Compositional, Structural, Morphological, and Optical Properties of ZnO Thin Films Prepared by PECVD Technique
Abstract
1. Introduction
2. Experimental Part
3. Results and Discussion
3.1. Chemical Composition Analysis
3.2. Structural and Morphological Investigation
3.2.1. XRD Characterization
3.2.2. SEM Results
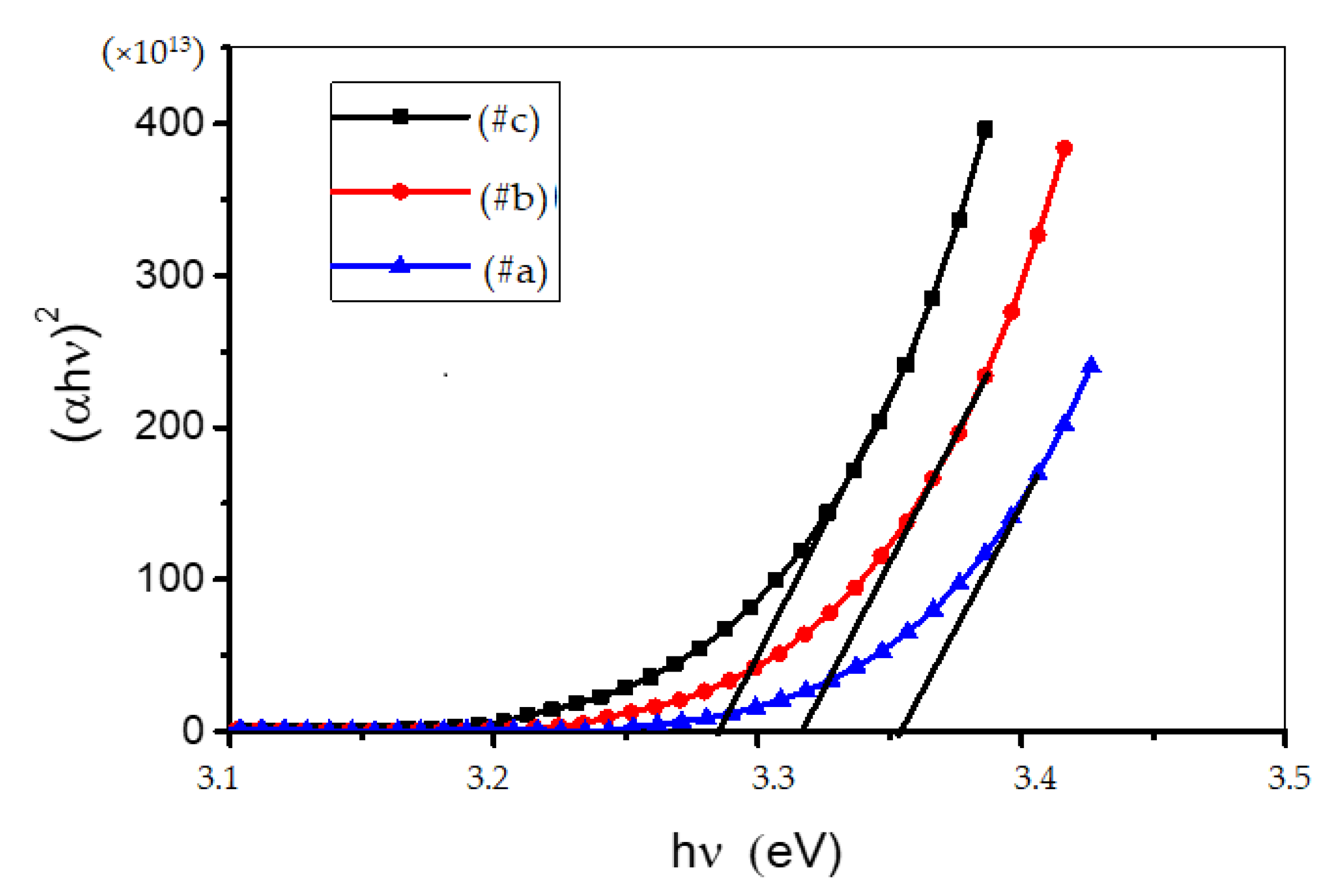
3.3. Optical Measurements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Choppali, U.; Kougianos, E.; Mohanty, S.P.; Gorman, B.P. Influence of annealing on polymeric precursor derived ZnO thinfilms on sapphire. Thin Solid Film. 2013, 545, 466–470. [Google Scholar] [CrossRef]
- Bedia, F.; Bedia, A.; Maloufi, N.; Aillerie, M.; Genty, F.; Benyoucef, B. Effect of tin doping on optical properties of nanostructured ZnO thin films grown by spray pyrolysis technique. J. Alloys Compd. 2014, 616, 312–318. [Google Scholar] [CrossRef]
- Hengehold, R.L.; Almassy, R.J.; Pedrotti, F.L. Electron energy loss and ultraviolet reflectivity spectra of crystalline ZnO. Phys. Rev. B 1970, 1, 4784–4791. [Google Scholar] [CrossRef]
- Gupta, T.K. Application of zinc oxide varistors. J. Am. Ceram. Soc. 1990, 73, 1817–1840. [Google Scholar] [CrossRef]
- Zeng, G.Y.; Nian, K.S.; Lee, K.Y. Characteristics of a dye-sensitized solar cell based on an anode combining ZnO nanostructures with vertically aligned carbon nanotubes. Diam. Relat. Mater. 2010, 19, 1457–1460. [Google Scholar] [CrossRef]
- Hassan, N.K.; Hashim, M.R. Structural and optical properties of ZnO thin film prepared by oxidation of Zn metal powders. In Proceedings of the 2012 International Conference on Enabling Science and Nanotechnology, Johor Bahru, Malaysia, 5–7 January 2012; Volume 42, pp. 1–2. [Google Scholar] [CrossRef]
