A Multi-Analytical Non-Invasive Approach to Aqueous Cleaning Systems in Treatments on Bowed String Musical Instruments
Abstract
1. Introduction
2. Materials and Methods
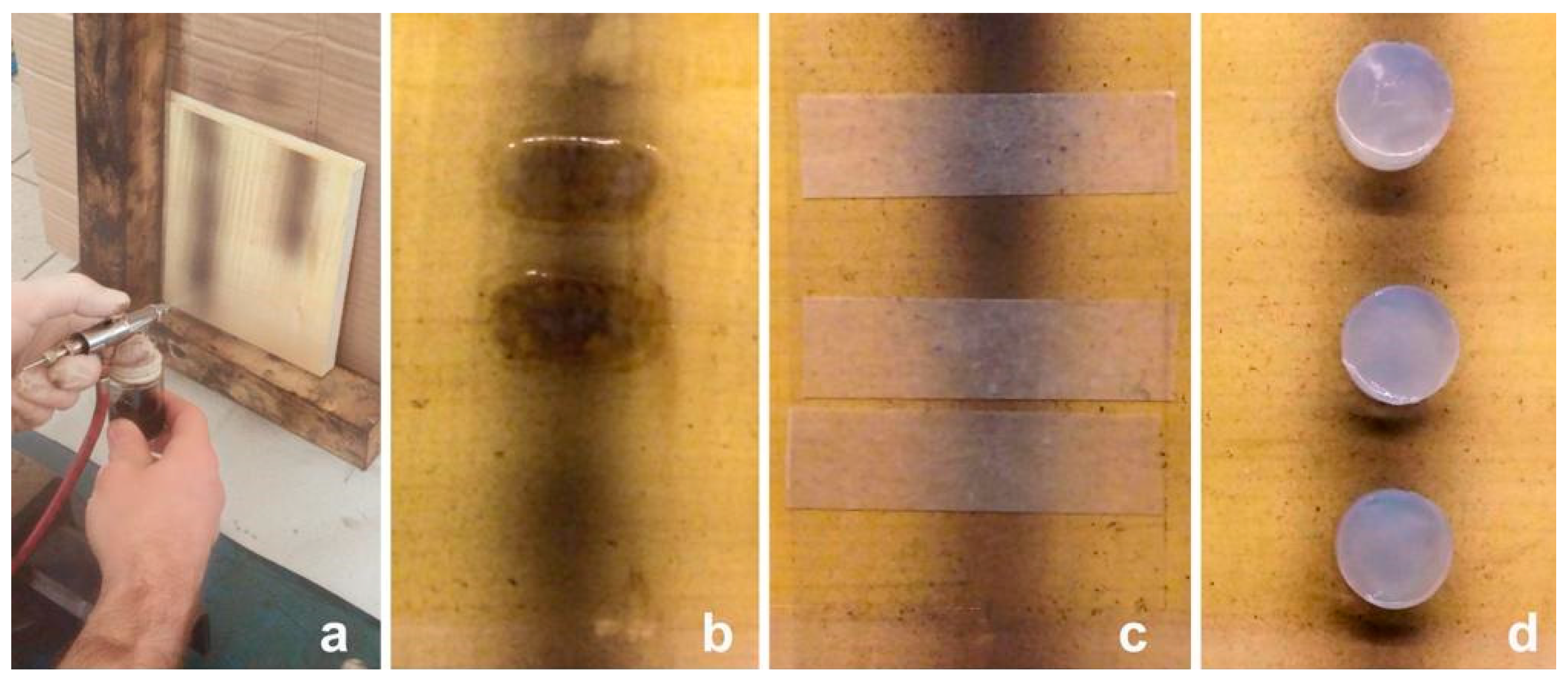
2.1. Mock-Ups
2.2. Cleaning Systems
- Korean paper: 2, 5, 10, and 20 min, respectively called K1, K2, K3, and K4;
- agarose gel: 10, 20, 30 min and 5 h, respectively called A1, A2, A3, and A4;
- w/o emulsion: 1, 2, 5, 10 min, respectively called E1, E2, E3, and E4.
