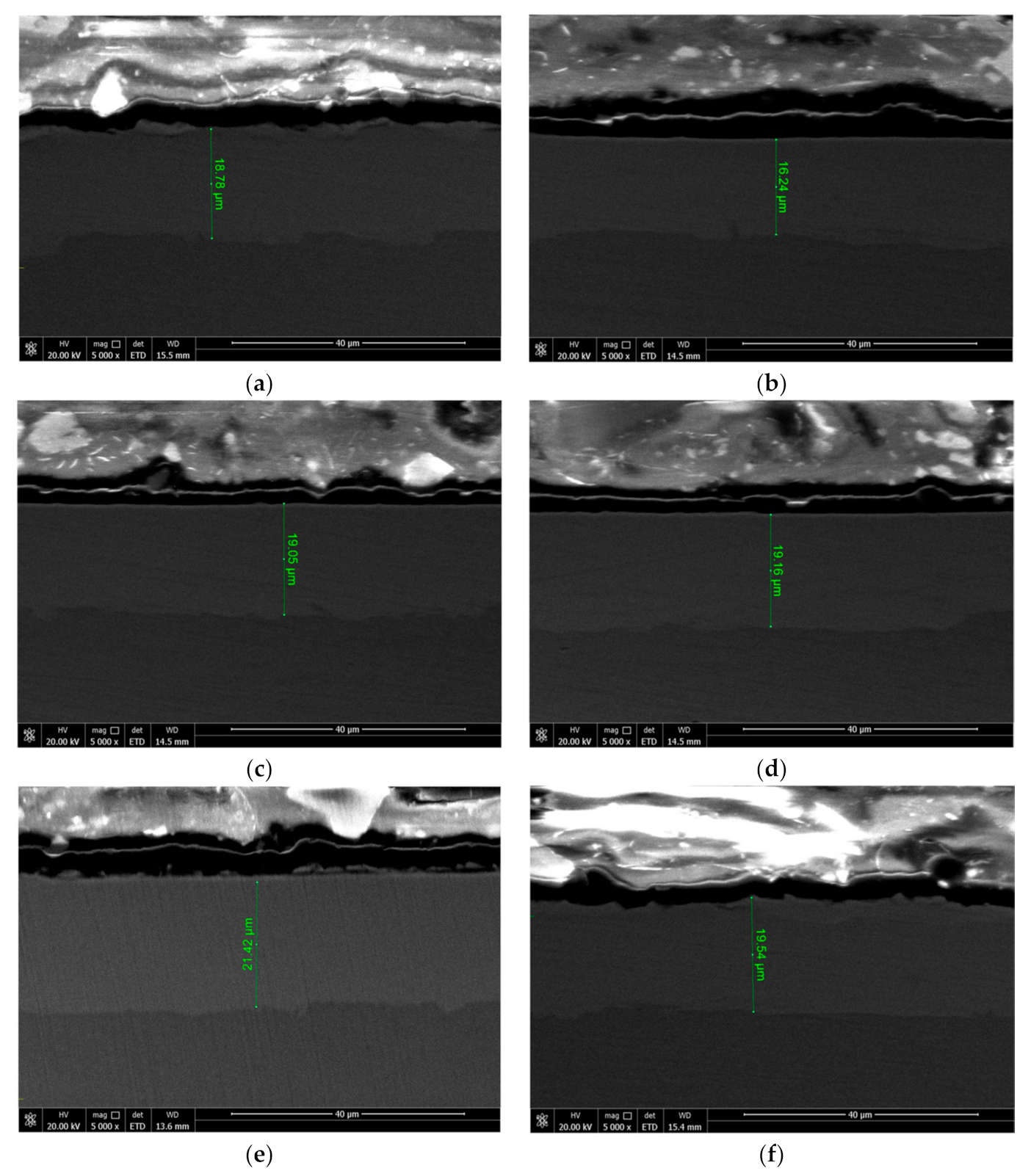
- The surface of the Ni−P alloy coating prepared by immersion-assisted jet-electrodeposition exhibits a typical cell structure. With increasing current density, the overpotential of cathode increases and the nucleation rate of surface cells of the subsequently increases. In addition, the surface quality and thickness of the Ni−P alloy coating increases first and then decreases. When the current density is 50 A·dm−2, the cell structure of the coating is uniform, the thickness of the Ni−P coating reaches the maximum, which is 21.42 μm, the boundary is blurred, and the smallest grain size is observed, i.e., the surface quality is the best;
- The grain size of the Ni−P alloy coating prepared through immersion-assisted jet-electrodeposition decreases first and then increases as current density increases. The reason is that the nucleation rate increases with current density, and this increase contributes to grain refinement. When the current density is 50 A·dm−2, the grain size reaches a minimum value of 6.6 nm, and the Ni element content reaches a maximum of 98.25%. When the current density continues to increase to 60 A·dm−2, the P content reaches a maximum of 3.45%;
- The microhardness of Ni−P alloy coatings prepared through immersion-assisted jet-electrodeposition presents a trend of increasing first and then decreasing as current density increases. The reasons are the increase in deposition rate and a dense coating surface without defects after the increase in current density. When the current density reaches the highest average microhardness (725.58 HV0.1), it is approximately 46.5% higher than that when the current density is 20 A·dm−2, and the effect is remarkable. The increase in hardness is beneficial to the improvement of wear resistance;
- With increasing current density, the improvement of microhardness of the coating can significantly improves the wear resistance of the coating, and the two are directly proportional. The Ni−P alloy coating prepared by immersion-assisted jet-electrodeposition has the best wear resistance when the current density is 50 A·dm−2, and the width, depth, and cross-sectional area of the wear scar of the coating are the smallest, which are 425.494 μm, 7.782 μm and 2266.533 μm2, respectively. No obvious furrow and shedding phenomenon or cracks are observed on the surface of the coating wear scar. The friction mechanism is mainly based on abrasive wear.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, W.S.; Cui, S.; He, L.; Li, Q.K. Electrochemically deposited Ni-based MoS2 composite coating and its wide temperature range friction properties. China Surf. Eng. 2017, 30, 97–103. [Google Scholar]
