Implantable Thin Film Devices as Brain-Computer Interfaces: Recent Advances in Design and Fabrication Approaches
Abstract
1. Introduction
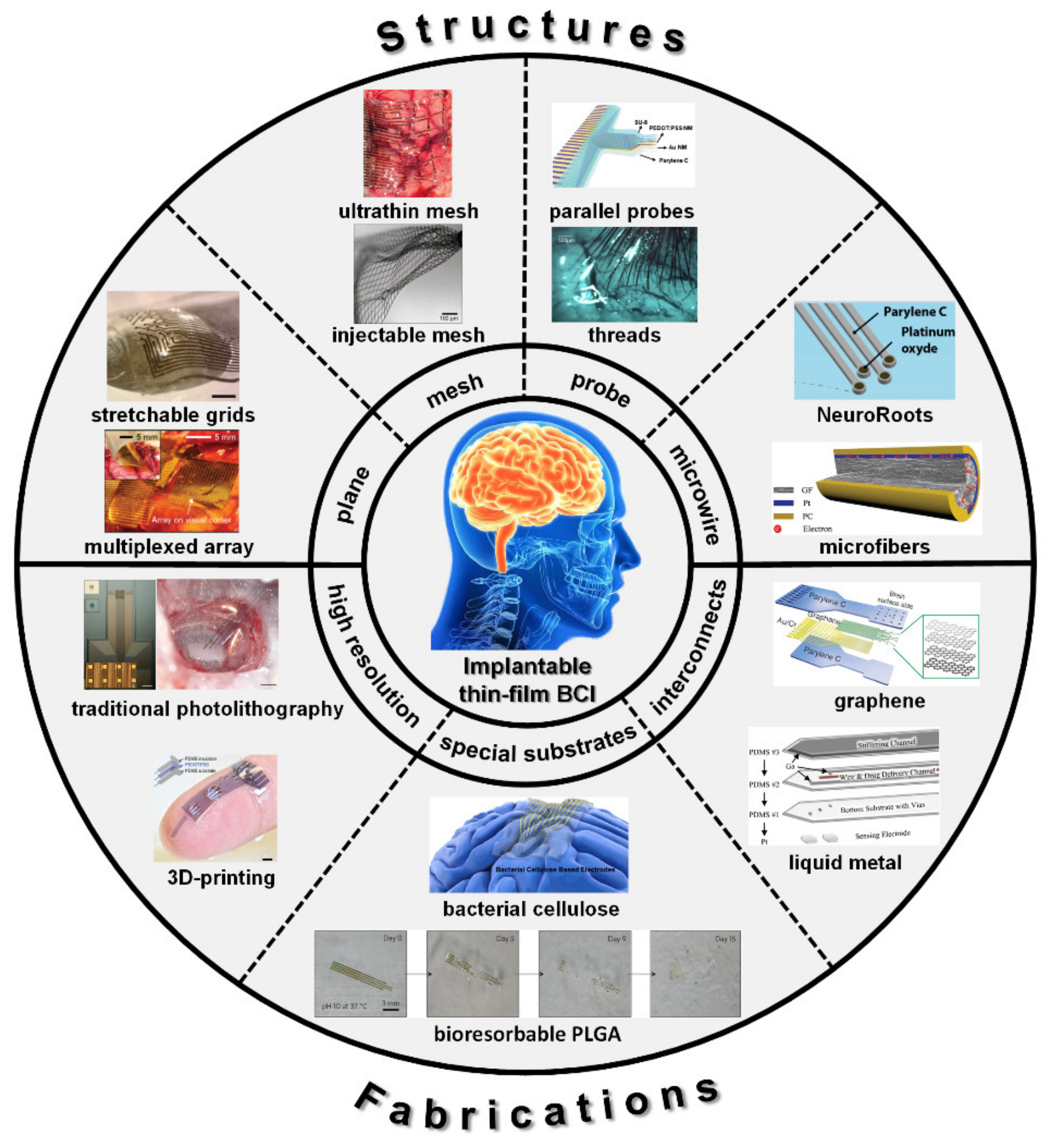
2. Design
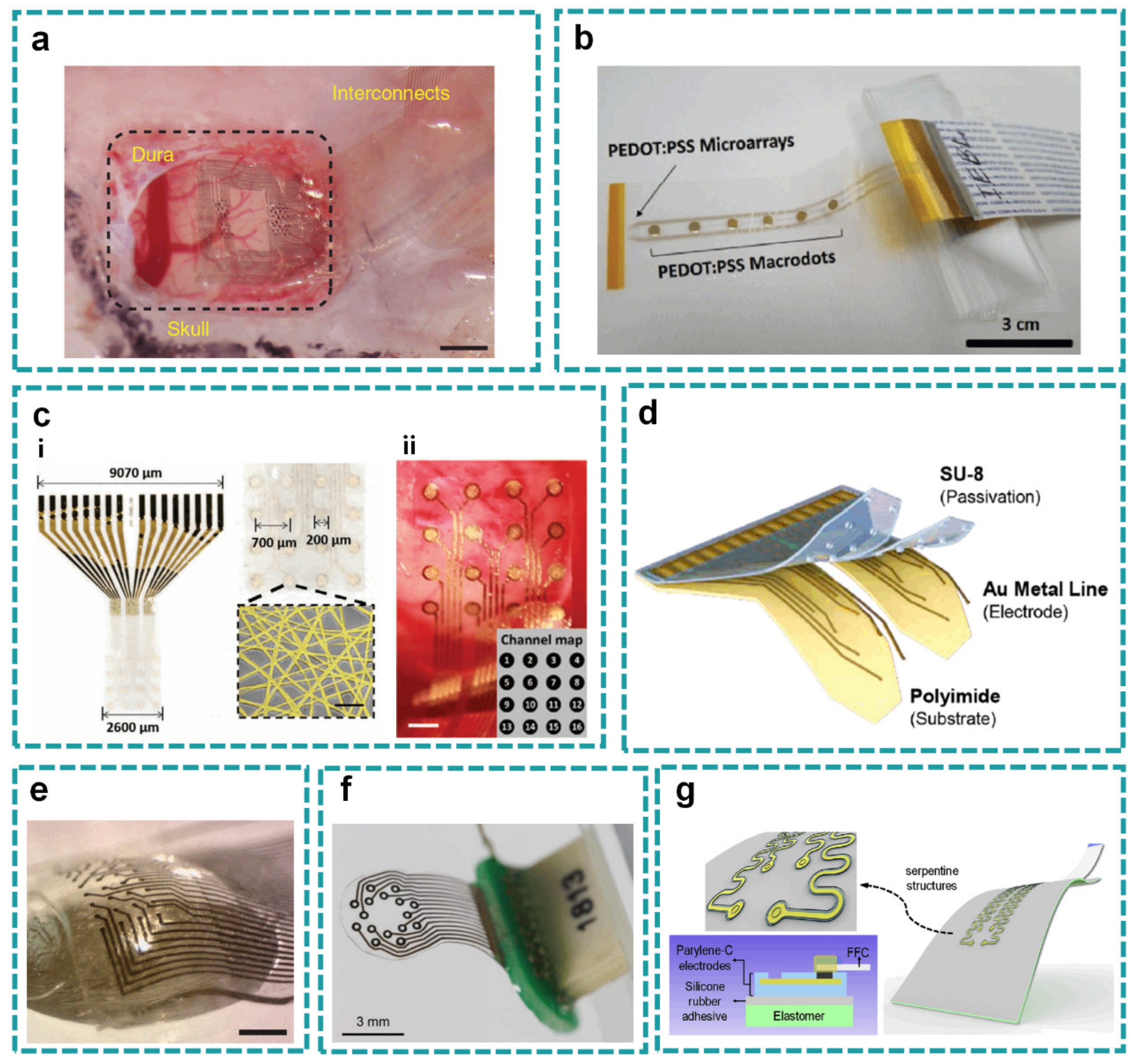
2.1. Planar Layout
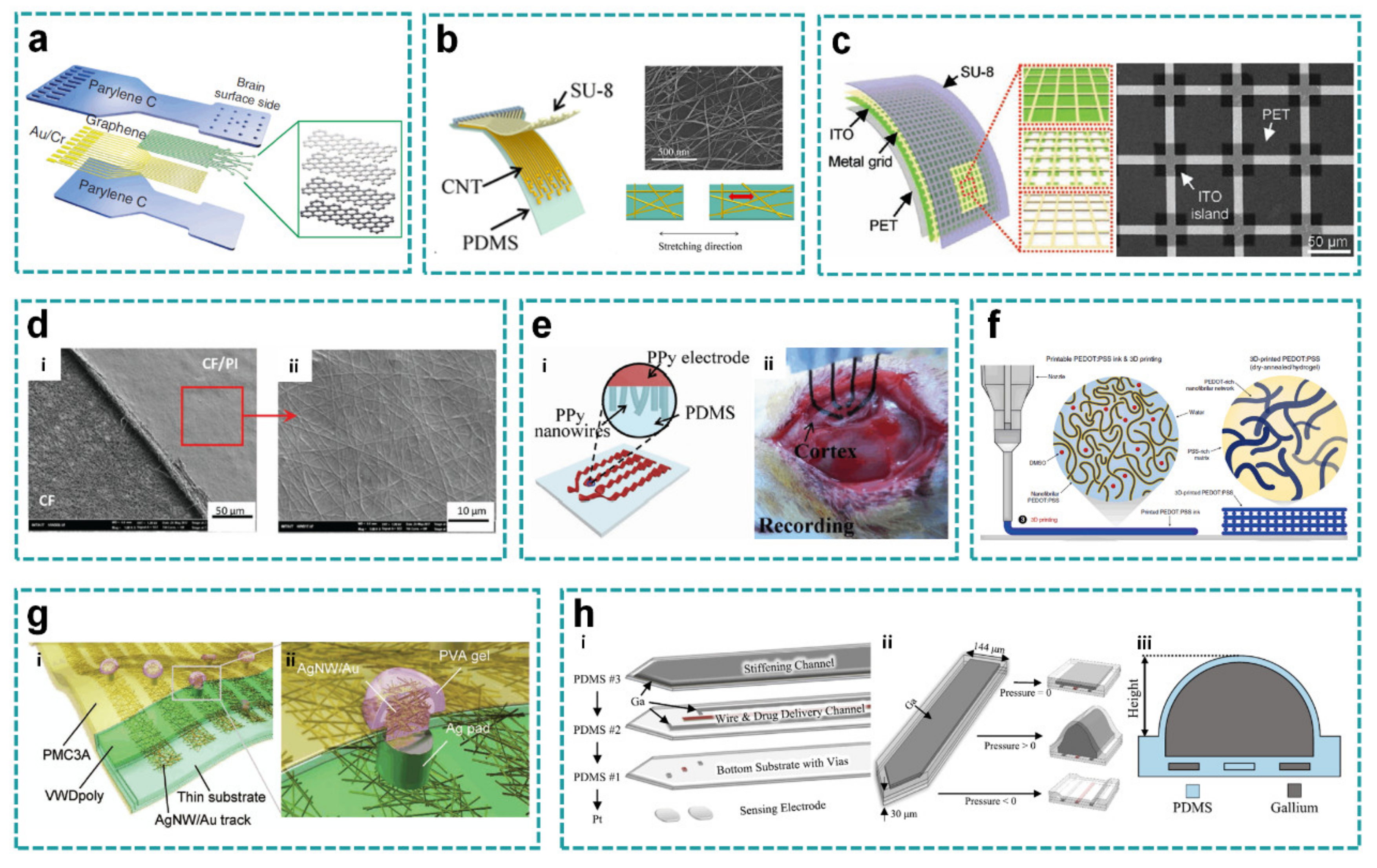
2.1.1. Flexible Planar Structure
2.1.2. Soft Planar Structure
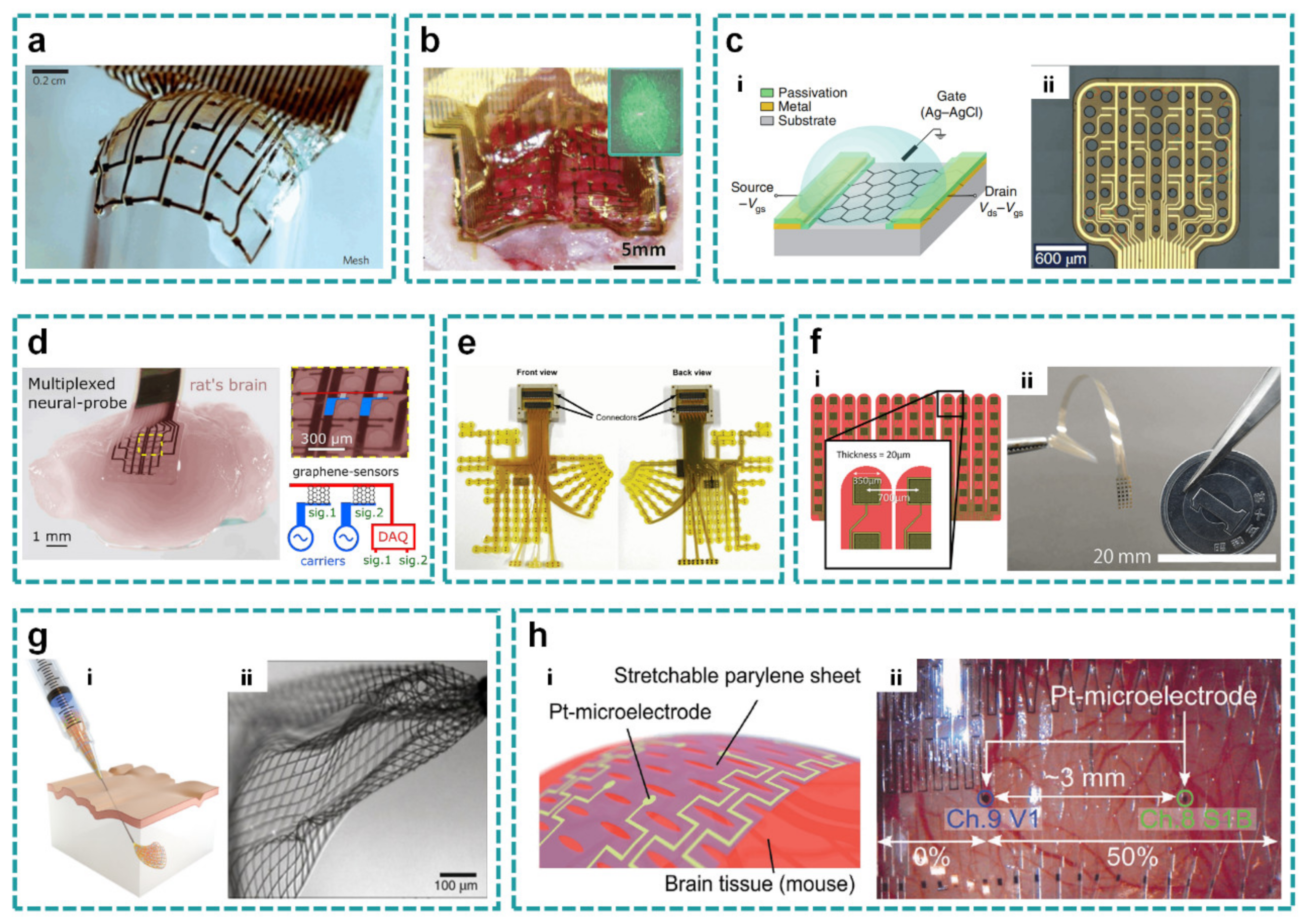
2.2. Open-Mesh Layout
2.2.1. Hole Mesh
2.2.2. Finger Mesh
2.2.3. Three-Dimensional Mesh
2.2.4. Kirigami Mesh
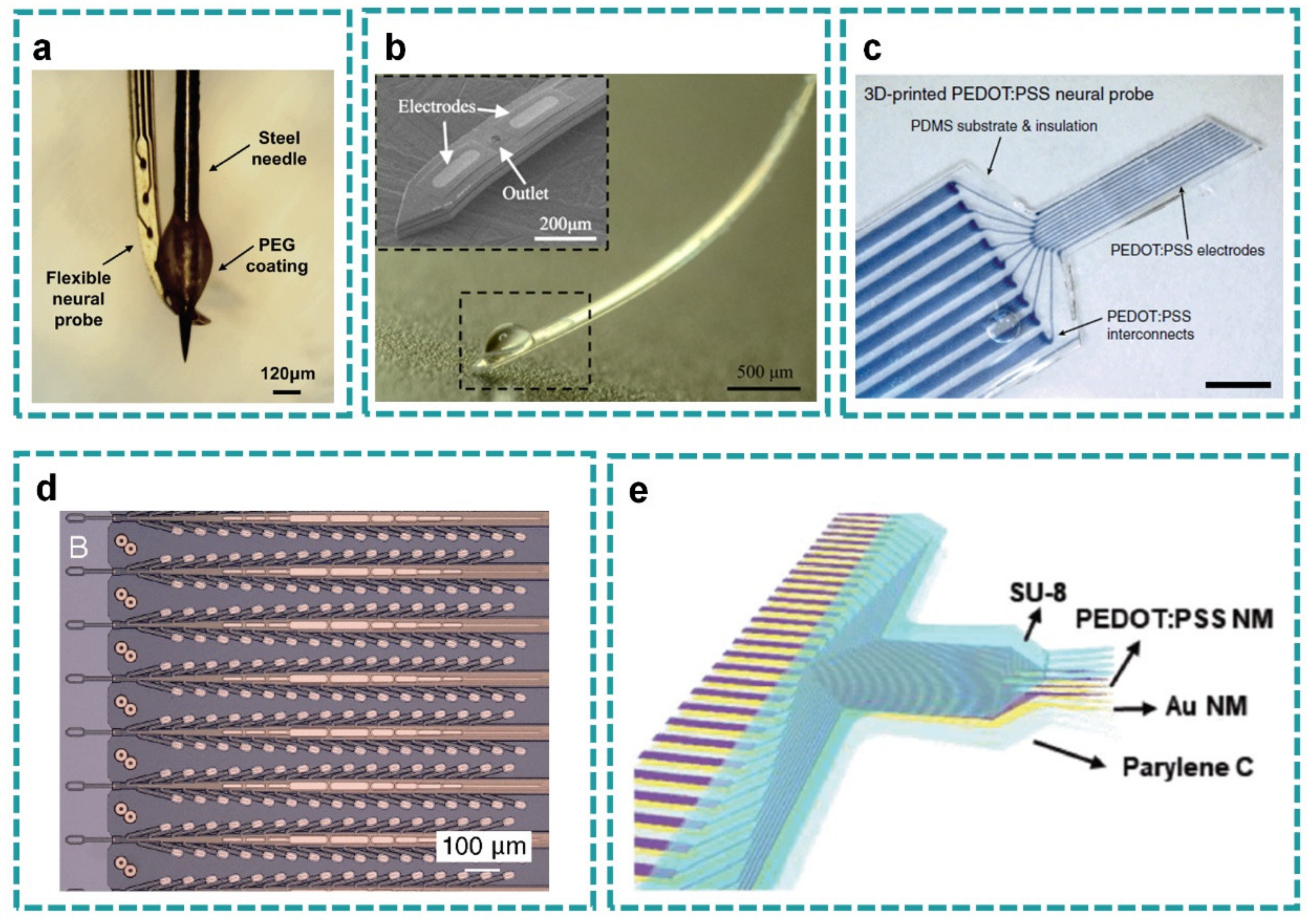
2.3. Probe Layout
2.4. Micro-Wire Layout
3. Fabrications
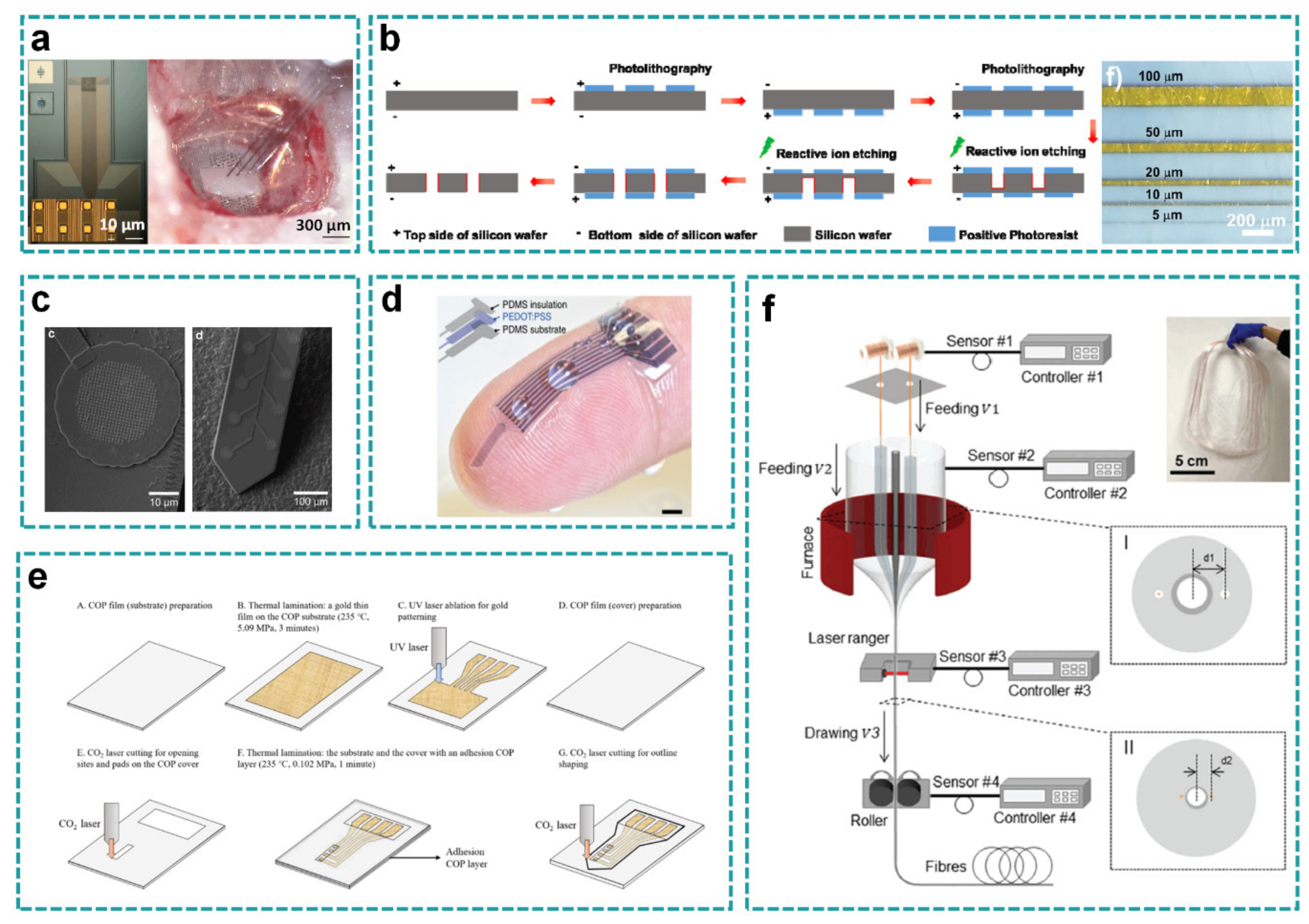
3.1. High Resolution
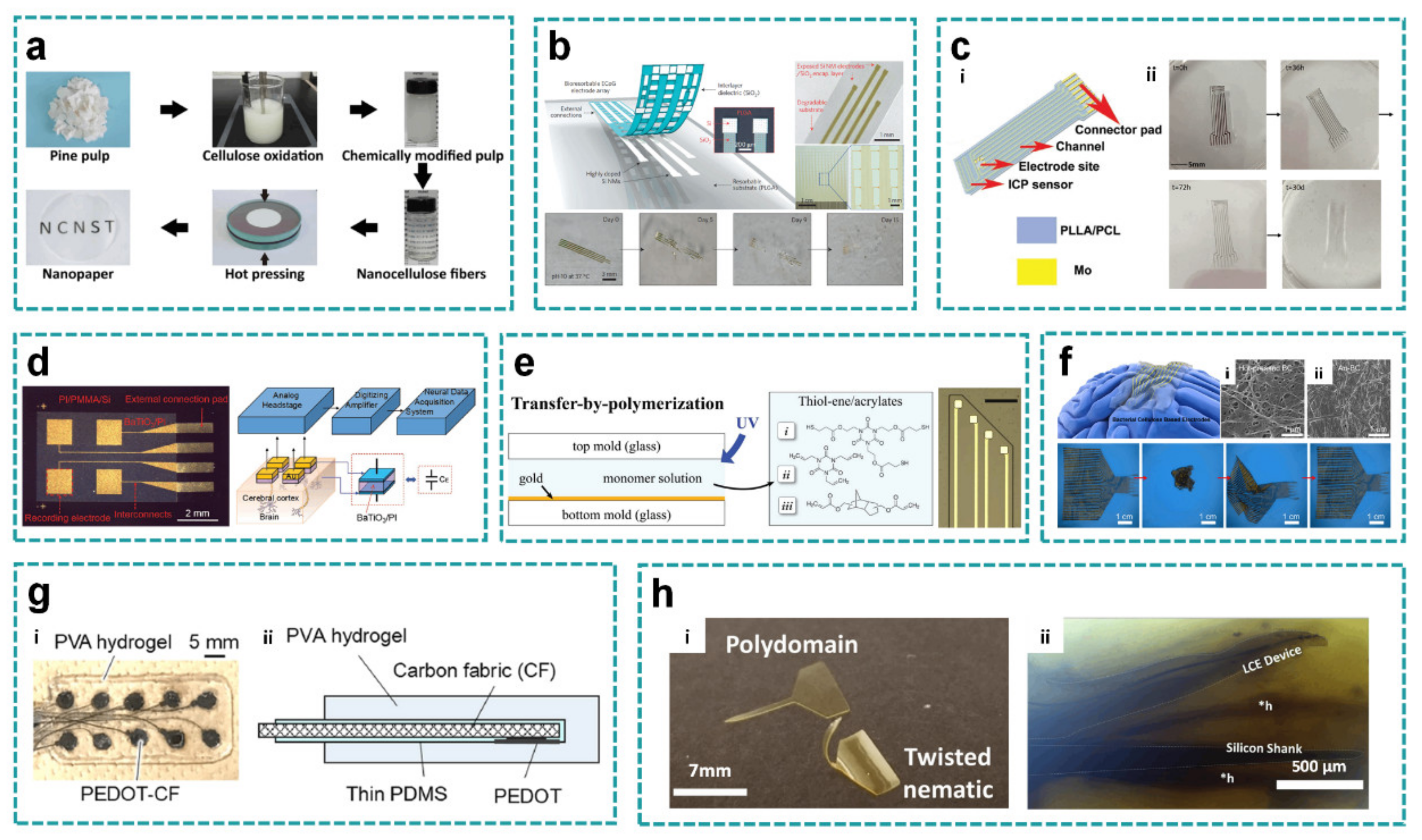
3.2. Special Substrates
3.3. Special Interconnects
4. Conclusions and Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Birbaumer, N. Breaking the silence: Brain–computer interfaces (BCI) for communication and motor control. Psychophysiology 2006, 43, 517–532. [Google Scholar] [CrossRef]
- Perlmutter, J.S.; Mink, J.W. Deep brain stimulation. Annu. Rev. Neurosci. 2006, 29, 229–257. [Google Scholar] [CrossRef]
- Fetz, E. Restoring motor function with bidirectional neural interfaces. Prog. Brain Res. 2015, 218, 241–252. [Google Scholar]
- Maharbiz, M.M.; Muller, R.; Alon, E.; Rabaey, J.M.; Carmena, J.M. Reliable next-generation cortical interfaces for chronic brain–machine interfaces and neuroscience. Proc. IEEE 2016, 105, 73–82. [Google Scholar] [CrossRef]
- Benabid, A.L.; Costecalde, T.; Eliseyev, A.; Charvet, G.; Verney, A.; Karakas, S.; Foerster, M.; Lambert, A.; Morinière, B.; Abroug, N.; et al. An exoskeleton controlled by an epidural wireless brain–machine interface in a tetraplegic patient: A proof-of-concept demonstration. Lancet Neurol. 2019, 18, 1112–1122. [Google Scholar] [CrossRef]
- Ganzer, P.D.; Colachis, S.C.; Schwemmer, M.A.; Friedenberg, D.A.; Dunlap, C.F.; Swiftney, C.E.; Jacobowitz, A.F.; Weber, D.J.; Bockbrader, M.A.; Sharma, G. Restoring the sense of touch using a sensorimotor demultiplexing neural interface. Cell 2020, 181, 763–773. [Google Scholar] [CrossRef] [PubMed]
- Engel, A.K.; Moll, C.K.E.; Fried, I.; Ojemann, G.A. Invasive recordings from the human brain: Clinical insights and beyond. Nat. Rev. Neurosci. 2005, 6, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Dimitriadis, G.; Fransen AM, M.; Maris, E. Sensory and cognitive neurophysiology in rats, Part 1: Controlled tactile stimulation and micro-ECoG recordings in freely moving animals. J. Neurosci. Methods 2014, 232, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Hakimian, S.; Schwartz, T.H. Intraoperative Electro CorticoGraphy (ECog): Indications, techniques, and utility in epilepsy surgery. Epileptic Disord 2014, 16, 271–279. [Google Scholar]
- Ji, B.; Ge, C.; Guo, Z.; Wang, L.; Wang, M.; Xie, Z.; Xu, Y.; Li, H.; Yang, B.; Wang, X.; et al. Flexible and stretchable opto-electric neural interface for low-noise electrocorticogram recordings and neuromodulation in vivo. Biosens. Bioelectron. 2020, 153, 112009. [Google Scholar] [CrossRef]
- Li, X.; Song, Y.; Xiao, G.; Xie, J.; Dai, Y.; Xing, Y.; He, E.; Wang, Y.; Xu, S.; Zhang, L.; et al. Flexible electrocorticography electrode array for epileptiform electrical activity recording under glutamate and GABA modulation on the primary somatosensory cortex of rats. Micromachines 2020, 11, 732. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Stark, E.; Ku, P.-C.; Wise, K.D.; Buzsáki, G.; Yoon, E. Monolithically integrated μLEDs on silicon neural probes for high-resolution optogenetic studies in behaving animals. Neuron 2015, 88, 1136–1148. [Google Scholar] [CrossRef]