2.3. Non-Invasive Techniques
Further Considerations on Imaging Analysis
3. Results and Discussion
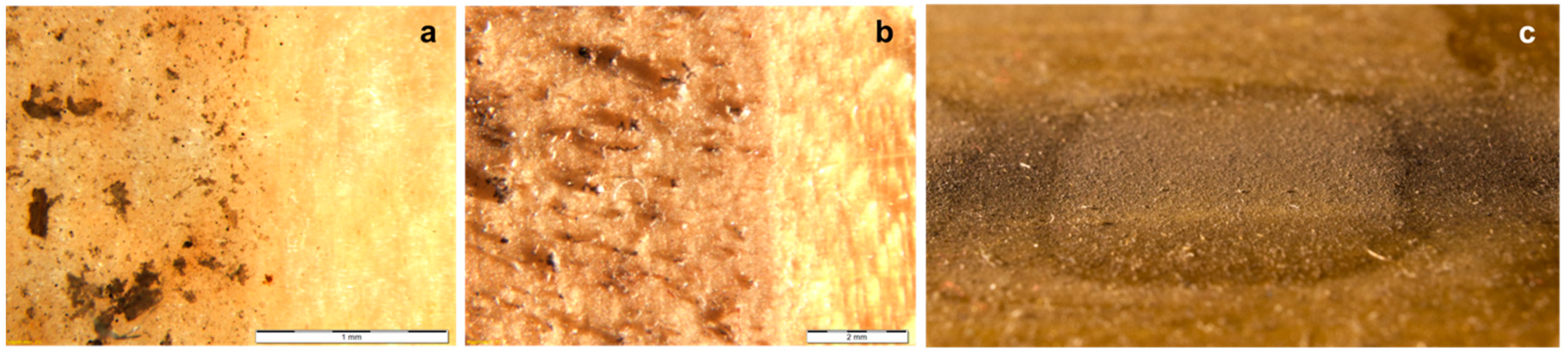
3.1. Stereomicroscopy
