High-Temperature Tribological Performance of Al2O3/a-C:H:Si Coating in Ambient Air
Abstract
1. Introduction
2. Materials and Methods
3. Results
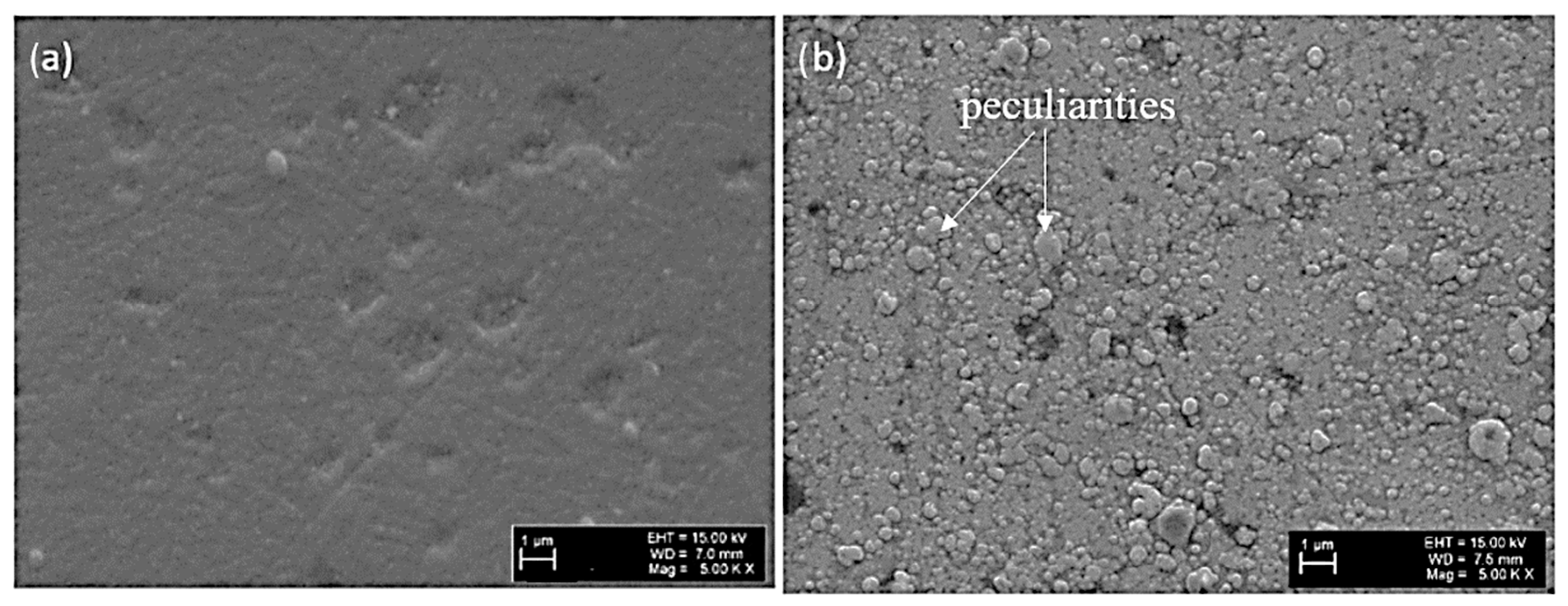
3.1. Morphology and Tribological Properties
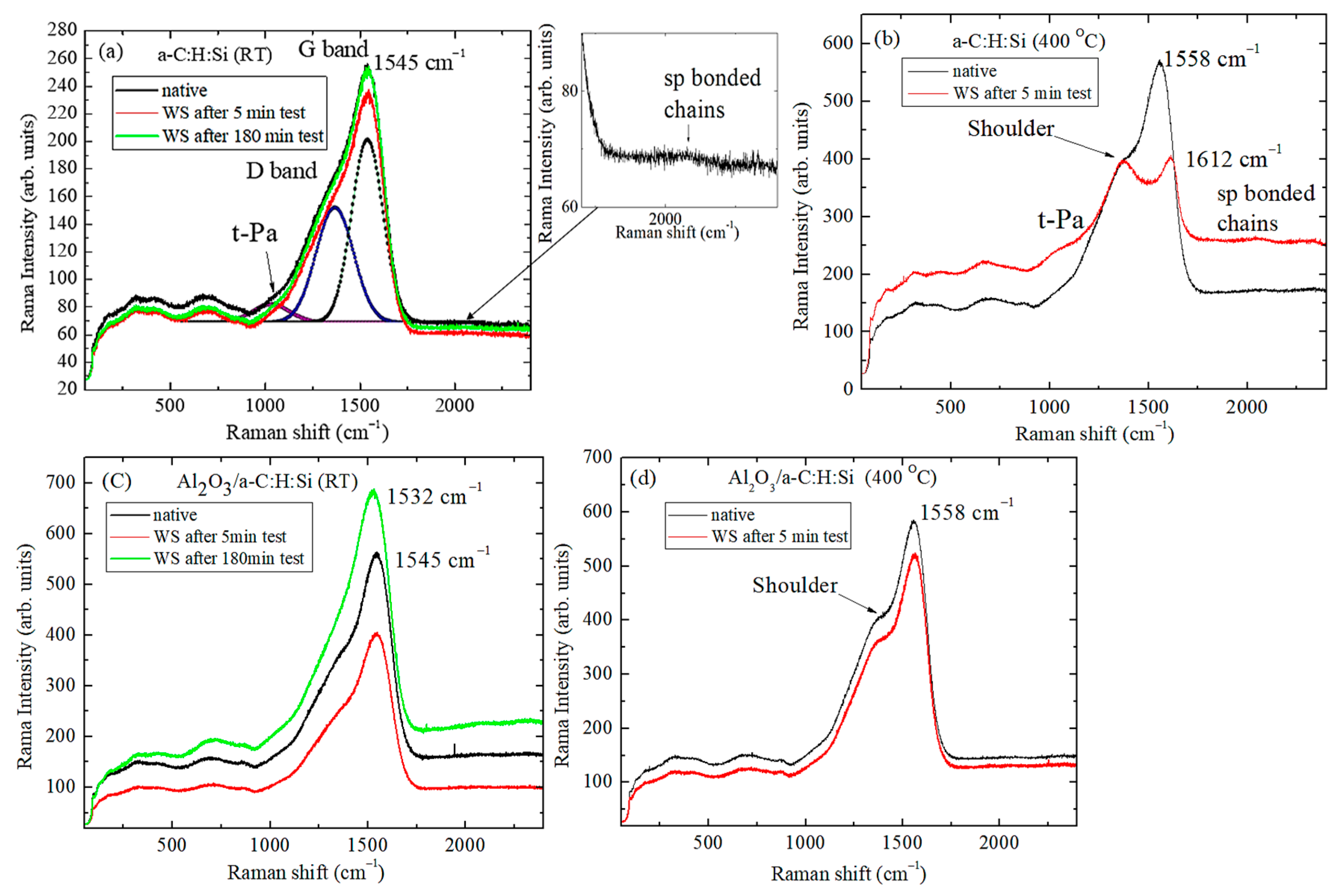
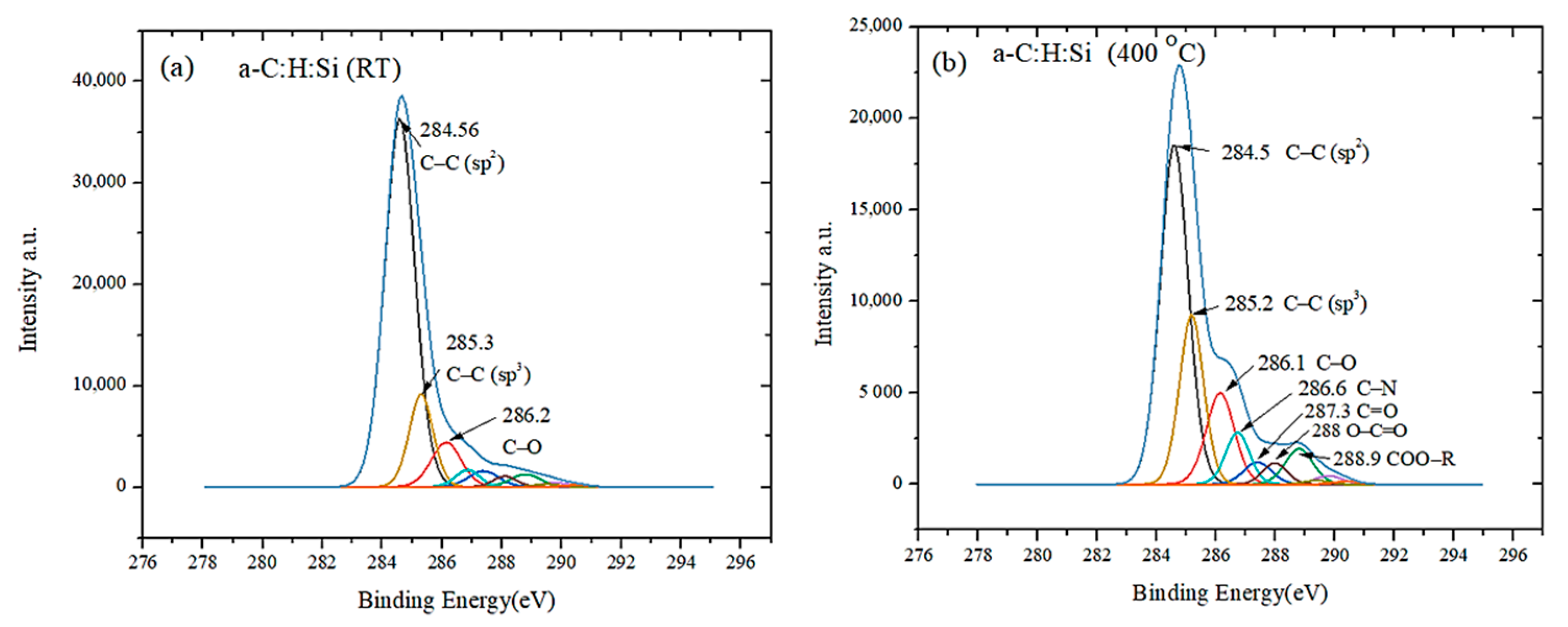
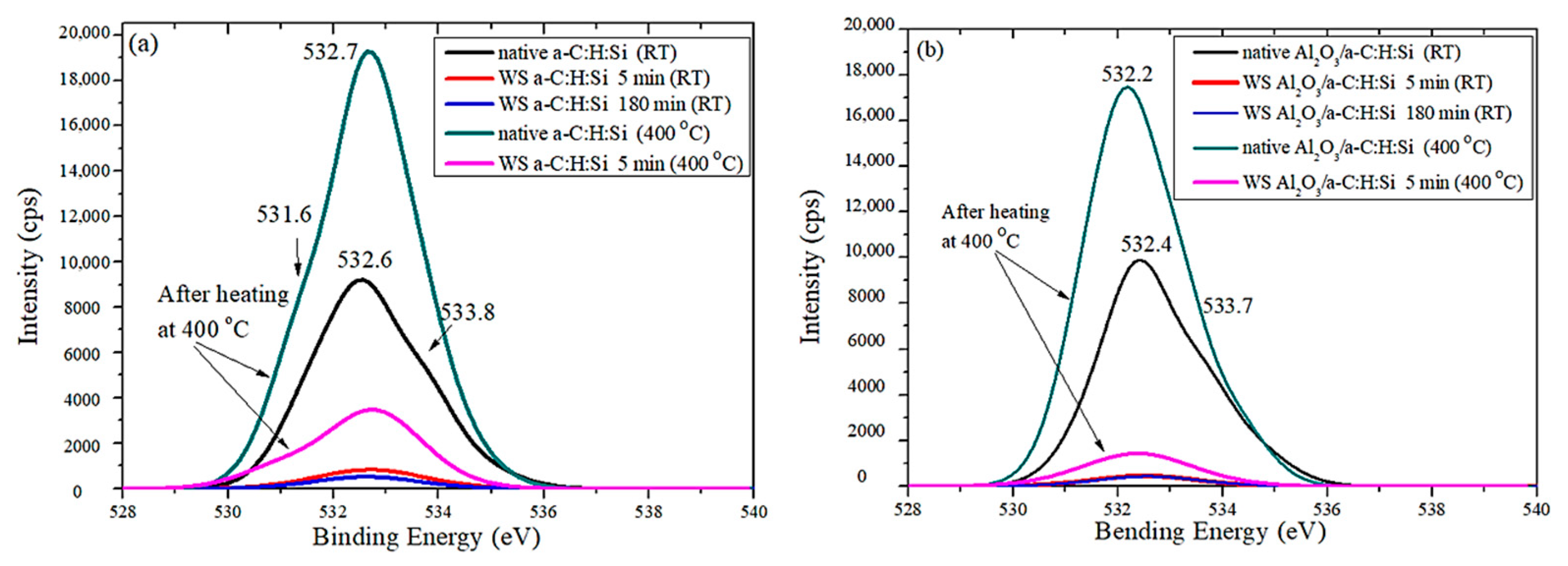
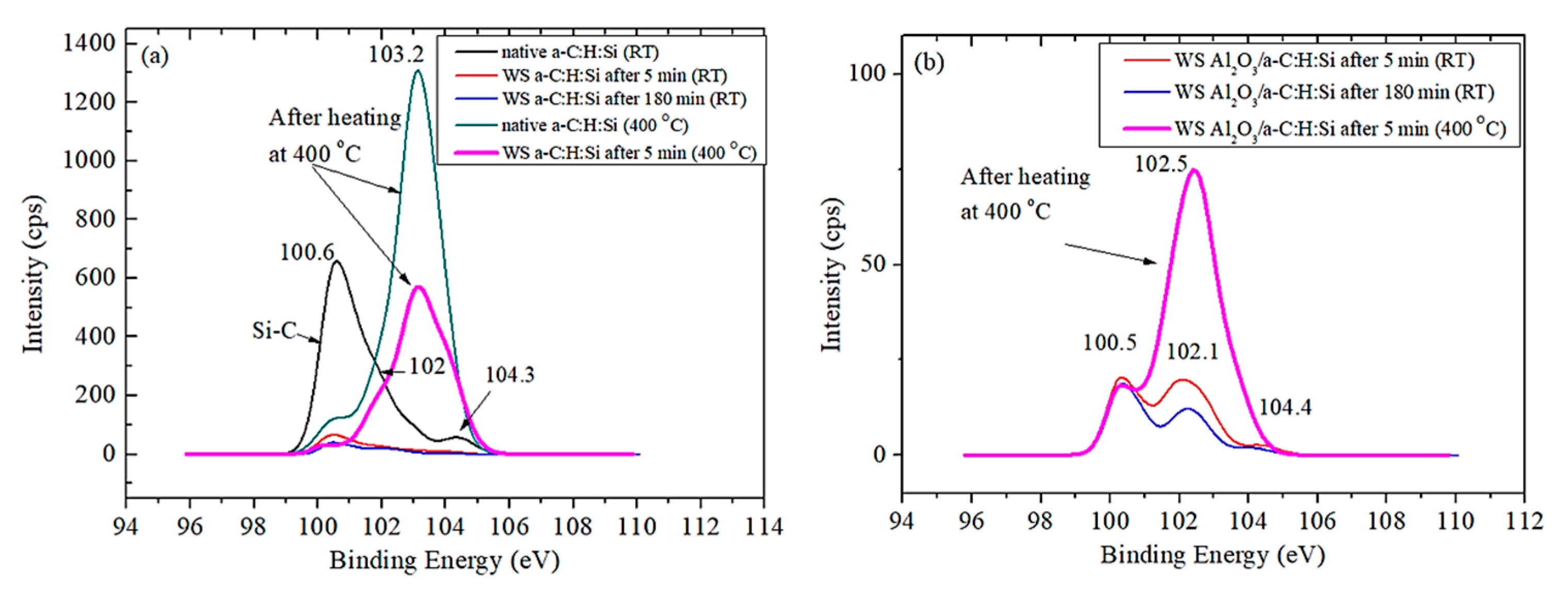
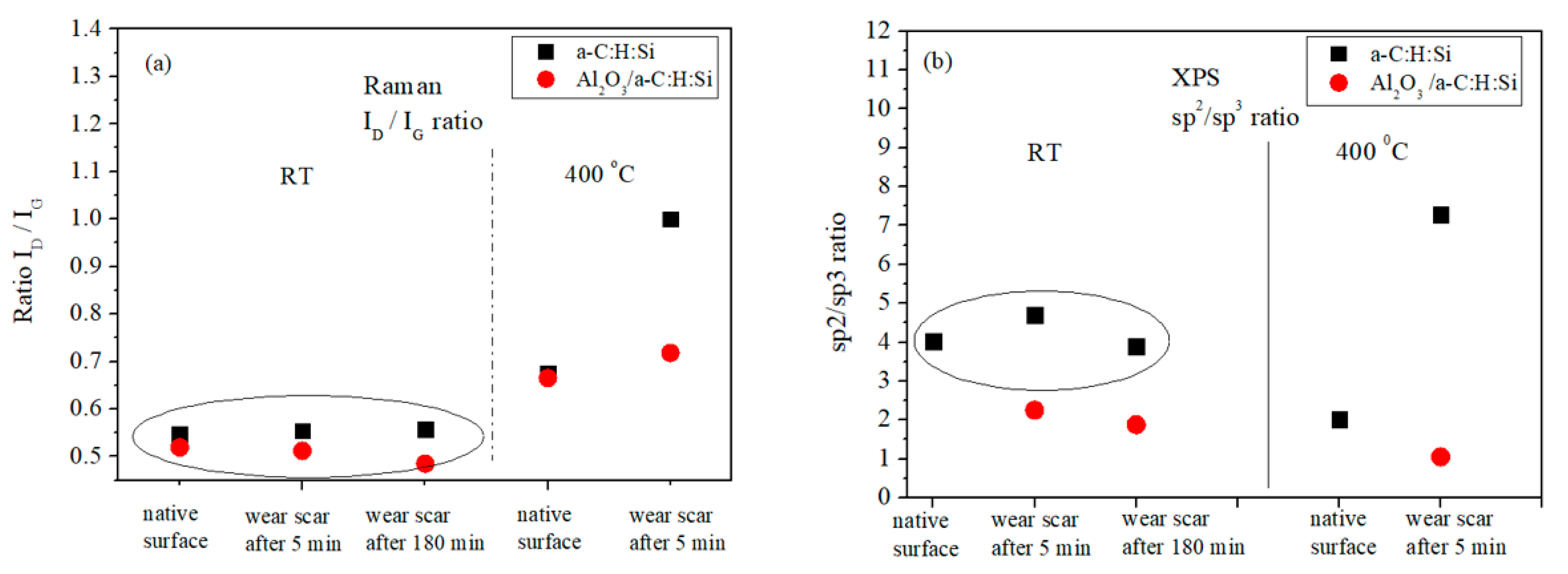
3.2. Raman and XPS Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Holmberg, K.; Erdemir, A. Influence of tribology on global energy consumption, costs and emissions. Friction 2017, 5, 263–284. [Google Scholar] [CrossRef]
- Kowalski, S. The influence of selected PVD coatings on fretting wear in a clamped joint based on the example of a rail vehicle wheel set. Ekspolatacja Niezawodn. Maint. Reliab. 2017, 20, 1–8. [Google Scholar] [CrossRef]
- Rybachuk, M.; Bell, J. Electronic states of trans-polyacetylene, poly(p-phenylene vinylene) and sp-hybridised carbon species in amorphous hydrogenated carbon probed by resonant Raman scattering. Carbon 2009, 47, 2481–2490. [Google Scholar] [CrossRef]
- Mulazzi, E.; Brivio, G.P.; Faulques, E.; Lefrant, S. Experimental and theoretical raman results in transpolyacetylene. Solid State Commun. 1983, 46, 851–855. [Google Scholar] [CrossRef]
- Ravagnan, L.; Manini, N.; Cinquanta, E.; Onida, G.; Sangalli, D.; Motta, C.; Devetta, M.; Bordoni, A.; Piseri, P.; Milani, P. Effect of axial torsion on sp carbon atomic wires. Phys. Rev. Lett. 2009, 102, 245502. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Raman spectroscopy of amorphous, nanostructured, diamond-like carbon, and nanodiamond. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2004, 362, 2477–2512. [Google Scholar] [CrossRef]