- Jun, J.J.; Steinmetz, N.A.; Siegle, J.H.; Denman, D.J.; Bauza, M.; Barbarits, B.; Lee, A.K.; Anastassiou, C.A.; Andrei, A.; Aydın, Ç.; et al. Fully integrated silicon probes for high-density recording of neural activity. Nature 2017, 551, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.E.; Joo, H.R.; Fan, J.L.; Liu, D.F.; Barnett, A.H.; Chen, S.; Geaghan-Breiner, C.; Karlsson, M.P.; Karlsson, M.; Lee, K.Y.; et al. High-density, long-lasting, and multi-region electrophysiological recordings using polymer electrode arrays. Neuron 2019, 101, 21–31. [Google Scholar] [CrossRef]
- Sahasrabuddhe, K.; Khan, A.A.; Singh, A.P.; Stern, T.M.; Ng, Y.; Tadić, A.; Orel, P.; LaReau, C.; Pouzzner, D.; Nishimura, K.; et al. The Argo: A 65,536 channel recording system for high density neural recording in vivo. bioRxiv 2020. [Google Scholar] [CrossRef]
- Obaid, A.; Hanna, M.-E.; Wu, Y.-W.; Kollo, M.; Racz, R.; Angle, M.R.; Müller, J.; Brackbill, N.; Wray, W.; Franke, F.; et al. Massively parallel microwire arrays integrated with CMOS chips for neural recording. Sci. Adv. 2020, 6, eaay2789. [Google Scholar] [CrossRef]
- Canales, A.; Jia, X.; Froriep, U.P.; Koppes, R.A.; Tringides, C.M.; Selvidge, J.; Lu, C.; Hou, C.; Wei, L.; Fink, Y.; et al. Multifunctional fibers for simultaneous optical, electrical and chemical interrogation of neural circuits in vivo. Nat. Biotechnol. 2015, 33, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.-I.; McCall, J.G.; Jung, Y.H.; Huang, X.; Siuda, E.R.; Li, Y.; Song, J.; Song, Y.M.; Pao, H.A.; Kim, R.-H.; et al. Injectable, cellular-scale optoelectronics with applications for wireless optogenetics. Science 2013, 340, 211–216. [Google Scholar] [CrossRef]
- Zhang, J.; Laiwalla, F.; Kim, J.A.; Urabe, H.; Wagenen, R.V.; Song, Y.K.; Connors, B.W.; Feng, Z.; Deisseroth, K.; Nurmikko, A.V. Integrated device for optical stimulation and spatiotemporal electrical recording of neural activity in light-sensitized brain tissue. J. Neural Eng. 2009, 6, 055007. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Yokota, T.; Suzuki, T.; Lee, S.; Someya, T. Ultraflexible organic light-emitting diodes for optogenetic nerve stimulation. Proc. Natl. Acad. Sci. USA 2020, 117, 21138–21146. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.A.; Wander, J.D.; Sarma, D.; Lee, S.; Someya, T. Direct electrical stimulation of the somatosensory cortex in humans using electrocorticography electrodes: A qualitative and quantitative report. J. Neural Eng. 2013, 10, 036021. [Google Scholar] [CrossRef] [PubMed]
- Hughes, C.L.; Flesher, S.N.; Weiss, J.M.; Downey, J.E.; Collinger, J.L.; Gaunt, R.A. Neural stimulation and recording performance in human somatosensory cortex over 1500 days. medRxiv 2020. [Google Scholar] [CrossRef]
- Parastarfeizabadi, M.; Kouzani, A.Z. Advances in closed-loop deep brain stimulation devices. J. Neuroeng. Rehabil. 2017, 14, 79. [Google Scholar] [CrossRef]
- Bonmassar, G.; Lee, S.W.; Freeman, D.K.; Polasek, M.; Gale, J.T. Microscopic magnetic stimulation of neural tissue. Nat. Commun. 2012, 3, 1–10. [Google Scholar] [CrossRef]
- Lee, S.W.; Fallegger, F.; Casse, B.D.F.; Fried, S.I. Implantable microcoils for intracortical magnetic stimulation. Sci. Adv. 2016, 2, e1600889. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.E.; Park, J.H.; Jang, D.; Shin, J.H.; Hwang, G.T. Optogenetic brain neuromodulation by stray magnetic field via flash-enhanced magneto-mechano-triboelectric nanogenerator. Nano Energy 2020, 75, 104951. [Google Scholar] [CrossRef]
- Lee, H.J.; Son, Y.; Kim, J.; Lee, C.J.; Yoon, E.S.; Cho, I.J. A multichannel neural probe with embedded microfluidic channels for simultaneous in vivo neural recording and drug delivery. Lab Chip 2015, 15, 1590–1597. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.W.; McCall, J.G.; Shin, G.; Zhang, Y.; AI-Hasani, R.; Kim, M.; Li, S.; Sim, J.Y.; Jang, K.; Shi, Y.; et al. Wireless optofluidic systems for programmable in vivo pharmacology and optogenetics. Cell 2015, 162, 662–674. [Google Scholar] [CrossRef]