3.2. Multispectral Imaging
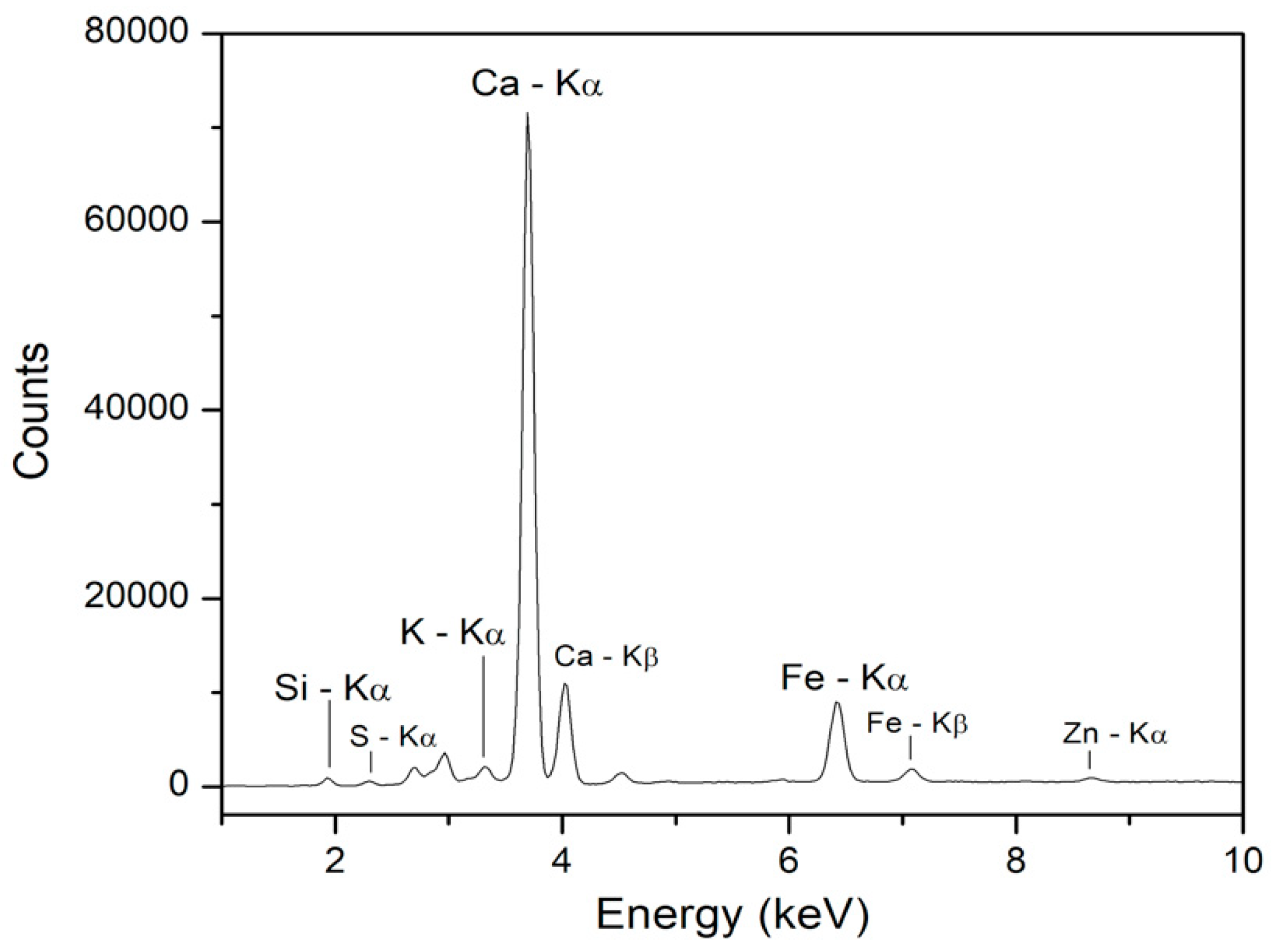
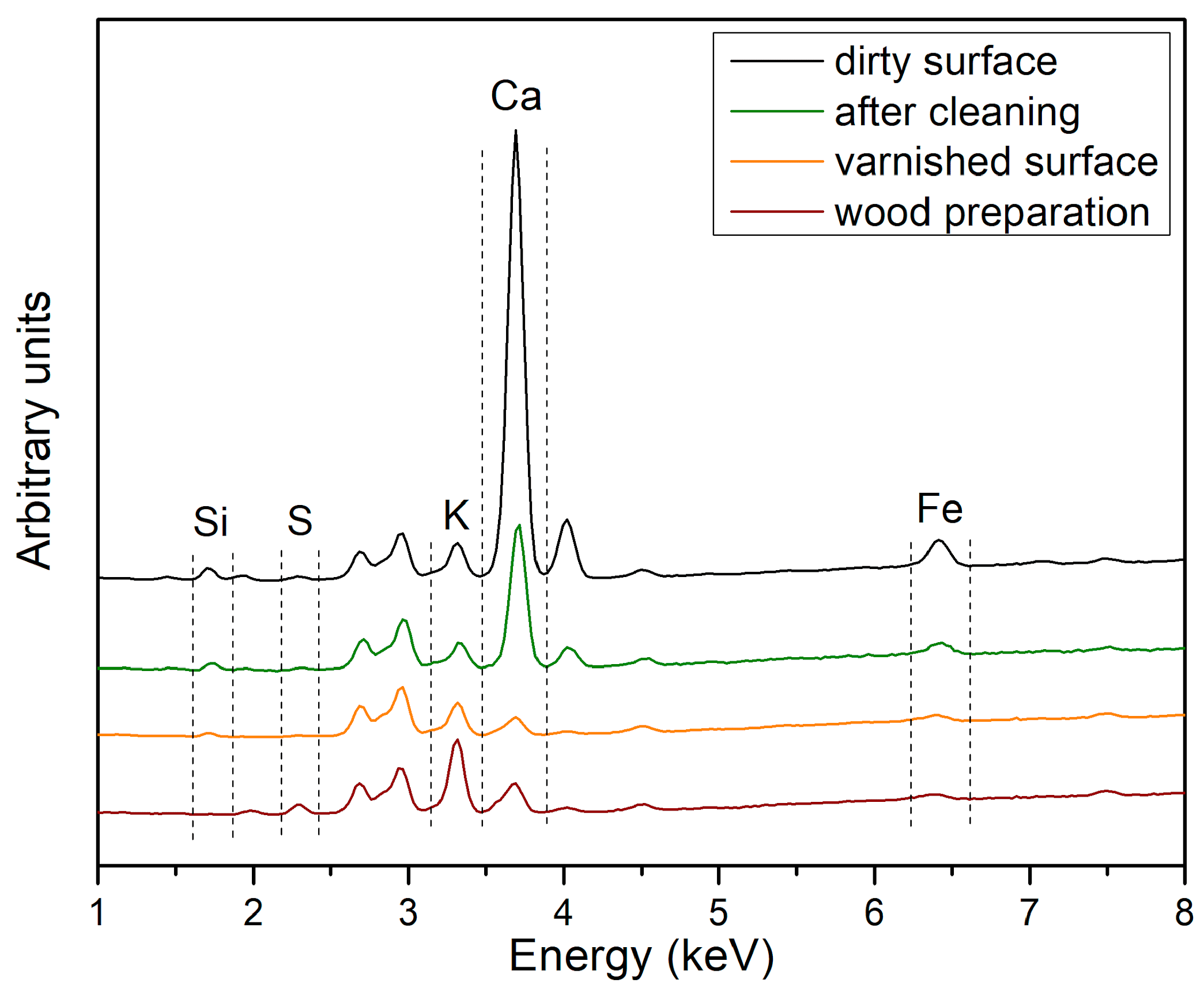
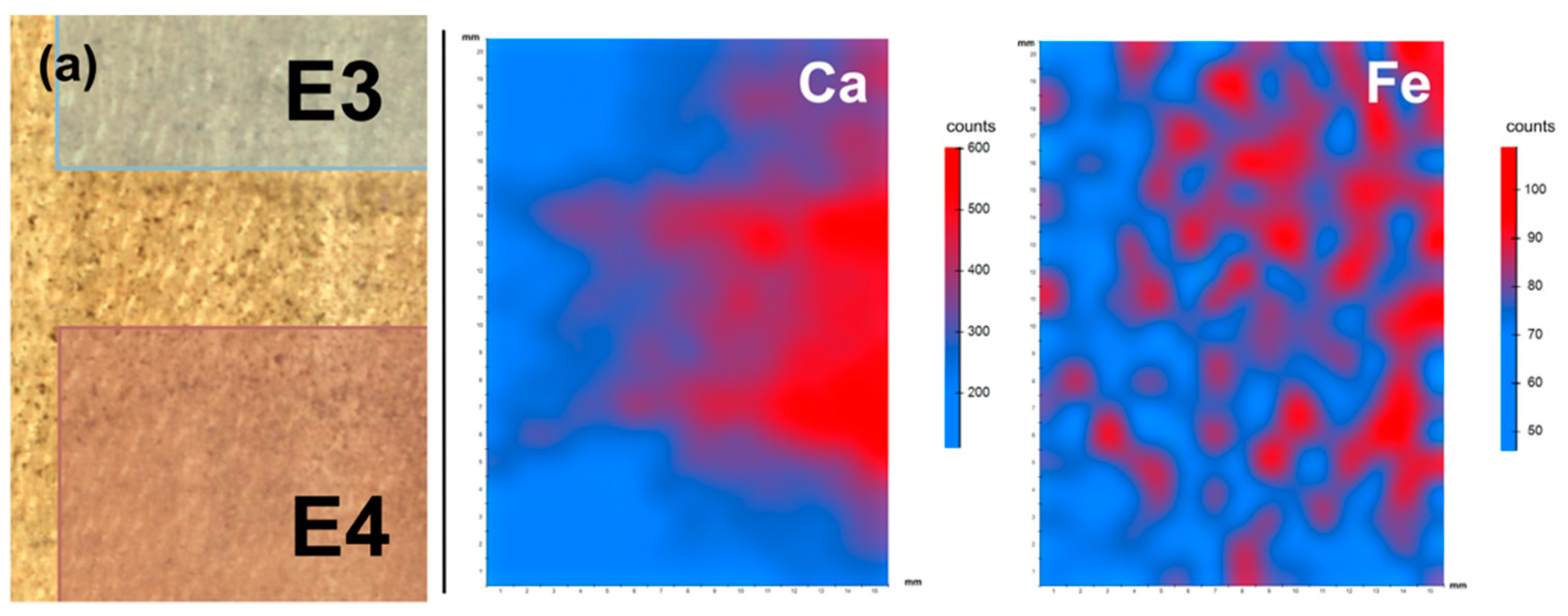