- Hilbert, J.; Mangolini, F.; McClimon, J.; Lukes, J.; Carpick, R. Si doping enhances the thermal stability of diamond-like carbon through reductions in carbon-carbon bond length disorder. Carbon 2018, 131, 72–78. [Google Scholar] [CrossRef]
- Dongping, L.; Baoxiang, C.; Yanhong, L. Structures of diamond-like carbon films. Plasma Sci. Technol. 2006, 8, 285–291. [Google Scholar] [CrossRef]
- Kalish, R.; Lifshitz, Y.; Nugent, K.; Prawer, S. Thermal stability and relaxation in diamond-like-carbon. A Raman study of films with different sp3 fractions (ta-C to a-C). Appl. Phys. Lett. 1999, 74, 2936–2938. [Google Scholar] [CrossRef]
- Rybachuk, M.; Bell, J.M. The effect of sp2 fraction and bonding disorder on micro-mechanical and electronic properties of a-C:H films. Thin Solid Film. 2007, 515, 7855–7860. [Google Scholar] [CrossRef][Green Version]
- Li, H.; Xu, T.; Wang, C.; Chen, J.; Zhou, H.; Liu, H. Annealing effect on the structure, mechanical and tribological properties of hydrogenated diamond-like carbon films. Thin Solid Film. 2006, 515, 2153–2160. [Google Scholar] [CrossRef]
- Tallant, D.; Parmeter, J.; Siegal, M.; Simpson, R. The thermal stability of diamond-like carbon. Diam. Relat. Mater. 1995, 4, 191–199. [Google Scholar] [CrossRef]
- Pastewka, L.; Moser, S.; Gumbsch, P.; Moseler, M. Anisotropic mechanical amorphization drives wear in diamond. Nat. Mater. 2010, 10, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Chen, X.; Zhang, C.; Luo, J. Influence factors on mechanisms of superlubricity in DLC films: A review. Front. Mech. Eng. 2020, 6, 65. [Google Scholar] [CrossRef]
