Gold, Silver, and Electrum Electroless Plating on Additively Manufactured Laser Powder-Bed Fusion AlSi10Mg Parts: A Review
Abstract
1. Introduction
1.1. Additive Manufacturing
1.2. Electroless Plating of Gold, Silver and Au–Ag Alloys
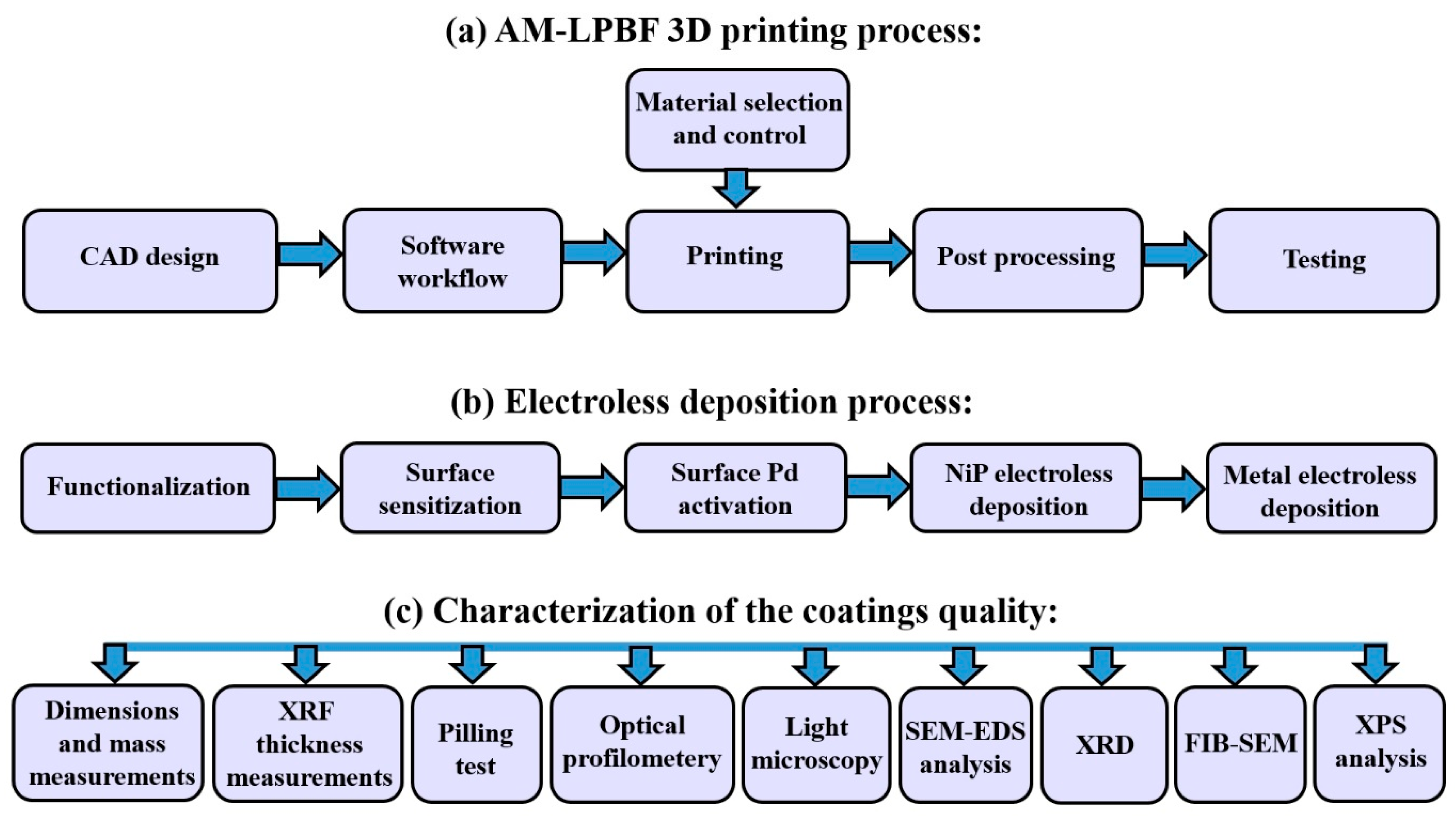
2. Materials and Experimental Methods
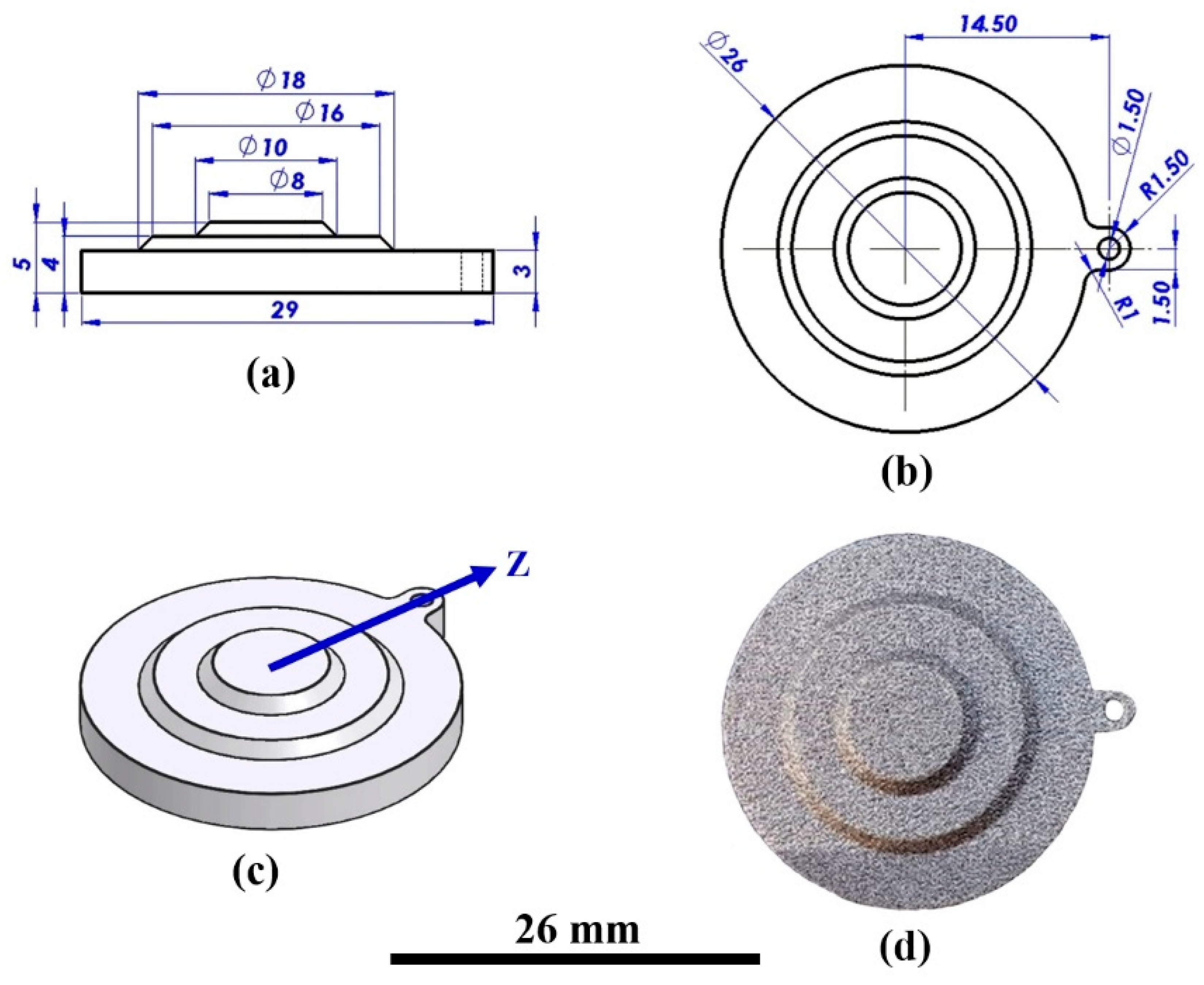
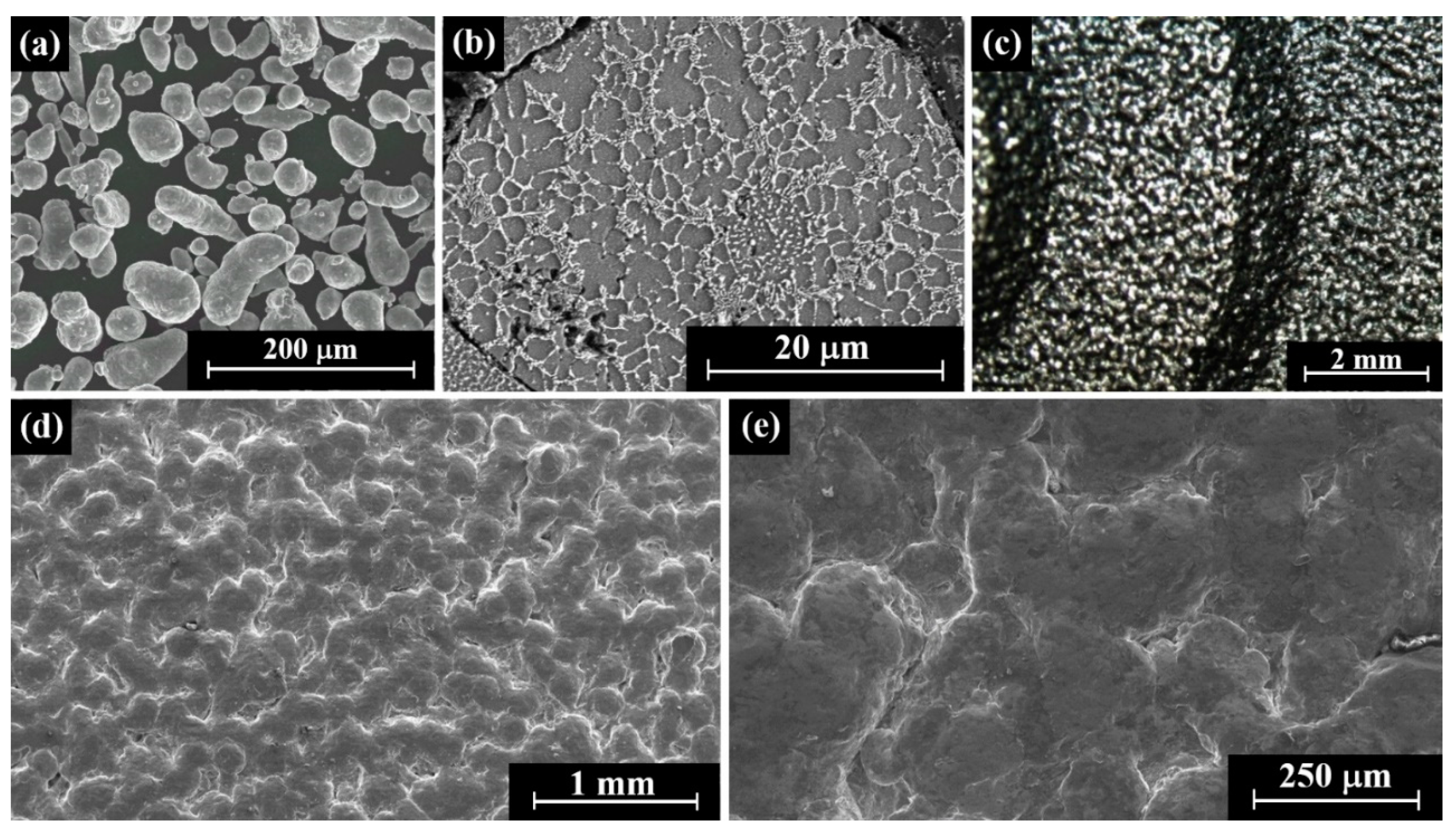
2.1. AM-LPBF AlSi10Mg Disk-Shaped Specimens
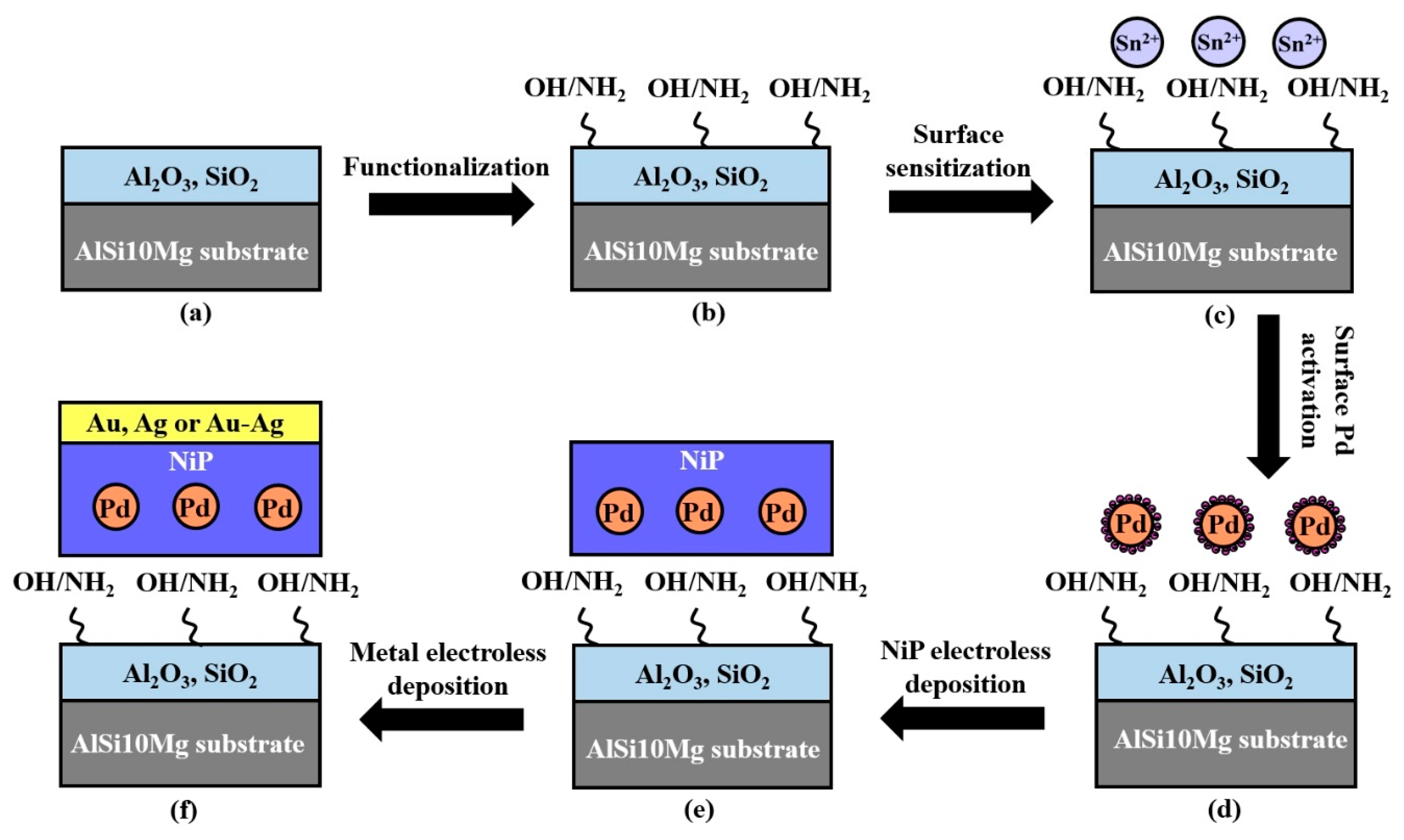
2.2. Silver Electroless Plating on AM-LPBF AlSi10Mg
2.3. Gold Electroless Plating on AM-LPBF AlSi10Mg
2.4. Gold–Silver Electroless Plating on AM-LPBF AlSi10Mg
2.5. Characterization Methods
- (a)
- The specimens were weighed in their as-printed condition, after cleaning, after etching, after coating by Ni–P interlayer, and after the final coating. The measurements were made with an analytical MRC ASB-220-C2 balance (MRC Laboratory Instruments, Holon, Israel) with a precision of ±0.0001 g [42,48].
- (b)
- A qualitative pilling test was performed on all coated specimens with transparent vinyl tape in order to evaluate the adhesion of the metal coatings to the printed substrate [42].
- (c)
- The thickness of the Ni–P interlayer and the electroless deposited Au, Ag, and Au-Ag films was measured by a calibrated high-resolution FISCHERSCOPE X-RAY XDL 230 X-ray fluorescence (XRF) instrument (Fischer Technology, Inc., Windsor, CT, USA), with an approximation error of ±0.1 μm [42]. Each measurement was performed in a circle of ≈1 mm in diameter for 30 s.