3.3. XRF Spectroscopy
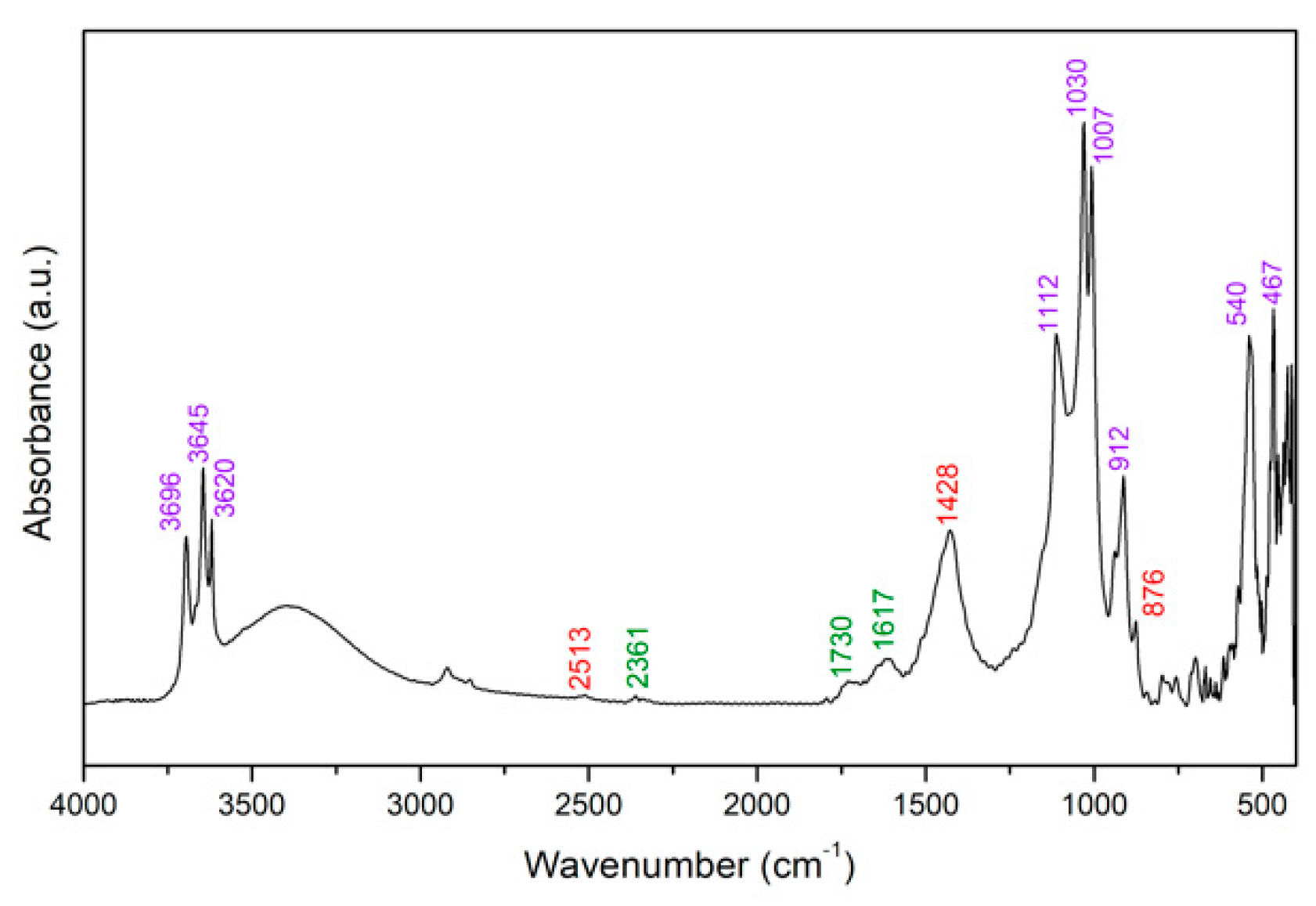
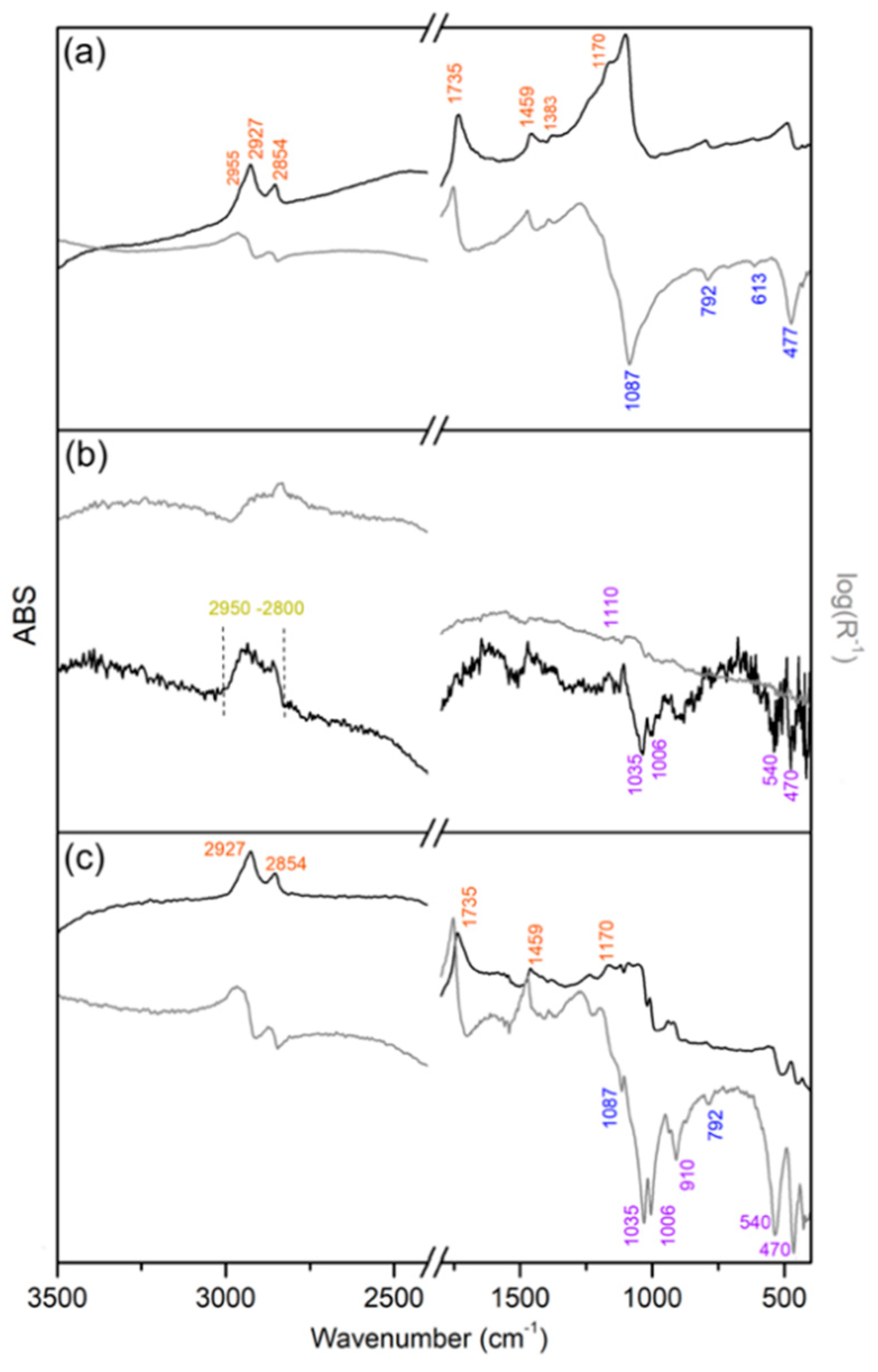
3.4. FTIR Spectroscopy
- broad ν(C–H) vibrations related to organic components (around 2900–2800 cm−1);
- ν(Si–O) and δ(Si–O) vibrations related to the kaolin (1035, 1006, 540, 468, 396 cm−1);
- sharp ν(C–H) vibrations due to colophony and linseed oil contributions (around 2900–2800 cm−1);
- ν(Si–O) and δ(Si–O) vibrations related to the diatomaceous earth (1087, 792, 613, 477 cm−1).