- Ţucureanu, V.; Matei, A.; Avram, A.M. FTIR spectroscopy for carbon family study. Crit. Rev. Anal. Chem. 2016, 46, 502–520. [Google Scholar] [CrossRef] [PubMed]
- Fontaine, J.; Loubet, J.L.; Mogne, T.L.; Grill, A. Superlow friction of diamond-like carbon films: A relation to viscoplastic properties. Tribol. Lett. 2004, 17, 709–714. [Google Scholar] [CrossRef]
- Chen, X.; Kato, T.; Kawaguchi, M.; Nosaka, M.; Choi, J. Structural and environmental dependence of superlow friction in ion vapour-deposited a-C:H:Si films for solid lubrication application. J. Phys. D Appl. Phys. 2013, 46, 255304. [Google Scholar] [CrossRef]
- Kano, M.; Yasuda, Y.; Okamoto, Y.; Mabuchi, Y.; Hamada, T.; Ueno, T.; Ye, J.; Konishi, S.; Takeshima, S.; Martin, J.M.; et al. Ultralow friction of DLC in presence of glycerol mono-oleate (GNO). Tribol. Lett. 2005, 18, 245–251. [Google Scholar] [CrossRef]
- Holmberg, K.; Ronkainen, H.; Laukkanen, A.; Wallin, K. Friction and wear of coated surfaces—Scales, modelling and simulation of tribomechanisms. Surf. Coat. Technol. 2007, 202, 1034–1049. [Google Scholar] [CrossRef]
- Erdemir, A. The role of hydrogen in tribological properties of diamond-like carbon films. Surf. Coat. Technol. 2001, 146–147, 292–297. [Google Scholar] [CrossRef]
- Zeng, Q.; Eryilmaz, O.; Erdemir, A. Superlubricity of the DLC films-related friction system at elevated temperature. RSC Adv. 2015, 5, 93147–93154. [Google Scholar] [CrossRef]
- Kano, M.; Martin, J.M.; Yoshida, K.; Bouchet, M.I.D.B. Super-low friction of ta-C coating in presence of oleic acid. Friction 2014, 2, 156–163. [Google Scholar] [CrossRef]
- Kuwahara, T.; Romero, P.A.; Makowski, S.; Weihnacht, V.; Moras, G.; Moseler, M. Mechano-chemical decomposition of organic friction modifiers with multiple reactive centres induces superlubricity of ta-C. Nat. Commun. 2019, 10, 151. [Google Scholar] [CrossRef]
