Evaluation of Antioxidant Activities, Oxidation Enzymes, and Quality of Nano-Coated Button Mushrooms (Agaricus Bisporus) during Storage
Abstract
1. Introduction
2. Materials and Methods
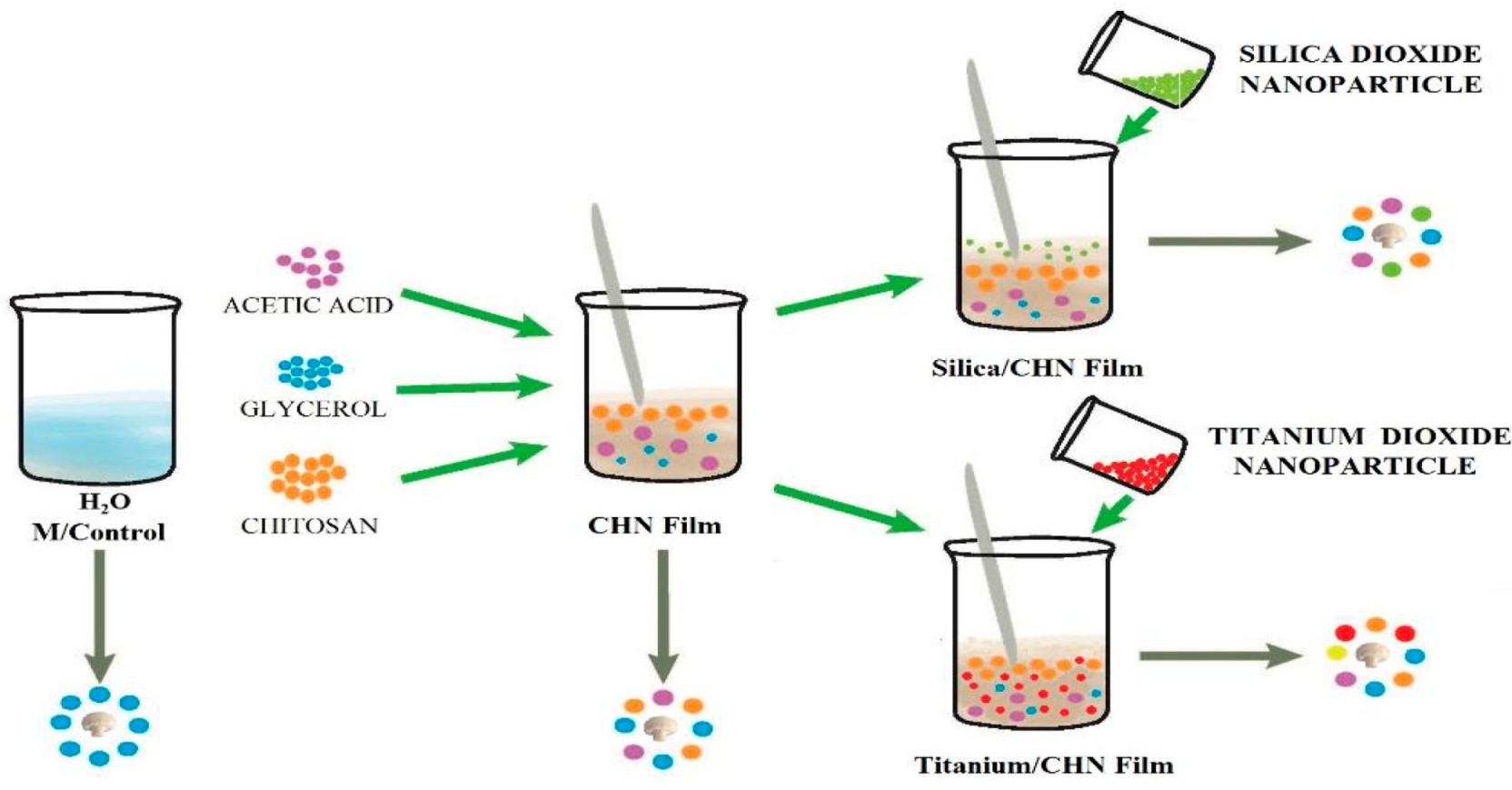
2.1. Materials and Films Preparation
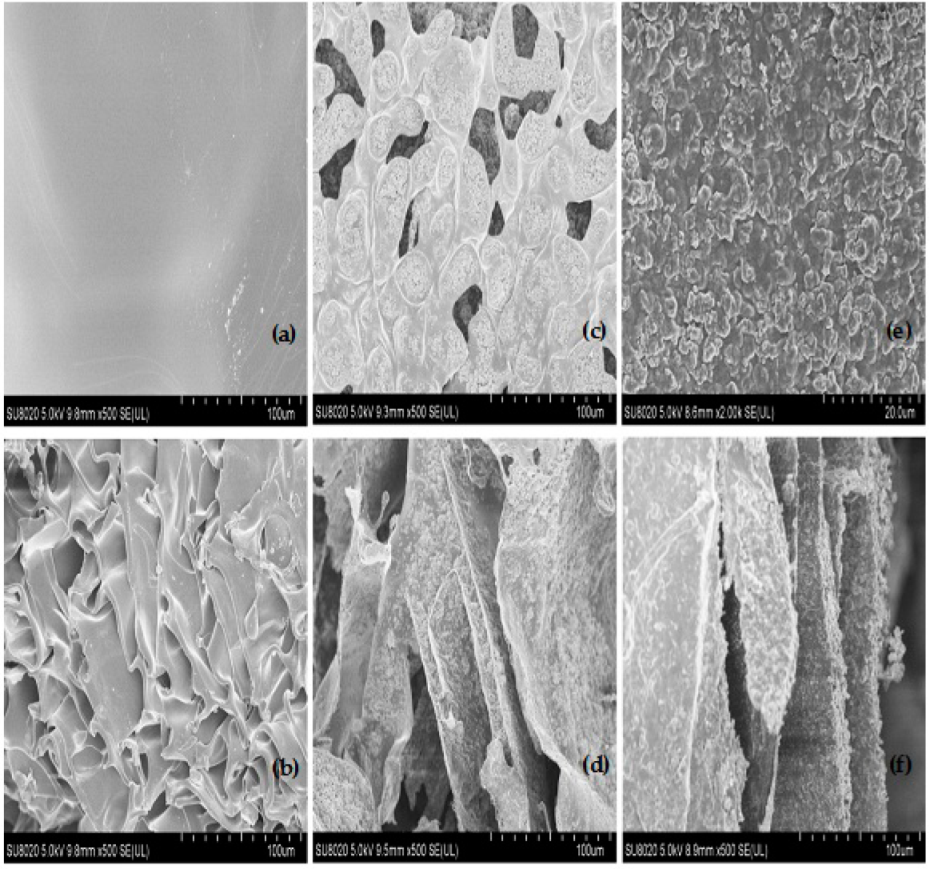
2.2. Scanning Electron Microscopy (SEM) Analysis
2.3. Mushrooms, Treatments, and Storage
2.4. Measurement of CO2 Respiration Rate
2.5. Measurement of O2 Production Rate
2.6. Bioactive Component and Antioxidant Activities
2.6.1. Total Polyphenols
2.6.2. Free Radical Scavenging Activity (DPPH) Assay
2.6.3. ABTS+ Radical Scavenging Assay
2.7. Oxidation Enzymes Determinations
2.8. Reactive Oxygen Species (ROS) Production
2.8.1. Hydrogen Peroxide (H2O2) Determination
2.8.2. Hydroxyl Radical (–OH) Determination
2.9. Thiobarbituric Acid-Reactive Substances (TBARS) Determination
2.10. Statistical Analysis
3. Results and Discussion
3.1. SEM Analysis
3.2. CO2 Respiration Rate
3.3. O2 Production Rate
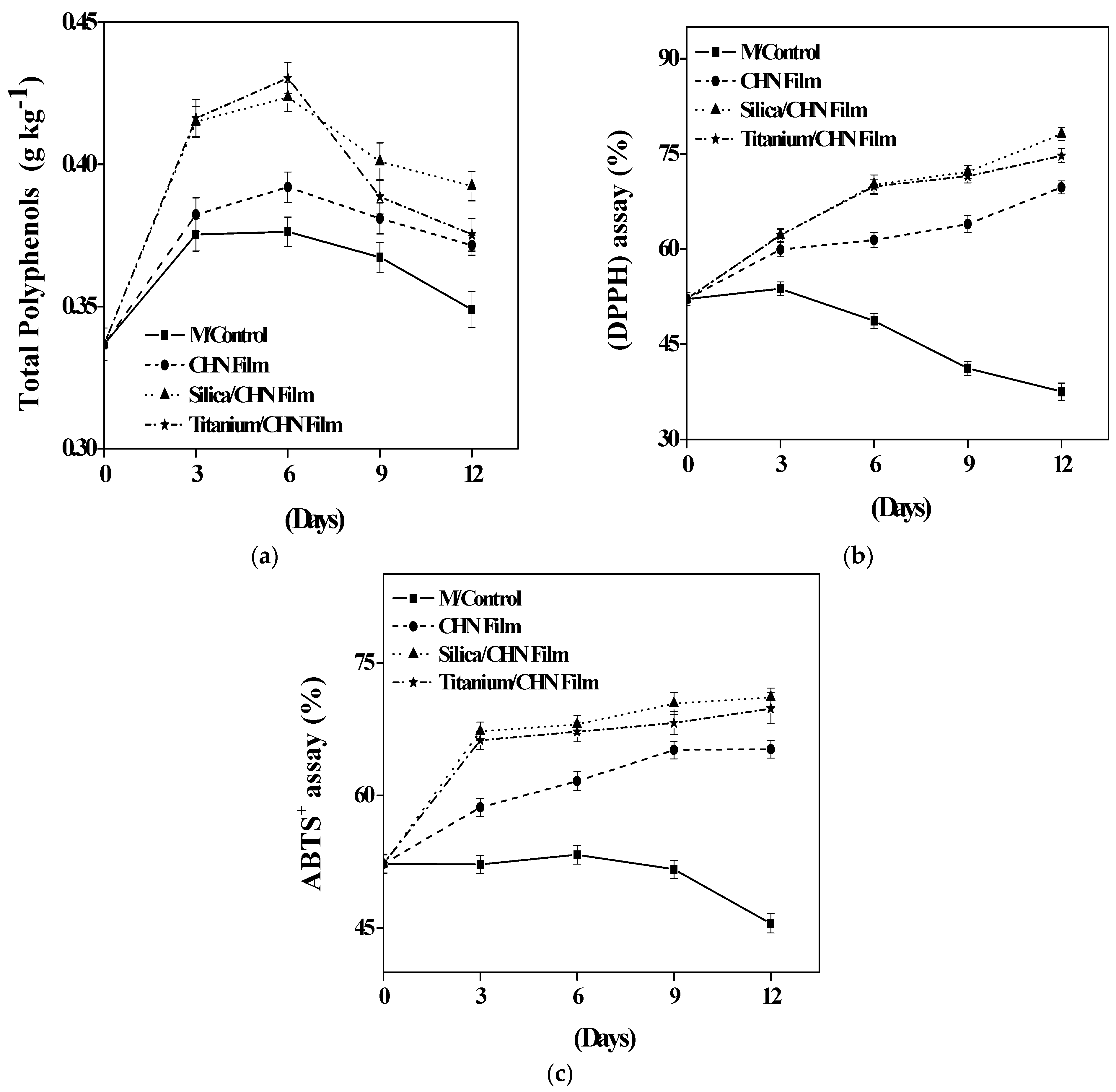
3.4. Total Polyphenols
3.5. DPPH Scavenging Activity
3.6. ABTS+ Radical Scavenging
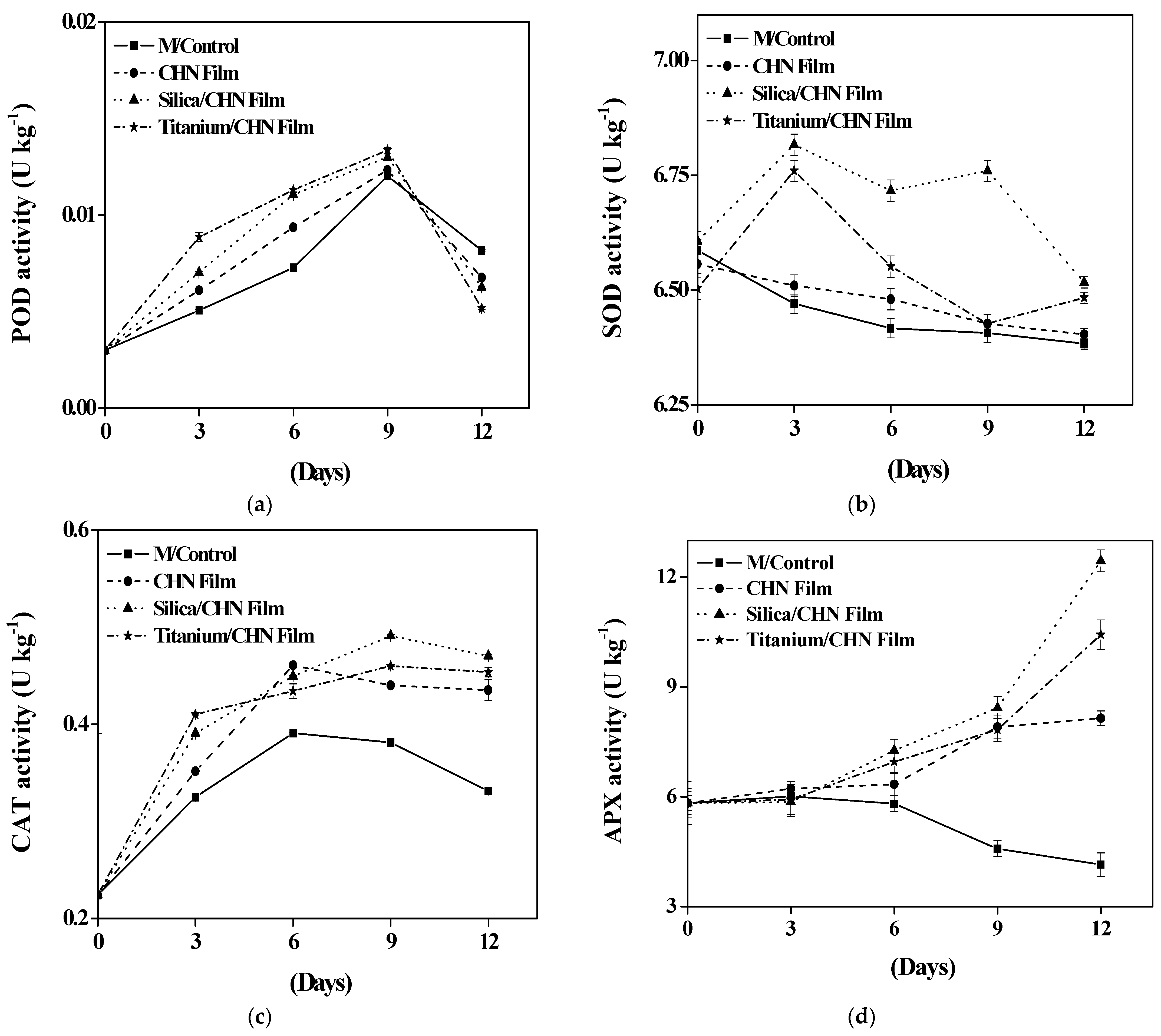
3.7. Effect of Coating on Oxidative Enzyme Activities
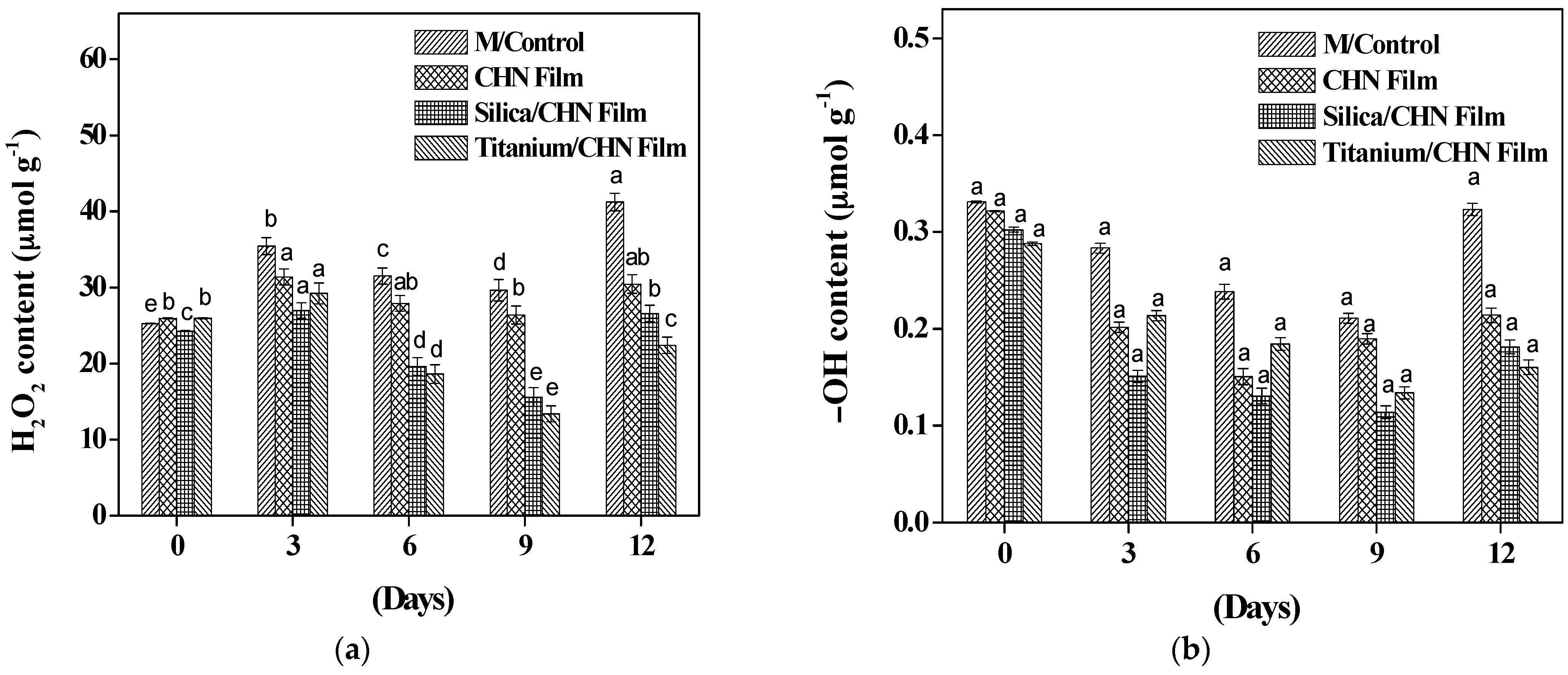
3.8. Effect of Coating on ROS Production
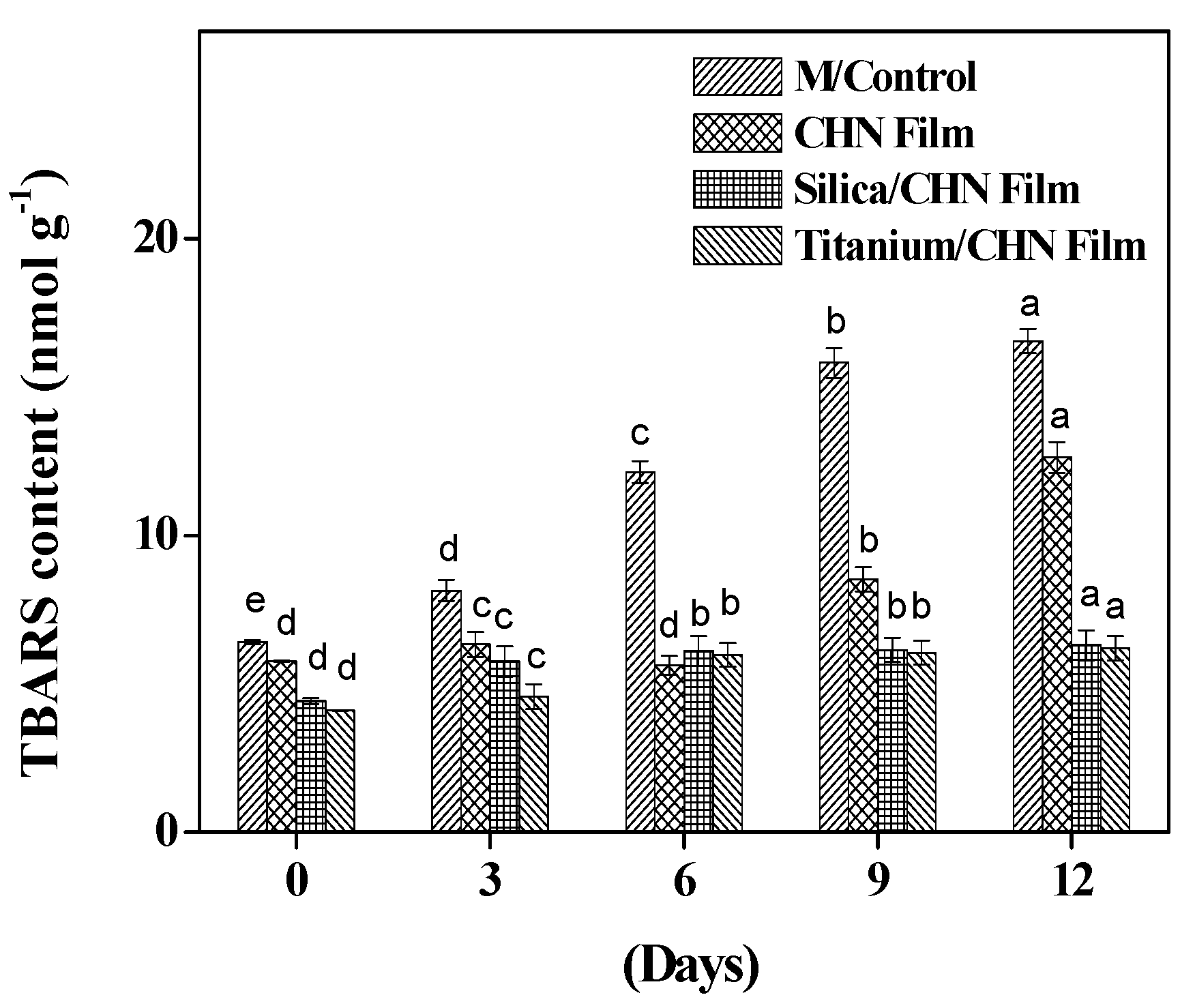
3.9. TBARS in Mushrooms
