Abstract
In the present article the doping of aluminide coatings by Pt/Pd as well as Hf or Pd using industrial processes was developed. The different combinations of doping elements were tested as well as their influence on chemical composition of coatings was initially investigated. The Pt and Pd and both Pt + Pd was electroplated on the surface of the MAR M247 nickel superalloy. The Zr or Hf was doped during low activity CVD aluminizing process using industrial Bernex BPX Pro 325S system. The conducted research showed that Pt and Pd formed the (Ni, Pd, Pt) Al solid solution in the outer additive layer. The higher concentration of palladium in the near surface and in the whole additive layer was detected. The platinum was presented below the surface of aluminide coating. The Zr or Hf was detected mainly in the diffusion zone. The low concentration of Zr (about 0.1 wt.%) in the outer zone was observed. The hafnium was detected mainly in the diffusion zone but in the outer additive layer a small concentration of this element was measured. The obtained results showed that formation of three elements (Pd, Pt) + Zr or Hf modified aluminide coating using proposed technology is possible. The structure of all obtained aluminide coatings was typical for a low-activity, high temperature (LAHT) formation process mainly by outward diffusion of Ni from base material.
Keywords:
modified aluminide coatings; CVD process; Pd; Pt; Hf; Zr; superalloys; oxidation resistance; NiAl 1. Introduction
Aluminizing is the primary process for production of oxidation resistant coatings for nickel superalloys [1,2]. Aluminide coatings are formed using pack cementation [3], out of pack [4] and chemical vapor deposition (CVD) [5] methods. Out of the pack, and its similar methods, vapor phase aluminizing were most common used in aerospace industry [6]. In this method, Cr-Al granules, placed min. 100 mm from the coated elements, are used as a source of aluminum. The process is performed using an AlF3 activator in a retort furnace in an argon or hydrogen atmosphere. Its main advantage is the fact that the coatings produced in this process are thicker than those deposited by CVD, its disadvantage is difficulty in coating formation into internal cooling channels of turbine blades [7]. In the CVD process, AlClx halides are formed in an external generator by passing HCl through aluminum pellets at a high temperature [8]. The deposited coating has greater purity and it is possible to coat internal cooling channels of turbine vanes or blades in this way [9]. The research on modifying aluminide coatings with other elements in order to extend their service life has been developed from over 30 years. The most common method is Pt incorporation by electroplating and its aluminizing using out of pack [10] or CVD methods [11]. Angenete et al. [12] showed that Pt-modified aluminide coatings formed by outward diffusion of Ni in the low-activity aluminizing process increases the oxide adhesion. The palladium might be used as an alternative to platinum in increasing oxidation resistance of aluminide coatings [13]. During oxidation, Pd stabilizes the β-NiAl phase and increases its oxidation resistance [14]. In research work [15,16] the use of both palladium and platinum for aluminide coating modification was proposed. Yavorska et al. [17] proposed the use of CVD aluminizing process coating formation after Pt and Pd electroplating. Reactive elements, such as Hf, can improve oxidation resistance of aluminide coatings. Pytel et al. [18] used the CVD low-activity process for doping by Hf produced in an external generator.
The influence of all the mentioned elements (Pt, Pd, Zr, Hf) on oxidation resistance of aluminide coatings is positive; however, the mechanisms of protection are different. Zhang et al. [19] showed that the presence of Pt into aluminide coatings eliminates the voids growth at the oxide/metal interface. Tawance et al. [20] explained that Pt improves thermal stability of aluminide coatings and its capability for selective oxidation of Al. Angenete et al. [12,21] suggested also that Pt suppressed the amount of Ni and Co as well as increasing the high concentration of Al at the surface area. Li et al. [22] concluded that palladium plays a significant role in the alumina layer stabilization and in the delay of NiAl phase degradation. The use of both Pd/Pt-modified aluminide coatings showed a less rumpled surface than Pt-modified aluminide coating due to its thicker coating layer formation. The pore formation in Pd-modified aluminide coating was observed as well [23]. The addition of Hf to aluminide coatings also dramatically improves its oxidation resistance [24]. The modification of Pt-modified aluminide coatings by Hf doping increases the cyclic oxidation resistance of TBCs. Hf diffuses to the surface of (Ni, Pt) Al coating, forms oxides and reduces the rumpling tendency of TBCs [25]. The modification of Pt-aluminide coating by Zr increases its oxidation resistance by retarding θ-Al2O3 to α-Al2O3 transformation segregates on alumina grains and reduces tendencies to rumpling [26].
Based on a conducted review as well as our previous works [6,18], the doping of aluminide coatings by Pt/Pd, as well as Hf or Pd using industrial processes, was tested. The different combinations of doping elements were tested and their influence on chemical composition of coatings was initially investigated.