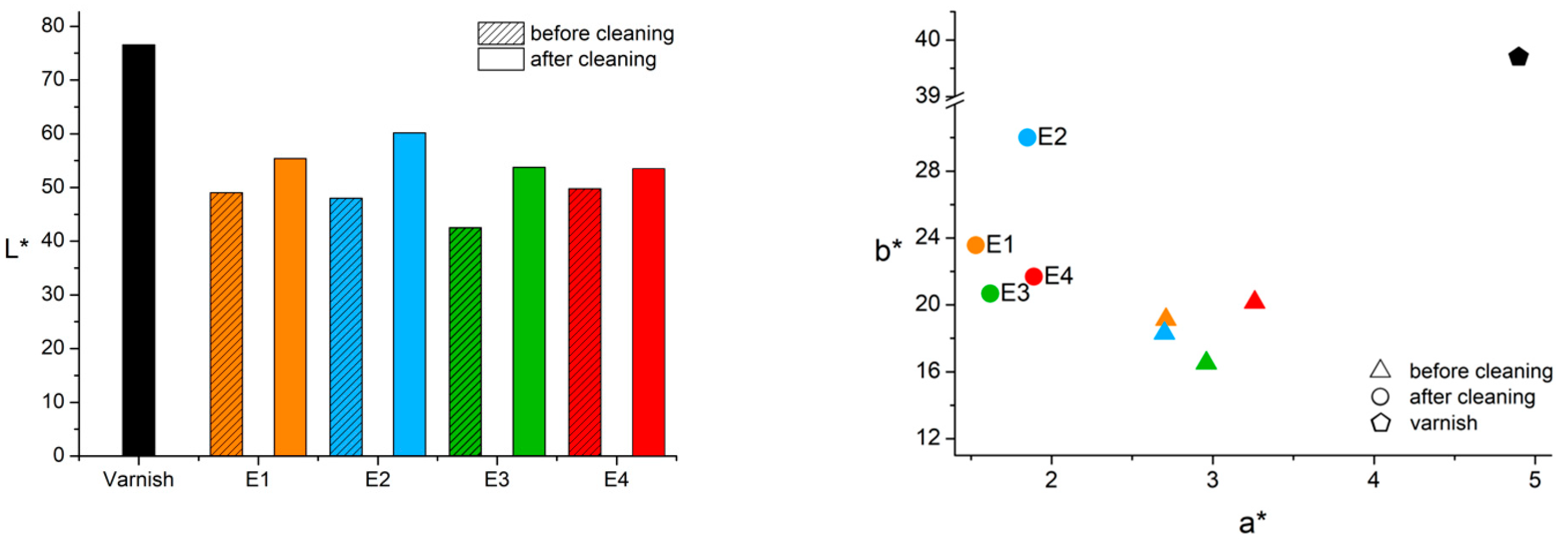
3.5. Evaluation of the Cleaning Systems Efficacy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Rhyne, C.S. Clean art? J. Am. Inst. Conserv. 2006, 45, 165–170. [Google Scholar] [CrossRef]
- Gritt, S. The removal of patina. In New Insights into the Cleaning of Paintings, Proceedings of the Cleaning 2010 International Conference, Universidad Politecnica de Valencia and Museum Conservation Institute; Smithsonian Institution: Washington, DC, USA, 2013. [Google Scholar]
- Camuffo, D. Microclimate for Cultural Heritage: Measurement, Risk Assessment, Conservation, Restoration, and Maintenance of Indoor and Outdoor Monuments; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 978-0-444-64107-6. [Google Scholar]
- Rovetta, T.; Invernizzi, C.; Licchelli, M.; Cacciatori, F.; Malagodi, M. The elemental composition of stradivari’s musical instruments: New results through non-invasive EDXRF analysis. X-ray Spectrom. 2018, 47, 159–170. [Google Scholar] [CrossRef]
- Holakooei, P.; Abed Esfahani, A. Conservation unit museums and galleries commission. In The Science For. Conservators Series: Volume 2: Cleaning; Routledge: Abingdon, UK, 2005; ISBN 978-1-134-90961-2. [Google Scholar]
- Wolbers, R.C. the use of a synthetic soiling mixture as a means for evaluating the efficacy of aqueous cleaning materials on painted surfaces. Conserv. Restaur. Des. Biens Cult. Rev. De L’araafu 1992, 4, 22–29. [Google Scholar]
- Florio, P.A.; Mersereau, E.P. Control of appearance changes due to soiling: The mechanism, measurement, and reduction of soiling changes in carpet during use. Text. Res. J. 1955, 25, 641–649. [Google Scholar] [CrossRef]
- Wolbers, R. Cleaning Painted Surfaces: Aqueous Methods; Archetype Publication: London, UK, 2000; ISBN 978-1-873132-36-4. [Google Scholar]
- Cavaleri, T.; Fiocco, G.; Rovetta, T.; Malagodi, M.; Piccirillo, A.; Pisani, M.; Zucco, M.; Gargano, M. A new imaging method of fluorescence induced by multispectral UV for studying historical musical instruments coatings. In UV-Vis Luminescence Imaging Techniques; López, L.F., Stols-Witlox, M., Picollo, M., Eds.; Editorial Universitat Politècnica de València: Valencia, Spain, 2020. [Google Scholar] [CrossRef]
- Signorini, E. Surface cleaning of paintings and polychrome objects in Italy: The last 15 years. In New Insights into the Cleaning of Paintings, Proceedings of the Cleaning 2010 International Conference, Universidad Politecnica de Valencia and Museum Conservation Institute; Smithsonian Institution: Washington, DC, USA, 2013. [Google Scholar]
- Cremonesi, P. L’uso Dei Tensioattivi e Chelanti Nella Pulitura Di Opere Policrome; Il Prato: Padova, Italy, 2004; ISBN 88-87243-84-0. [Google Scholar]