- Ikeyama, M.; Nakao, S.; Miyagawa, Y. Effects of Si content in DLC films on their friction and wear properties. Surf. Coat. Technol. 2005, 191, 38–42. [Google Scholar] [CrossRef]
- Baba, K.; Hatada, R.; Flege, S.; Ensinger, W. Deposition of silicon-containing diamond-like carbon films by plasma-enhanced chemical vapour deposition. Surf. Coat. Technol. 2009, 203, 2747–2750. [Google Scholar] [CrossRef]
- Zhang, T.F.; Wan, Z.X.; Ding, J.C.; Zhang, S.; Wang, Q.M.; Kim, K.H. Microstructure and high-temperature tribological properties of Si-doped hydrogenated diamond-like carbon films. Appl. Surf. Sci. 2018, 435, 963–973. [Google Scholar] [CrossRef]
- Wang, J.; Pu, J.; Zhang, G.; Wang, L. Interface architecture for superthick carbon-based films toward low internal stress and ultrahigh load-bearing capacity. ACS Appl. Mater. Interfaces 2013, 5, 5015–5024. [Google Scholar] [CrossRef] [PubMed]
- Moolsradoo, N.; Abe, S.; Watanabe, S. Thermal stability and tribological performance of DLC-Si–O films. Adv. Mater. Sci. Eng. 2011, 2011, 1–7. [Google Scholar] [CrossRef]
- Mangolini, F.; Krick, B.A.; Jacobs, T.D.; Khanal, S.R.; Streller, F.; McClimon, J.B.; Hilbert, J.; Prasad, S.V.; Scharf, T.W.; Ohlhausen, J.A.; et al. Effect of silicon and oxygen dopants on the stability of hydrogenated amorphous carbon under harsh environmental conditions. Carbon 2018, 130, 127–136. [Google Scholar] [CrossRef]
- Zhang, D.; Li, S.; Zuo, X.; Guo, P.; Ke, P.; Wang, A. Structural and mechanism study on enhanced thermal stability of hydrogenated diamond-like carbon films doped with Si/O. Diam. Relat. Mater. 2020, 108, 107923. [Google Scholar] [CrossRef]