- (d)
- Average roughness (Ra) and root mean square (RMS) roughness measurements were accomplished with Alpha-Step D-500 Stylus profilometer (KLA Corporation, Milpitas, CA, USA), with vertical high-resolution profiling of 0.1 nm. The average surface roughness was calculated considering seven to 10 measurements [42].
- (e)
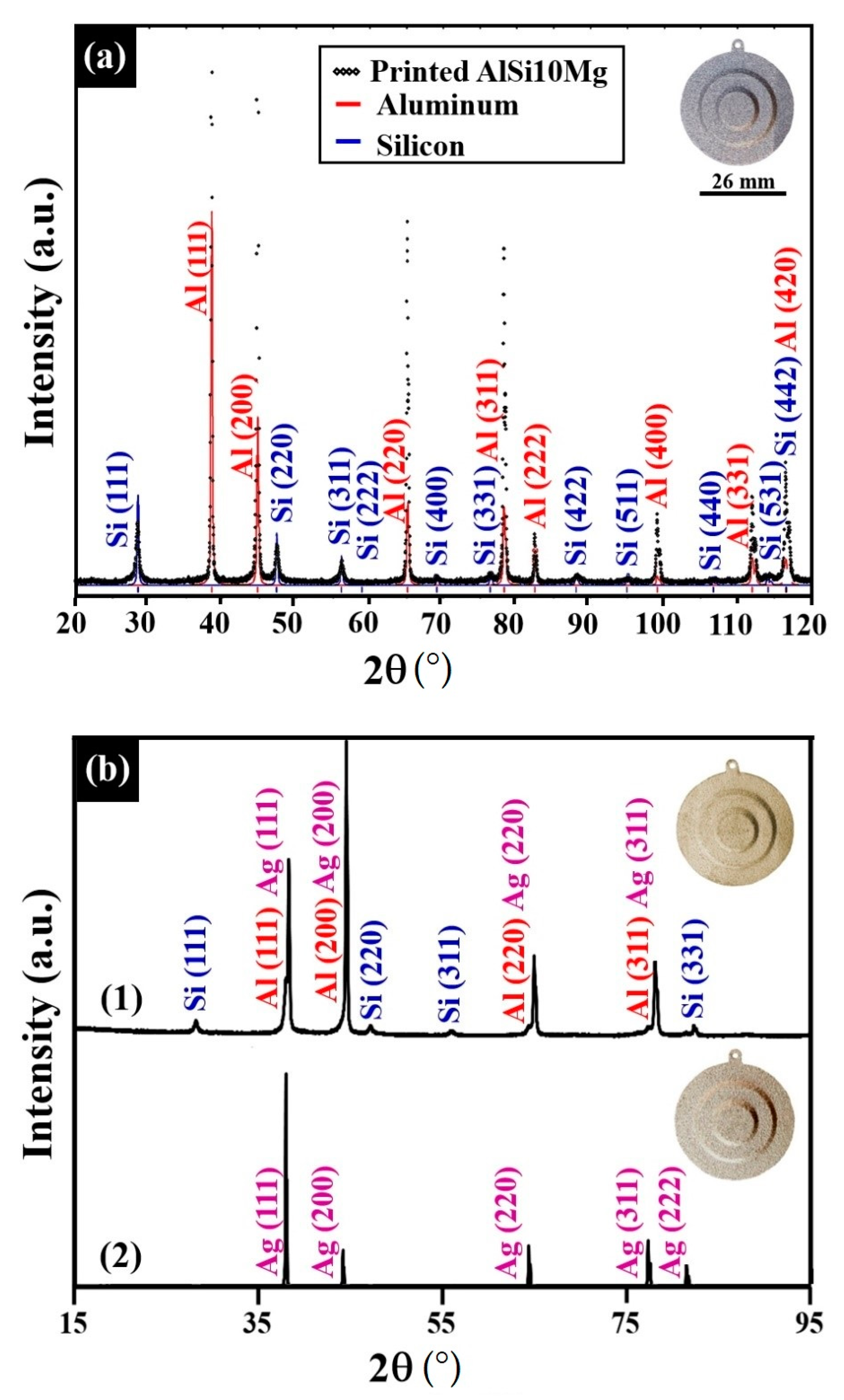
- X-ray diffraction (XRD) analysis was performed before and after electroless coating with a powder PANalytical Empyrean X-Ray Diffractometer (Malvern Panalytical Company, Malvern, UK) to determine the crystal structure and lattice constants of the coated layers. Data were collected in the common symmetrical Bragg–Brentano geometry (θ/θ) using a Cu Kα radiation (wavelength of λ = 1.541 Å) operating at 40 kV and 30 mA. Phase identification from the XRD results was done by using International Centre for Diffraction Data (ICDD) databases and Pearson’s handbook with the assistance of a PowderCell program for powder pattern calculation [48,56].
- (f)
- HIROX RH-2000 digital 3D multi-focal light microscope (LM) (HIROX Company, Tokyo, Japan), equipped with different optical lenses, a high-intensity LED lighting source, and powerful 3D software, was used to characterize the specimens’ general surface quality before and after coating and to identify microscopic discontinuities and defects [43,57].