2. Materials and Methods
The MAR M247 nickel superalloy (chemical composition is presented in Table 1) was used as a base material. Cylindrical samples with a diameter of 14 mm and a height of 4 mm were cut from a cast rod. Samples were grinded, sandblasted and degreased in isopropanol. The samples were not heat-treated. The palladium and platinum of approximately 3 μm thick layers were electroplated at Silesian University of Technology, Gliwice, Poland. After the electroplating process, the low-activity aluminizing process based on parameters described in ref. [15] was conducted: HCl flow: 0.2 NLPM (normal liters per minute), H2 flow 10.5 NLPM, temperature 1040 °C, pressure 150 mbar, process time 8 h. The doping by Hf and Zr was conducted during aluminizing process using parameters selected in ref. [5]: HCl flow 0.4 NLPM, Ar flow 2.5 NLPM, process time 2 h. The Bernex BPX Pro 325 S CVD industrial system (Ion Bond, Olten, Switzerland) was used for all aluminizing processes. The seven types of aluminide coatings were produced: simple Pt- (1), Pd-modified (2), Pt and Pd modified (3), Pd and Zr (4), Pd and Hf (5), Pd, Pt and Hf (6) as well Pt, Pd and Zr (7). The microstructure was analyzed using S-3400 Scanning electron microscope (SEM, Hitachi, Tokyo, Japan) equipped with an EDS analyzer (Thermo Fisher, Waltham, MA, USA).

Table 1.
Nominal composition of MAR M247 nickel superalloy.
3. Results
3.1. The Simple Pt-Al Aluminide Coating (1)
The platinum-modified aluminide coating was produced as a reference coating for comparison with other developed modified aluminide coatings. It was conventionally produced by platinum electroplating and low-activity CVD aluminizing process. The obtained coating had a typical single-phase structure (Ni, Pt) Al solid solution. The presence of bright PtAl2 precipitations was not observed. The thickness of Pt-modified aluminide coating was 46 μm (Figure 1, Table 2). The analysis of average chemical composition (area 4 on Figure 1) showed the concentration of Al—19 wt.% and Pt—15.8 wt.%. The concentration of Al was changed from 24.1 wt.% (area 1 on Figure 1) on the near-surface area to 16.8 wt.% close to the diffusion zone (area 3 on Figure 1). The amount of platinum in the near-surface area was lower (10 wt.%) than in near diffusion-zone area (areas 2 and 3, Figure 1, Table 2)—17 wt.%. The presence of hafnium, molybdenum, tungsten and titanium in the outer additive layer was noted. Chromium and cobalt were detected in this area as well. In the diffusion zone, except for the mentioned elements, the presence of platinum (12.9 wt.%) and aluminum (12.7 wt.%) was detected.
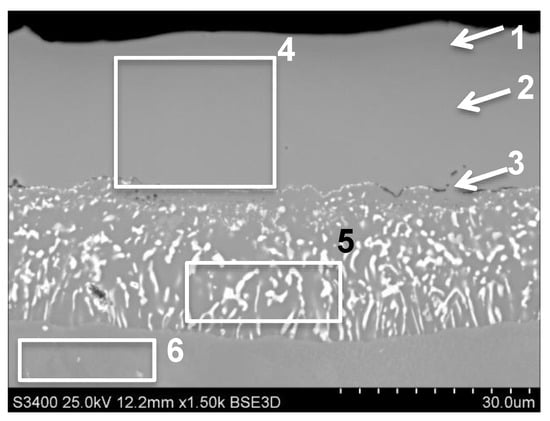
Figure 1.
The reference Pt-modifed aluminide coating produced on MAR M247 nickel superalloy with marked areas of chemical composition analysis.

Table 2.
Results of chemical composition analysis from areas presented on Figure 1.
3.2. The Simple Pd-Al Coating (2)
The Pt-modified aluminide coating obtained on MAR M247 alloy had an average thickness of 50.36 μm. Its microstructure was typical for aluminide coatings formed during low-activity aluminizing with dominated outward diffusion of nickel from base material (Figure 2). Chemical composition analysis from the area marked as “4” on Figure 2 showed the average amount of Al to be about 20 wt.% (Table 3). The average concentration of palladium in this area was about 24 wt.%. In this area, the presence of chromium, cobalt, zirconium and hafnium from base material was observed as well. The amount of Al on the cross-section of aluminide coatings was changed from 20 wt.% (area 1 on Figure 2) on the near-surface area to 18 wt.% near the diffusion zone. Similarly, the amount of palladium was changed from 24 wt.% on area 1 to 17 wt.% close to diffusion zone (Figure 2, Table 3). Into diffusion zone (areas 5, 6, Figure 2, Table 3), the larger amount of tungsten, tantalum and titanium was noted. The presence of chromium and cobalt was detected as well. On area marked 5 and 6 (Figure 2) the precipitations of hafnium were observed.