- Safaie, P.; Eshaghi, A.; Bakhshi, S.R. Structure and mechanical properties of oxygen doped diamond-like carbon thin films. Diam. Relat. Mater. 2016, 70, 91–97. [Google Scholar] [CrossRef]
- Wild, C.; Koidl, P. Thermal gas effusion from hydrogenated amorphous carbon films. Appl. Phys. Lett. 1987, 51, 1506–1508. [Google Scholar] [CrossRef]
- Yang, B.; Zheng, Y.; Zhang, B.; Wei, L.; Zhang, J. The high-temperature tribological properties of Si-DLC films. Surf. Interface Anal. 2012, 44, 1601–1605. [Google Scholar] [CrossRef]
- Kim, J.-I.; Jang, Y.-J.; Kim, J.; Jeong, J.H. Improvement of running-in process of tetrahedral amorphous carbon film sliding against Si3N4 under humid air by O2 plasma post-irradiation. Appl. Surf. Sci. 2021, 538, 147957. [Google Scholar] [CrossRef]
- Fredriksson, H.; Chakarov, D.; Kasemo, B. Patterning of highly oriented pyrolytic graphite and glassy carbon surfaces by nanolithography and oxygen plasma etching. Carbon 2009, 47, 1335–1342. [Google Scholar] [CrossRef]
- Guo, M.; Diao, D.; Fan, X.; Yang, L.; Yu, L. Scratch behavior of re-structured carbon coating by oxygen plasma etching technology for magnetic disk application. Surf. Coat. Technol. 2014, 251, 128–134. [Google Scholar] [CrossRef]
- Choi, J.; Nakao, S.; Ikeyama, M.; Kato, T. Effect of oxygen plasma treatment on the tribological properties of Si-DLC coatings. Phys. Status Solid 2008, 5, 956–959. [Google Scholar] [CrossRef]
- Jongwannasiri, C.; Watanabe, S. Tribological behavior of O2 and CF4 plasma post-treated diamond-like carbon films under dry air and in a high relative humidity environment. Surf. Coat. Technol. 2016, 306, 200–204. [Google Scholar] [CrossRef]