- Saito, N.; Haneda, H.; Sekiguchi, T.; Ohashi, N.; Sakaguchi, I.; Koumoto, K. low-temperature fabrication of light-emitting zinc oxide micropatterns using self-assembled monolayers. Adv. Mater. 2002, 14, 418–421. [Google Scholar] [CrossRef]
- Chen, Y.; Bagnall, D.M.; Koh, H.-J.; Park, K.-T.; Hiraga, K.; Zhu, Z.; Yao, T. Plasma assisted molecular beam epitaxy of ZnO on c-plane sapphire: Growth and characterization. J. Appl. Phys. 1998, 84, 3912–3918. [Google Scholar] [CrossRef]
- Hvam, J.M. Temperature-induced wavelength shift of electron-beam-pumped lasers from CdSe, CdS and ZnO. Phys. Rev. B 1971, 4, 4459–4464. [Google Scholar] [CrossRef]
- Wang, L.-H.; Liang, J.; Ma, S.-F.; Liu, X.-G. Synthesis and characterization of the GaN film. Chin. J. Lumin 2008, 29, 152–155. [Google Scholar]
- Choudhary, S.; Gangopadhyay, S. Zincoxidenano-structures:Fromnano-walltonano-rodgrowthmorphology. AIP Conf. Proc. 2018, 1989, 020007–020012. [Google Scholar]
- Guileen, C.; Herrero, J. Structure, optical and electrical properties of indium tin oxide thin films prepared by sputtering at room temperature and annealed in air or nitrogen. J. Appl. Phys. 2007, 101, 073514–073521. [Google Scholar] [CrossRef]
- Chen, M.; Pei, Z.; Sun, C.; Wen, L.; Wang, X. Surface characterization of transparent conductive oxide Al-doped ZnO films. J. Cryst. Growth 2000, 220, 254–262. [Google Scholar] [CrossRef]