- Dagdeviren, C.; Ramadi, K.B.; Joe, P.; Spencer, K.; Schwerdt, H.N.; Shimazu, H.; Delcasso, S.; Amemori, K.; Nunez-Lopez, C.; Graybiel, A.M.; et al. Miniaturized neural system for chronic, local intracerebral drug delivery. Sci. Transl. Med. 2018, 10, 425. [Google Scholar] [CrossRef]
- Bruno, G.; Colistra, N.; Melle, G.; Cerea, A.; Dipalo, M. Microfluidic multielectrode arrays for spatially localized drug delivery and electrical recordings of primary neuronal cultures. Front. Bioeng. Biotechnol. 2020, 8, 626. [Google Scholar] [CrossRef]
- Cotler, M.J.; Rousseau, E.B.; Ramadi, K.B.; Fang, J.; Graybiel, A.M.; Langer, R.; Cima, M.J. Steerable microinvasive probes for localized drug delivery to deep tissue. Small 2019, 15, 1901459. [Google Scholar] [CrossRef]
- Maynard, E.M.; Nordhausen, C.T.; Normann, R.A. The Utah intracortical electrode array: A recording structure for potential brain-computer interfaces. Electroencephalogr. Clin. Neurophysiol. 1997, 102, 228–239. [Google Scholar] [CrossRef]
- Rousche, P.J.; Normann, R.A. Chronic recording capability of the Utah intracortical electrode array in cat sensory cortex. J. Neurosci. Methods 1998, 82, 1–15. [Google Scholar] [CrossRef]
- Normann, R.A.; Fernandez, E. Clinical applications of penetrating neural interfaces and Utah electrode array technologies. J. Neural Eng. 2016, 13, 061003. [Google Scholar] [CrossRef] [PubMed]
- Kipke, D.R.; Vetter, R.J.; Williams, J.C.; Hetke, J.F. Silicon-substrate intracortical microelectrode arrays for long-term recording of neuronal spike activity in cerebral cortex. IEEE Trans. Neural Syst. Rehabil. Eng. 2003, 11, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Vetter, R.J.; Williams, J.C.; Hetke, J.F.; Nunamaker, E.A.; Kipke, D.R. Chronic neural recording using silicon-substrate microelectrode arrays implanted in cerebral cortex. IEEE Trans. Biomed. Eng. 2004, 51, 896–904. [Google Scholar] [CrossRef]
- Ruther, P.; Herwik, S.; Kisban, S.; Seidl, K.; Paul, O. Recent progress in neural probes using silicon MEMS technology. IEEE Trans. Electr. Electron. Eng. 2010, 5, 505–515. [Google Scholar] [CrossRef]
- Wang, M.H.; Gu, X.W.; Ji, B.W.; Wang, L.C.; Guo, Z.J.; Yang, B.; Wang, X.L.; Li, C.Y.; Liu, J.Q. Three-dimensional drivable optrode array for high-resolution neural stimulations and recordings in multiple brain regions. Biosens. Bioelectron. 2019, 131, 9–16. [Google Scholar] [CrossRef]
- Hubel, D.H. Tungsten microelectrode for recording from single units. Science 1957, 125, 549–550. [Google Scholar] [CrossRef] [PubMed]
- Tseng, W.T.; Yen, C.T.; Tsai, M.L. A bundled microwire array for long-term chronic single-unit recording in deep brain regions of behaving rats. J. Neurosci. Methods 2011, 201, 368–376. [Google Scholar] [CrossRef]
- Zhao, S.; Liu, X.; Xu, Z.; Ren, H.; Deng, B.; Tang, M.; Lu, L.; Fu, X.; Peng, H.; Liu, Z. Graphene encapsulated copper microwires as highly MRI compatible neural electrodes. Nano Lett. 2016, 16, 7731–7738. [Google Scholar] [CrossRef]
- Elkin Benjamin, S.; Azeloglu, E.U.; Costa, K.D.; Morrison, B. Mechanical heterogeneity of the rat hippocampus measured by atomic force microscope indentation. J. Neurotrauma 2007, 24, 812–822. [Google Scholar] [CrossRef]
- Budday, S.; Nay, R.; De Rooij, R.; Steinmann, P.; Wyrobek, T.; Ovaert, T.C.; Kuhl, E. Mechanical properties of gray and white matter brain tissue by indentation. J. Mech. Behav. Biomed. Mater. 2015, 46, 318–330. [Google Scholar] [CrossRef]
- Biran, R.; Martin, D.C.; Tresco, P.A. Neuronal cell loss accompanies the brain tissue response to chronically implanted silicon microelectrode arrays. Exp. Neurol. 2005, 195, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Bellamkonda, R.V.; Sun, W.; Levenston, M.E. Biomechanical analysis of silicon microelectrode-induced strain in the brain. J. Neural Eng. 2005, 2, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Polikov, V.S.; Tresco, P.A.; Reichert, W.M. Response of brain tissue to chronically implanted neural electrodes. J. Neurosci. Methods 2005, 148, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Köhler, P.; Wolff, A.; Ejserholm, F.; Wallman, L.; Schouenborg, J.; Linsmeier, C.E. Influence of probe flexibility and gelatin embedding on neuronal density and glial responses to brain implants. PLoS ONE 2015, 10, e0119340. [Google Scholar] [CrossRef]