- (g)
- Scanning electron microscopy (SEM) observation and energy-dispersive spectroscopy (EDS) analysis were performed with an ESEM FEI Quanta 200 FEG instrument (FEI Company, Hillsboro, OR, USA) in the high vacuum mode, before and after the electroless coating. An Everhart–Thonley secondary electron liquid-nitrogen-cooled Si(Li) X-ray EDS detector (Oxford Instruments Company, Abingdon, UK), calibrated with standard specimens received from the instrument manufacturer, was utilized to measure the chemical composition of the substrate and the coating. The EDS analysis provided results with an estimated error of 1% [42].
- (h)
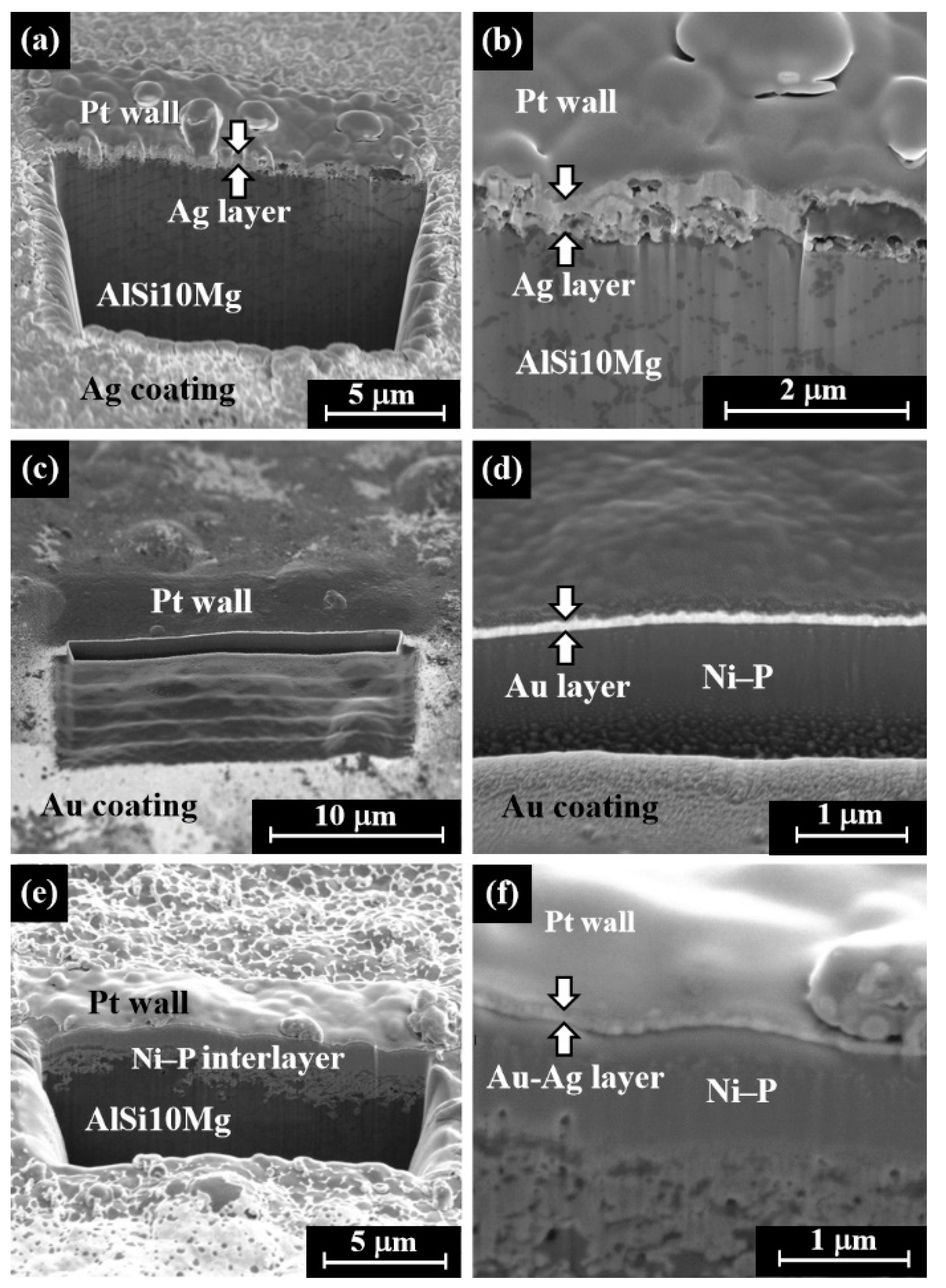
- Focused ion beam (FIB) technology with an accelerated high-energy gallium ions source, equipped with high-resolution SEM Helios 600 SEM/FIB dual beam (Thermo Fisher Scientific, Waltham, MA, USA), was applied in order to observe the cross-section of the silver, gold, and electrum plated specimens. Before digging into the specimen, a thin layer of platinum was deposited (voltage of 2.0 kV and current of 2.7 nA) on the surface of the samples to protect the deposited films during the milling processes. The digging was performed with a high voltage of 30.0 kV and a beam current of 21.0 nA, which was followed by etching with a 6.5 nA beam current. The FIB’s milled cut dimensions were 15 μm × 15 μm, with a depth of ≈7 μm [42].
- (i)
- X-ray photoelectron spectroscopy (XPS) profiling with an Al Kα monochromatic irradiation source (1486.6 eV) was performed to the electrum coated specimens by using 5600 Multi-Technique System (PHI Inc., Chanhassen, MN, USA) in an ultra-high vacuum (UHV) with a base pressure of 2.5 × 10−10 Torr. The specimens were not charged during the analysis. The outcome electrons were examined with a Spherical Capacitor Analyzer (SCA), using a 0.8 mm slit aperture. The sputtering was performed with a 4kV Ar+ Ion Gun using 17 Å/min sputter rate on SiO2/Si and was assumed to be approximately three times higher on Au–Ag [48].
3. Results
3.1. AM-LPBF AlSi10Mg Disk-Shaped Specimens
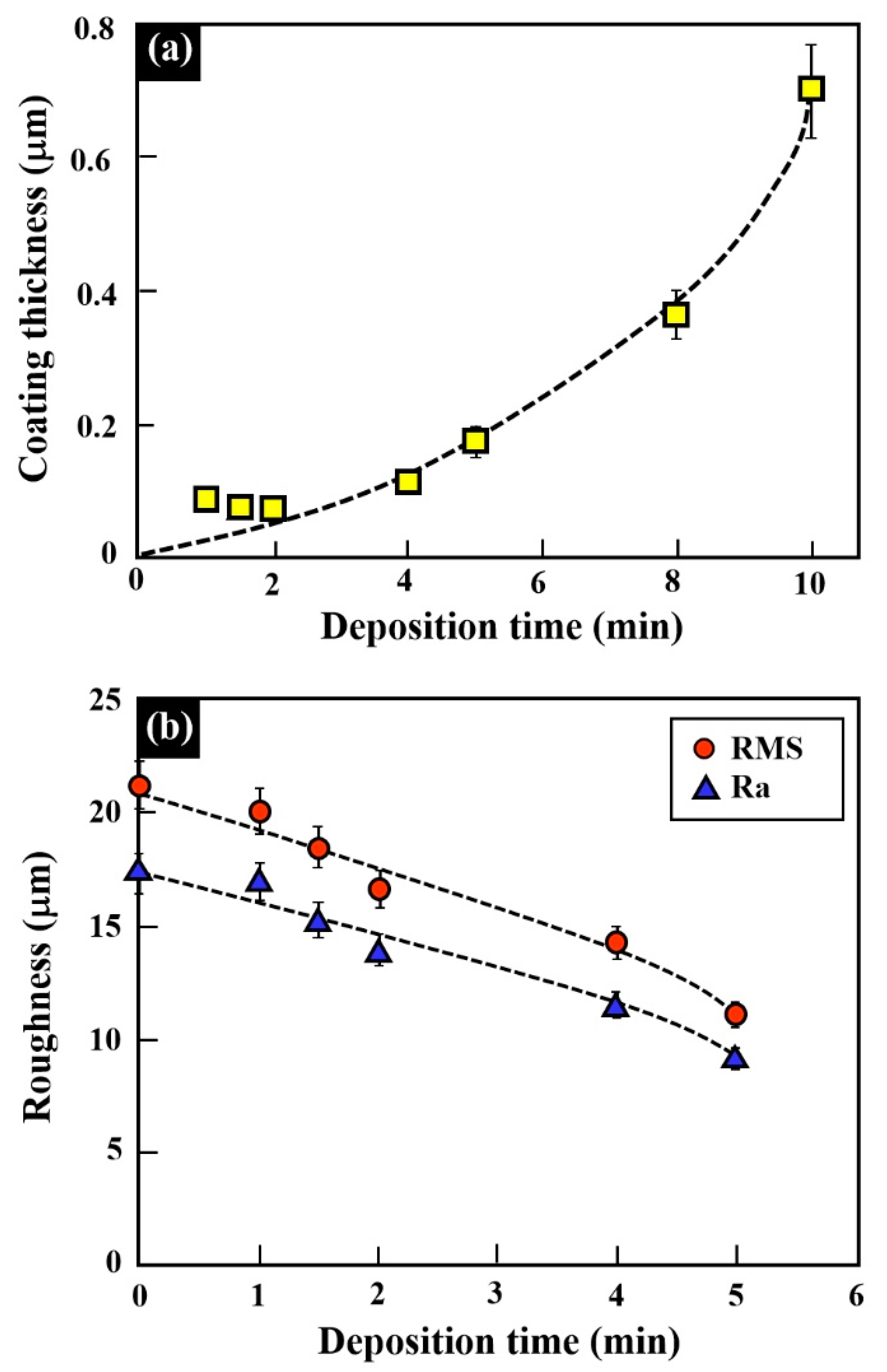
3.2. Silver Electroless Plating on AM-LPBF AlSi10Mg Disk-Shaped Specimens
3.3. Gold Electroless Plating on AM-LPBF AlSi10Mg Disk-Shaped Specimens
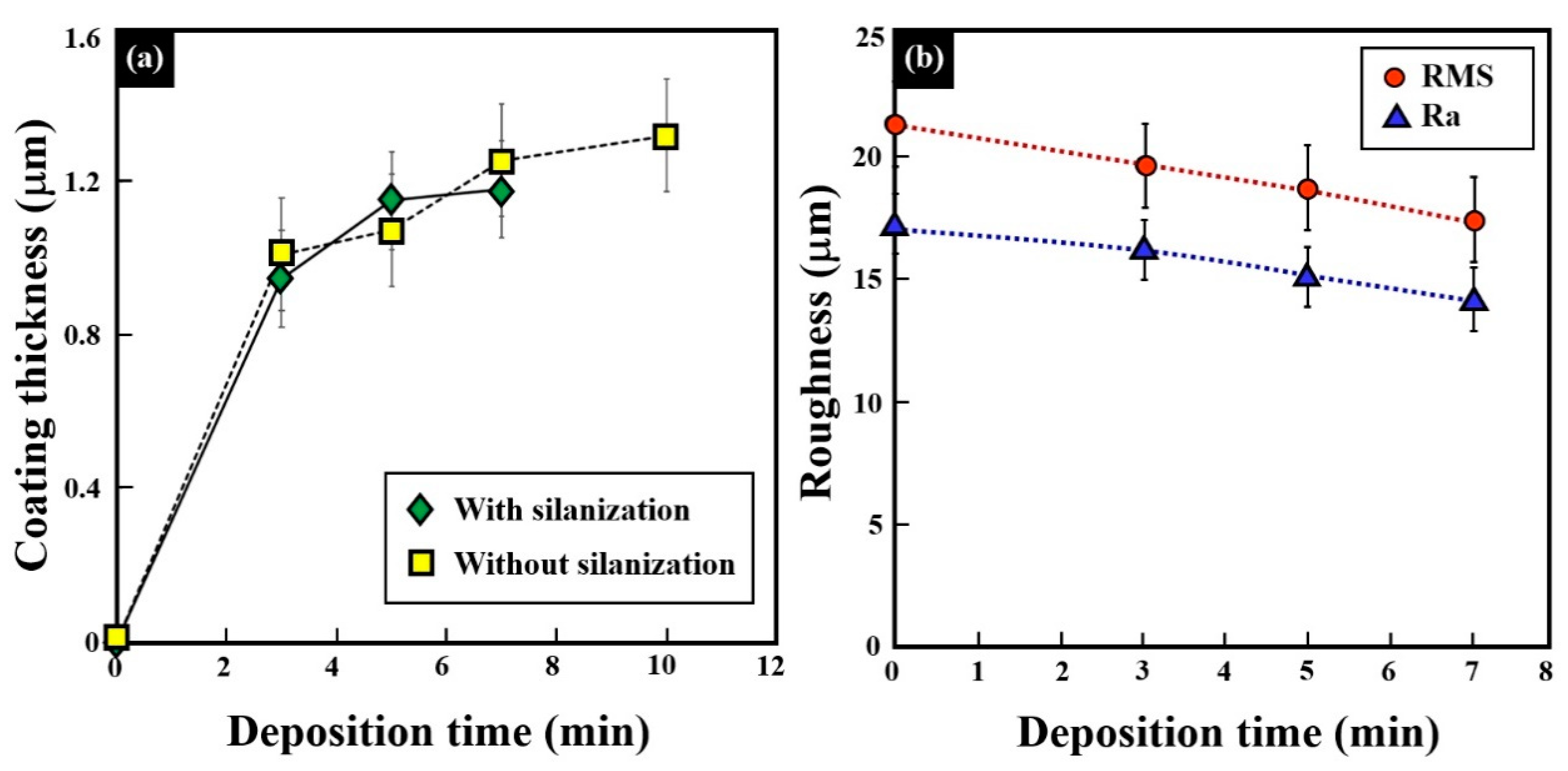
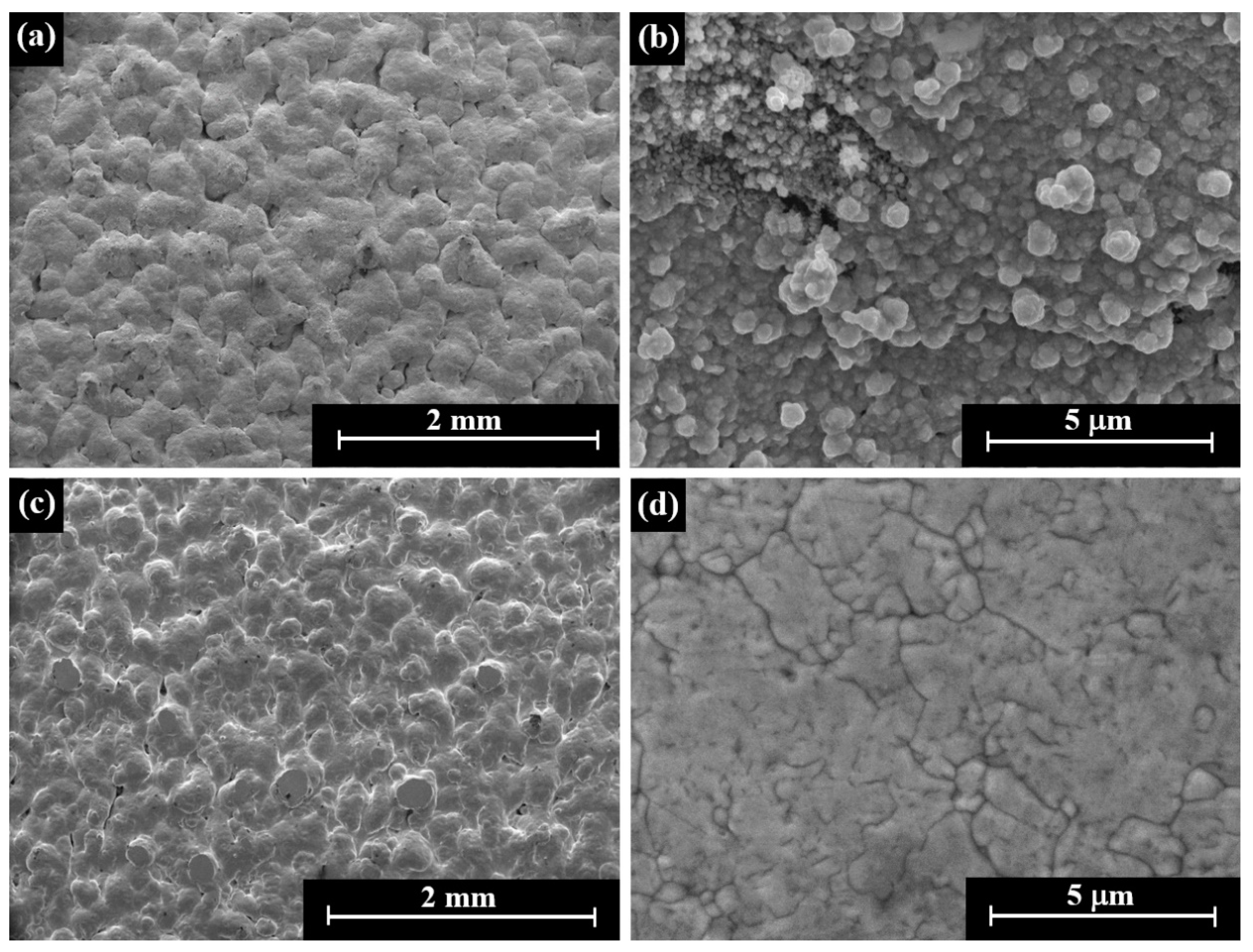
3.4. Gold–Silver Electroless Plating on AM-LPBF AlSi10Mg Disk-Shaped Specimens
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, W.; Li, S.; Liu, J.; Zhang, A.; Zhou, Y.; Wei, Q.; Yan, C.; Shi, Y. Effect of heat treatment on AlSi10Mg alloy fabricated by selective laser melting: Microstructure evolution, mechanical properties and fracture mechanism. Mater. Sci. Eng. A 2016, 663, 116–125. [Google Scholar] [CrossRef]
- Aboulkhair, N.T.; Tuck, C.; Ashcroft, I.; Maskery, I.; Everitt, N.M. On the precipitation hardening of selective laser melted AlSi10Mg. Met. Mater. Trans. A 2015, 46, 3337–3341. [Google Scholar] [CrossRef]
- Zhou, L.; Mehta, A.; Schulz, E.; McWilliams, B.; Cho, K.; Sohn, Y. Microstructure, precipitates and hardness of selectively laser melted AlSi10Mg alloy before and after heat treatment. Mater. Charact. 2018, 143, 5–17. [Google Scholar] [CrossRef]