- Grundmann, M.; Rahm, A.; Nobis, N.; Lorenz, M.; Czekalla, C.; Evgeni, M.K.; Lenzner, J.; Boukos, N.; Travlos, A.; HeniniIn, M. Handbook of Self Assembled Semiconductor Nanostructures for Novel Devices in Photonics and Electronics; Elsevier: Amsterdam, The Netherlands, 2008; pp. 293–319. [Google Scholar]
- Ezema, F.I.; Nwankwo, U.O.A. Effect of annealing temperature on the structural and optical propertises of Al-doped zinc oxide (ZnO) crystals. J. Optoelectron. Biomed. Mat. 2010, 1, 167–173. [Google Scholar]
- Kluth, O.; Loffl, A.; Wieder, S.; Beneking, C.; Appenzeller, W.; Houben, L.; Rech, B.; Wagner, H.; Hoffmann, S.; Waser, R. Texture etched Al-doped ZnO: A new material for enhanced light trapping in thin film solar cells. In Proceedings of the Twenty Sixth IEEE Photovoltaic Specialists Conference—1997, Anaheim, CA, USA, 30 September–3 October 1997; pp. 715–718. [Google Scholar]
- Groenen, R.; Löffler, J.; Sommeling, P.; Linden, J.; Hamers, E.; Schropp, R.E.I.; VanDe Sanden, M. Surface textured ZnO films for thin film solar cell applications by expanding thermal plasma CVD. Thin Solid Film. 2001, 392, 226–230. [Google Scholar] [CrossRef]
- Löffler, J.; Groenen, R.; Linden, J.L.; Van de Sanden, M.C.M.; Schropp, R.E.I. Amorphous silicon solar cells on natively textured ZnO grown by PECVD. Thin Solid Film. 2001, 392, 315–319. [Google Scholar]
- Ouhaibi, A.; Ghamnia, M.; Dahamni, M.A.; Heresanu, V.; Fauquet, C.; Tonneau, D. The effect of strontium doping on structural and morphological properties of ZnO nanofilms synthesized by ultrasonic spray pyrolysis method. J. Sci. Adv. Mater. Devices 2018, 3, 29–36. [Google Scholar] [CrossRef]
- Hamzaoui, N.; Boukhachem, A.; Ghamnia, M.; Fauquet, C. Investigation of some physical properties of ZnO nanofilms synthesized by micro-droplet technique. Results Phys. 2017, 7, 1950–1958. [Google Scholar] [CrossRef]