- Ahmadkhaniha, D.; Zanella, C. The Effects of Additives, Particles Load and Current Density on Codeposition of SiC Particles in NiP Nanocomposite Coatings. Coatings 2019, 9, 554. [Google Scholar] [CrossRef]
- Benea, L.; Celis, J.P. Effect of Nano-TiC Dispersed Particles and Electro-Codeposition Parameters on Morphology and Structure of Hybrid Ni/TiC Nanocomposite Layers. Materials 2016, 9, 269. [Google Scholar] [CrossRef] [PubMed]
- Rosolymou, E.; Spanou, S.; Zanella, C.; Tsoukleris, D.S.; Köhler, S.; Leisner, P.; Pavlatou, E.A. Electrodeposition of Photocatalytic Sn-Ni Matrix Composite Coatings Embedded with Doped TiO2 Particles. Coatings 2020, 10, 775. [Google Scholar] [CrossRef]
- Jiang, W.; Shen, L.; Qiu, M.; Wang, X.; Fan, M.; Tian, Z. Preparation of Ni-SiC composite coatings by magnetic field-enhanced jet electrodeposition. J. Alloys Compd. 2018, 762, 115–124. [Google Scholar] [CrossRef]
- Xue, Y.J.; Si, D.H.; Liu, H.B. The influence of electrodeposition methods on the friction and wear properties of Ni-CeO2 nanocomposite coatings. Chin. J. Nonferrous Met. 2011, 21, 2157–2162. [Google Scholar]
- Hessam, R.; Najafisayar, P. The effects of applied current density and bath concentration on the morphology, crystal structure and optical properties of electrodeposited hematite thin films. Thin Solid Films 2019, 692, 137633. [Google Scholar] [CrossRef]
- Shourije, S.J.; Bahrololoom, M.E. Effect of current density, MoS2 content and bath agitation on tribological properties of electrodeposited nanostructured Ni-MoS2 composite coatings. Tribol. Mater. Surf. Interfaces 2019, 13, 76–87. [Google Scholar] [CrossRef]
- Fan, H.; Zhao, Y.P.; Jiang, J.; Wang, S.; Shan, W.; Ma, R. Pulse jet electrodeposition of nanocrystal line copper and its application as an electrical discharge machining electrode. Int. J. Electrochem. Sci. 2020, 15, 2648–2658. [Google Scholar] [CrossRef]
- Song, Z.Y.; Zhang, H.W.; Fu, X.Q. Effect of Current Density on the Performance of Ni-P-ZrO2-CeO2 Composite Coatings Prepared by Jet-Electrodeposition. Coatings 2020, 10, 616. [Google Scholar] [CrossRef]
- Qiao, G.Y.; Jing, T.F.; Wang, N.; Gao, Y.; Zhao, X.; Zhou, J.; Wang, W. High-speed jet electrodeposition and microstructure of nanocrystalline Ni–Co alloys. Electrochim. Acta 2005, 51, 85–92. [Google Scholar] [CrossRef]