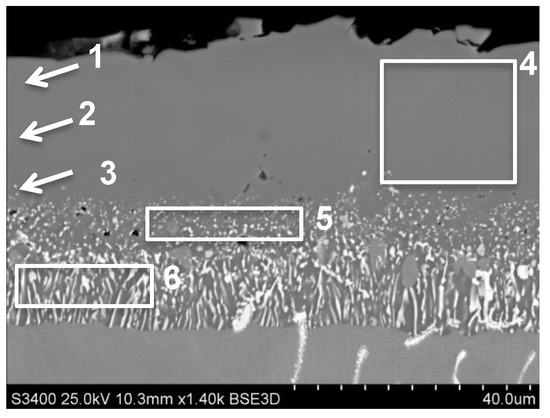
Figure 2.
Aluminide coating produced by Pd electroplating and low activity aluminizing deposited by CVD method on MAR M247 nickel superalloy with marked areas of chemical composition analysis.

Table 3.
Results of chemical composition analysis from areas presented on Figure 2.
3.3. The (Pt + Pd)-Al Coating (3)
The palladium and platinum-modified coating was about 55 μm thick. It was produced by electroplating of platinum and palladium and low activity CVD aluminizing after that. On SEM microphotographs, the two different areas in the additive layer were observed (Figure 3). Chemical composition analysis from near-surface zone (areas 1 and 2 on Figure 3, Table 4) showed the presence of aluminum of 21 wt.% and 18 wt.% respectively. The amount of Pd in outer zone was high and was in area 1—22.7 wt.% and 23.2 wt.% in area 2 (Figure 3). The concentration of platinum in near surface area (marked as “1” on Figure 3) was 10 wt.% and below, in zone 2—approx. 15 wt.%. In the second visible layer concentration of aluminum was lower—approx. 16–17 wt.% in areas 3 and 4 (Figure 3, Table 4). The content of Pd in this zone was lower: in area 3—about 20 wt.% and in area 4—17 wt.%. The concentration Pt in area 3 was highest—18 wt.% and in area marked as 4—14 wt.%. In outer zone the molybdenum and tungsten were not present. The titanium, cobalt and chromium were only detected. The chemical composition analysis in the diffusion zone (area marked as “5” on Figure 3, Table 4) showed the low concentration of aluminum (10 wt.%) and the presence of tungsten, titanium and molybdenum. In the diffusion zone (area 5 on Figure 3, Table 4), the platinum (13 wt.%) and palladium (8.8 wt.%) were detected as well.
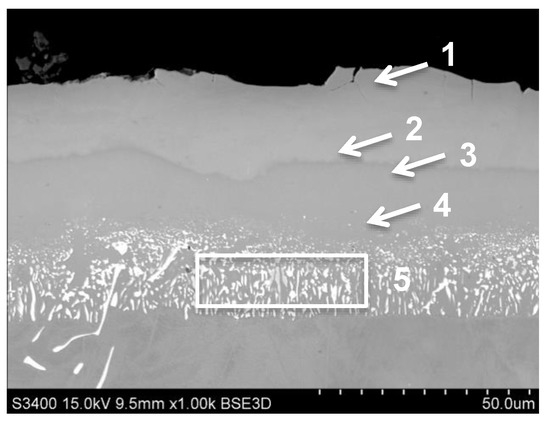
Figure 3.
The aluminide coating produced by Pt and Pd electroplating and CVD low-activity aluminizing on MAR M247 nickel superalloy with marked areas of EDS analysis.

Table 4.
Results of chemical composition analysis from areas presented on Figure 3.
3.4. The (Pd + Zr)-Al Coating (4)
The aluminide coating modified by palladium and zirconium was characterized by a thickness approx. 51 μm (Figure 4). This coating was produced by palladium electroplating and a low activity CVD aluminizing process. The Zr was doped from an external reactor by passing of HCl though Zr granules. The measurement of an average concentration of elements into an additive layer (area 4 on Figure 4, Table 5) showed the concentration of Al—21.2 wt.% and palladium—17.4 wt.%. The concentration of Zr was very low (0.1–0.2 wt.%). The presence of Cr and Co in this zone was noted as well (area 4, Figure 4, Table 5). The Hf in this zone was not presented. The concentration of Al was changed in the additive layer from 25.8 wt.% (area 1 marked on Figure 4) by 21 wt.% in the middle to 19 wt.% close to the diffusion zone. The highest amount of palladium was measured in the middle of the additive layer (18.6 wt.% in area marked 2 on Figure 4). The EDS chemical analysis in the diffusion zone (area 5 on Figure 4, Table 5) showed the presence of palladium (approx. 6 wt.%) and refractory metals: titanium, molybdenum, tantalum and tungsten. The amount of Al in the diffusion zone (area 5 on Figure 4) was approx. 11 wt.%.