- Bekhti, W.; Ghamnia, M.; Guerbous, L. Effect of some amines, dodecylamine (DDA) and hexadecyldimethylamine (DMHA), on the formation of ZnO nanorods synthesized by hydrothermal route. Philos. Mag. 2014, 94, 2886–2899. [Google Scholar] [CrossRef]
- Matsuo, T.; Okuda, S.; Washio, K. Growth and characterization of ZnO thin film by RF magnetron sputtering for photoacoustic tomography sensor. MRS Online Proc. Libr. Arch. 2013, 1494, 19–24. [Google Scholar] [CrossRef]
- Srinivasarao, K.; Srinivasarao, G.; Madhuri, K.V.; Murthy, K.K.; Mukhopadhyay, P.K. Preparation and characterization of RF magnetron sputtered Mo:ZnO. Thin Films Indian J. Mat. Sci. 2013, 2013, 1–7. [Google Scholar]
- Al Asmar, R.; Zaouk, D.; Bahouth, P.; Podleki, J.; Foucaran, A. Characterization of electron beam evaporated ZnO thin films and stacking ZnO fabricated by e-beam evaporation and RF magnetron sputtering for the realization of resonators. Microelectron. Eng. 2006, 83, 393–398. [Google Scholar] [CrossRef]
- Agarwal, D.C.; Chauhan, R.; Kumar, A.; Kabiraj, D.; Singh, F. Synthesis and characterization of ZnO thin film grown by electron beam evaporation. J. Appl. Phys. 2006, 99, 123105. [Google Scholar] [CrossRef]
- Vincze, A.; Bruncko, J.; Michalka, M.; Figura, D. Growth and characterization of pulsed laser deposited ZnO thin films. Open Phys. 2007, 5, 385–397. [Google Scholar] [CrossRef]
- Wisz, G.; Virt, I.; Sagan, P.; Potera, P.; Yavorskyi, R. Structural, optical and electrical properties of Zinc oxide layers produced by pulsed laser deposition method. Nanoscale Res. Lett. 2017, 12, 253. [Google Scholar] [CrossRef]
