Modeling of Poly(methylmethacrylate) Viscous Thin Films by Spin-Coating
Abstract
1. Introduction
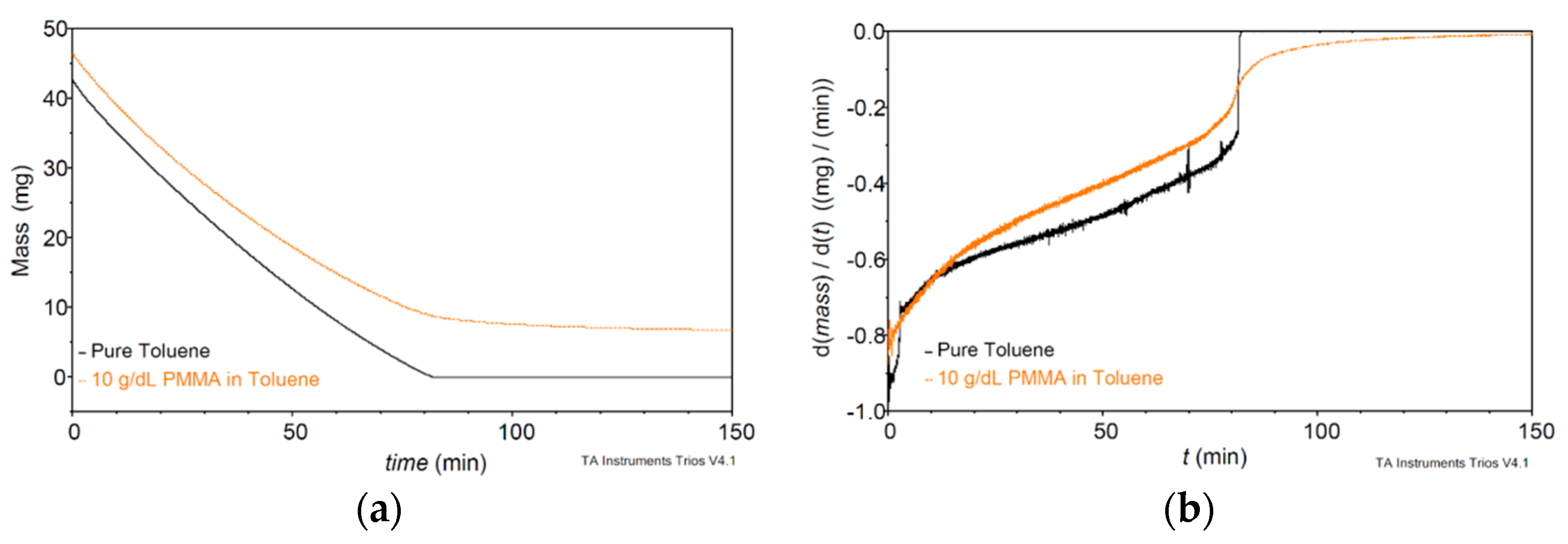
2. Materials and Methods
3. Results
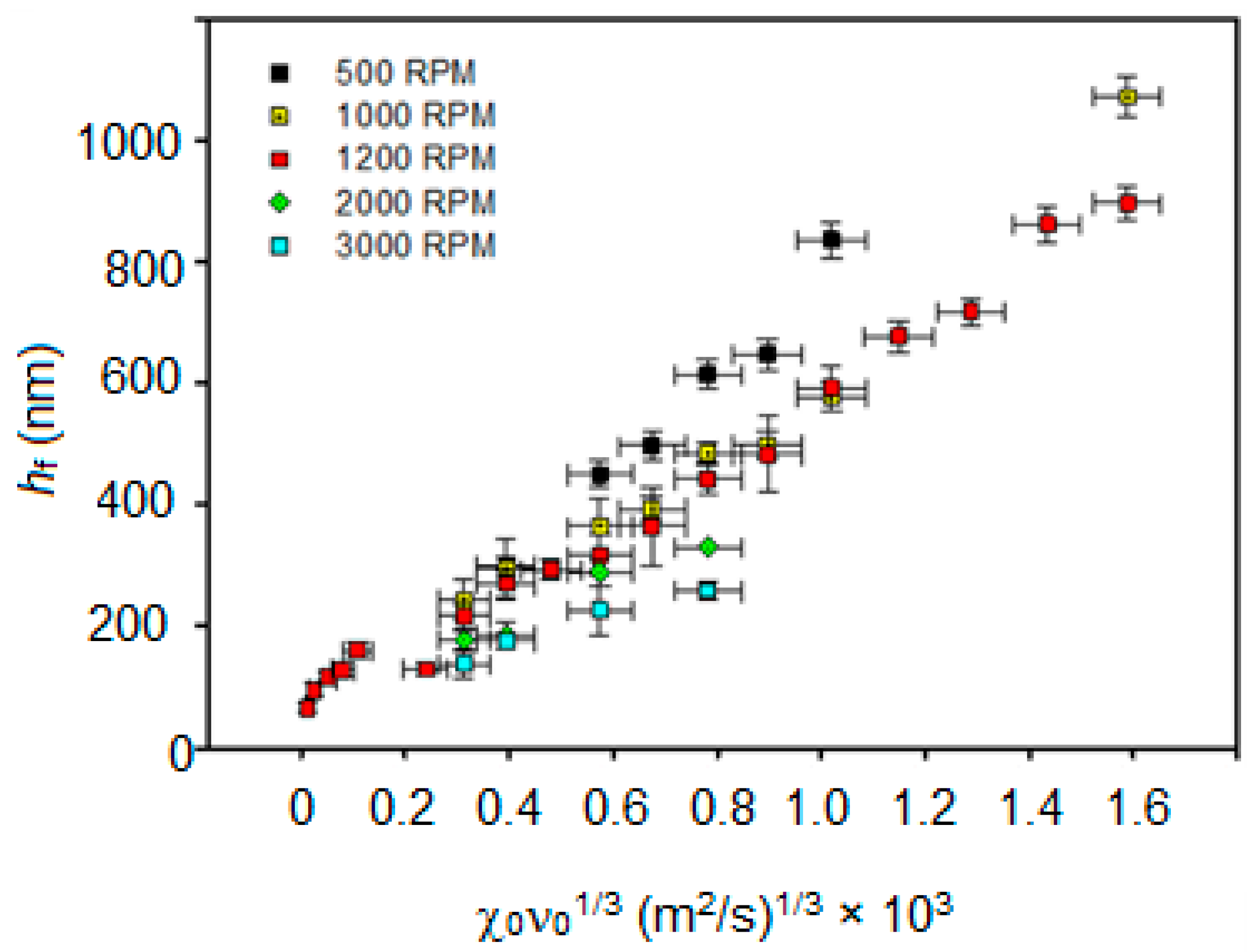
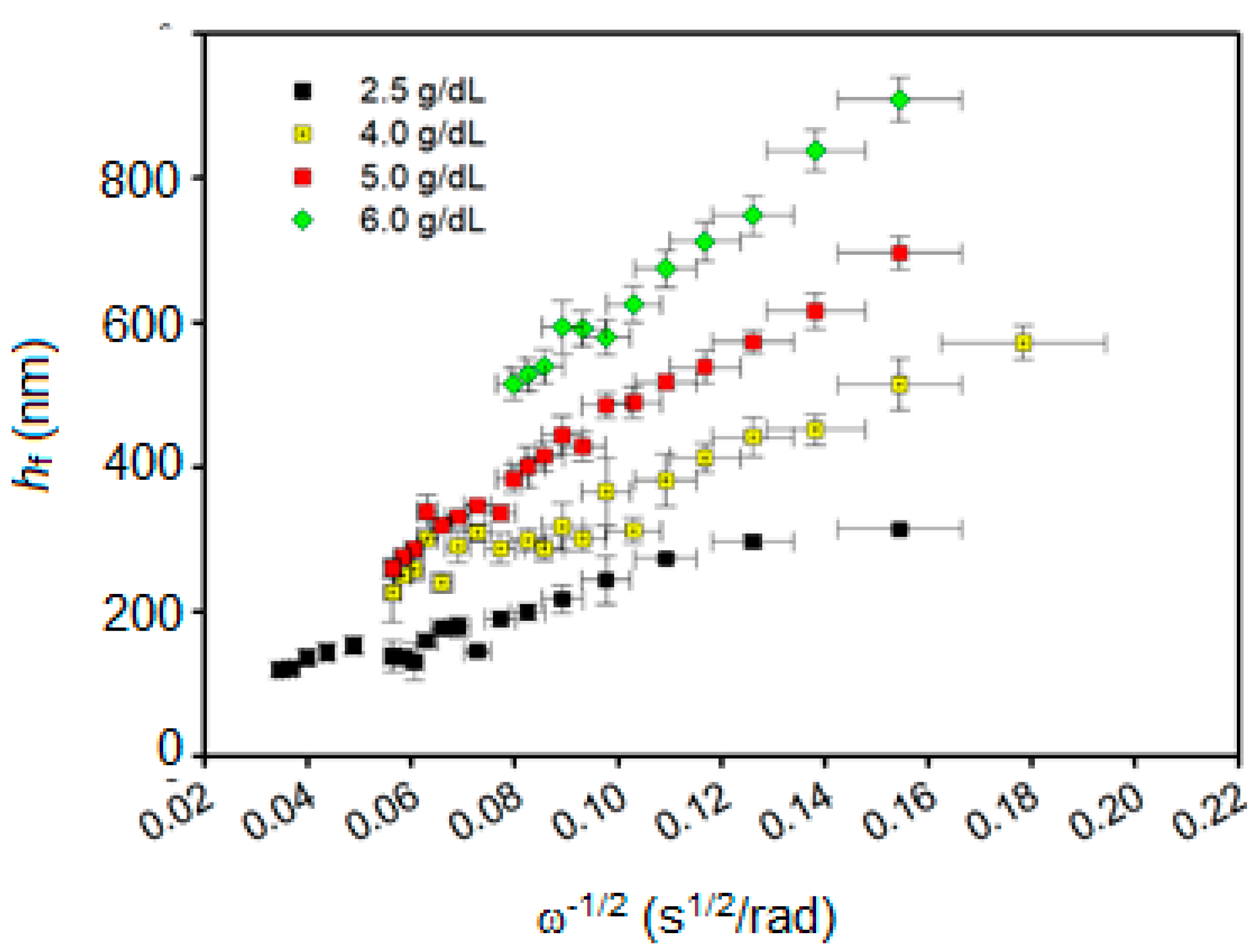
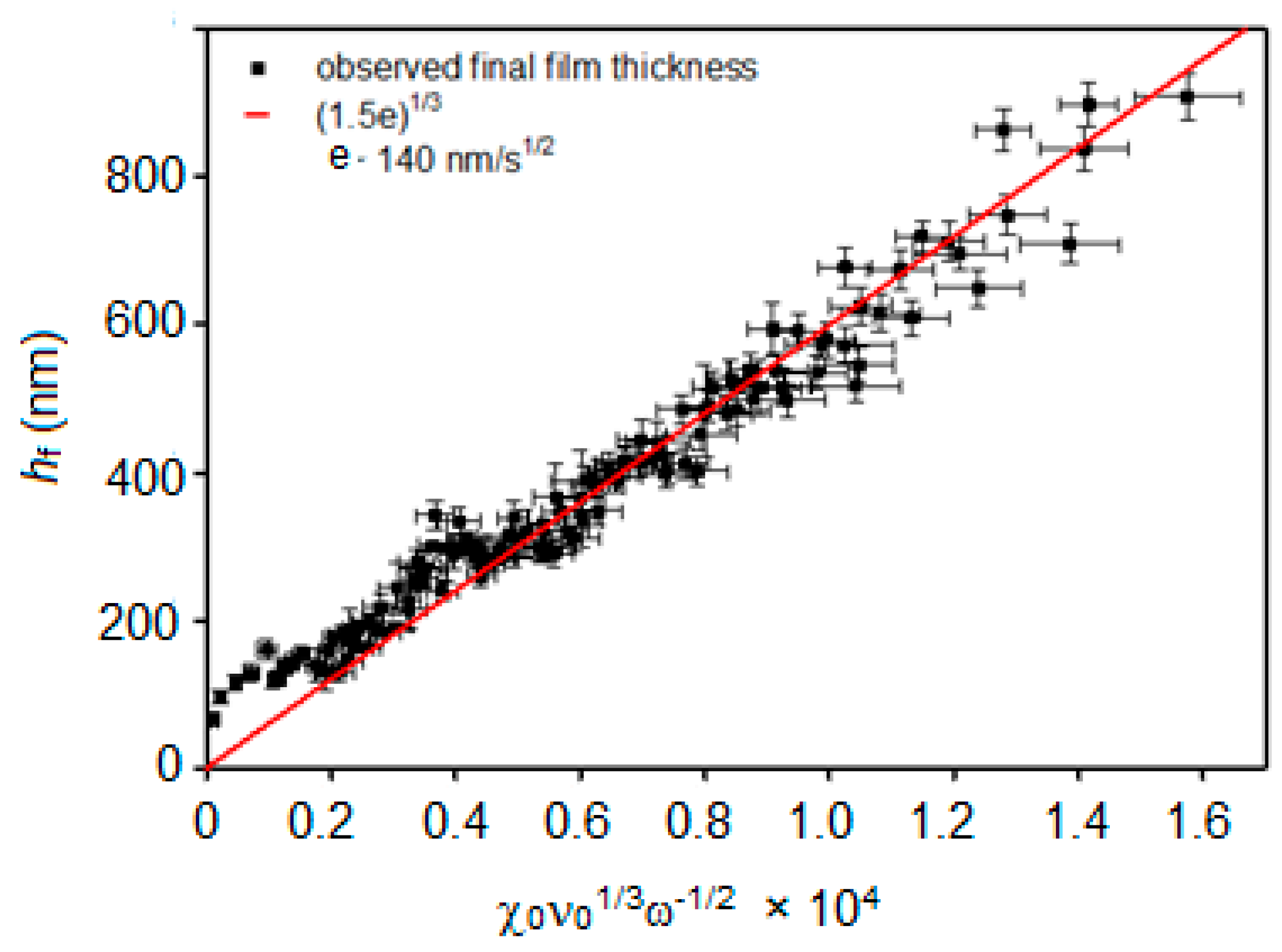
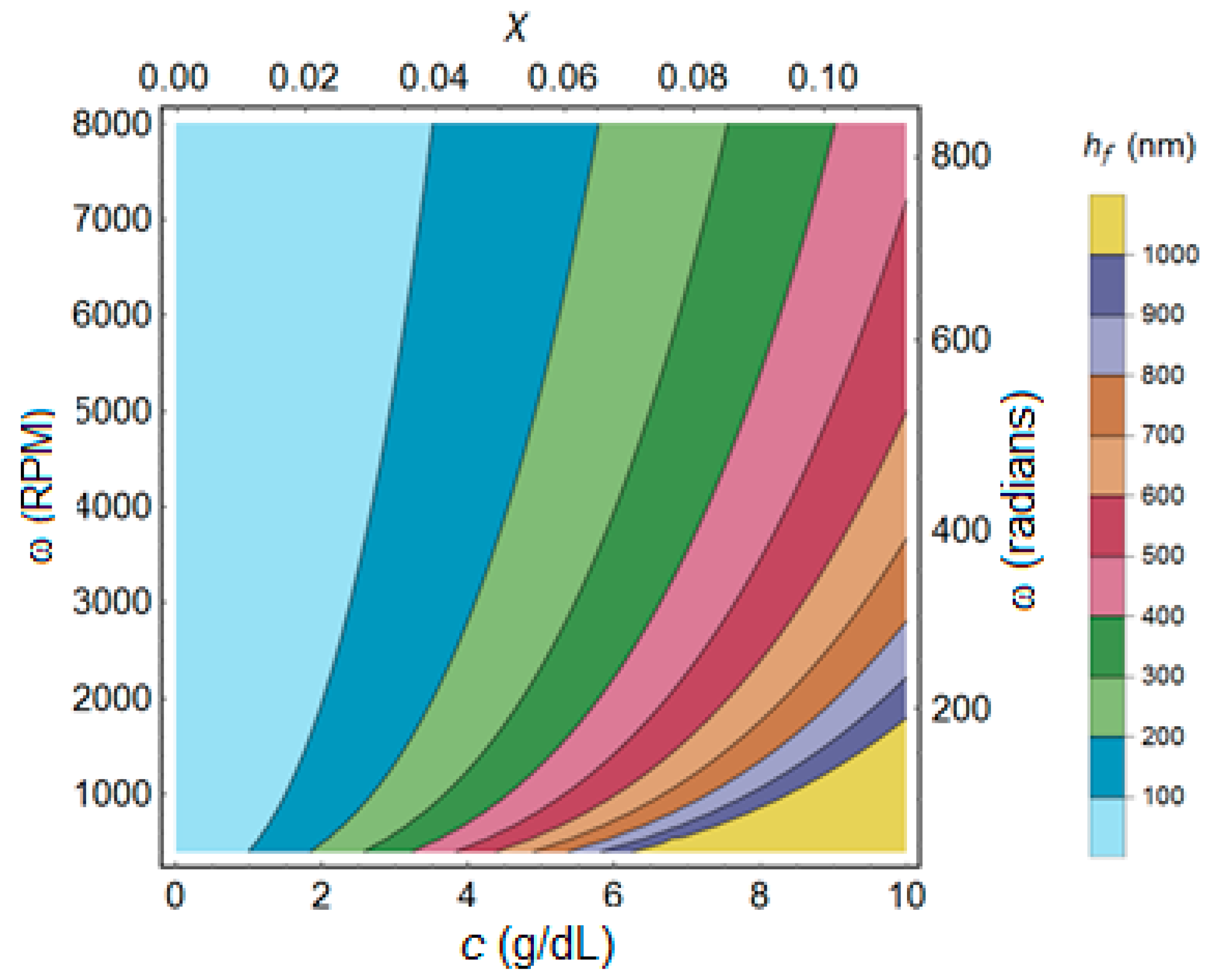
3.1. Spin-Coating Theory
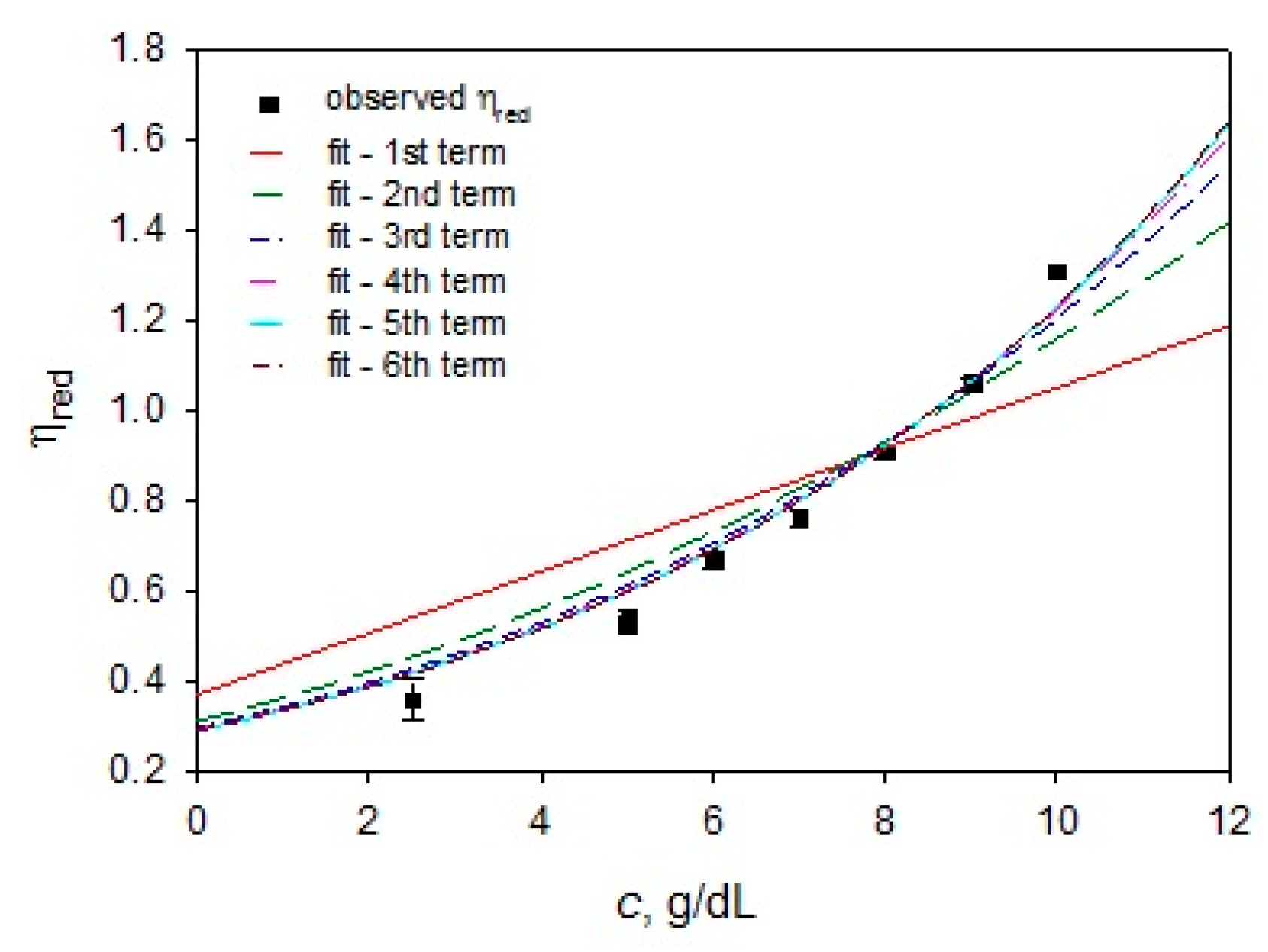
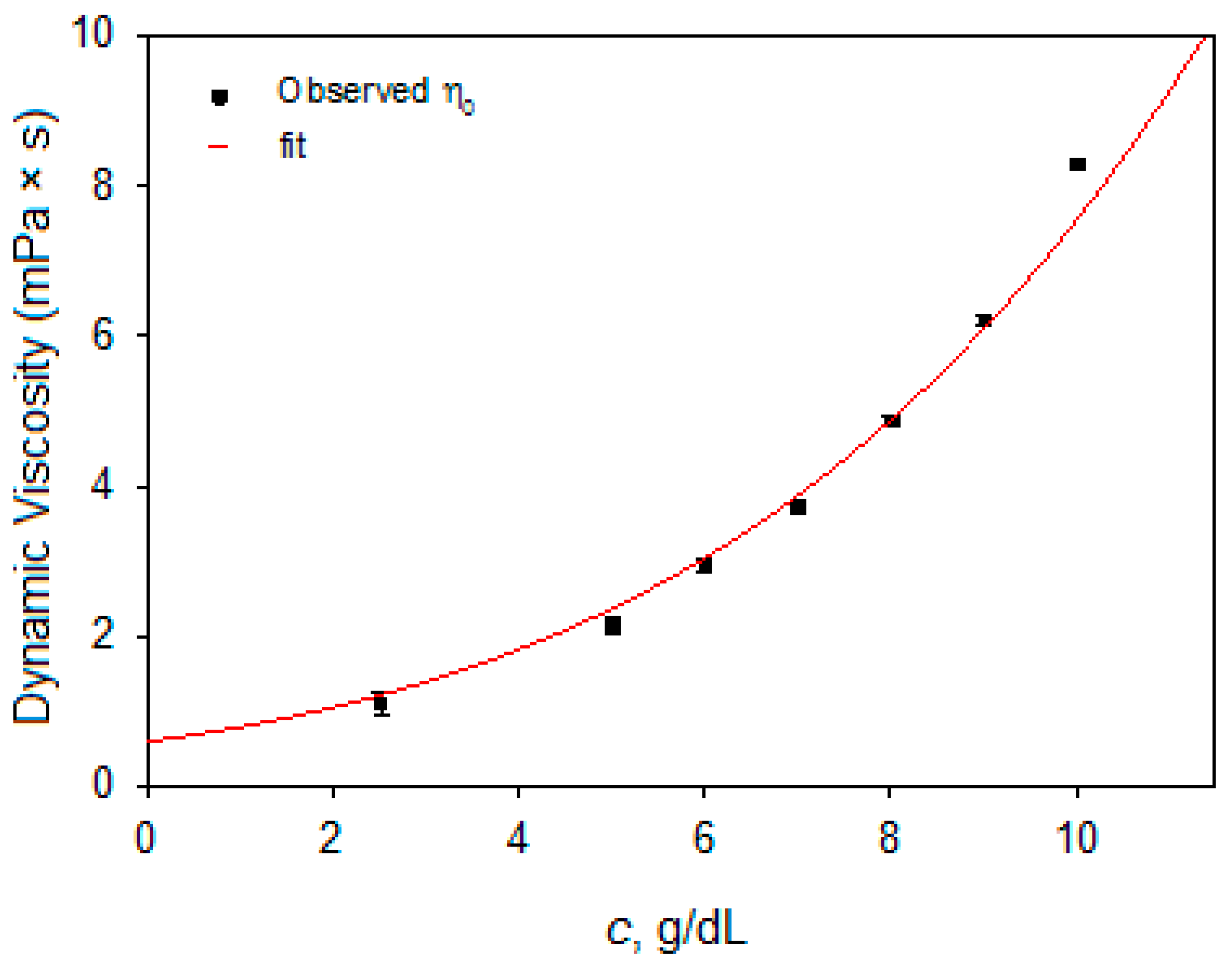
3.2. PMMA Solution Characterization
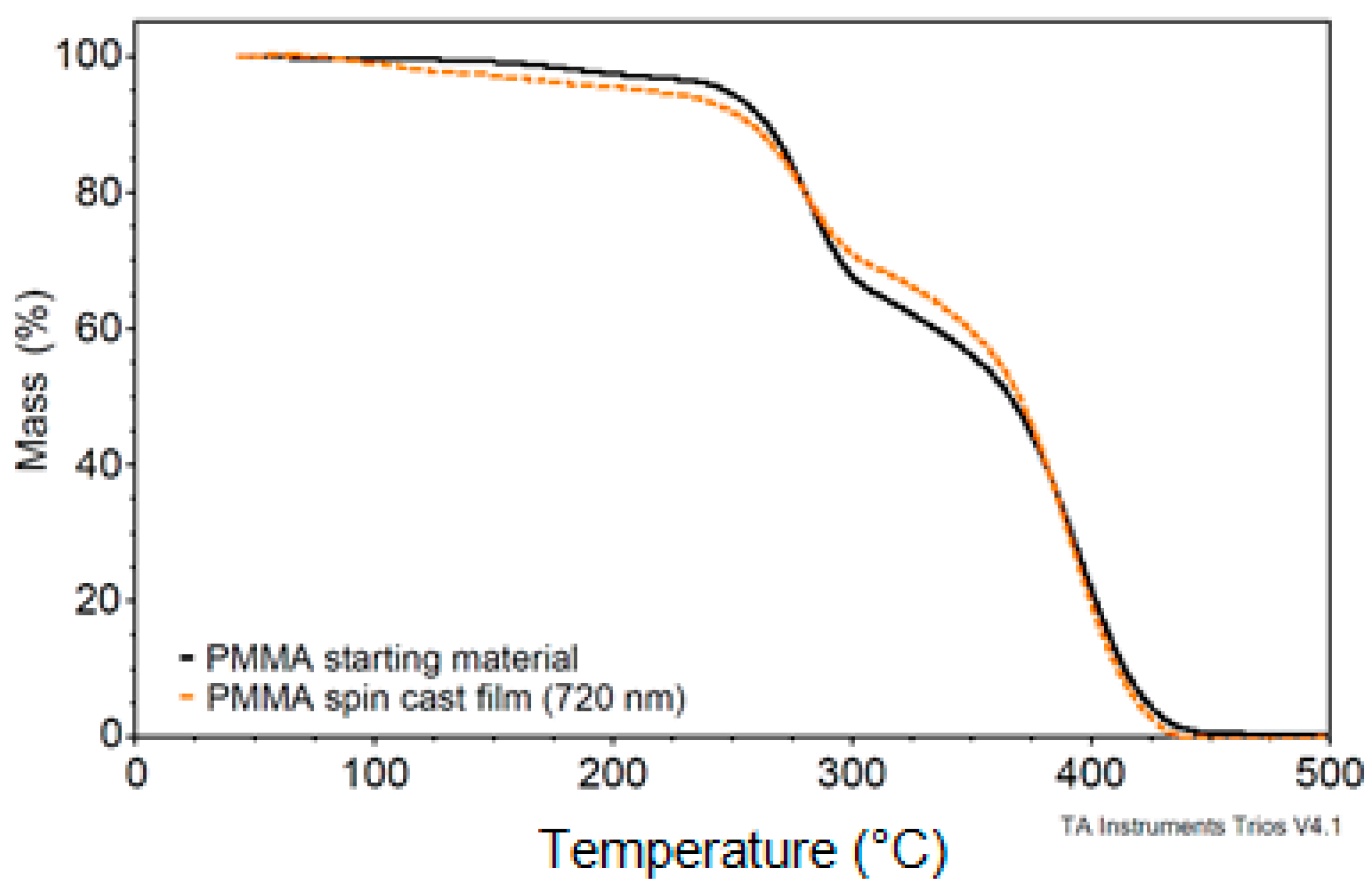
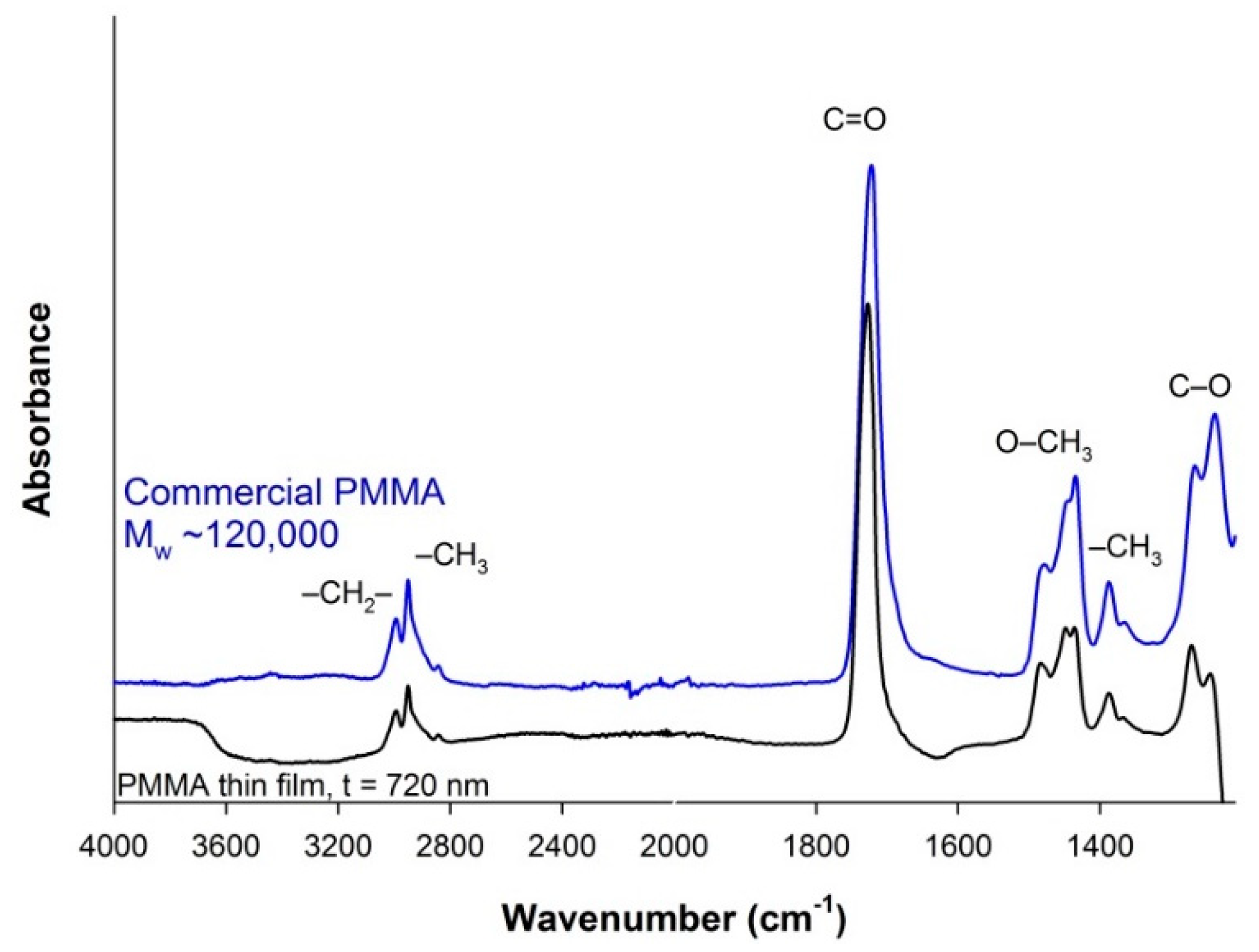
3.3. PMMA Thin Film Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lalova, A.; Todorov, R.; Malinowski, A.J. Optical properties of thin PMMA films for sensor application. Bulg. Chem. Commun. 2015, 47, 29–34. [Google Scholar]
- Martínez, V.M.; Arbeloa, F.L.; Prieto, J.B.; Arbeloa, I.L. Characterization of rhodamine 6G aggregates intercalated in solid thin films of laponite clay. 2 fluorescence spectroscopy. J. Phys. Chem. B 2005, 109, 7443–7450. [Google Scholar] [CrossRef]
- Danglad-Flores, J.; Eickelmann, S.; Riegler, H. Deposition of polymer films by spin casting: A quantitative analysis. Chem. Eng. Sci. 2018, 179, 257–264. [Google Scholar] [CrossRef]
- Mouhamad, Y.; Mokarian-Tabari, P.; Clarke, N.; Jones, R.A.L.; Geoghegan, M. Dynamics of polymer film formation during spin coating. J. Appl. Phys. 2014, 116, 123513. [Google Scholar] [CrossRef]
- Bistac, S.; Schultz, J. Study of solution-cast films of PMMA by dielectric spectroscopy: Influence of the nature of the solvent on α and β relaxations. Int. J. Adhes. Adhes. 1997, 17, 197–201. [Google Scholar] [CrossRef]
- Hall, D.B.; Underhill, P.; Torkelson, J.M. Spin coating of thin and ultrathin polymer films. Polym. Eng. Sci. 1998, 38, 2039–2045. [Google Scholar] [CrossRef]
- Patra, N.; Barone, A.C.; Salerno, M. Solvent effects on the thermal and mechanical properties of poly(methyl methacrylate) casted from concentrated solutions. Adv. Polym. Technol. 2011, 30, 12–20. [Google Scholar] [CrossRef]
- Van Dyk, J.W.; Frisch, H.L.; Wu, D.T. Solubility, solvency, and solubility parameters. Ind. Eng. Chem. Prod. Res. Dev. 1985, 24, 473–478. [Google Scholar] [CrossRef]
- Vashishtha, S.R.; Chand, N.; Hashmi, S.A.R. Morphology of PS/PMMA blends and their solution rheology. Indian J. Chem. Technol. 2002, 9, 316–323. [Google Scholar]
- Zhang, H.Q.; Euler, W.B. Detection of gas-phase explosive analytes using fluorescent spectroscopy of thin films of xanthene dyes. Sens. Actuators B. Chem. 2016, 225, 553–562. [Google Scholar] [CrossRef]
- Penzkofer, A.; Drotleff, E.; Holzer, W. Optical constants measurement of single-layer thin films on transparent substrates. Opt. Commun. 1998, 158, 221–230. [Google Scholar] [CrossRef]
- Emslie, A.G.; Bonner, F.T.; Peck, L.G. Flow of a viscous liquid on a rotating disk. J. Appl. Phys. 1958, 29, 858–862. [Google Scholar] [CrossRef]
- Meyerhofer, D. Characteristics of resist films produced by spinning. J. Appl. Phys. 1978, 49, 3993–3997. [Google Scholar] [CrossRef]
- Haas, D.E.; Quijada, J.N.; Picone, S.J.; Birnie, D.P., III. Effect of solvent evaporation rate on skin formation during spin coating of complex solutions. In Sol-Gel Optics V; Dunn, B.S., Pope, E.J.A., Schmidt, H.K., Yamane, M., Eds.; SPIE: Bellingham, WA, USA, 2000; pp. 280–284. [Google Scholar] [CrossRef]
- Weissberg, S.G.; Simha, R.; Rothman, S. Viscosity of dilute to moderately concentrated polymer solutions. J. Res. Natl. Bur. Stand. 1951, 47, 298–314. [Google Scholar] [CrossRef]
- Matsuoka, S.; Cowman, M.K. Equation of state for polymer solution. Polymer 2002, 43, 3447–3453. [Google Scholar] [CrossRef]
- Patrone, E.; Bianchi, U. Viscosity-molecular weight relationships for low molecular weight polymers. II. poly(vinyl acetate) and poly(methyl methacrylate). Die Makromol. Chem. 1966, 94, 52–59. [Google Scholar] [CrossRef]
- Brandrup, J.; Immergut, E.H.; Grulke, E.A.; Abe, A.; Bloch, D.R.; Wiley, J.; Chichester, N.Y.; Brisbane, W.; Toronto, S. Polymer Handbook, 4th ed.; Wiley-Interscience: Hoboken, NJ, USA, 1999. [Google Scholar]
- Cohn-Ginsberg, E.; Fox, T.; Mason, H. Properties of dilute polymer solutions II: Light scattering and viscometric properties of solutions of conventional polymethyl methacrylate. Polymer 1962, 3, 97–109. [Google Scholar] [CrossRef]
- Moore, W.R.; Fort, R.J. Viscosities of dilute solutions of polymethyl methacrylate. J. Polym. Sci. Part A Gen. Pap. 1963, 1, 929–942. [Google Scholar] [CrossRef]
- Matsuda, H.; Yamano, K.; Inagaki, H. Styrene-methyl acrylate copolymers and acrylate homopolymers in solution. J. Polym. Sci. Part A-2 Polym. Phys. 1969, 7, 609–633. [Google Scholar] [CrossRef]
- Yamamoto, H. Hansen Solubility Parameter(HSP)-HSP Application Note #2. 2009. Available online: https://pirika.com/NewHP/PirikaE/polymer-solvent.html (accessed on 28 December 2020).
- Hansen, C.; Hansen, K. Solubility parameter prediction of the barrier properties of chemical protective clothing. In Performance of Protective Clothing: Second Symposium; ASTM International: West Conshohocken, PA, USA, 1988; pp. 197–208. [Google Scholar] [CrossRef]
- Hansen, C.M. Hansen Solubility Parameters: A User’s Handbook, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Mokarian-Tabari, P.; Geoghegan, M.; Howse, J.R.; Heriot, S.Y.; Thompson, R.L.; Jones, R.A.L. Quantitative evaluation of evaporation rate during spin-coating of polymer blend films: Control of film structure through defined-atmosphere solvent-casting. Eur. Phys. J. E 2010, 33, 283–289. [Google Scholar] [CrossRef]
- Gałka, P.; Kowalonek, J.; Kaczmarek, H. Thermogravimetric analysis of thermal stability of poly(methyl methacrylate) films modified with photoinitiators. J. Therm. Anal. Calorim. 2014, 115, 1387–1394. [Google Scholar] [CrossRef]
- Ferriol, M.; Gentilhomme, A.; Cochez, M.; Oget, N.; Mieloszynski, J.L. Thermal degradation of poly(methyl methacrylate) (PMMA): Modelling of DTG and TG curves. Polym. Degrad. Stab. 2003, 79, 271–281. [Google Scholar] [CrossRef]
- Kashiwagi, T.; Inaba, A.; Brown, J.E.; Hatada, K.; Kitayama, T.; Masuda, E. Effects of weak linkages on the thermal and oxidative degradation of poly(methyl methacrylates). Macromolecules 1986, 19, 2160–2168. [Google Scholar] [CrossRef]
- Peng, J.; Yang, F.; Chiang, D.; Lee, S. Kinetics of field-induced surface patterns on PMMA. Langmuir 2016, 32, 4602–4609. [Google Scholar] [CrossRef] [PubMed]
- Chapman, M.; Mullen, M.; Novoa-Ortega, E.; Alhasani, M.; Elman, J.F.; Euler, W.B. Structural evolution of ultrathin films of rhodamine 6G on glass. J. Phys. Chem. C 2016, 120, 8289–8297. [Google Scholar] [CrossRef]
| [η] (dL/g) | MMH (10−3 mL/g) | α | T (°C) | Measurement | Reference |
|---|---|---|---|---|---|
| 0.292 ± 0.031 | – | – | 20 | Viscosity | this work |
| 0.28 | 7.0 | 0.71 | 30 | Viscosity | [20] |
| 0.33 | 8.12 | 0.71 | 25 | Osmometry | [21] |
| 0.27 | 7.79 | 0.697 | 25 | light scattering | [19] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chapman, N.; Chapman, M.; Euler, W.B. Modeling of Poly(methylmethacrylate) Viscous Thin Films by Spin-Coating. Coatings 2021, 11, 198. https://doi.org/10.3390/coatings11020198
Chapman N, Chapman M, Euler WB. Modeling of Poly(methylmethacrylate) Viscous Thin Films by Spin-Coating. Coatings. 2021; 11(2):198. https://doi.org/10.3390/coatings11020198
Chicago/Turabian StyleChapman, Navid, Mingyu Chapman, and William B. Euler. 2021. "Modeling of Poly(methylmethacrylate) Viscous Thin Films by Spin-Coating" Coatings 11, no. 2: 198. https://doi.org/10.3390/coatings11020198
APA StyleChapman, N., Chapman, M., & Euler, W. B. (2021). Modeling of Poly(methylmethacrylate) Viscous Thin Films by Spin-Coating. Coatings, 11(2), 198. https://doi.org/10.3390/coatings11020198