- Williams, J.C.; Hippensteel, J.A.; Dilgen, J. Complex impedance spectroscopy for monitoring tissue responses to inserted neural implants. J. Neural Eng. 2007, 4, 410. [Google Scholar] [CrossRef] [PubMed]
- Musk, E. Neuralink an integrated brain-machine interface platform with thousands of channels. J. Med. Internet Res. 2019, 21, e16194. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.J.; Kolarcik, C.L.; Kozai, T.D.; Luebben, S.D.; Sapp, S.A.; Zheng, X.S.; Nabity, J.A.; Cui, X.T. Ultrasoft microwire neural electrodes improve chronic tissue integration. Acta Biomater. 2017, 53, 46–58. [Google Scholar] [CrossRef]
- Luan, L.; Wei, X.; Zhao, Z.; Siegel, J.J.; Potnis, O.; Tuppen, C.A.; Lin, S.; Kazmi, S.; Fowler, R.A.; Holloway, S.; et al. Ultraflexible nanoelectronic probes form reliable, glial scar–free neural integration. Sci. Adv. 2017, 3, e1601966. [Google Scholar] [CrossRef]
- Vomero, M.; Cruz, M.F.P.; Zucchini, E.; Ciarpella, F.; Delfino, E.; Carli, S.; Boehler, C.; Asplund, M.; Ricci, D.; Fadiga, L.; et al. Conformable polyimide-based µECoGs: Bringing the electrodes closer to the signal source. Biomaterials 2020, 255, 120178. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Hirschberg, A.W.; Xu, H.; Slingsby-Smith, Z.; LeComte, A.; Scholten, K.; Song, D.; Meng, E. A Parylene neural probe array for multi-region deep brain recordings. J. Microelectromechanical Syst. 2020, 29, 499–513. [Google Scholar] [CrossRef]
- Zatonyi, A.; Madarasz, M.; Szabo, G.; Lrincz, T.; Fekete, Z. Transparent, low-autofluorescence microECoG device for simultaneous Ca2+ imaging and cortical electrophysiology in vivo. J. Neural Eng. 2020, 17, 016062. [Google Scholar] [CrossRef]
- Fu, T.M.; Hong, G.; Viveros, R.D.; Zhou, T.; Lieber, C.M. Highly scalable multichannel mesh electronics for stable chronic brain electrophysiology. Proc. Natl. Acad. Sci. USA 2017, 114, E10046–E10055. [Google Scholar] [CrossRef] [PubMed]
- Park, A.H.; Lee, S.H.; Lee, C.; Kim, J.; Lee, H.E.; Paik, S.B.; Lee, J. Optogenetic mapping of functional connectivity in freely moving mice via insertable wrapping electrode array beneath the skull. ACS Nano 2016, 10, 2791–2802. [Google Scholar] [CrossRef]
- Byun, S.H.; Sim, J.Y.; Zhou, Z.; Lee, J.; Jeong, J.W. Mechanically transformative electronics, sensors, and implantable devices. Sci. Adv. 2019, 5, eaay0418. [Google Scholar] [CrossRef]
- Jimbo, Y.; Matsuhisa, N.; Lee, W.; Zalar, P.; Jinno, H.; Yokota, T.; Sekino, M.; Someya, T. Ultraflexible transparent oxide/metal/oxide stack electrode with low sheet resistance for electrophysiological measurements. ACS Appl. Mater. Interfaces 2017, 9, 34744–34750. [Google Scholar] [CrossRef]
- Liu, X.; Lu, Y.; Ege, I.; Shi, Y.; Duygu, K. A compact closed-loop optogenetics system based on artifact-free transparent graphene electrodes. Front. Neurosci. 2018, 12, 132. [Google Scholar] [CrossRef]
- Yamagiwa, S.; Ishida, M.; Kawano, T. Flexible optrode array: Parylene-film waveguide arrays with microelectrodes for optogenetics. In 2015 Transducers-2015 18th International Conference on Solid-State Sensors, 63 Actuators and Microsystems (TRANSDUCERS); IEEE: Piscataway, NJ, USA, 2015; pp. 277–280. [Google Scholar]
- Reddy, J.W.; Lassiter, M.; Chamanzar, M. Parylene photonics: A flexible, broadband optical waveguide platform with integrated micromirrors for biointerfaces. Microsystems Nanoeng. 2020, 6, 1–14. [Google Scholar] [CrossRef]