- Asghar, M.; Mahmood, K.; Raja, M.Y.; Hasan, M. Synthesis and characterization of ZnO nanorods using molecular beam epitaxy. Adv. Mat. Res. 2013, 622, 919–924. [Google Scholar] [CrossRef]
- Zhou, Y.; Yu, G.; Shi, X.; Zhang, X.; Yan, W.; Wei, S.; Xie, Y. Growth and characterization of ZnO thin film prepared by molecular beam epitaxy on Si(100). Nucl. Tech. 2003, 26, 9–12. [Google Scholar]
- Ilican, S.; Caglar, Y.Y.; Caglar, M. Preparation and characterization of ZnO thin films deposited by sol-gel spin coating method. J. Optoelectr. Adv. Mat. 2008, 10, 2578–2583. [Google Scholar]
- Firdaus, C.; Rizam, M.; Rusop, M.; Hidayah, S. Characterization of ZnO and ZnO: TiO2 thin films prepared by sol-gel spray-spin coating technique. Procedia Eng. 2012, 41, 1367–1373. [Google Scholar] [CrossRef]
- Cruz, M.R.A.; Zarzosa, G.O.; Castañón, G.M.; Martinez, J.R. Characterization of ZnO threads obtained using dipcoatingmethod at room temperature. Mat. Lett. 2012, 78, 159–161. [Google Scholar] [CrossRef]
- Rani, R.A.; Ab Ghafar, S.; Zoolfakar, A.S.; Rusop, M. Growth of ZnO nanorods on glass substrate deposited using dipcoatingmethod. Nanosci. Nanotechnol. Nano-Scitech 2018, 1963, 02007. [Google Scholar] [CrossRef]
- Ergin, B.; Ketenci, E.; Atay, F. Characterization of ZnO films obtained by ultrasonic spray pyrolysis technique. Int. J. Hydrog. Energy 2009, 34, 5249–5254. [Google Scholar] [CrossRef]
- Alver, U.; Solakyildirim, T.K.; Bacaksız, E.; Kucukomeroglu, T.; Nezir, S.; Mutlu, I.H.; Aslan, F. Synthesis and characterization of spray pyrolysis zinc oxide microrods. Thin Sol. Film. 2007, 515, 3448–3451. [Google Scholar] [CrossRef]
- Chen, Z.; Salagaj, K.S.; Strob, W.Z.K. ZnO thin films synthesized by chemical vapor deposition. In Proceedings of the 2010 IEEE Long Island Systems, Applications and Technology Conference, Farmingdale, NY, USA, 7 May 2010. [Google Scholar]
- Reuge, N.; Bacsa, R.R.; Serp, P.; Caussat, B. Chemical vapor synthesis of zinc oxide nanoparticles: Experimental and preliminary modeling studies. J. Phys. Chem. C 2009, 113, 19845–19852. [Google Scholar] [CrossRef]
- Salar, E.; Ghoranneviss, M. Growth and characterization of carbon nanotubes and zinc oxide nanocomposite with the PECVD technique. Results Phys. 2017, 7, 1006–1009. [Google Scholar] [CrossRef]
- Chao, H.; Wei, D.H. Synthesis and Characterization of high c-axis ZnO thin film by plasma enhanced chemical vapor deposition, system and its UV photodetector application. J. Vis. Exp. 2015, 104, e53097. [Google Scholar] [CrossRef] [PubMed]
- Vimalkumar, T.; Poornima, N.; Kartha, C.S.; Vijayakumar, K. On tuning the orientation of grains of spray pyrolysed ZnO thin films. Appl. Surf. Sci. 2010, 256, 6025–6028. [Google Scholar] [CrossRef]
- Khoshhesab, Z.M.; Sarfaraz, M.; Asadabad, M.A. Preparation of ZnO nanostructures by chemical precipitation method. Synth. React. Inorg. Met. Chem. 2011, 41, 814–819. [Google Scholar] [CrossRef]
- Bindu, P.; Thomas, S. Estimation of lattice strain in ZnO nanoparticles: X-ray peak profile analysis. J. Appl. Phys. 2014, 8, 123–134. [Google Scholar] [CrossRef]
- Marouf, S.; Beniaiche, A.; Guessas, H.; Azizi, A. Morphological, structural and optical properties of ZnO thin films deposited by dip coating method. Mater. Res. 2016, 20, 88–95. [Google Scholar] [CrossRef]
- Cullity, B.D.; Weymouth, J.W. Elements of X-Ray Diffraction. Am. J. Phys. 1957, 25, 394–395. [Google Scholar] [CrossRef]
- Tauc, J.; Menth, A. States in the gap. J. Non-Cryst. Solids 1972, 8, 569–585. [Google Scholar] [CrossRef]
- Shinde, S.; Oh, Y.; Haranath, D.; Bhosale, C.; Rajpure, K.; Shinde, P.S. Structural, optoelectronic, luminescence and thermalproperties of Ga-doped zinc oxide thin films. Appl. Surf. Sci. 2012, 258, 9969–9976. [Google Scholar] [CrossRef]
- Wang, Z.L. Zinc oxide nanostructures: Growth, properties and applications. J. Phys. Condens. Matter 2004, 16, R829–R858. [Google Scholar] [CrossRef]
- Zargar, R.A.; Hassan, M.M.; Boora, N.; Ahmed, I.; Ahmed, S.; Nissa, K.H.; Kumari, S.; Hafiz, A.K. A comparative study of microand nano ZnO films fabricated by sol-gel syringe spray method. Int. J. Ceram. Eng. Sci. 2020, 2, 169–176. [Google Scholar] [CrossRef]
- Khan, M.; Bhatti, K.; Qindeel, R.; Alonizan, N.; Althobaiti, H.S. Characterizations of multilayer ZnO thin films deposited bysol-gel spin coating technique. Results Phys. 2017, 7, 651–655. [Google Scholar] [CrossRef]
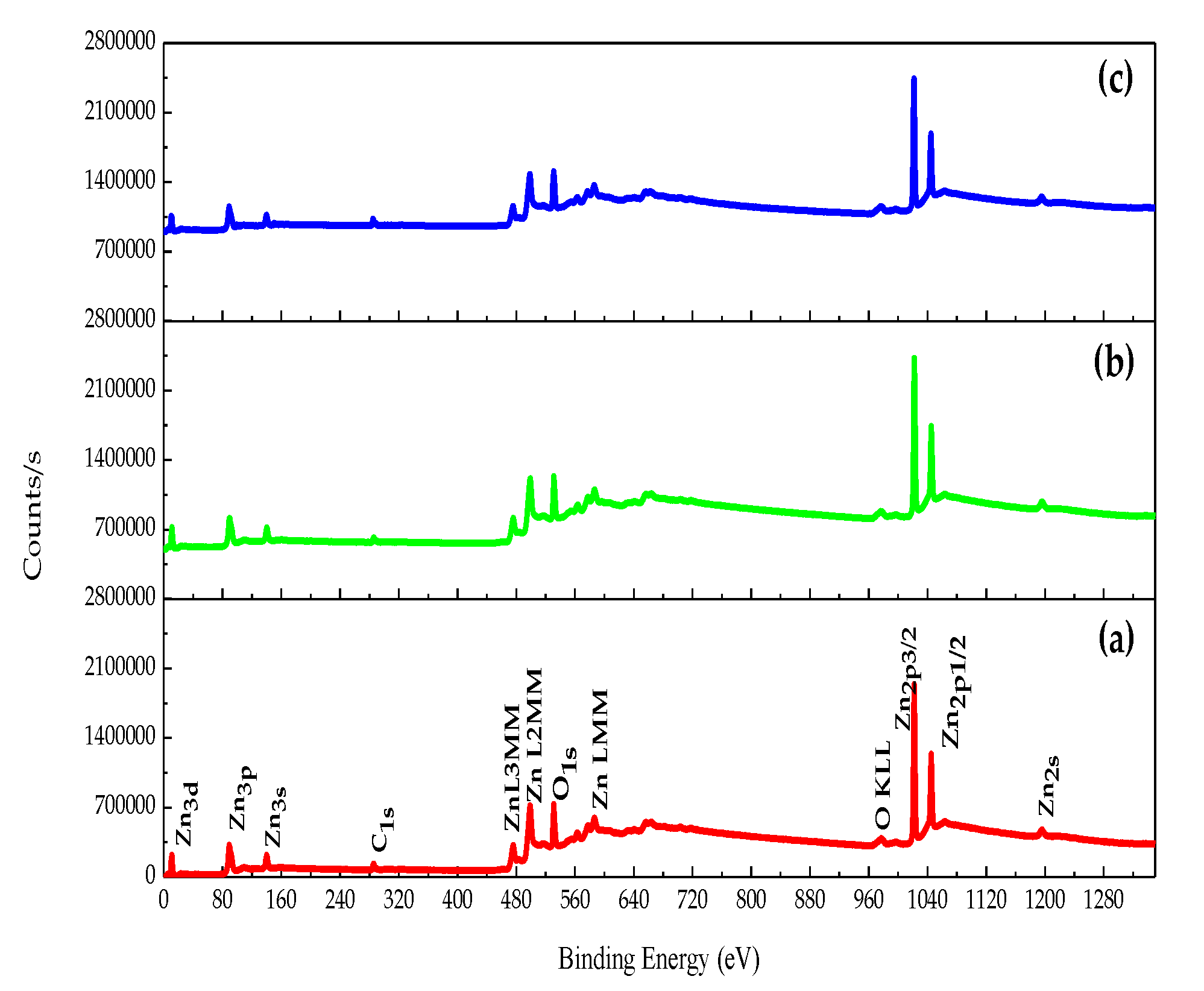
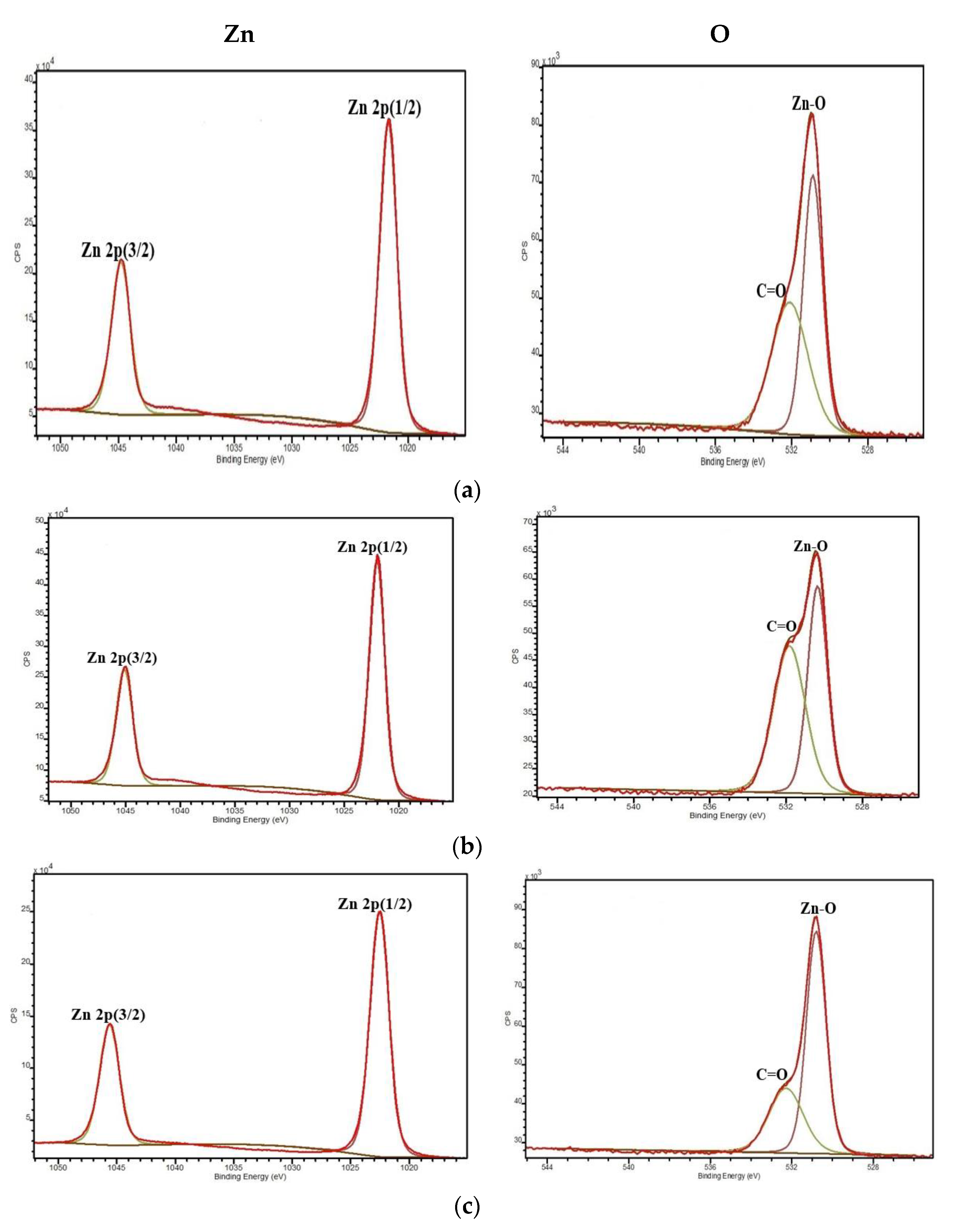

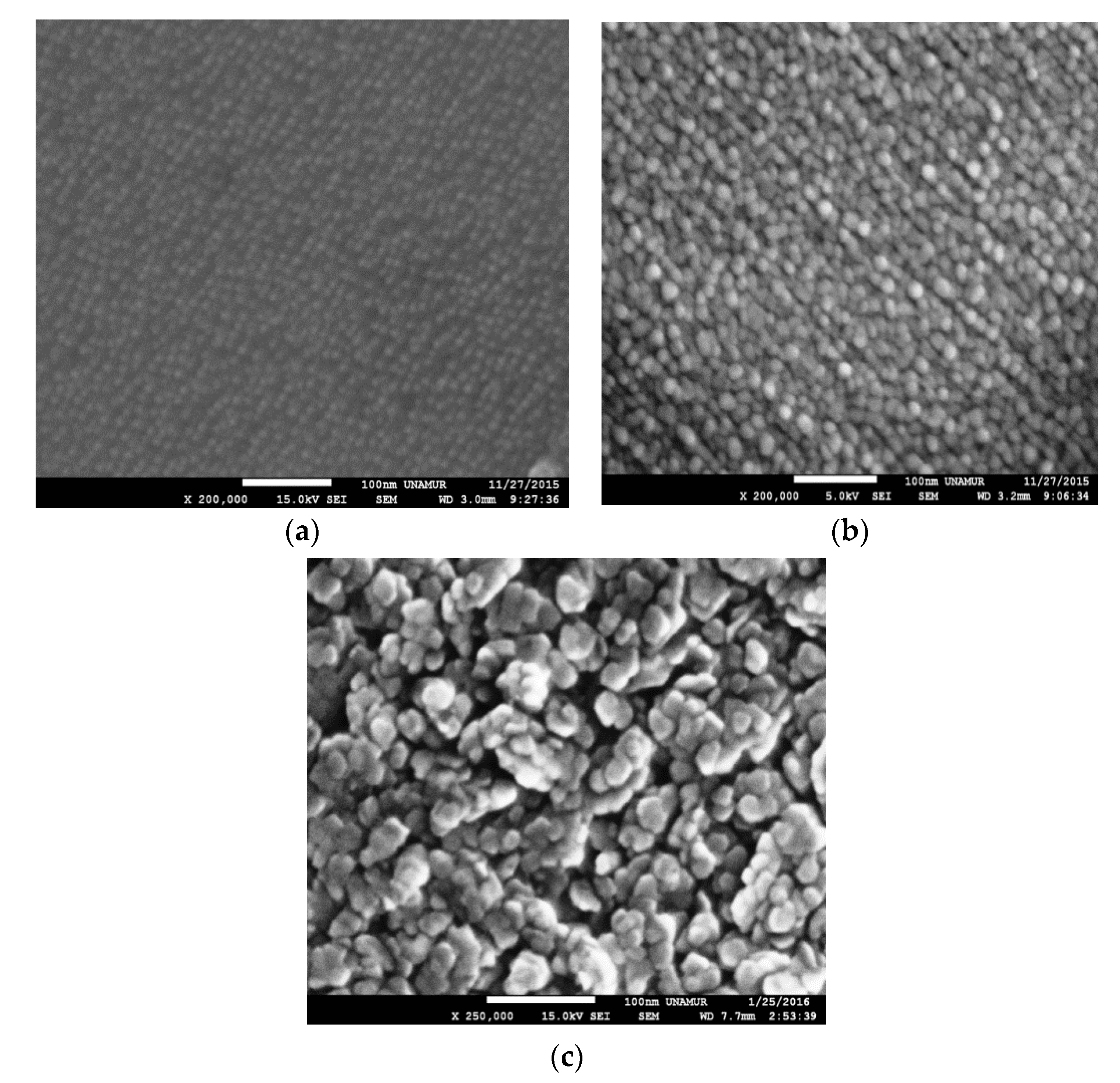
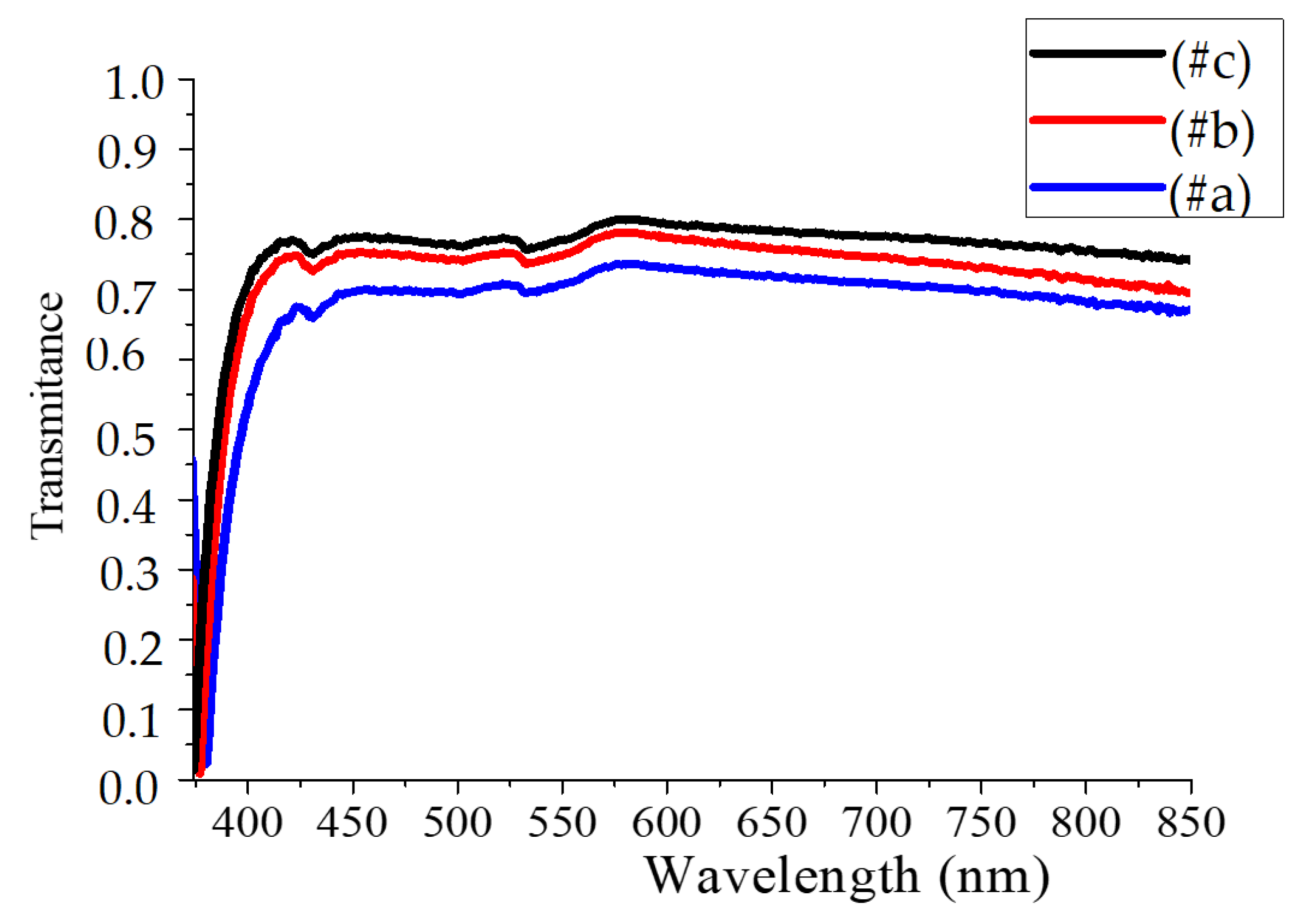
| Samples | Temperature (°C) |
|---|---|
| (#a) | 200 |
| (#b) | 300 |
| (#c) | 400 |
| Sample | 2θ | (hkl) | d (Å) | a (Å) | a (Å) in Literature | c (Å) | c (Å) in Literature |
|---|---|---|---|---|---|---|---|