- Van Cauwenbergh, P.; Samaee, V.; Thijs, L.; Nejezchlebová, J.; Sedlák, P.; Iveković, A.; Schryvers, D.; Van Hooreweder, B.; Vanmeensel, K. Unravelling the multi-scale structure–property relationship of laser powder bed fusion processed and heat-treated AlSi10Mg. Sci. Rep. 2021, 11, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Macías, J.G.S.; Douillard, T.; Zhao, L.; Maire, E.; Pyka, G.; Simar, A. Influence on microstructure, strength and ductility of build platform temperature during laser powder bed fusion of AlSi10Mg. Acta Mater. 2020, 201, 231–243. [Google Scholar] [CrossRef]
- Kusoglu, I.M.; Gökce, B.; Barcikowski, S. Research trends in laser powder bed fusion of Al alloys within the last decade. Addit. Manuf. 2020, 36, 101489. [Google Scholar] [CrossRef]
- Rosenthal, I.; Stern, A.; Frage, N. Strain rate sensitivity and fracture mechanism of AlSi10Mg parts produced by selective laser melting. Mater. Sci. Eng. A 2017, 682, 509–517. [Google Scholar] [CrossRef]
- Zaretsky, E.; Stern, A.; Frage, N. Dynamic response of AlSi10Mg alloy fabricated by selective laser melting. Mater. Sci. Eng. A 2017, 688, 364–370. [Google Scholar] [CrossRef]
- Yang, T.; Liu, T.; Liao, W.; MacDonald, E.; Wei, H.; Chen, X.; Jiang, L. The influence of process parameters on vertical surface roughness of the AlSi10Mg parts fabricated by selective laser melting. J. Mater. Process. Technol. 2019, 266, 26–36. [Google Scholar] [CrossRef]
- Steuben, J.C.; Birnbaum, A.J.; Michopoulos, J.G.; Iliopoulos, A.P. Enriched analytical solutions for additive manufacturing modeling and simulation. Addit. Manuf. 2019, 25, 437–447. [Google Scholar] [CrossRef]
- Whip, B.; Sheridan, L.; Gockel, J. The effect of primary processing parameters on surface roughness in laser powder bed additive manufacturing. Int. J. Adv. Manuf. Technol. 2019, 103, 4411–4422. [Google Scholar] [CrossRef]
- Boschetto, A.; Bottini, L.; Veniali, F. Roughness modeling of AlSi10Mg parts fabricated by selective laser melting. J. Mater. Process. Technol. 2017, 241, 154–163. [Google Scholar] [CrossRef]
- Nahmany, M.; Hadad, Y.; Aghion, E.; Stern, A.; Frage, N. Microstructural assessment and mechanical properties of electron beam welding of AlSi10Mg specimens fabricated by selective laser melting. J. Mater. Process. Technol. 2019, 270, 228–240. [Google Scholar] [CrossRef]
- Park, T.H.; Baek, M.S.; Sohn, Y.; Lee, K.A. Effect of post-heat treatment on the wear properties of AlSi10Mg alloy manufactured by selective laser melting. Arch. Metall. Mater. 2020, 65, 1073–1080. [Google Scholar] [CrossRef]
- Han, Q.; Jiao, Y. Effect of heat treatment and laser surface remelting on AlSi10Mg alloy fabricated by selective laser melting. Int. J. Adv. Manuf. Technol. 2019, 102, 3315–3324. [Google Scholar] [CrossRef]
- Lee, S.; Wajahat, M.; Kim, J.H.; Pyo, J.; Chang, W.S.; Cho, S.H.; Kim, J.T.; Seol, S.K. Electroless Deposition-assisted 3D printing of micro circuitries for structural electronics. ACS Appl. Mater. Interfaces 2019, 11, 7123–7130. [Google Scholar] [CrossRef]
- Kuo, C.-C.; Wang, C.-W.; Lee, Y.-F.; Liu, Y.-L.; Qiu, Q.-Y. A surface quality improvement apparatus for ABS parts fabricated by additive manufacturing. Int. J. Adv. Manuf. Technol. 2016, 89, 635–642. [Google Scholar] [CrossRef]
- Han, Q.; Gu, H.; Soe, S.; Setchi, R.; Lacan, F.; Hill, J. Manufacturability of AlSi10Mg overhang structures fabricated by laser powder bed fusion. Mater. Des. 2018, 160, 1080–1095. [Google Scholar] [CrossRef]
- Inberg, A.; Shacham-Diamand, Y.; Rabinovich, E.; Golan, G.; Croitoru, N. Electroless-deposited Ag–W films for microelectronics applications. Thin Solid Films 2001, 389, 213–218. [Google Scholar] [CrossRef]
- Estrada-Raygoza, I.; Sotelo-Lerma, M.; Ramírez-Bon, R. Structural and morphological characterization of chemically deposited silver films. J. Phys. Chem. Solids 2006, 67, 782–788. [Google Scholar] [CrossRef]
- Asher, T.; Inberg, A.; Glickman, E.; Fishelson, N.; Shacham-Diamand, Y. Formation and characterization of low resistivity sub-100nm copper films deposited by electroless on SAM. Electrochim. Acta 2009, 54, 6053–6057. [Google Scholar] [CrossRef]
- Inberg, A.; Livshits, P.; Zalevsky, Z.; Shacham-Diamand, Y. Electroless deposition of silver thin films on gold nanoparticles catalyst for micro and nanoelectronics applications. Microelectron. Eng. 2012, 98, 570–573. [Google Scholar] [CrossRef]
- Duhin, A.; Inberg, A.; Eliaz, N.; Gileadi, E. Electroless plating of rhenium-based alloys with nickel, cobalt and iron. Electrochim. Acta 2015, 174, 660–666. [Google Scholar] [CrossRef]
- Lelevic, A.; Walsh, F.C. Electrodeposition of NiP composite coatings: A review. Surf. Coat. Tech. 2019, 378, 124803. [Google Scholar] [CrossRef]
- Paunovic, M. Electrochemical aspects of electroless deposition of metals. Plating 1968, 55, 1161–1167. [Google Scholar]
- Shacham-Diamand, Y.; Inberg, A.; Sverdlov, Y.; Croitoru, N. Electroless silver and silver with tungsten thin films for microelectronics and microelectromechanical system applications. J. Electrochem. Soc. 2000, 147, 3345–3349. [Google Scholar] [CrossRef]
- Shukla, S.; Gomathi, N.; George, R. Autocatalytic silver-plating of aluminum radio frequency waveguides with autocatalytic nickel as the undercoat for space applications. Surf. Topogr. Metrol. Prop. 2014, 2, 045004. [Google Scholar] [CrossRef]
- Shacham-Diamand, Y.; Osaka, T.; Okinaka, Y.; Sugiyama, A.; Dubin, V. 30 years of electroless plating for semiconductor and polymer micro-systems. Microelectron. Eng. 2015, 132, 35–45. [Google Scholar] [CrossRef]
- Brenner, A.; Riddell, G. Nickel plating on steel by chemical reduction. J. Res. Natl. Inst. Stand. Technol. 1946, 37, 31. [Google Scholar] [CrossRef]
- Wang, C.; Farhat, Z.; Jarjoura, G.; Hassan, M.K.; Abdullah, A.M. Indentation and erosion behavior of electroless Ni–P coating on pipeline steel. Wear 2017, 376–377, 1630–1639. [Google Scholar] [CrossRef]
- Cui, C.; Du, H.; Liu, H.; Xiong, T. Corrosion behavior of the electroless Ni–P coating on the pore walls of the lotus-type porous copper. Corros. Sci. 2020, 162, 108202. [Google Scholar] [CrossRef]
- Guo, Z.; Keong, K.; Sha, W. Crystallisation and phase transformation behaviour of electroless nickel phosphorus platings during continuous heating. J. Alloys Compd. 2003, 358, 112–119. [Google Scholar] [CrossRef]
- Wang, J.; Wu, Q. The effects of anodic interlayer on the morphology and mechanical performances of electroless Ni–P coating on Al alloy. Appl. Phys. A 2017, 123, 435. [Google Scholar] [CrossRef]
- Tsaia, T.K.; Chao, C.G. The growth morphology and crystallinity of electroless NiP deposition on silicon. Appl. Surf. Sci. 2004, 233, 180–190. [Google Scholar] [CrossRef]
- Ashkenazi, D.; Gitler, H.; Stern, A.; Tal, O. Metallurgical investigation on fourth century BCE silver jewellery of two hoards from Samaria. Sci. Rep. 2017, 7, 40659. [Google Scholar] [CrossRef] [PubMed]
- Fishelson, N.; Inberg, A.; Croitoru, N.; Shacham-Diamand, Y. Highly corrosion resistant bright silver metallization deposited from a neutral cyanide-free solution. Microelectron. Eng. 2012, 92, 126–129. [Google Scholar] [CrossRef]
- Zhang, H.; Shen, L.; Chang, J. Comparative study of electroless Ni–P, Cu, Ag, and Cu–Ag plating on polyamide fabrics. J. Ind. Text. 2010, 41, 25–40. [Google Scholar] [CrossRef]
- Liu, S.; Chen, G.; Prasad, P.N.; Swihart, M.T. Synthesis of monodisperse Au, Ag, and Au–Ag alloy nanoparticles with tunable size and surface plasmon resonance frequency. Chem. Mater. 2011, 23, 4098–4101. [Google Scholar] [CrossRef]
- Hough, R.M.; Butt, C.R.M.; Fischer-Bühner, J. The crystallography, metallography and composition of gold. Elements 2009, 5, 297–302. [Google Scholar] [CrossRef]
- Troalen, L.G.; Tate, J.; Guerra, M.F. Goldwork in ancient Egypt: Workshop practices at Qurneh in the 2nd Intermediate Period. J. Archaeol. Sci. 2014, 50, 219–226. [Google Scholar] [CrossRef]
- Suematsu, Y.; Saito, K.; Koyama, M.; Enokida, Y.; Okura, Y.; Nakayasu, T.; Sukegawa, T. Development of micro-mirror slicer integral field unit for space-borne solar spectrographs. CEAS Space J. 2017, 9, 421–431. [Google Scholar] [CrossRef]
- Dresler, N.; Inberg, A.; Ashkenazi, D.; Shacham-Diamand, Y.; Stern, A. Silver electroless finishing of selective laser melting 3D-printed AlSi10Mg artifacts. Met. Microstruct. Anal. 2019, 8, 678–692. [Google Scholar] [CrossRef]
- Inberg, A.; Ashkenazi, D.; Kimmel, G.; Shacham-Diamand, Y.; Stern, A. Gold plating of AlSi10Mg parts produced by a laser powder-bed fusion additive manufacturing technique. Prog. Addit. Manuf. 2020, 5, 395–404. [Google Scholar] [CrossRef]
- Akben, K.; Timur, S. A study of gold–silver alloy electrodeposited from pyrophosphate-cyanide electrolyte using polyethylenimine-KSeCN additives. Int. J. Electrochem. Sci. 2018, 13, 3855–3873. [Google Scholar] [CrossRef]
- Huang, T. Electrodeposited silver-gold alloy as a sensor for paracetamol determination. Int. J. Electrochem. Sci. 2017, 12, 11419–11427. [Google Scholar] [CrossRef]
- Márquez, K.; Staikov, G.; Schultze, J.W. Electrochemical deposition of Ag, Au and Ag–Au alloys on n-Si(111). Trans. IMF 2002, 80, 73–78. [Google Scholar] [CrossRef]
- Stansfield, G.L.; Johnston, H.M.; Baxter, S.N.; Thomas, P.J. Deposition of Ag and Ag–Au nanocrystalline films with tunable conductivity at the water–toluene interface. RSC Adv. 2018, 8, 6225–6230. [Google Scholar] [CrossRef]
- Inberg, A.; Ashkenazi, D.; Kimmel, G.; Shacham-Diamand, Y.; Stern, A. Gold–silver electroless plating on laser powder-bed fusion additively printed AlSi10Mg parts. Metals 2020, 10, 557. [Google Scholar] [CrossRef]
- Shani, D.; Inberg, A.; Ashkenazi, D.; Shacham-Diamand, Y.; Stern, A. Gold plating on AM-FDM ABS components. Ann. Dunarea Jos Univ. Galati. Fascicle XII Weld. Equip. Technol. 2019, 30, 43–50. [Google Scholar] [CrossRef]
- Olivera, S.; Muralidhara, H.B.; Venkatesh, K.; Gopalakrishna, K.; Vivek, C.S. Plating on acrylonitrile–butadiene–styrene (ABS) plastic: A review. J. Mater. Sci. 2016, 51, 3657–3674. [Google Scholar] [CrossRef]
- Yoshino, M.; Masuda, T.; Yokoshima, T.; Sasano, J.; Shacham-Diamand, Y.; Matsuda, I.; Osaka, T.; Hagiwara, Y.; Sato, I. Electroless diffusion barrier process using SAM on Low-k dielectrics. J. Electrochem. Soc. 2007, 154, D122–D125. [Google Scholar] [CrossRef]
- Ulman, A. Formation and structure of self-assembled monolayers. Chem. Rev. 1996, 96, 1533–1554. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, F. Structure and growth of self-assembling monolayers. Prog. Surf. Sci. 2000, 65, 151–257. [Google Scholar] [CrossRef]
- Ashkenazi, D.; Nusbaum, I.; Shacham-Diamand, Y.; Cvikel, D.; Kahanov, Y.; Inberg, A. A method of conserving ancient iron artefacts retrieved from shipwrecks using a combination of silane self-assembled monolayers and wax coating. Corros. Sci. 2017, 123, 88–102. [Google Scholar] [CrossRef]
- Krusenstern, A. Galvanotechnik of Noble Metals; Metallurgy: Moscow, Russia, 1974; p. 91. (In Russian) [Google Scholar]
- Werner, K.; Nolze, G. Powder cell—A program for the representation and manipulation of crystal structures and calculation of the resulting X-ray powder patterns. J. Appl. Cryst. 1996, 29, 301–303. [Google Scholar]
- Ashkenazi, D.; Cvikel, D. A journey into the microstructure: Using a multifocal 3D digital light microscope to study archaeological artefacts retrieved from shipwrecks. Digit. Appl. Archaeol. Cult. Heritage 2020, 16, e00129. [Google Scholar] [CrossRef]
- Reiher, T.; Lindemann, C.; Jahnke, U.; Deppe, G.; Koch, R. Holistic approach for industrializing AM technology: From part selection to test and verification. Prog. Addit. Manuf. 2017, 2, 43–55. [Google Scholar] [CrossRef]
- Ngo, T.D.; Kashani, A.; Imbalzano, G.; Nguyen, K.T.; Hui, D. Additive manufacturing (3D printing): A review of materials, methods, applications and challenges. Compos. Part. B Eng. 2018, 143, 172–196. [Google Scholar] [CrossRef]
- Silbernagel, C.; Ashcroft, I.; Dickens, P.; Galea, M. Electrical resistivity of additively manufactured AlSi10Mg for use in electric motors. Addit. Manuf. 2018, 21, 395–403. [Google Scholar] [CrossRef]
- Duhin, A.; Inberg, A.; Eliaz, N.; Gileadi, E. Electroless plating of rhenium–nickel alloys. Electrochim. Acta 2011, 56, 9637–9643. [Google Scholar] [CrossRef]
- Guisbiers, G.; Mendoza-Cruz, R.; Bazán-Díaz, L.; Velázquez-Salazar, J.J.; Mendoza-Perez, R.; Robledo-Torres, J.A.; Rodriguez-Lopez, J.-L.; Montejano-Carrizales, J.M.; Whetten, R.L.; José-Yacamán, M. Electrum, the Gold–Silver Alloy, from the Bulk Scale to the Nanoscale: Synthesis, Properties, and Segregation Rules. ACS Nano 2016, 10, 188–198. [Google Scholar] [CrossRef] [PubMed]


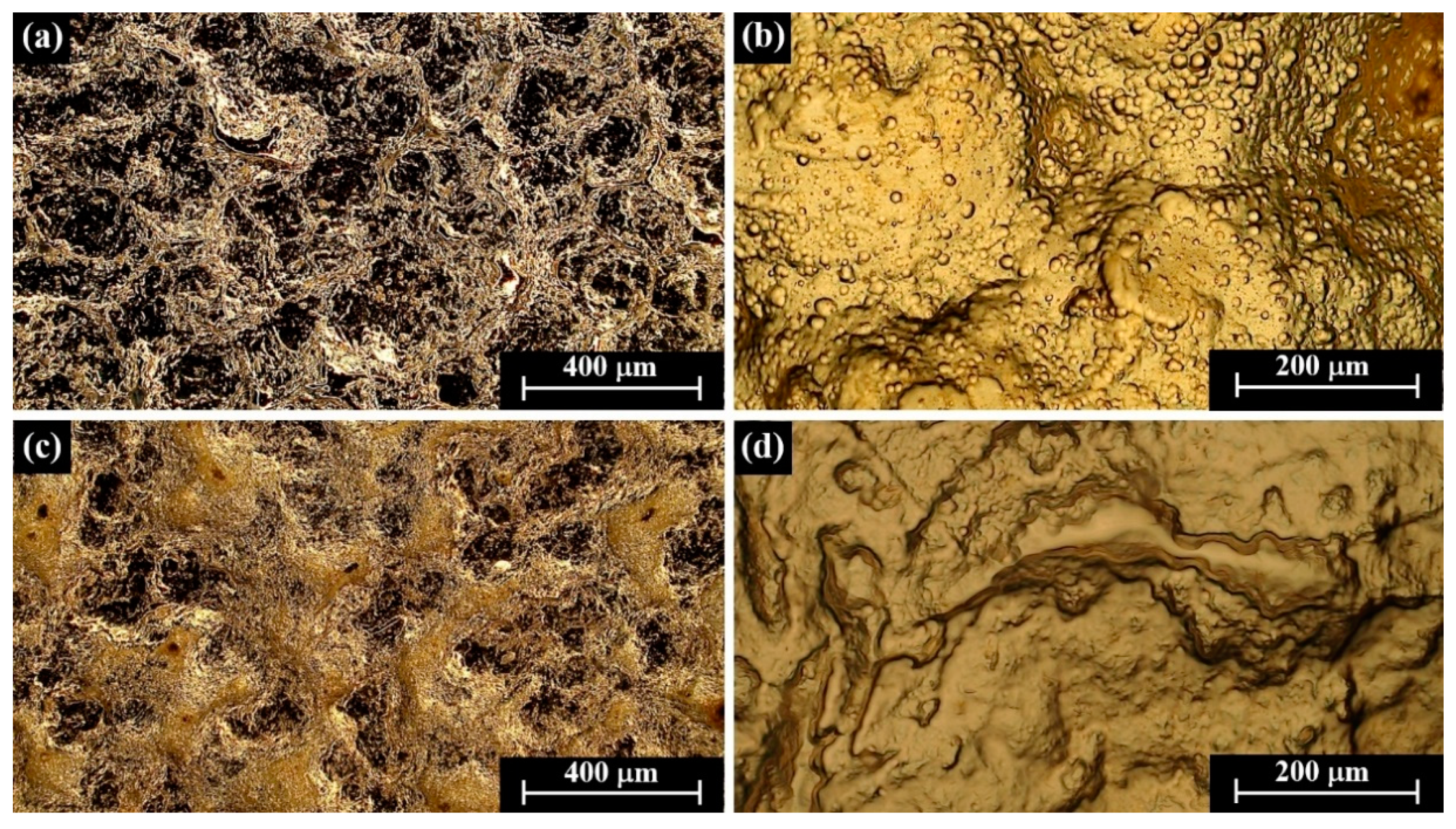
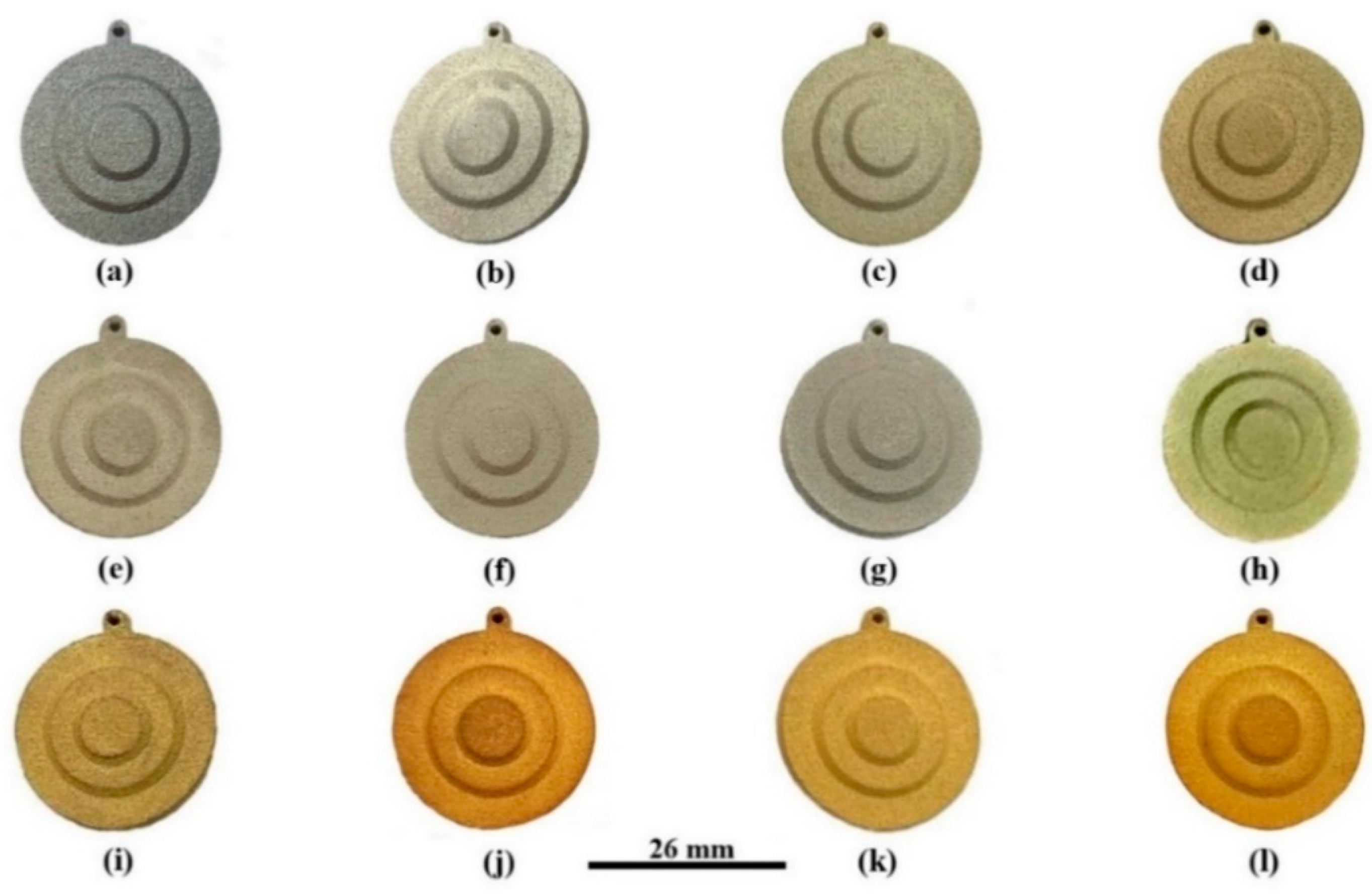
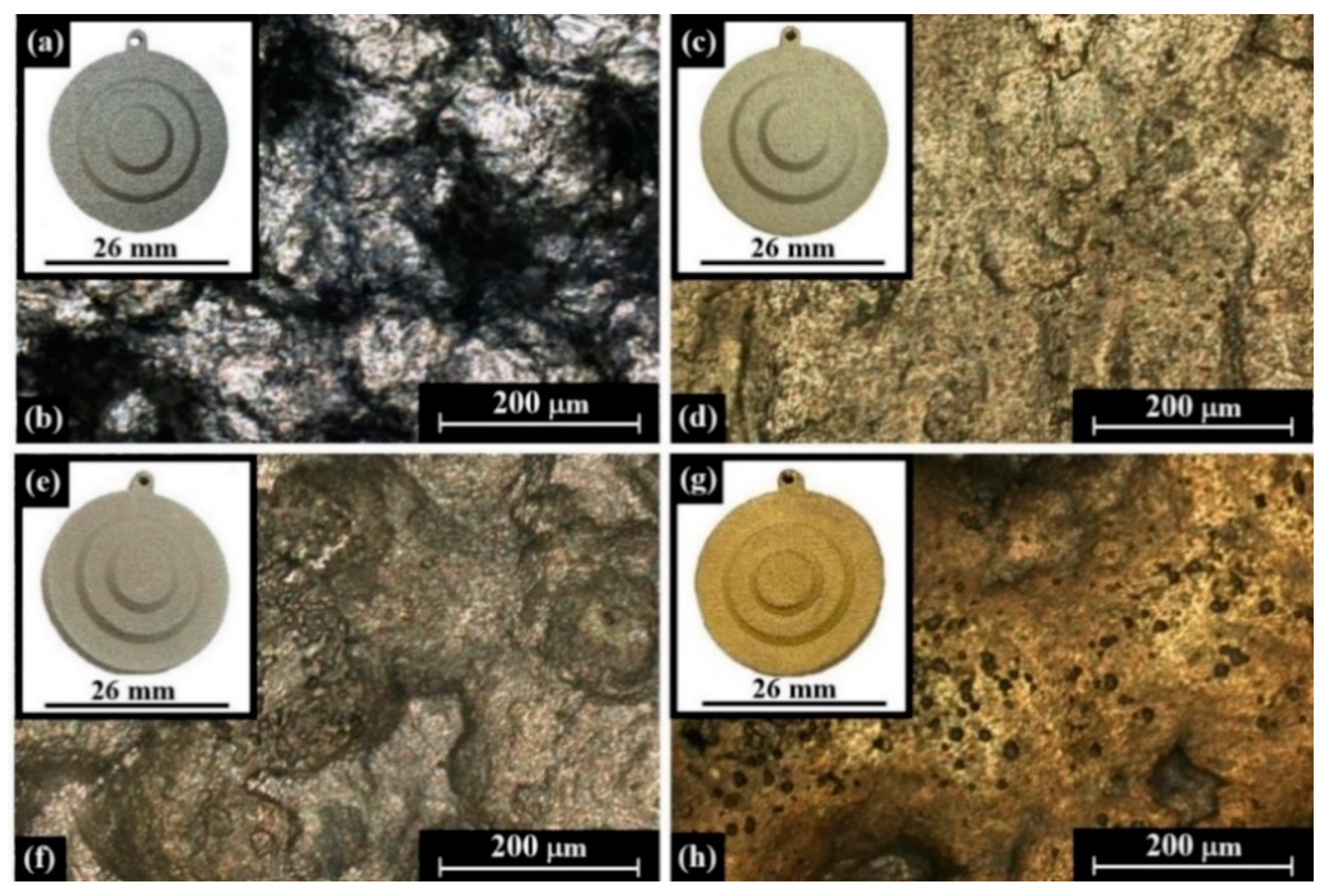
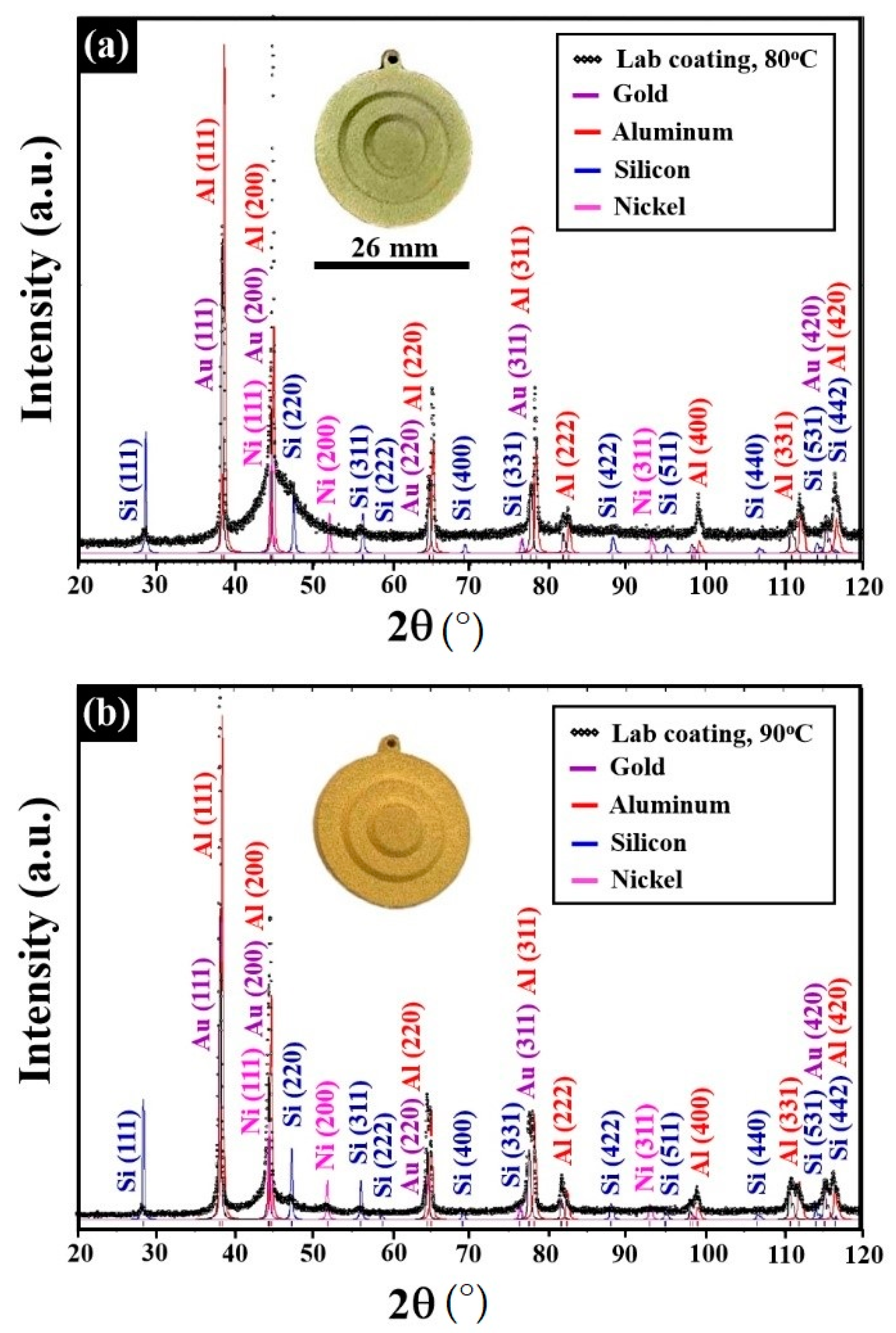
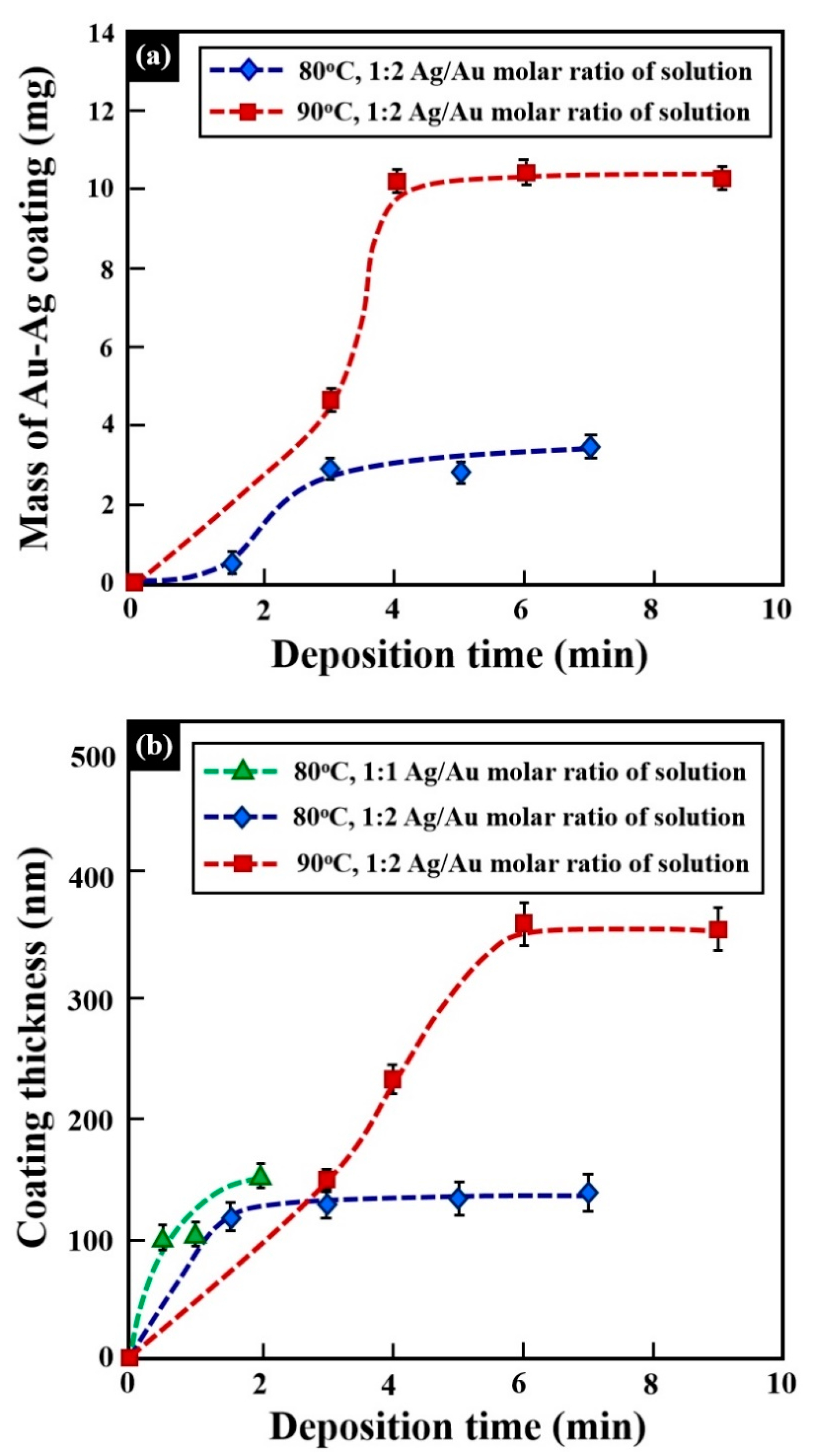
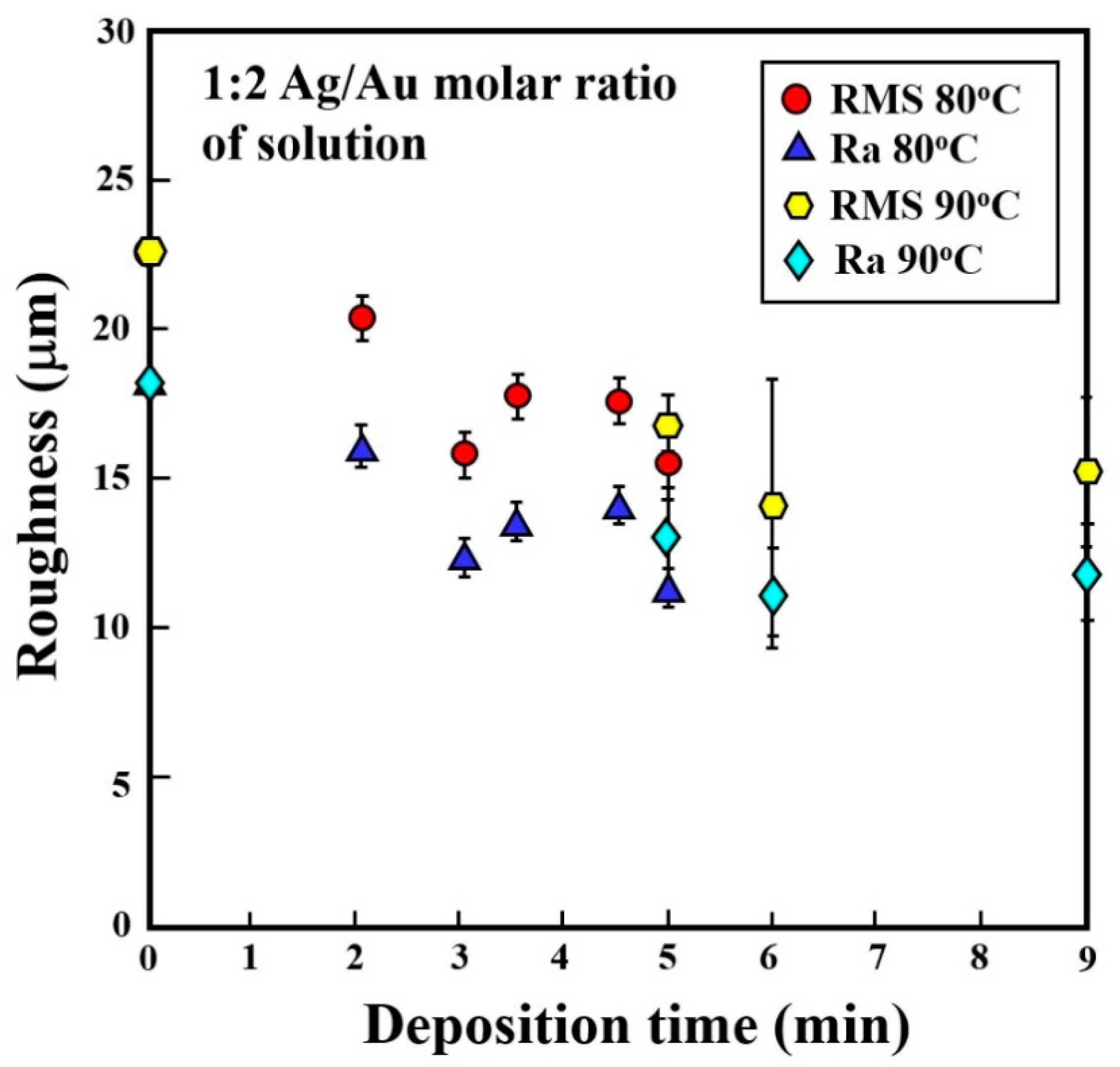
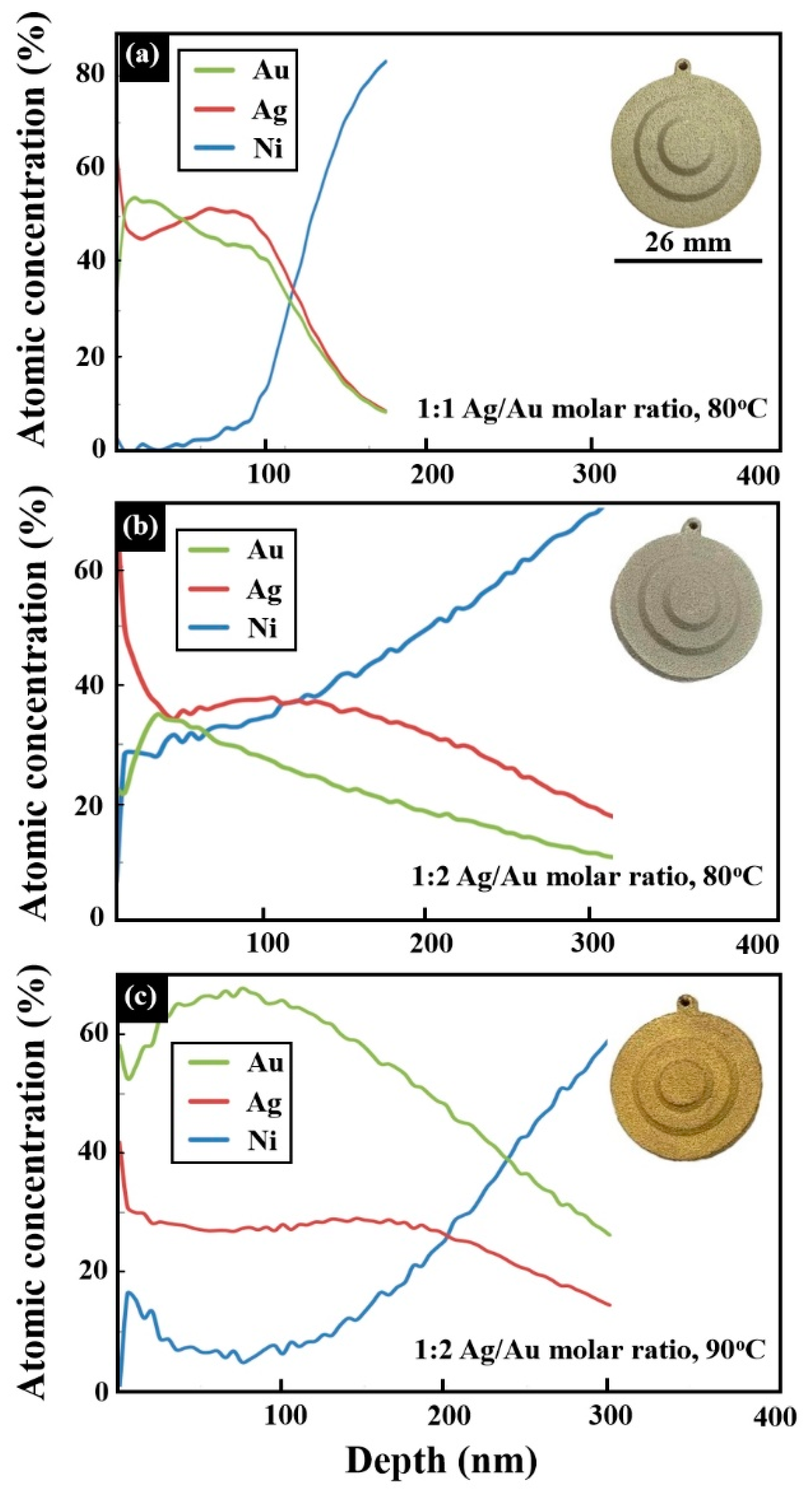
| Alloy | Composition (wt %) | |||||||
|---|---|---|---|---|---|---|---|---|
| Si | Mg | Fe | Cu, Mn, Zn, Ni, Pn, Sn | Ti | Al | O | N | |
| 1706 | 9.0–11.0 | 0.20–0.45 | ≤0.55 | ≤0.1 | ≤0.15 | Bal. | N.A. | N.A. |
| Component | Concentration (M) |
|---|---|
| Silver nitrate (AgNO3) | 0.03 |
| Ammonia (NH4OH) | 1.0 |
| Acetic acid (CH3COOH) | 0.5 |
| Component | Concentration (M) |
|---|---|
| Nickel(II) sulfate hexahydrate (NiSO4·6H2O) | 0.114 |
| 3-Na-citrate (Na3C6H5O7) | 0.043 |
| Sodium hypophosphite (NaH2PO2) | 0.094 |
| Sodium acetate (NaCH3COO) | 0.037 |
| Component | Concentration (M) |
|---|---|
| Potassium dicyanoaurate [KAu(CN)2] | 0.007–0.010 |
| Ammonium chloride (NH4Cl) | 1.3–1.4 |
| 3-Na-citrate (Na3C6H5O7) | 0.155–0.170 |
| Sodium hypophosphite (NaH2PO2) | 0.075–0.094 |
| Component | Concentration (M) |
|---|---|
| Potassium dicyanoaurate [KAu(CN)2] | 0.007 |
| Potassium silver cyanide [KAg(CN)2] | 0.0035 |
| 3-Na-citrate (Na3C6H5O7) | 0.170 |
| Hydrazine hydrate (H6N2O) | 0.015 |
| Polyethylene glycol 1500 (PEG 1500) | 0.0003 |
| Sodium saccharin (C7H4NNaO3S) | 0.002 |
| Specimen | Lattice Parameter (Å) | Characteristics of the Au–Ag Coating | Characteristics of the Ni–P Interlayer | Characteristics of the Substrate |
|---|---|---|---|---|
| 1:1 Ag/Au molar ratio at 80 °C | 4.080 (±0.001) | Rich in Ag, solid-solution crystalline phase | Quasi-amorphous | Major crystalline phase AlMinor crystalline phase Si |
| 1:2 Ag/Au molar ratio at 80 °C | 4.079 (±0.001) | |||
| 1:2 Ag/Au molar ratio at 90 °C | 4.076 (±0.001) | Rich in Au, solid-solution crystalline phase | Mix of quasi-amorphous and nanocrystalline |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ashkenazi, D.; Inberg, A.; Shacham-Diamand, Y.; Stern, A. Gold, Silver, and Electrum Electroless Plating on Additively Manufactured Laser Powder-Bed Fusion AlSi10Mg Parts: A Review. Coatings 2021, 11, 422. https://doi.org/10.3390/coatings11040422
Ashkenazi D, Inberg A, Shacham-Diamand Y, Stern A. Gold, Silver, and Electrum Electroless Plating on Additively Manufactured Laser Powder-Bed Fusion AlSi10Mg Parts: A Review. Coatings. 2021; 11(4):422. https://doi.org/10.3390/coatings11040422
Chicago/Turabian StyleAshkenazi, Dana, Alexandra Inberg, Yosi Shacham-Diamand, and Adin Stern. 2021. "Gold, Silver, and Electrum Electroless Plating on Additively Manufactured Laser Powder-Bed Fusion AlSi10Mg Parts: A Review" Coatings 11, no. 4: 422. https://doi.org/10.3390/coatings11040422
APA StyleAshkenazi, D., Inberg, A., Shacham-Diamand, Y., & Stern, A. (2021). Gold, Silver, and Electrum Electroless Plating on Additively Manufactured Laser Powder-Bed Fusion AlSi10Mg Parts: A Review. Coatings, 11(4), 422. https://doi.org/10.3390/coatings11040422