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sami, R.; Khojah, E.; Elhakem, A.; Benajiba, N.; Chavali, M.; Vivek, K.; Iqbal, A.; Helal, M. Investigating the nano-films effect on physical, mechanical properties, chemical changes, and microbial load contamination of white button mushrooms during storage. Coatings 2021, 11, 44. [Google Scholar]
- Sami, R.; Elhakem, A.; Alharbi, M.; Benajiba, N.; Almatrafi, M.; Jing, J.; Helal, M. Effect of Titanium Dioxide Nanocomposite Material and Antimicrobial Agents on Mushrooms Shelf-Life Preservation. Processes 2020, 8, 1632. [Google Scholar] [CrossRef]
- Singh, N.; Vaidya, D.; Mishra, V.; Thakur, K. Shelf life and storage quality of white button mushrooms (Agaricus bisporus) as affected by packaging material. Int. J. Adv. Res 2016, 4, 1790–1799. [Google Scholar] [CrossRef]
- Sedaghat, N.; Vahedi, N. Evaluation of several methods for preservation and packaging of the button white mushroom to increase its shelf life. Iran. Food Sci. Technol. 2015, 11, 22–30. [Google Scholar]
- Gholami, R.; Ahmadi, E.; Farris, S. Shelf life extension of white mushrooms (Agaricus bisporus) by low temperatures conditioning, modified atmosphere, and nanocomposite packaging material. Food Packag. Shelf Life 2017, 14, 88–95. [Google Scholar] [CrossRef]
- Qiao, G.; Xiao, Z.; Ding, W.; Sami, R. Effect of Chitosan/Nano-Titanium Dioxide/Thymol and Tween Films on Ready-to-Eat Cantaloupe Fruit Quality. Coatings 2019, 9, 828. [Google Scholar] [CrossRef]
- Srivastava, P.; Prakash, P.; Bunkar, D. Enhancement in physiological and sensory attributes of button mushroom (Agaricus bisporus) as influenced by chemical and modified atmospheric packaging (MAP) treatments at low temperature storage. Int. J. Chem. Stud 2020, 8, 2059–2064. [Google Scholar] [CrossRef]
- Mirshekari, A.; Madani, B.; Golding, J.B. Aloe vera gel treatment delays postharvest browning of white button mushroom (Agaricus bisporus). J. Food Meas. Charact. 2019, 13, 1250–1256. [Google Scholar] [CrossRef]
- Sami, R.; Jia, F.; Li, Y.; Nie, X.; Xu, J.; Han, R.; Yu, H.; Amanullah, S.; Almatrafi, M.M.; Helal, M. Application of nano-titanum dioxide coating on fresh Highbush blueberries shelf life stored under ambient temperature. LWT 2020, 110422. [Google Scholar]
- Maneerat, C.; Hayata, Y. Antifungal activity of TiO2 photocatalysisagainst Penicilliumexpansum in vitro and in fruit tests. Int. J. Food Microbiol. 2006, 107, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Sami, R.; Khojah, E.; Elhakem, A.; Benajiba, N.; Helal, M. Chitosan, Nisin, Silicon Dioxide nanoparticles coating films effects on blueberry (Vaccinium myrtillus) quality. Coatings 2020, 10, 962. [Google Scholar]
- Li, Y.; Sami, R.; Jia, F.; Nie, X.; Xu, J.; Elhakem, A.; Almatrafi, M.; Benajiba, N.; Helal, M. Shelf-Life, Quality, Safety Evaluations of Blueberry Fruits Coated with Chitosan Nano-Material Films. Sci. Rep. 2021, 11, 55. [Google Scholar] [CrossRef] [PubMed]
- Sakinah, M.; Misran, A.; Mahmud, T.M.; Abdullah, S.; Azhar, M. Evaluation of storage temperature, packaging system and storage duration on postharvest quality of straw mushroom (Volvariella volvacea). Food Res. 2020, 4, 679–689. [Google Scholar]
- Hu, Y.-H.; Chen, C.-M.; Xu, L.; Cui, Y.; Yu, X.-Y.; Gao, H.-J.; Wang, Q.; Liu, K.; Shi, Y.; Chen, Q.-X. Postharvest application of 4-methoxy cinnamic acid for extending the shelf life of mushroom (Agaricus bisporus). Postharvest Biol. Technol. 2015, 104, 33–41. [Google Scholar] [CrossRef]
- Peng, Y.; Li, T.; Jiang, H.; Gu, Y.; Chen, Q.; Yang, C.; Liang, Q.W.; Liu, S.-Q.; Zhang, X. Postharvest biochemical characteristics and ultrastructure of Coprinus comatus. PeerJ 2020, 8, e8508. [Google Scholar] [CrossRef] [PubMed]
- Qiao, G.; Wenxin, D.; Zhigang, X.; Sami, R.; Khojah, E.; Amanullah, S. Antioxidant and anti-inflammatory capacities of pepper tissues. Ital. J. Food Sci. 2020, 32. [Google Scholar] [CrossRef]
- Abeer, H.E.; Almatrafi, M.M.; Benajiba, N.; Koko, M.Y.; Sami, R. Comparative analysis of bioactive compounds, antioxidant and anti-inflammatory activities of apple varieties. Asian J. Plant Sci. 2021, 20, 61–66. [Google Scholar]
- Sami, R.; Li, C.-J.; Zhao, Y.; Li, Y.; Sun, C.-H. Cabbage (Brassica oleracea L. var. capitata) phytochemicals with antioxidant and anti-inflammatory potential. Asian Pac. J. Cancer Prev. 2013, 14, 6657–6662. [Google Scholar]
- Elhakem, A.H.; Benajiba, N.; Khojah, E.; Koko, M.Y.; Sami, R. DPPH, FRAP and TAEC Assays with postharvest cabbage (Brassica Oleracca) parameters during the packaging process. Pak. J. Agric. Sci. 2020, 24, 182–187. [Google Scholar]
- Liu, J.; Liu, S.; Zhang, X.; Kan, J.; Jin, C. Effect of gallic acid grafted chitosan film packaging on the postharvest quality of white button mushroom (Agaricus bisporus). Postharvest Biol. Technol. 2019, 147, 39–47. [Google Scholar] [CrossRef]
- Dokhanieh, A.Y.; Aghdam, M.S. Postharvest browning alleviation of Agaricus bisporus using salicylic acid treatment. Sci. Hortic. 2016, 207, 146–151. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Z.; Wang, X.; Dong, S.; Sun, Y.; Zhao, Z. The properties of chitosan/zein blend film and effect of film on quality of mushroom (Agaricus bisporus). Postharvest Biol. Technol. 2019, 155, 47–56. [Google Scholar] [CrossRef]
- Xu, Y.; Tian, Y.; Ma, R.; Liu, Q.; Zhang, J. Effect of plasma activated water on the postharvest quality of button mushrooms, Agaricus bisporus. Food Chem. 2016, 197, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Liu, D.; Wu, Y.; Yuan, M.; Li, L.; Yang, J. Effect of PLA/PCL/cinnamaldehyde antimicrobial packaging on physicochemical and microbial quality of button mushroom (Agaricus bisporus). Postharvest Biol. Technol. 2015, 99, 73–79. [Google Scholar] [CrossRef]
- Zhang, L.; Li, S.; Wang, A.; Li, J.; Zong, W. Mild heat treatment inhibits the browning of fresh-cut Agaricus bisporus during cold storage. LWT Food Sci. Technol. 2017, 82, 104–112. [Google Scholar] [CrossRef]
- Camargo, J.M.; Dunoyer, A.T.; García-Zapateiro, L.A. The effect of storage temperature and time on total phenolics and enzymatic activity of sapodilla (Achras sapota L.). Rev. Fac. Nac. Agron. Medellín 2016, 69, 7955–7963. [Google Scholar] [CrossRef]
- Thakur, R.R.; Shahi, N.C.; Mangaraj, S.; Lohani, U.C.; Chand, K. Effect of apple peel based edible coating material on physicochemical properties of button mushrooms under ambient condition. Int. J. Chem. Stud. 2020, 8, 2362–2370. [Google Scholar] [CrossRef]
- Walkowiak-Tomczak, D.; Idaszewska, N.; Bieńczak, K.; Kómoch, W. The effect of mechanical actions occurring during transport on physicochemical changes in Agaricus bisporus mushrooms. Sustainability 2020, 12, 4993. [Google Scholar] [CrossRef]
- Eissa, H.A. Effect of chitosan coating on shelf-life and quality of fresh-cut mushroom. Pol. J. Food Nutr. Sci. 2008, 58, 95–105. [Google Scholar] [CrossRef]
- Hosseini, A.; Moradinezhad, F. Effect of short-term high CO2 treatment on quality and shelf life of button mushroom (Agaricus bisporus) at refrigerated storage. J. Hortic. Postharvest Res. 2018, 1, 37–48. [Google Scholar]
- Karimirad, R.; Behnamian, M.; Dezhsetan, S. Development and characterization of nano biopolymer containing cumin oil as a new approach to enhance antioxidant properties of button mushroom. Int. J. Biol. Macromol. 2018, 113, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Sami, R. Application of Nano-coating and Chitosan Combination Films on Cantaloupe Preservation. Pak. J. Biol. Sci. PJBS 2020, 23, 1037–1043. [Google Scholar]
- Karimirad, R.; Behnamian, M.; Dezhsetan, S. Application of chitosan nanoparticles containing Cuminum cyminum oil as a delivery system for shelf life extension of Agaricus bisporus. LWT 2019, 106, 218–228. [Google Scholar] [CrossRef]
- Song, H.; Yuan, W.; Jin, P.; Wang, W.; Wang, X.; Yang, L.; Zhang, Y. Effects of chitosan/nano-silica on postharvest quality and antioxidant capacity of loquat fruit during cold storage. Postharvest Biol. Technol. 2016, 119, 41–48. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, L.; Yang, H.; Wang, A. Effect of exogenous glycine betaine on qualities of button mushrooms (Agaricus bisporus) during postharvest storage. Eur. Food Res. Technol. 2015, 240, 41–48. [Google Scholar] [CrossRef]
- Zeb, A.; Ullah, F. A simple spectrophotometric method for the determination of thiobarbituric acid reactive substances in fried fast foods. J. Anal. Methods Chem. 2016, 2016, 9412767. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sami, R.; Elhakem, A.; Alharbi, M.; Benajiba, N.; Almatrafi, M.; Abdelazez, A.; Helal, M. Evaluation of Antioxidant Activities, Oxidation Enzymes, and Quality of Nano-Coated Button Mushrooms (Agaricus Bisporus) during Storage. Coatings 2021, 11, 149. https://doi.org/10.3390/coatings11020149
Sami R, Elhakem A, Alharbi M, Benajiba N, Almatrafi M, Abdelazez A, Helal M. Evaluation of Antioxidant Activities, Oxidation Enzymes, and Quality of Nano-Coated Button Mushrooms (Agaricus Bisporus) during Storage. Coatings. 2021; 11(2):149. https://doi.org/10.3390/coatings11020149
Chicago/Turabian StyleSami, Rokayya, Abeer Elhakem, Mona Alharbi, Nada Benajiba, Manal Almatrafi, Amro Abdelazez, and Mahmoud Helal. 2021. "Evaluation of Antioxidant Activities, Oxidation Enzymes, and Quality of Nano-Coated Button Mushrooms (Agaricus Bisporus) during Storage" Coatings 11, no. 2: 149. https://doi.org/10.3390/coatings11020149
APA StyleSami, R., Elhakem, A., Alharbi, M., Benajiba, N., Almatrafi, M., Abdelazez, A., & Helal, M. (2021). Evaluation of Antioxidant Activities, Oxidation Enzymes, and Quality of Nano-Coated Button Mushrooms (Agaricus Bisporus) during Storage. Coatings, 11(2), 149. https://doi.org/10.3390/coatings11020149