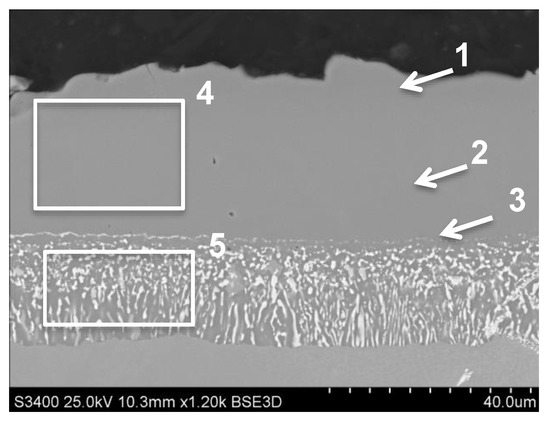
Figure 4.
The aluminide coating produced by Pd electroplating and CVD low-activity aluminizing with Zr doping on MAR M247 nickel superalloy with marked areas of chemical composition analysis.

Table 5.
Results of chemical composition analysis from areas presented on Figure 4.
3.5. The (Pd + Hf)-Al Coating (5)
The (Pd + Hf)-Al aluminide coating was obtained by palladium electroplating and a low-activity CVD aluminizing process. The hafnium was moped from an external reactor during aluminizing. The obtained coating was 47 μm trick and had a typical structure of coating produced in a low-activity aluminizing process (Figure 5). The average amount of aluminum in the outer additive layer was about 20 wt.%. (area 4 on Figure 5 and in Table 6). The concentration of Al in the coating was changed from 22 wt.% on the near-surface zone, by 20 wt.% in the middle of additive layer to 18 wt.% near the diffusion zone. The amount of palladium was increased from 22.8 wt.% in the near-surface area marked “1” to 25.4 wt.% in the middle (marked “2”) of additive layer into 19 wt.% close to diffusion zone (marked “3” on Figure 5, Table 6). The average concentration of Pd in outer zone was approx. 25 wt.% (areas 1–4 on Figure 5, Table 6). In outer zone the presence of chromium, cobalt and titanium was also observed (areas 1–4 on Figure 5, Table 6). The concentration of hafnium was very low (up to 0.3 wt.%). The higher concentration of this element was noted in diffusion zone (approx. 2.3% in area 5, Figure 5) and was higher than the presence of high melting elements: tungsten, titanium and molybdenum were noted (area 5 on Figure 5, Table 6).
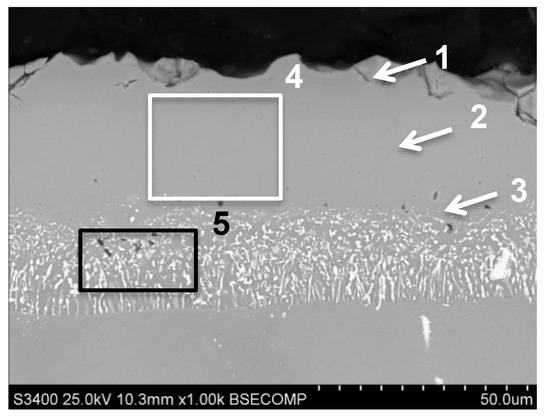
Figure 5.
The aluminide coating produced by Pd electroplating and CVD low-activity aluminizing with Hf doping on MAR M247 nickel superalloy with marked areas of chemical composition analysis.

Table 6.
Results of chemical composition analysis from areas presented on Figure 5.
3.6. The (Pt + Pd + Zr)-Al Coating (6)
The aluminide coatings modified by Pt, Pt and Zr was produced by electroplating of palladium and platinum as well as doping of zirconium during low-activity CVD aluminizing process. The thickness of obtained coating was 51.7 μm. Its structure was typical for low-activity high temperature (HTLA) model of coating growth by outward diffusion of nickel (Figure 6). Analysis of the average chemical composition from diffusion zone (marked as “4” on Figure 6, Table 7) showed that the concentration of aluminum was 18.7 wt.%, platinum—12.2 wt.% and palladium—18.2 wt.%. Average content of Zr in this zone was low 0.1 wt.% (areas 3 and 4 on Figure 6, Table 7). In the outer layer, the presence of refractory metals: (tungsten, molybdenum, titanium) in small contents was detected (area 1–4 on Figure 6, Table 7). The chromium and cobalt were found in this zone as well. The aluminum concentration in this outer, additive layer was changed from 22.8 wt.% in the near-surface area (marked “1”), by 17.9 wt.% in the middle (area 2 on Figure 6) of zone to 16.6 wt.% near the diffusion zone (area 3, Figure 6, Table 7). The highest concentration of palladium (19.2 wt.%) and platinum (13.3 wt.%) was detected in the middle of additive layer (area 2, Figure 6, Table 7). In the diffusion zone (area 5 on Figure 6), the Al concentration decreased to 12.2 wt.%, palladium to 7.3 wt.% and platinum to 8 wt.% (Table 7). In this zone the concentration of zirconium was highest (2.6 wt.%) in all cross-sections of coating. The presence of chromium, cobalt and refractory metals increased in this zone (area 5, Figure 6).