- Wennerström, H. Micelles. physical chemistry of surfactant association. Phys. Rep. 1979, 52, 1–86. [Google Scholar] [CrossRef]
- Baglioni, P.; Berti, D.; Bonini, M.; Carretti, E.; Dei, L.; Fratini, E.; Giorgi, R. Micelle, microemulsions, and gels for the conservation of cultural heritage. Adv. Colloid Interface Sci. 2014, 205, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Invernizzi, C.; Daveri, A.; Rovetta, T.; Vagnini, M.; Licchelli, M.; Cacciatori, F.; Malagodi, M. A multi-analytical non-invasive approach to violin materials: The case of Antonio Stradivari “Hellier” (1679). Microchem. J. 2016, 124, 743–750. [Google Scholar] [CrossRef]
- Poggialini, F.; Fiocco, G.; Campanella, B.; Legnaioli, S.; Palleschi, V.; Iwanicka, M.; Targowski, P.; Sylwestrzak, M.; Invernizzi, C.; Rovetta, T.; et al. Stratigraphic analysis of historical wooden samples from ancient bowed string instruments by laser induced breakdown spectroscopy. J. Cult. Herit. 2020, 44, 275–284. [Google Scholar] [CrossRef]
- Fiocco, G.; Rovetta, T.; Malagodi, M.; Licchelli, M.; Gulmini, M.; Lanzafame, G.; Zanini, F.; Lo Giudice, A.; Re, A. Synchrotron radiation micro-computed tomography for the investigation of finishing treatments in historical bowed string instruments: Issues and perspectives. Eur. Phys. J. Plus 2018, 133, 525. [Google Scholar] [CrossRef]
- Weththimuni, M.L.; Canevari, C.; Legnani, A.; Licchelli, M.; Malagodi, M.; Ricca, M.; Zeffiro, A. Experimental characterization of oil-colophony varnishes: A preliminary study. Int. J. Conserv. Sci. 2016, 2, 813–826. [Google Scholar]
- Robson, J. Burnishing Varnish to a Fine Polish. Strad 2007, 64–67. [Google Scholar]
- Cremonesi, P. Rigid gels and enzyme cleaning. New Insights into the Cleaning of Paintings: Proceedings from the Cleaning 2010 International Conference Universidad Politecnica de Valencia and Museum Conservation Institute; Smithsonian Institution: Washington, DC, USA, 2013. [Google Scholar]
- Campani, E. L’uso di Agarosio e Agar per la Preparazione di gel Rigidi = Use of Agarose and Agar for Preparing Rigid Gels; Il Prato: Padova, Italy, 2007; ISBN 978-88-89566-65-7. [Google Scholar]
- Gorel, F. Assessment of agar gel loaded with micro-emulsion for the cleaning of porous surfaces. Ceroart. Conserv. Expo. Restaur. D’objets D’art 2010. [Google Scholar] [CrossRef]
- Rasband, W.S. ImageJ.; U.S. National Institutes of Health: Bethesda, MD, USA, 1997. [Google Scholar]
- CIE ILV CIE Publication S 017/E:2011. ILV: International Lighting Vocabulary. CIE Central Bureau, Kegelgasse 27, A-1030 Vienna, Austria (2011). Available online: http://eilv.cie.co.at/termlist (accessed on 2 September 2020).
- Oleari, C. Standard Colorimetry: Definitions, Algorithms and Software; John Wiley & Sons: Hoboken, NJ, USA, 2016; ISBN 978-1-118-89444-6. [Google Scholar]
- