Superhydrophobic Sands for the Preservation and Purification of Water
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Sand
2.2. TiO2 Sol-Gel Coating
2.3. ZnO Hydrothermal Coating
2.4. Hydrophobic Coating
3. Results and Discussion
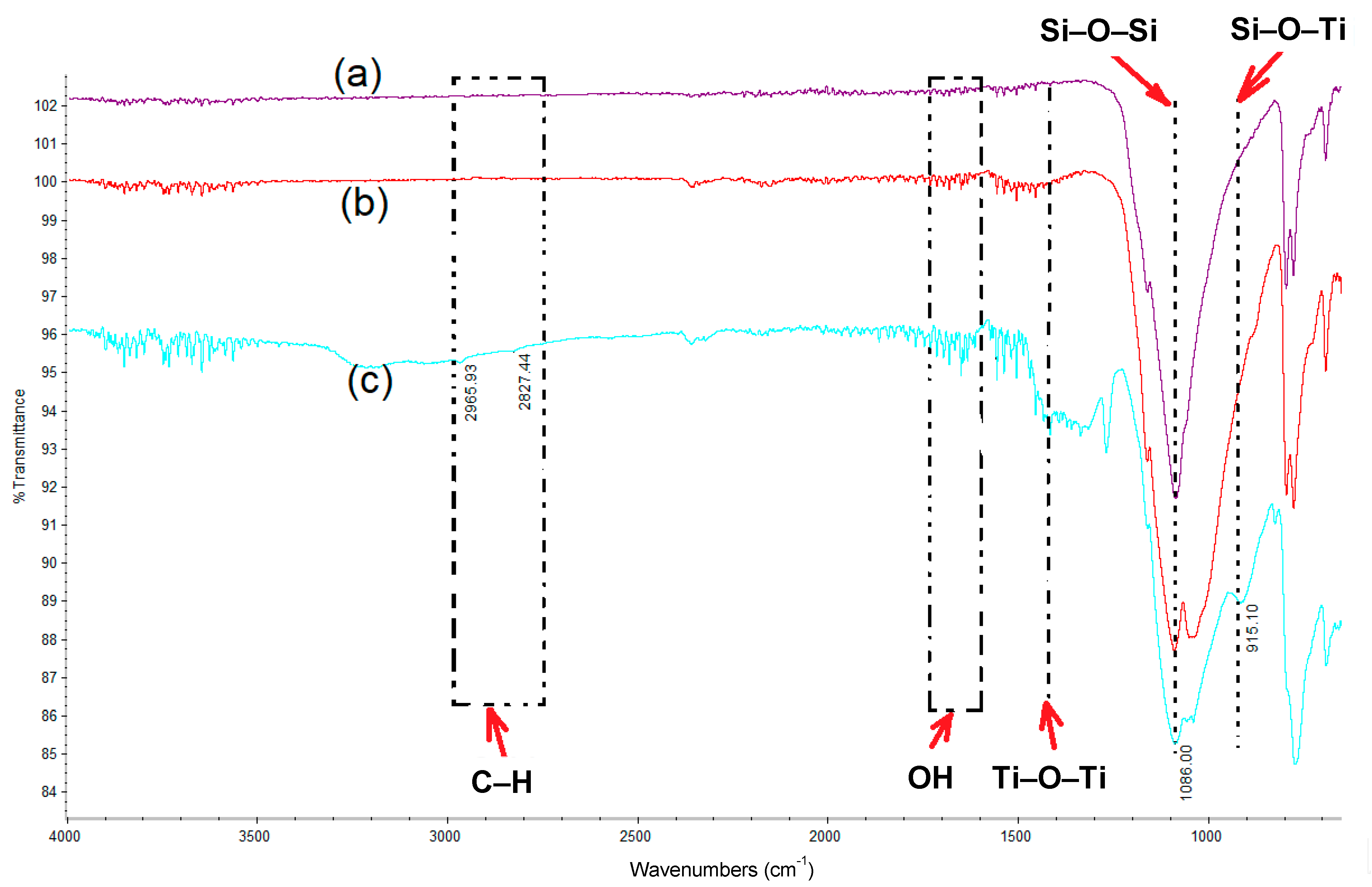
3.1. Fourier-Transform Infrared Spectroscopy (FTIR) Analysis
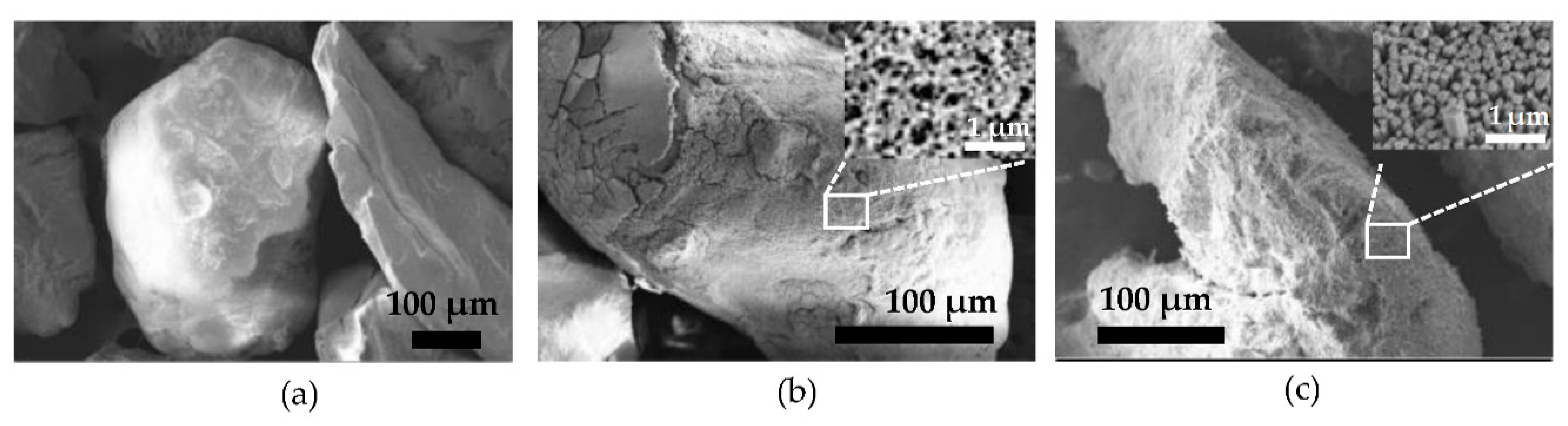
3.2. Surface Morphology
3.3. Superhydrophobicity
3.4. Gateable Property for Water Penetration
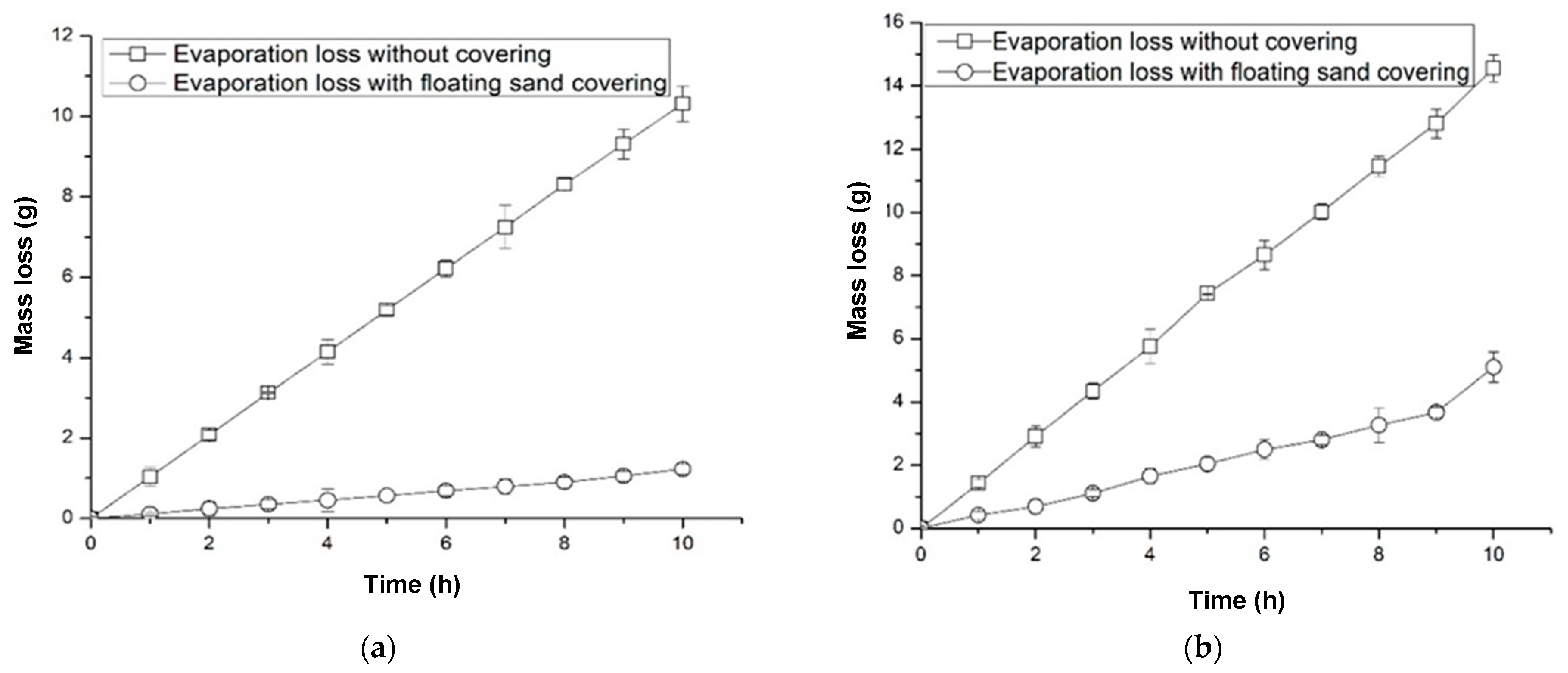
3.5. Reduction in Evaporation Loss
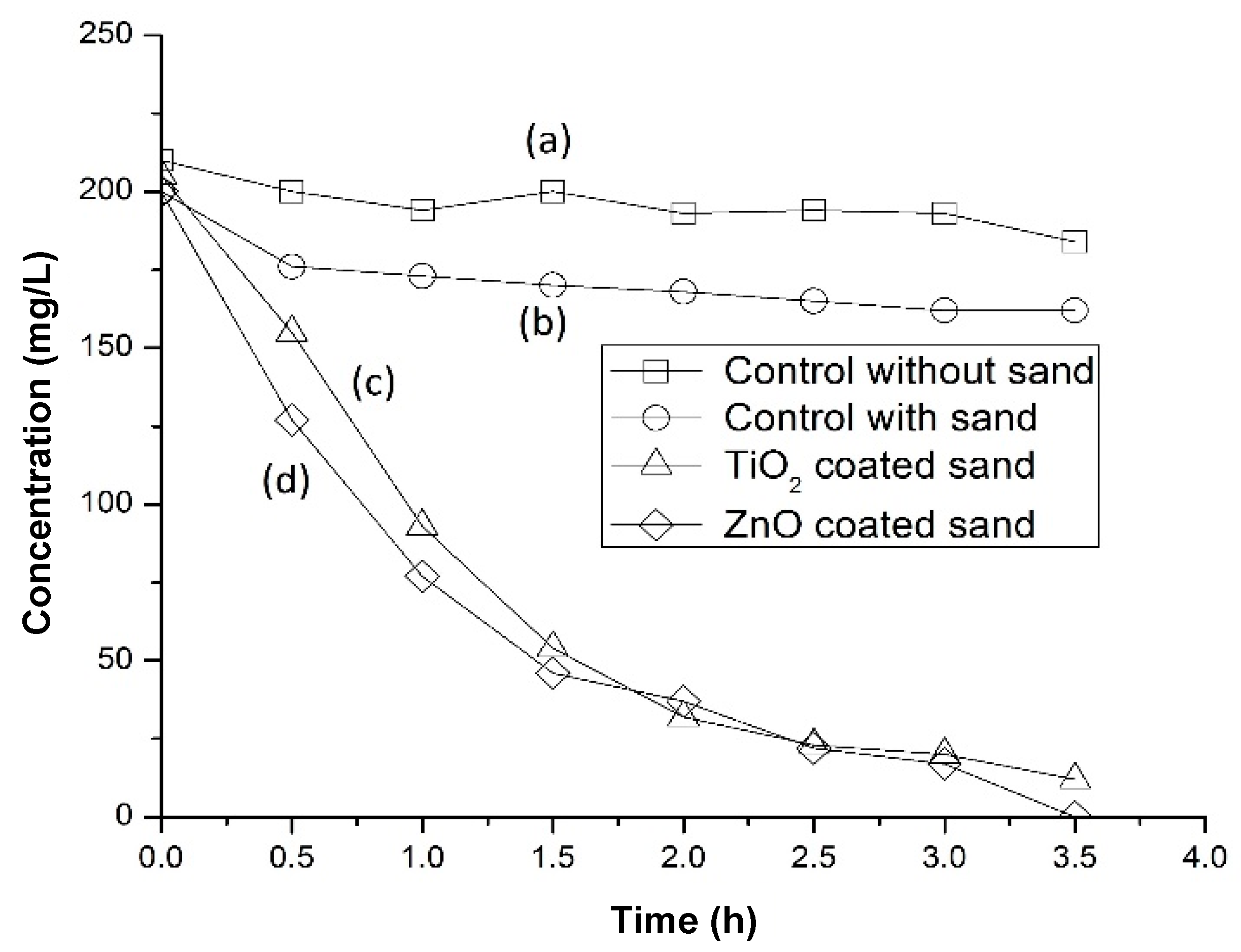
3.6. Photocatalytic Water Purification
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gleick, P.H. The World’s Water; Island Press: Washington, DC, USA, 2011; Volume 3. [Google Scholar]
- Craig, I.; Green, A.; Scobie, M.; Schmidt, E. Controlling Evaporation Loss from Water Storages; National Center for Engineering in Agriculture Publication: Toowoomba, Australia, 2005.
- Assouline, S.; Narkis, K.; Or, D. Evaporation suppression from water reservoirs: Efficiency considerations of partial covers. Water Resour. Res. 2011, 47, W07506. [Google Scholar] [CrossRef]
- Yao, X.; Zhang, H.; Lemckert, C.; Brook, A.; Schouten, P. Evaporation Reduction by Suspended and Floating Covers: Over-View, Modelling and Efficiency; Technical Report No. 28; Urban Water Security Research Alliance: Brisbane, Australia, 2011. [Google Scholar]
- Mittal, D.; Saxena, B.K.; Rao, K.V.S. Potential of floating photovoltaic system for energy generation and reduction of water evaporation at four different lakes in Rajastha. In Proceedings of the 2017 International Conference On Smart Technologies For Smart Nation (SmartTechCon), Bangalore, India, 17–19 August 2017; pp. 238–243. [Google Scholar]
- La Mer, V.K. Retardation of Evaporation by Monolayers; Elsevier Inc.: New York, NY, USA, 1962. [Google Scholar]
- Roberts, W.J. Evaporation suppression from water surfaces. Trans. Am. Geophys. Union 1957, 38, 740–744. [Google Scholar] [CrossRef]
- Roberts, W.J. Reducing lake evaporation in the midwest. J. Geophys. Res. 2012, 64, 1605–1610. [Google Scholar] [CrossRef]
- Frenkiel, J. Evaporation Reduction: Physical and Chemical Principles and Review of Experiments; UNESCO: Paris, France, 1965.
- Barnes, G. The potential for monolayers to reduce the evaporation of water from large water storages. Agric. Water Manag. 2008, 95, 339–353. [Google Scholar] [CrossRef]
- Henry, D.J.; Dewan, V.I.; Prime, E.L.; Qiao, G.G.; Solomon, D.H.; Yarovsky, I. Monolayer structure and evaporation resistance: A molecular dynamics study of octadecanol on water. J. Phys. Chem. B 2010, 114, 3869–3878. [Google Scholar] [CrossRef] [PubMed]
- Mansfield, W.W. Influence of monolayers on the natural rate of evaporation of water. Nature 1955, 175, 247–249. [Google Scholar] [CrossRef]
- Aminzadeh, M. Evaporation suppression and energy balance of water reservoirs covered with self-assembling floating elements. Hydrol. Earth Syst. Sci. 2018, 22, 4015–4032. [Google Scholar] [CrossRef]
- Simko, A.J.; Dressler, R.G. Investigation of C20 to C25 fatty alcohols and blends as water evaporation retardants. Ind. Eng. Chem. Prod. Res. Dev. 1969, 8, 446–450. [Google Scholar] [CrossRef]
- Fukuda, K.; Kato, T.; Machida, S.; Shimizu, Y. Binary mixed monolayers of polyvinyl stearate and simple long-chain compounds at the air/water interface. J. Colloid Interface Sci. 1979, 68, 82–95. [Google Scholar] [CrossRef]
- Wenzel, R.N. Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]
- Lafuma, A.; Quéré, D. Superhydrophobic states. Nat. Mater. 2003, 2, 457–460. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.-D.; Nakajima, A.; Fujishima, A.; Watanabe, T.; Hashimoto, K. Photoinduced surface wettability conversion of ZnO and TiO2 thin films. J. Phys. Chem. B 2001, 105, 1984–1990. [Google Scholar] [CrossRef]
- Fujishima, A.; Zhang, X.; Tryk, D.A. TiO2 photocatalysis and related surface phenomena. Surf. Sci. Rep. 2008, 63, 515–582. [Google Scholar] [CrossRef]
- Wang, R.; Hashimoto, K.; Fujishima, A.; Chikuni, M.; Kojima, E.; Kitamura, A.; Shimohigoshi, M.; Watanabe, T. Light-induced amphiphilic surfaces. Nature 1997, 388, 431–432. [Google Scholar] [CrossRef]
- Sakai, N.; Fujishima, A.; Watanabe, T.; Hashimoto, K. Quantitative evaluation of the photoinduced hydrophilic conversion properties of TiO2 thin film surfaces by the reciprocal of contact angle. J. Phys. Chem. B 2003, 107, 1028–1053. [Google Scholar] [CrossRef]
- Machida, M.; Norimoto, K.; Watanabe, T.; Hashimoto, K.; Fujishima, A. The effect of SiO2 addition in super-hydrophilic property of TiO2 photocatalyst. J. Mater. Sci. 1999, 34, 2569–2574. [Google Scholar] [CrossRef]
- Gao, W.; Majumder, M.; Alemany, L.B.; Narayanan, T.N.; Ibarra, M.A.; Pradhan, B.K.; Ajayan, P.M. Engineered graphite oxide materials for application in water purification. ACS Appl. Mater. Interfaces 2011, 3, 1821–1826. [Google Scholar] [CrossRef]
- Hoffman, J.F.; Vergara, V.B.; Mog, S.R.; Kalinich, J.F. Hydrophobic sand is a non-toxic method of urine collection, appropriate for urinary metal analysis in the rat. Toxics 2017, 5, 25. [Google Scholar] [CrossRef]
- Chen, L.; Si, Y.; Guo, Z.; Liu, W. Superhydrophobic sand: A hope for desert water storage and transportation projects. J. Mater. Chem. A 2017, 5, 6416–6423. [Google Scholar] [CrossRef]
- Chen, L.; Wu, Y.; Guo, Z. Superhydrophobic sand grains structured with aligned Cu(OH)2 nano-needles for efficient oily water treatment. Mater. Des. 2017, 135, 377–384. [Google Scholar] [CrossRef]
- Liu, S.; Cai, T.; Shen, X.; Huang, E.; Wang, Z.; Sun, Q. Superhydrophobic sand with multifunctionalities by TiO2-incorporated mussel-inspired polydopamine. Ceram. Int. 2019, 45, 21263–21269. [Google Scholar] [CrossRef]
- Qi, K.; Chen, X.; Liu, Y.; Xin, J.H.; Mak, C.L.; Daoud, W.A. Facile preparation of anatase/SiO2 spherical nanocomposites and their application in self-cleaning textiles. J. Mater. Chem. 2007, 17, 3504–3508. [Google Scholar] [CrossRef]
- Ebert, D.; Bhushan, B. Transparent, superhydrophobic, and wear-resistant coatings on glass and polymer substrates using SiO2, ZnO, and ITO nanoparticles. Langmuir 2012, 28, 11391–11399. [Google Scholar] [CrossRef]
- Srinivasan, U.; Houston, M.; Howe, R.T.; Maboudian, R. Alkyltrichlorosilane-based self-assembled monolayer films for stiction reduction in silicon micromachines. J. Microelectromechanical Syst. 1998, 7, 252–260. [Google Scholar] [CrossRef]
- Mattle, M.J.; Thampi, K.R. Photocatalytic degradation of Remazol Brilliant Blue® by sol–gel derived carbon-doped TiO2. Appl. Catal. B Environ. 2013, 140–141, 348–355. [Google Scholar] [CrossRef]
- Tsai, C.-Y.; Kuo, T.-H.; Hsi, H.-C. Fabrication of Al-doped TiO2 visible-light photocatalyst for low-concentration mercury removal. Int. J. Photoenergy 2012, 2012, 1–8. [Google Scholar] [CrossRef]
- Dimapilis, E.A.; Hsu, C.S.; Mendoza, R.M.; Lu, M.C. Zinc oxide nanoparticles for water disinfection. Sustain. Environ. Res. 2018, 28, 47–56. [Google Scholar]
- Stylidi, M.; Kondarides, D.I.; Verykios, X.E. Mechanistic and kinetic study of solar-light induced photocatalytic degradation of Acid Orange 7 in aqueous TiO2 suspensions. Int. J. Photoenergy 2003, 5, 59–67. [Google Scholar] [CrossRef]
- Lu, Y.; Xiao, S.; Gao, R.; Li, J.; Sun, Q. Improved weathering performance and wettability of wood protected by CeO2 coating deposited onto the surface. Holzforschung 2014, 68, 345–351. [Google Scholar] [CrossRef]
- Buzea, C.; Pacheco, I.I.; Robbie, K. Nanomaterials and nanoparticles: Sources and toxicity. Biointerphases 2007, 2, MR17–MR71. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Xie, H. Nanoparticles in daily life: Applications, toxicity and regulations. J. Environ. Pathol. Toxicol. Oncol. 2018, 37, 209–230. [Google Scholar] [CrossRef] [PubMed]
- Sengul, A.B.; Asmatulu, E. Toxicity of metal and metal oxide nanoparticles: A review. Environ. Chem. Lett. 2020, 18, 1659–1683. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Choi, C.-H. Superhydrophobic Sands for the Preservation and Purification of Water. Coatings 2021, 11, 151. https://doi.org/10.3390/coatings11020151
Liu Y, Choi C-H. Superhydrophobic Sands for the Preservation and Purification of Water. Coatings. 2021; 11(2):151. https://doi.org/10.3390/coatings11020151
Chicago/Turabian StyleLiu, Yuyang, and Chang-Hwan Choi. 2021. "Superhydrophobic Sands for the Preservation and Purification of Water" Coatings 11, no. 2: 151. https://doi.org/10.3390/coatings11020151
APA StyleLiu, Y., & Choi, C.-H. (2021). Superhydrophobic Sands for the Preservation and Purification of Water. Coatings, 11(2), 151. https://doi.org/10.3390/coatings11020151