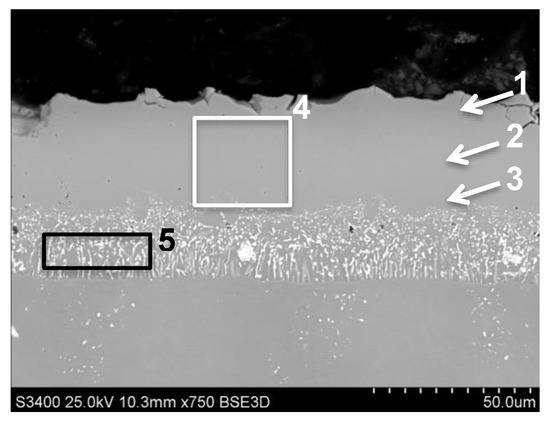
Figure 6.
The aluminide coating produced by Pt and Pd electroplating and CVD low-activity aluminizing with Zr doping on MAR M247 nickel superalloy with marked areas of EDS chemical composition analysis.

Table 7.
Results of chemical composition analysis from areas presented in Figure 6.
3.7. The (Pt + Pd + Hf)-Al Coating (7)
During conducted research the three-element (hafnium, palladium, platinum) modified aluminide coating was developed. It was produced by electroplating of platinum and palladium then the low-activity CVD aluminizing with doping of hafnium during process. The obtained coating had thickness approx. 46 μm (Figure 7). The conducted analysis of chemical composition in the outer zone (area 3 on Figure 7, Table 8) showed the presence of aluminum (18.1 wt.%), platinum (16.2 wt.%) and palladium (16.2 wt.%). Additionally, the presence of cobalt (4.4 wt.%) and chromium (1.9 wt.%) was also confirmed. In the outer zone, tungsten was not detected (area 3). Hafnium, molybdenum and titanium were observed in the additive layer, close to the diffusion zone (area 2 on Figure 7, Table 8). The Al content in the additive layer was changed from 19.5 wt.% in the near-surface zone to 16.5 wt.% near the diffusion zone (areas 1, 2, Figure 7). The concentration of palladium was decreased from 24 wt.% to 20.7 wt.% on the cross-section of the additive layer. The platinum content was highest close to the diffusion zone (17.9 wt.%, area 2 on Figure 7) compared with the near-surface area (14.7 wt.%, area 1 on Figure. 7). In the diffusion zone (areas 4, 5 on Figure 7, Table 8), the Al content was 11 wt.%. In this zone the palladium (8–11 wt.%) and platinum (13–15 wt.%) were detected. The elements from the base material were presented as well: tungsten, titanium, molybdenum, zirconium and hafnium (Table 8).
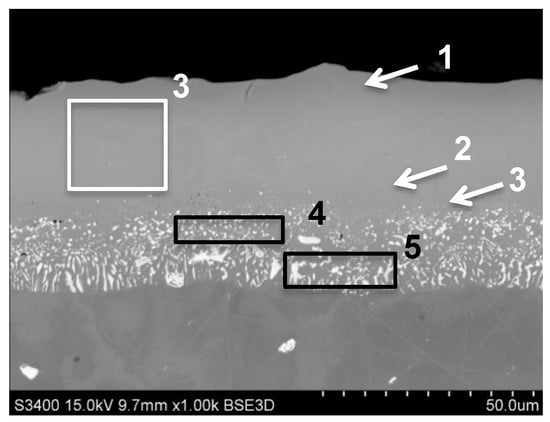
Figure 7.
The aluminide coating produced by Pt and Pd electroplating and CVD low-activity aluminizing with Hf doping on MAR M247 nickel superalloy with marked areas of chemical composition analysis.

Table 8.
Results of chemical composition analysis from areas presented on Figure 7.
4. Discussion
In the present research, the modified aluminide coatings containing different combinations of Pt, Pd, Zr, Hf were formed in the industrial-scale CVD process. All the obtained coatings were characterized by structure formed in a low-activity high temperature process with the dominating outward diffusion of nickel. Their thickness was in the range 45–55 μm, which is acceptable for application in the aerospace industry. They were characterized by the formation of an outer additive layer containing an NiAl solid solution with different elements, depending on used elements (Pt, Pd, Zr, Hf). The “state of art” simple Pt-modified aluminide coating (1) was characterized by the formation of (Ni, Pt) an Al solid solution in the outer zone. The concentration of Pt was lower on the near surface area and was higher near the diffusion zone. It is a typical structure for commercial Pt-Al coatings [12,21] produced, for example, in the MDC 150L process developed by Howmet [9,11]. The simple Pd-Al modified aluminide coating (2) had a typical single-phase structure. The highest concentration of palladium was observed in the near-surface area—opposite to the concentration of Pt in aluminide coating. Some voids were observed on the interface outer additive layer/diffusion zone reported by Hong et al. [15] The (Pt + Pd)-Al coating (3) was characterized by differences in this element concentration in the outer additive layer. According to what was observed in the Pt or Pd modified coating the highest concentration of Pt in additive layer was observed close to diffusion zone, but Pd concentrated in near surface area of coating. The Pt/Pd content ratio was similar to that obtained by Swadzba et al. [16] However, the presence of Cr and Co (about 2–5 at.%) in this zone reported by this author was also observed in the obtained coating. The concentration of Pd in (Pd + Zr)-Al aluminide coating (4) was approx. 2 wt.% lower in comparison with Pd-modified aluminide coating. The zirconium was observed in whole areas of obtained aluminide coatings regardless of its doping during aluminizing process [27]. It might be connected with some concentration of this element from base alloy. According to our previous results [18], the formation of small precipitations containing Zr was observed in Pd/Zr modified aluminide coatings. The presence of small Zr-precipitations was confirmed by Yavorska et al. [28] The concentration of Pd in (Pd + Hf)-Al (5) coating was similar to that measured in Pd-only modified coating. The concentration of Hf did not exceed 0.3 wt.% in the outer additive layer and was approx. 2.3 wt.% in the diffusion zone. The presence of hafnium is possible by doping during CVD aluminizing as well by outward diffusion from base material containing Hf [29]. The concentration of palladium and platinum in (Pd + Pt + Hf)-Al aluminide coating (6) was approx. 2–3% lower in comparison with (Pd + Pt)-Al (3) coating, but distribution of these elements in the outer additive layer was similar. Zirconium was detected mainly in the diffusion zone of the coating (2.6 wt.%). In the outer additive layer and in selected areas, the concentration of Zr did not exceed 0.1 wt.%. The presence of Hf in this coating was detected as result of outward diffusion of this element from the base material. There are no references describing this type of modified aluminide coatings. The concentration of Pt and Pd in aluminide coating modified by (Pt + Pd + Hf) Al (7) was higher than that observed in (Pt + Pd + Zr) Al (6) coating. The concentration of Hf in an additive outer layer was very low. The highest amount of Hf in the inner diffusion zone was observed. The presence of Hf must be considered as both a result of doping during the CVD process and its outward diffusion from the base material (MAR M247 contains Hf, Table 1). Based on the structure of all three element modified aluminide coatings, the difficulties of introduction of Zr as well Hf were noted. Based on the obtained results as well as on previous work [18], the Zr probably forms small precipitations in the aluminide coating. Higher concentrations of Hf in aluminide coatings might be a result of its outward diffusion from the MAR M247 superalloy. According to Romanowska et al. [30], it forms an Hf-rich belt between outer additive layer and the diffusion zone. Based on the obtained results, it is possible to form three-element Pt + Pd + (Hf or Zr) aluminide coatings using proposed technology on the industrial scale. They have a typical structure formed by an outward diffusion of nickel (LAHT). Furthermore, deeper microstructural investigation and cyclic oxidation tests are necessary for analysis of their corrosion resistance.
5. Conclusions
The obtained results showed that three-element Pt + Pd + (Hf or Zr) modified aluminide coatings have a typical structure formed by an outward diffusion of nickel (LAHT, low activity high temperature). The results of chemical composition analysis on the cross-section of obtained coatings enables us to conclude that all doping elements were incorporated into the coating. Electroplated platinum and palladium-formed (Ni, Pd, Pt) Al solid solution in the outer additive layer in aluminide coating. The concentration of other doping elements such as Zr was very low. Analysis of its concentration suggests that Zr probably forms small precipitations in the additive layer. On the other hand, hafnium diffuses both from the base material and by co-doping during the CVD aluminizing process. The effect of Hf addition requires a deeper analysis. In further work, the oxidation resistance of obtained coatings must be investigated.
Author Contributions
M.G.: preparation of experimental plan, analysis of results, preparation of article text, M.P.: preparation of coatings concept, SEM observations and EDS analysis, K.O.: conducting of CVD process, M.D.: analysis of results, T.K.: preparation of samples for coating deposition, W.S.: analysis and preparation of Pt and Pd electroplating process, L.N.: electroplating of Pt and Pd on samples. All authors have read and agreed to the published version of the manuscript.
Funding
Project co-financed by the European Areal Development Fund under the Operational Programme Innovative Economy and the National Centre for Research and Development Poland (NCBR)—Grant No. INNOLOT/I/7/NCBR/2013—TED.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yu, C.; Yang, Y.; Bao, Z.; Zhu, S. Research progress in preparation and development of excellent bond coats for advanced thermal barrier coatings. J. Chin. Soc. Corros. Prot. 2019, 39, 395–403. [Google Scholar] [CrossRef]
- Swadzba, R. Interfacial phenomena and evolution of modified aluminide bondcoatings in Thermal Barrier Coatings. Appl. Surf. Sci. 2018, 445, 133–144. [Google Scholar] [CrossRef]
- Pauletti, E.; D’Oliveira, A.S.C.M. Study on the mechanisms of formation of aluminized diffusion coatings on a Ni-base superalloy using different pack aluminization procedures. J. Vac. Sci. Technol. A Vac. Surf. Film. 2018, 36, 041504. [Google Scholar] [CrossRef]
- Rannou, B.; Bouchaud, B.; Balmain, J.; Bonnet, G.; Pedraza, F. Comparative isothermal oxidation behaviour of new aluminide coatings from slurries containing Al particles and conventional out-of-pack aluminide coatings. Oxid. Met. 2014, 81, 139–149. [Google Scholar] [CrossRef]
- Kopec, M.; Kukla, D.; Yuan, X.; Rejmer, W.; Kowalewski, Z.L.; Senderowski, C. Aluminide thermal barrier coating for high temperature performance of MAR 247 nickel based superoy. Coatings 2021, 11, 48. [Google Scholar] [CrossRef]
- Góral, M.; Sieniawski, J.; Kotowski, S.; Pytel, M.; Masłyk, M. Influence of turbine blade geometry on thickness of TBCs deposited by VPA and PS-PVD methods. Arch. Mater. Sci. Eng. 2012, 54, 22–28. [Google Scholar]
- Squillace, A.; Bonetti, R.; Archer, N.J.; Yeatman, J.A. The control of the composition and structure of aluminide layers formed by vapour aluminizing. Surf. Coat. Technol. 1999, 120–121, 118–123. [Google Scholar] [CrossRef]
- Adamiak, S.; Bochnowski, W.; Dziedzic, A.; Filip, R.; Szeregij, E. Structure and Properties of the Aluminide Coatings on the Inconel 625 Superalloy. High Temp. Mater. Process. 2016, 35, 103–112. [Google Scholar] [CrossRef]
- Warnes, B.M.; Punola, D.C. Clean diffusion coatings by chemical vapor deposition. Surf. Coat. Technol. 1997, 94–95. [Google Scholar] [CrossRef]
- Swadzba, L.; Nawrat, G.; Mendala, B.; Goral, M. The influence of deposition process on structure of platinum-modifed aluminide coatings o Ni-base superalloy. Key Eng. Mater. 2011, 465, 247–250. [Google Scholar] [CrossRef]
- Purvis, A.L.; Warnes, B.M. The effects of platinum concentration on the oxidation resistance of superalloys coated with wingle-phase platinum aluminide. Surf. Coat. Technol. 2001, 146–147, 1–6. [Google Scholar] [CrossRef]
- Angenete, J.; Stiller, K. Comparison of inward and outward grown Pt modified aluminide diffusion coatings on a Ni based single crystal superalloy. Surf. Coat. Technol. 2002, 150, 107–118. [Google Scholar] [CrossRef]
- Li, M.J.; Sun, X.F.; Guan, H.R.; Jiang, X.X.; Hu, Z.Q. Oxidation kinetics and scale morphology of a palladium-modified-aluminide coating at high temperature in air. Oxid. Met. 2004, 61, 91–104. [Google Scholar] [CrossRef]
- Li, M.J.; Sun, X.F.; Guan, H.R.; Jiang, X.X.; Hu, Z.Q. The degradation of (Ni,Pd)Al coatings on superalloy IN738 during isothermal oxidation. Surf. Coat. Technol. 2004, 185, 172–177. [Google Scholar] [CrossRef]