| (#a) | 34.9 36.0 | (0 0 2) (1 0 1) | 2.5989 2.4801 | 3.2586 | 3.2400 [41] | 5.1979 | 5.2100 [41] |
| (#b) | 35.0 36.5 | (0 0 2) (1 0 1) | 2.6055 2.4758 | 3.2489 | 3.2229 [42] | 5.2109 | 5.1755 [42] |
| (#c) | 34.9 36.0 | (0 0 2) (1 0 1) | 2.5989 2.4801 | 3.2586 | 2.9950 [43] | 5.1979 | 5.1890 [43] |
| Sample | 2θ (°) | β (°) | D (nm) | δ (10−4·nm−2) |
|---|---|---|---|---|
| (#a) | 34.9 | 0.2342 | 72.18 | 1.919 |
| (#b) | 35.0 | 0.2900 | 58.13 | 2.959 |
| (#c) | 34.9 | 0.5353 | 31.57 | 9.960 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hacini, N.; Ghamnia, M.; Dahamni, M.A.; Boukhachem, A.; Pireaux, J.-J.; Houssiau, L. Compositional, Structural, Morphological, and Optical Properties of ZnO Thin Films Prepared by PECVD Technique. Coatings 2021, 11, 202. https://doi.org/10.3390/coatings11020202
Hacini N, Ghamnia M, Dahamni MA, Boukhachem A, Pireaux J-J, Houssiau L. Compositional, Structural, Morphological, and Optical Properties of ZnO Thin Films Prepared by PECVD Technique. Coatings. 2021; 11(2):202. https://doi.org/10.3390/coatings11020202
Chicago/Turabian StyleHacini, Noureddine, Mostefa Ghamnia, Mohamed Amine Dahamni, Abdelwaheb Boukhachem, Jean-Jacques Pireaux, and Laurent Houssiau. 2021. "Compositional, Structural, Morphological, and Optical Properties of ZnO Thin Films Prepared by PECVD Technique" Coatings 11, no. 2: 202. https://doi.org/10.3390/coatings11020202
APA StyleHacini, N., Ghamnia, M., Dahamni, M. A., Boukhachem, A., Pireaux, J.-J., & Houssiau, L. (2021). Compositional, Structural, Morphological, and Optical Properties of ZnO Thin Films Prepared by PECVD Technique. Coatings, 11(2), 202. https://doi.org/10.3390/coatings11020202