- López-Santos, C.; Yubero, F.; Cotrino, J.; González-Elipe, A. Lateral and in-depth distribution of functional groups on diamond-like carbon after oxygen plasma treatments. Diam. Relat. Mater. 2011, 20, 49–56. [Google Scholar] [CrossRef]
- Corona-Gomez, J.; Shiri, S.; Mohammadtaheri, M.; Yang, Q. Adhesion enhancement of DLC on CoCrMo alloy by diamond and nitrogen incorporation for wear resistant applications. Surf. Coat. Technol. 2017, 332, 120–127. [Google Scholar] [CrossRef]
- Rao, X.; Yang, J.; Chen, Z.; Yuan, Y.; Chen, Q.; Feng, X.; Qin, L.; Zhang, Y. Tuning C–C sp2/sp3 ratio of DLC films in FCVA system for biomedical application. Bioact. Mater. 2020, 5, 192–200. [Google Scholar] [CrossRef]
- Fox-Rabinovich, G.S. Principles of friction control for surface-engineered materials. In Self-Organization during Friction. Advanced Surface-Engineered Materials and Systems Design; Fox-Rabinovich, G.S., Totten, G.E., Eds.; CRC Press: Boca Raton, FL, USA, 2006; pp. 3–12. [Google Scholar]
- Podgursky, V.; Bogatov, A.; Yashin, M.; Sobolev, S.; Gershman, I.S. Relation between self-organization and wear mechanisms of diamond films. Entropy 2018, 20, 279. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Vlassak, J.J. Determining the elastic modulus and hardness of an ultra-thin film on a substrate using nanoindentation. J. Mater. Res. 2009, 24, 1114–1126. [Google Scholar] [CrossRef]
- Jõgiaas, T.; Zabels, R.; Tamm, A.; Merisalu, M.; Hussainova, I.; Heikkilä, M.; Mändar, H.; Kukli, K.; Ritala, M.; Leskelä, M. Mechanical properties of aluminum, zirconium, hafnium and tantalum oxides and their nanolaminates grown by atomic layer deposition. Surf. Coat. Technol. 2015, 282, 36–42. [Google Scholar] [CrossRef]
- Rouhani, M.; Hobley, J.; Hong, F.C.-N.; Jeng, Y.-R. Spectroscopic investigation of thermally induced structural evolution of a-C:H:Si film. Appl. Surf. Sci. 2020, 541, 148413. [Google Scholar] [CrossRef]
- Åstrand, M.; Selinder, T.; Fietzke, F.; Klostermann, H. PVD-Al2O3-coated cemented carbide cutting tools. Surf. Coat. Technol. 2004, 188–189, 186–192. [Google Scholar] [CrossRef]
- Podgursky, V.; Yashin, M.; Jõgiaas, T.; Viljus, M.; Alamgir, A.; Danilson, M.; Bogatov, A. high temperature tribological properties of Al2O3/NCD films investigated under ambient air conditions. Coatings 2020, 10, 175. [Google Scholar] [CrossRef]
- Alamgir, A.; Yashin, M.; Bogatov, A.; Viljus, M.; Traksmaa, R.; Sondor, J.; Lümkemann, A.; Sergejev, F.; Podgursky, V. High-temperature tribological performance of hard multilayer TiN-AlTiN/nACo-CrN/AlCrN-AlCrO-AlTiCrN coating deposited on WC-Co Substrate. Coatings 2020, 10, 909. [Google Scholar] [CrossRef]
- Mangolini, F.; Hilbert, J.; McClimon, J.B.; Lukes, J.R.; Carpick, R.W. Thermally induced structural evolution of silicon- and oxygen-containing hydrogenated amorphous carbon: A combined spectroscopic and molecular dynamics simulation investigation. Langmuir 2018, 34, 2989–2995. [Google Scholar] [CrossRef] [PubMed]
- Scharf, T.W.; Ott, R.D.; Yang, D.; Barnard, J.A. Structural and tribological characterization of protective amorphous diamond-like carbon and amorphous CNx overcoats for next generation hard disks. J. Appl. Phys. 1999, 85, 3142–3154. [Google Scholar] [CrossRef]
- XPS Interpretation of Silicon. Available online: https://xpssimplified.com/elements/silicon.php (accessed on 1 April 2021).