- Kwon, K.Y.; Sirowatka, B.; Weber, A.; Li, W. Opto-μECoG array: A hybrid neural interface with transparent μECoG electrode array and integrated LEDs for optogenetics. IEEE Trans. Biomed. Circuits Syst. 2013, 7, 593–600. [Google Scholar] [CrossRef]
- Ji, B.; Guo, Z.; Wang, M.; Yang, B.; Wang, X.; Li, W.; Liu, J. Flexible polyimide-based hybrid opto-electric neural interface with 16 channels of micro-LEDs and electrodes. Microsyst. Nanoeng. 2018, 4, 1–11. [Google Scholar] [CrossRef]
- Guan, S.; Wang, J.; Gu, X.; Zhao, Y.; Hou, R.; Fan, H.; Zou, L.; Gao, L.; Du, M.; Li, C. Elastocapillary self-assembled neurotassels for stable neural activity recordings. Sci. Adv. 2019, 5, eaav2842. [Google Scholar] [CrossRef]
- Zhou, H.L.; Zhang, Y.; Qiu, Y.; Wu, H.P.; Qin, W.Y.; Liao, Y.B.; Yu, Q.; Cheng, H.Y. Stretchable piezoelectric energy harvesters and self-powered sensors for wearable and implantable devices. Biosens. Bioelectron. 2020, 168, 112569. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, Y.; Ayub, S.; Paul, O.; Kawano, T.; Ruther, P. Highly stretchable kirigami structure with integrated LED chips and electrodes for optogenetic experiments on perfused hearts. In 2019 20th International Conference on Solid-State Sensors, Actuators and Microsystems & Eurosensors XXXIII (TRANSDUCERS & EUROSENSORS XXXIII); IEEE: Piscataway, NJ, USA, 2019; pp. 2484–2487. [Google Scholar]
- Vahidi, N.W.; Rudraraju, S.; Castagnola, E.; Cea, C.; Nimbalkar, S.; Hanna, R.; Arvizu, R.; A Dayeh, S.; Gentner, T.Q.; Kassegne, S. Epi-Intra neural probes with glassy carbon microelectrodes help elucidate neural coding and stimulus encoding in 3D volume of tissue. J. Neural Eng. 2020, 17, 046005. [Google Scholar] [CrossRef] [PubMed]
- Khodagholy, D.; Gelinas, J.N.; Thesen, T.; Doyle, W.K.; Devinsky, O.; Malliaras, G.G.; Buzsáki, G. NeuroGrid: Recording action potentials from the surface of the brain. Nat. Neurosci. 2015, 18, 310–315. [Google Scholar] [CrossRef]
- Viventi, J.; Kim, D.-H.; Vigeland, L.; Frechette, E.S.; Blanco, J.A.; Kim, Y.-S.; Avrin, A.E.; Tiruvadi, V.R.; Hwang, S.-W.; Vanleer, A.C.; et al. Flexible, foldable, actively multiplexed, high-density electrode array for mapping brain activity in vivo. Nat. Neurosci. 2011, 14, 1599–1605. [Google Scholar] [CrossRef] [PubMed]
- Woods, V.; Trumpis, M.; Bent, B.; Palopoli-Trojani, K.; Chiang, C.-H.; Wang, C.; Yu, C.; Insanally, M.N.; Froemke, R.C.; Viventi, J. Long-term recording reliability of liquid crystal polymer µECoG arrays. J. Neural Eng. 2018, 15, 066024. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Fang, Y. Flexible and implantable microelectrodes for chronically stable neural interfaces. Adv. Mater. 2019, 31, e1804895. [Google Scholar] [CrossRef] [PubMed]
- Tybrandt, K.; Khodagholy, D.; Dielacher, B.; Stauffer, F.; Renz, A.F.; Buzsáki, G.; Vörös, J. High-density stretchable electrode grids for chronic neural recording. Adv. Mater. 2018, 30, e1706520. [Google Scholar] [CrossRef]
- Liu, J.; Fu, T.M.; Cheng, Z.; Hong, G.S.; Zhou, T. Syringe-injectable electronics. Nat. Nanotechnol. 2015, 10, 629–636. [Google Scholar] [CrossRef]
- Kim, D.-H.; Viventi, J.; Amsden, J.J.; Xiao, J.; Vigeland, L.; Kim, Y.-S.; Blanco, J.A.; Panilaitis, B.; Frechette, E.S.; Contreras, D.; et al. Dissolvable films of silk fibroin for ultrathin conformal bio-integrated electronics. Nat. Mater. 2010, 9, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Li, X.; He, F.; Wei, X.; Lin, S.; Xie, C. Parallel, minimally-invasive implantation of ultra-flexible neural electrode arrays. J. Neural Eng. 2019, 16, 035001. [Google Scholar] [CrossRef]
- Wang, K.; Frewin, C.L.; Esrafilzadeh, D.; Yu, C.; Wang, C.; Pancrazio, J.J.; Romero-Ortega, M.; Jalili, R.; Wallace, G. High-performance graphene-fiber-based neural recording microelectrodes. Adv. Mater. 2019, 31, 1805867. [Google Scholar] [CrossRef] [PubMed]
- Yuk, H.; Lu, B.; Lin, S.; Qu, K.; Xu, J.; Luo, J.; Zhao, X. 3D printing of conducting polymers. Nat. Commun. 2020, 11, 1–8. [Google Scholar] [CrossRef]
- Yang, J.; Du, M.; Wang, L.; Li, S.; Wang, G.; Yang, X.; Zhang, L.; Fang, Y.; Zheng, W.; Yang, G.; et al. Bacterial cellulose as a supersoft neural interfacing substrate. ACS Appl. Mater. Interfaces 2018, 10, 33049–33059. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.J.; Kuzum, D.; Hwang, S.W.; Kim, B.H.; Juul, H.; Kim, N.H.; Won, S.M.; Chiang, K.; Trumpis, M.; Richardson, A.G. Bioresorbable silicon electronics for transient spatiotemporal mapping of electrical activity from the cerebral cortex. Nat. Mater. 2016, 15, 782–791. [Google Scholar] [CrossRef] [PubMed]
- Park, D.-W.; Schendel, A.A.; Mikael, S.; Brodnick, S.K.; Richner, T.J.; Ness, J.P.; Hayat, M.R.; Atry, F.; Frye, S.T.; Pashaie, R.; et al. Graphene-based carbon-layered electrode array technology for neural imaging and optogenetic applications. Nat. Commun. 2014, 5, 5258. [Google Scholar] [CrossRef]
- Ximiao, W.; Wang, B.; Huang, S.; Liu, T.L.; Lee, M.S.; Chung, P.S.; Chow, Y.T.; Huang, I.W.; Monbouquette, H.G.; Maidment, N.T.; et al. Flexible, multifunctional neural probe with liquid metal enabled, ultra-large tunable stiffness for deep-brain chemical sensing and agent delivery. Biosens. Bioelectron. 2019, 131, 37–45. [Google Scholar]
- Seo, K.J.; Artoni, P.; Qiang, Y.; Han, X.; Shi, Z.; Yao, W.H.; Fagiolini, M.; Fang, H. Transparent, flexible, penetrating microelectrode arrays with capabilities of single-unit electrophysiology. Adv. Biosyst. 2019, 3, 1800276. [Google Scholar] [CrossRef]
- Ferro, M.; Proctor, C.M.; Gonzalez, A.; Zhao, E.; Melosh, N.A. NeuroRoots, a bio-inspired, seamless brain machine interface device for long-term recording. BioRxiv 2018. [Google Scholar] [CrossRef]
- Ganji, M.; Kaestner, E.; Hermiz, J.; Rogers, N.; Tanaka, A.; Cleary, D.; Lee, S.H.; Snider, J.; Halgren, M.; Cosgrove, G.R.; et al. Development and translation of PEDOT:PSS microelectrodes for intraoperative monitoring. Adv. Funct. Mater. 2018, 28. [Google Scholar] [CrossRef]
- Seo, J.; Kim, K.; Kim, M.K.; Jeong, S.; Kim, H.; Ghim, J.; Lee, J.H.; Choi, N.; Lee, J.; Lee, H.J. Artifact-free 2D mapping of neural activity in vivo through transparent gold nanonetwork array. Adv. Funct. Mater. 2020, 30, 2000896. [Google Scholar] [CrossRef]
- Renz, A.F.; Lee, J.; Tybrandt, K.; Brzezinski, M.; Lorenzo, D.A.; Cheraka, M.C.; Lee, J.; Helmchen, F.; Vörös, J.; Lewis, C.M. Opto-E-Dura: A soft, stretchable ECoG array for multimodal, multiscale neuroscience. Adv. Heal. Mater. 2020, 9, 2000814. [Google Scholar] [CrossRef] [PubMed]
- Ji, B.; Xie, Z.; Hong, W.; Jiang, C.; Guo, Z.; Wang, L.; Wang, X.; Yang, B.; Liu, J. Stretchable parylene-C electrodes enabled by serpentine structures on arbitrary elastomers by silicone rubber adhesive. J. Materiomics 2020, 6, 330–338. [Google Scholar] [CrossRef]
- Chiang, C.-H.; Sang, M.W.; Orsborn, A.L.; Yu, K.J.; Trumpis, M.; Bent, B.; Wang, C.; Xue, Y.; Min, S.; Woods, V. Development of a neural interface for high-definition, long-term recording in rodents and nonhuman primates. Sci. Transl. Med. 2020, 12, eaay4682. [Google Scholar] [CrossRef] [PubMed]
- Insanally, M.; Trumpis, M.; Wang, C.; Chiang, C.H.; Woods, V.; Palopoli-Trojani, K.; Bossi, S.; Froemke, R.; Viventi, J. A low-cost, multiplexed μECoG system for high-density recordings in freely moving rodents. J. Neural Eng. 2016, 13, 026030. [Google Scholar] [CrossRef]
- Shi, Z.; Zheng, F.M.; Zhou, Z.T.; Li, M.; Fan, Z.; Ye, H.P.; Zhang, S.; Xiao, T.; Chen, L.; Tao, T.H.; et al. Silk-enabled conformal multifunctional bioelectronics for investigation of spatiotemporal epileptiform activities and multimodal neural encoding/decoding. Adv. Sci. 2019, 6, 1801617. [Google Scholar] [CrossRef] [PubMed]
- Masvidal-Codina, E.; Illa, X.; Dasilva, M.; Calia, A.B.; Dragojević, T.; Vidal-Rosas, E.E.; Prats-Alfonso, E.; Martínez-Aguilar, J.; Cruz, J.M.D.; Garcia-Cortadella, R.; et al. High-resolution mapping of infraslow cortical brain activity enabled by graphene microtransistors. Nat. Mater. 2019, 18, 280–288. [Google Scholar] [CrossRef]
- Garcia-Cortadella, R.; Schäfer, N.; Cisneros-Fernandez, J.; Ré, L.; Illa, X.; Schwesig, G.; Moya, A.; Santiago, S.; Guirado, G.; Villa, R.; et al. Switchless multiplexing of graphene active sensor arrays for brain mapping. Nano Lett. 2020, 20, 3528–3537. [Google Scholar] [CrossRef]
- Fukushima, M.; Saunders, R.C.; Mullarkey, M.; Doyle, A.M.; Mishkin, M.; Fujii, N. An electrocorticographic electrode array for simultaneous recording from medial, lateral, and intrasulcal surface of the cortex in macaque monkeys. J. Neurosci. Methods 2014, 233, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Kaiju, T.; Keiichi, D.; Masashi, Y.; Kei, W.; Masato, I.; Hiroshi, A.; Kazutaka, T.; Fumiaki, Y.; Masayuki, H.; Takafumi, S. High spatiotemporal resolution ECoG recording of somatosensory evoked potentials with flexible micro-electrode arrays. Front. Neural Circuits 2017, 11, 20. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, Y.; Yamagiwa, S.; Sawahata, H.; Numano, R.; Koida, K.; Ishida, M.; Kawano, T. Ultrastretchable kirigami bioprobes. Adv. Healthc. Mater. 2017, 7, 1701100. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Jia, L.; Fu, T.M.; Dai, X.; Wei, Z.; Lieber, C.M. Three-dimensional macroporous nanoelectronic networks as minimally invasive brain probes. Nat. Mater. 2015, 14, 1286–1292. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, C.; Gao, H.; Yu, C.; Song, J. A Removable insertion shuttle for ultraflexible neural probe implantation with stable chronic brain electrophysiological recording. Adv. Mater. Interfaces 2020, 7, 1901775. [Google Scholar] [CrossRef]
- Guo, Z.; Ji, B.; Wang, L.; Yang, B.; Liu, J. A flexible and stretchable kirigami-inspired implantable neural probe with floating microsites for electrophysiology recordings. In 2020 IEEE 33rd International Conference on Micro Electro Mechanical Systems (MEMS); IEEE: Piscataway, NJ, USA, 2020; pp. 350–353. [Google Scholar]
- Lu, L.; Fu, X.; Liew, Y.; Zhang, Y.; Zhao, S.; Xu, Z.; Zhao, J.; Li, D.; Li, Q.; Stanley, G.B.; et al. Soft and MRI compatible neural electrodes from carbon nanotube fibers. Nano Lett. 2019, 19, 1577–1586. [Google Scholar] [CrossRef]
- Du, M.; Huang, L.; Zheng, J.; Xi, Y.; Dai, Y.; Zhang, W.D.; Yan, W.; Tao, G.M.; Qiu, J.R.; So, K.F.; et al. Flexible fiber probe for efficient neural stimulation and detection. Adv. Sci. 2020, 7, 2001410. [Google Scholar] [CrossRef]
- Wang, L.L.; Xie, Z.X.; Zhong, C.; Tang, Y.Q.; Ye, F.M.; Wang, L.P.; Lu, Y. Self-spreadable Octopus-like Electrode Arrays for Long-term Neural Recordings. Acta Phys. Chim. Sin. 2019. [Google Scholar] [CrossRef]
- Scholten, K.; Meng, E. Electron-beam lithography for polymer bioMEMS with submicron features. Microsyst. Nanoeng. 2016, 2, 16053. [Google Scholar] [CrossRef] [PubMed]
- Shim, S.; Park, H.Y.; Choi, G.J.; Shin, H.C.; Kim, S.J. A simply fabricated neural probe by laser machining of a thermally laminated gold thin film on transparent cyclic olefin polymer. ACS Omega 2019, 4, 2590–2595. [Google Scholar] [CrossRef]
- Guo, Y.; Fang, Z.; Du, M.; Yang, L.; Shao, L.; Zhang, X.; Li, L.; Shi, J.; Tao, J.; Wang, J. Flexible and biocompatible nanopaper-based electrode arrays for neural activity recording. Nano Res. 2018, 11, 5604–5614. [Google Scholar] [CrossRef]
- Kedi, X.; Li, S.J.; Dong, S.R.; Zhang, S.M.; Pan, G.; Wang, G.M.; Shi, L.; Guo, W.; Yu, C.N.; Luo, J.K. Bioresorbable electrode array for electrophysiological and pressure signal recording in the brain. Adv. Healthc. Mater. 2019, 8, 1801649. [Google Scholar]
- Chen, C.; Xue, M.M.; Wen, Y.G.; Yao, G.; Cui, Y.; Liao, F.Y.; Yan, Z.C.; Huang, L.; Khan, S.A.; Gao, M.; et al. A ferroelectric ceramic/polymer composite-based capacitive electrode array for in vivo recordings. Adv. Healthc. Mater. 2017, 6, 1700305. [Google Scholar] [CrossRef]
- Ware, T.; Simon, D.; Liu, C.; Musa, T.; Vasudevan, S.; Sloan, A.; Keefer, E.W.; Rennaker, R.L.; Voit, W. Thiol-ene/acrylate substrates for softening intracortical electrodes. J. Biomed. Mater. Res. Part B Appl. Biomater. 2014, 102, 1–11. [Google Scholar] [CrossRef]
- Oribe, S.; Yoshida, S.; Kusama, S.; Osawa, S.I.; Nishizawa, M. Hydrogel-based organic subdural electrode with high conformability to brain surface. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Rihani, R.; Stiller, A.M.; Usoro, J.O.; Lawson, J.; Pancrazio, J.J. Deployable, liquid crystal elastomer-based intracortical probes. Acta Biomater. 2020, 111, 54–64. [Google Scholar] [CrossRef]
- Ware, T.; Simon, D.; Hearon, K.; Liu, C.; Shah, S.; Reeder, J.; Khodaparast, N.; Kilgrad, M.P.; Maitland, D.J.; Rennaker, R.L., II; et al. Three-dimensional flexible electronics enabled by shape memory polymer substrates for responsive neural interfaces. Macromol. Mater. Eng. 2012, 297, 1193–1202. [Google Scholar] [CrossRef] [PubMed]
- Shoffstall, A.J.; Ecker, M.; Danda, V.; Joshi-Imre, A.; Stiller, A.; Yu, M.; Paiz, J.E.; Mancuso, E.; Bedell, H.W.; Voit, W.E.; et al. Characterization of the neuroinflammatory response to thiol-ene shape memory polymer coated intracortical microelectrodes. Micromachines 2018, 9, 486. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, Q.; Wang, Y.; Zeng, Q.; Du, X. Self-unfolding flexible microelectrode arrays based on shape memory polymers. Adv. Mater. Technol. 2019, 4, 1900566. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, X.J.; Xu, W.J.; Luo, W.H.; Li, M. Stretchable transparent electrode arrays for simultaneous electrical and optical interrogation of neural circuits in vivo. Nano Lett. 2018, 18, 2903–2911. [Google Scholar] [CrossRef]
- Chen, Z.; Yin, R.T.; Obaid, S.N.; Tian, J.; Lu, L. Flexible and transparent metal oxide/metal grid hybrid interfaces for electrophysiology and optogenetics. Adv. Mater. Technol. 2020, 5, 2000322. [Google Scholar] [CrossRef]
- Vomero, M.; Gueli, C.; Zucchini, E.; Fadiga, L.; Stieglitz, T. Flexible bioelectronic devices based on micropatterned monolithic carbon fiber materials. Adv. Mater. Technol. 2019, 5, 1900713. [Google Scholar] [CrossRef]
- Qi, D.; Liu, Z.; Liu, Y.; Jiang, Y.; Leow, W.R.; Pal, M.; Pan, S.; Yang, H.; Wang, Y.; Zhang, X. Highly stretchable, compliant, polymeric microelectrode arrays for in vivo electrophysiological interfacing. Adv. Mater. 2017, 29, 1702800. [Google Scholar] [CrossRef]
- Araki, T.; Yoshida, F.; Uemura, T.; Noda, Y.; Sekitani, T. Long-term implantable, flexible, and transparent neural interface based on Ag/Au core–shell nanowires. Adv. Healthc. Mater. 2019, 8, e1900130. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Ji, B.; Wang, M.; Zhang, K.; Huangfu, S.; Feng, H.; Chang, H.; Yuan, X. Implantable Thin Film Devices as Brain-Computer Interfaces: Recent Advances in Design and Fabrication Approaches. Coatings 2021, 11, 204. https://doi.org/10.3390/coatings11020204
Zhou Y, Ji B, Wang M, Zhang K, Huangfu S, Feng H, Chang H, Yuan X. Implantable Thin Film Devices as Brain-Computer Interfaces: Recent Advances in Design and Fabrication Approaches. Coatings. 2021; 11(2):204. https://doi.org/10.3390/coatings11020204
Chicago/Turabian StyleZhou, Yuhao, Bowen Ji, Minghao Wang, Kai Zhang, Shuaiqi Huangfu, Huicheng Feng, Honglong Chang, and Xichen Yuan. 2021. "Implantable Thin Film Devices as Brain-Computer Interfaces: Recent Advances in Design and Fabrication Approaches" Coatings 11, no. 2: 204. https://doi.org/10.3390/coatings11020204
APA StyleZhou, Y., Ji, B., Wang, M., Zhang, K., Huangfu, S., Feng, H., Chang, H., & Yuan, X. (2021). Implantable Thin Film Devices as Brain-Computer Interfaces: Recent Advances in Design and Fabrication Approaches. Coatings, 11(2), 204. https://doi.org/10.3390/coatings11020204