- Rajput, M.S.; Pandey, P.M.; Jha, S. Modelling of high speed selective jet electrodeposition process. J. Manuf. Process. 2015, 17, 98–107. [Google Scholar] [CrossRef]
- Tian, J.; Song, H.; Zheng, X.H.; Zhang, Q.; Wang, M. High performance Co–Cr3C2 composite coating by jet electrodeposition. Surf. Eng. 2017, 34, 861–869. [Google Scholar]
- Ning, D.D.; Zhang, A. Enhanced Wear Performance of Cu-Carbon Nanotubes Composite Coatings Prepared by Jet Electrodeposition. Materials 2019, 12, 392. [Google Scholar] [CrossRef]
- Fu, X.Q.; Shen, Z.Y.; Chen, X.X. Influence of Element Penetration Region on Adhesion and Corrosion Performance of Ni-Base Coatings. Coatings 2020, 10, 895. [Google Scholar] [CrossRef]
- Singh, C.; Tiwari, S.K.; Singh, R. Exploring environment friendly nickel electrodeposition on AZ91 magnesium alloy: Effect of prior surface treatments and temperature of the bath on corrosion behaviour. Corros. Sci. 2019, 151, 1–19. [Google Scholar] [CrossRef]
- Li, T.T.; Ling, L.; Lin, M.C.; Jiang, Q.; Lin, Q.; Lou, C.W.; Lin, J.H. Effects of ultrasonic treatment and current density on the properties of hydroxyapatite coating via electrodeposition and its in vitro biomineralization behavior. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 105, 110062. [Google Scholar] [CrossRef]
- Li, N.B.; Xu, W.H.; Zhao, J.H.; Xiao, G.Y.; Lu, Y.P. The significant influence of ionic concentrations and immersion temperatures on deposition behaviors of hydroxyapatite on alkali- and heat-treated titanium in simulated body fluid. Thin Solid Films 2018, 646, 163–172. [Google Scholar] [CrossRef]
- Li, Y.D.; Jiang, H.; Huang, W.H.; Tian, H. Effects of peak current density on the mechanical properties of nanocrystalline Ni–Co alloys produced by pulse electrodeposition. Appl. Surf. Sci. 2008, 254, 6865–6869. [Google Scholar] [CrossRef]
- Lelevic, A.; Walsh, F.C. Electrodeposition of Ni P composite coatings: A review. Surf. Coat. 2019, 378, 124803. [Google Scholar] [CrossRef]
- He, Y.; Wang, S.C.; Walsh, F.C.; Chiu, Y.L.; Reed, P.A.S. Self-lubricating Ni-P-MoS2 composite coatings. Surf. Coat. Technol. 2016, 307, 926–934. [Google Scholar] [CrossRef]
- Shui, Y. The research status and development trend of Ni-P coating. Surf. Technol. 2002, 1, 1–4. [Google Scholar]
- Li, L.; Lu, Z.X.; Pu, J.B.; Wang, H.; Li, Q.; Chen, S.; Zhang, Z.; Wang, L. The superlattice structure and self-adaptive performance of C–Ti/MoS2 composite coatings. Ceram. Int. 2020, 46, 5733–5744. [Google Scholar] [CrossRef]
- Anas, N.S.; Krishna, L.R.; Dash, R.K.; Vijay, R. Tribological Performance of Al Alloys Dispersed with Carbon Nanotubes or Ni-Coated Carbon Nanotubes Produced by Mechanical Milling and Extrusion. J. Mater. Eng. Perform. 2020, 29, 1630–1639. [Google Scholar] [CrossRef]



| Composition | Content/(g·L−1) |
|---|---|
| NiSO4·6H2O | 200 |
| NiCl2·6H2O | 30 |
| H3PO3 | 20 |
| H3BO3 | 30 |
| C6H8O7 | 60 |
| CH4N2S | 0.01 |
| C12H25SO4Na | 0.08 |
| Element | C | Si | Mn | Cr | Ni | Cu |
|---|---|---|---|---|---|---|
| Content | 0.42−0.50 | 0.17–0.37 | 0.50–0.80 | ≤0.25 | ≤0.30 | ≤0.25 |
| Current Density/(A·dm−2) | Ni/(wt.%) | P/(wt.%) |
|---|---|---|
| 30 (J) | 70.78 | 1.94 |
| 20 | 79.26 | 0.77 |
| 30 | 84.32 | 1.98 |
| 40 | 89.84 | 1.38 |
| 50 | 98.25 | 1.73 |
| 60 | 82.28 | 3.45 |
| Current Density/(A·dm−2) | 2θ/(o) | D/(nm) |
|---|---|---|
| 30 (J) | 44.759 | 6.6 |
| 20 | 44.836 | 8.8 |
| 30 | 44.973 | 7.0 |
| 40 | 44.807 | 6.7 |
| 50 | 44.900 | 6.6 |
| 60 | 44.794 | 7.2 |
| Current Density/(A·dm−2) | Width/ (μm) | Height/ (μm) | Scratch/ Area (μm2) |
|---|---|---|---|
| 30 (J) | 603.140 ± 15.372 | 13.620 ± 1.232 | 5039.194 ± 105.577 |
| 20 | 592.257 ± 18.266 | 11.623 ± 1.839 | 3922.100 ± 152.228 |
| 30 | 516.905 ± 18.952 | 11.191 ± 1.325 | 3623.217 ± 131.174 |
| 40 | 510.434 ± 14.275 | 10.607 ± 0.923 | 3204.018 ± 138.380 |
| 50 | 425.494 ± 10.338 | 7.782 ± 0.574 | 2266.533 ± 101.909 |
| 60 | 465.117 ± 16.159 | 8.441 ± 1.391 | 2593.077 ± 146.263 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xian, J.; Shen, Z.; Zhang, Z.; Wu, H.; Jin, M.; Jiang, M. Effect of Current Density on the Wear Resistance of Ni–P Alloy Coating Prepared through Immersion-Assisted Jet-Electrodeposition. Coatings 2021, 11, 527. https://doi.org/10.3390/coatings11050527
Xian J, Shen Z, Zhang Z, Wu H, Jin M, Jiang M. Effect of Current Density on the Wear Resistance of Ni–P Alloy Coating Prepared through Immersion-Assisted Jet-Electrodeposition. Coatings. 2021; 11(5):527. https://doi.org/10.3390/coatings11050527
Chicago/Turabian StyleXian, Jieyu, Zhenyu Shen, Zhengwei Zhang, Hongbin Wu, Meifu Jin, and Minjie Jiang. 2021. "Effect of Current Density on the Wear Resistance of Ni–P Alloy Coating Prepared through Immersion-Assisted Jet-Electrodeposition" Coatings 11, no. 5: 527. https://doi.org/10.3390/coatings11050527
APA StyleXian, J., Shen, Z., Zhang, Z., Wu, H., Jin, M., & Jiang, M. (2021). Effect of Current Density on the Wear Resistance of Ni–P Alloy Coating Prepared through Immersion-Assisted Jet-Electrodeposition. Coatings, 11(5), 527. https://doi.org/10.3390/coatings11050527