- Hong, S.-J.; Hwang, G.-H.; Han, W.-K.; Kang, S.-G. Effect of Pd and Ru on the structural and mechanical properties of a Pt-modified aluminide coating. J. Korean Phys. Soc. 2009, 54, 1191–1197. [Google Scholar] [CrossRef]
- Swadzba, R.; Hetmanczyk, M.; Sozanska, M.; Witala, B.; Swadzba, L. Structure and cyclic oxidation resistance of Pt, Pt/Pd-modified and simple aluminide coatings on CMSX-4 superalloy. Surf. Coat. Technol. 2011, 206, 1538–1544. [Google Scholar] [CrossRef]
- Zagula-Yavorska, M.; Sieniawski, J.; Gancarczyk, T. Some properties of platinum and palladium modified aluminide coatings deposited by CVD method on nickel-base superalloys. Arch. Metall. Mater. 2012, 57, 503–509. [Google Scholar] [CrossRef]
- Pytel, M.; Tokarski, M.; Goral, M.; Filip, R. Structure of Pd-Zr and Pt-Zr modified aluminide coatings deposited by a CVD method on nickel superalloys. Kov. Mater. 2019, 57, 343–354. [Google Scholar] [CrossRef]
- Zhang, Y.; Haynes, J.; Wright, G.; Pint, B.A.; Cooley, K.M.; Lee, W.Y.; Liaw, P.K. Effects of Pt incorporation on the isothermal oxidation behavior of chemical vapor deposition aluminide coatings. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2001, 32, 1727–1741. [Google Scholar] [CrossRef]
- Tawancy, H.M.; Sridhar, N.; Abbas, N.M.; Rickerby, D. Comparative thermal stability characteristics and isothermal oxidation behavior of an aluminized and a Pt-aluminized Ni-base superalloy. Scr. Metall. Mater. 1995, 33, 1431–1438. [Google Scholar] [CrossRef]
- Angenete, J.; Stiller, K. A comparative study of two inward grown Pt modified Al diffusion coatings on a single crystal Ni base superalloy. Mat. Sci. Eng. A 2001, 316, 182–194. [Google Scholar] [CrossRef]
- Li, M.J.; Sun, X.F.; Guan, H.R.; Jiang, X.X.; Hu, Z.Q. Cyclic oxidation behavior of palladium-modified aluminide coating. Surf. Coat. Technol. 2003, 167, 106–111. [Google Scholar] [CrossRef]
- Hong, S.J.; Hwang, G.H.; Han, W.K.; Kang, S.G. Cyclic oxidation of Pt/Pd-modified aluminide coating on a nickel-based superalloy at 1150 °C. Intermetallics 2009, 17, 381–386. [Google Scholar] [CrossRef]
- Pint, B.A. The role of chemical composition on the oxidation performance of aluminide coatings. Surf. Coat. Technol. 2004, 188–189, 71–78. [Google Scholar]
- Jiang, C.; Li, S.; Liu, H.; Bao, Z.; Zhang, J.; Zhu, S.; Wang, F. Effect of Hf addition in (Ni,Pt)Al bond coat on thermal cycling behavior of a thermal barrier coating system at 1100 °C. Corr. Sci. 2020, 166, 108424. [Google Scholar] [CrossRef]
- Jiang, C.; Qian, L.; Feng, M.; Liu, H.; Bao, Z.; Chen, M.; Zhu, S.; Wang, F. Benefits of Zr addition to oxidation resistance of a single-phase (Ni, Pt) Al coating at 1373 K. J. Mater. Sci. Technol. 2019, 35, 1334–1344. [Google Scholar] [CrossRef]
- Filip, R.; Nowotnik, A.; Goral, M. Zirconia modified aluminide coatings deposited by VPA and CVD methods. Solid State Phenom. 2013, 203–204, 220–223. [Google Scholar] [CrossRef]
- Zagula-Yavorska, M.; Morgiel, J.; Romanowska, J.; Sieniawski, J. Nanoparticles in zirconium-doped aluminide coatings. Mater. Lett. 2015, 139, 50–54. [Google Scholar] [CrossRef]
- Filip, R.; Góral, M.; Zawadzki, M.; Nowotnik, A.; Pytel, M. The influence of long-term heat treatment on microstructure of Zr-modified aluminide coating deposited by CVD method on MAR M200+Hf nickel superalloy. Key Eng. Mater. 2014, 592–593, 469–472. [Google Scholar] [CrossRef]
- Romanowska, J.; Morgiel, J.; Kolek, Ł.; Kwolek, P.; Zagula-Yavorska, M. Effect of Pd and Hf co-doping of aluminide coatings on pure nickel and CMSX-4 nickel superalloy. Arch. Civ. Mech. Eng. 2018, 18, 1421–1429. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).