- Pu, J.-C.; Wang, S.-F.; Sung, J.C. High-temperature oxidation behavior of nanocrystalline diamond films. J. Alloy. Compd. 2010, 489, 638–644. [Google Scholar] [CrossRef]
- Kudin, K.N.; Ozbas, B.; Schniepp, H.C.; Prud’Homme, R.K.; Aksay, I.A.; Car, R. Raman spectra of graphite oxide and functionalized graphene sheets. Nano Lett. 2008, 8, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Doremus, R.H. Diffusion in alumina. J. Appl. Phys. 2006, 100, 101301. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Podgursky, V.; Alamgir, A.; Yashin, M.; Jõgiaas, T.; Viljus, M.; Raadik, T.; Danilson, M.; Sergejev, F.; Lümkemann, A.; Kluson, J.; et al. High-Temperature Tribological Performance of Al2O3/a-C:H:Si Coating in Ambient Air. Coatings 2021, 11, 495. https://doi.org/10.3390/coatings11050495
Podgursky V, Alamgir A, Yashin M, Jõgiaas T, Viljus M, Raadik T, Danilson M, Sergejev F, Lümkemann A, Kluson J, et al. High-Temperature Tribological Performance of Al2O3/a-C:H:Si Coating in Ambient Air. Coatings. 2021; 11(5):495. https://doi.org/10.3390/coatings11050495
Chicago/Turabian StylePodgursky, Vitali, Asad Alamgir, Maxim Yashin, Taivo Jõgiaas, Mart Viljus, Taavi Raadik, Mati Danilson, Fjodor Sergejev, Andreas Lümkemann, Jan Kluson, and et al. 2021. "High-Temperature Tribological Performance of Al2O3/a-C:H:Si Coating in Ambient Air" Coatings 11, no. 5: 495. https://doi.org/10.3390/coatings11050495
APA StylePodgursky, V., Alamgir, A., Yashin, M., Jõgiaas, T., Viljus, M., Raadik, T., Danilson, M., Sergejev, F., Lümkemann, A., Kluson, J., Sondor, J., & Bogatov, A. (2021). High-Temperature Tribological Performance of Al2O3/a-C:H:Si Coating in Ambient Air. Coatings, 11(5), 495. https://doi.org/10.3390/coatings11050495






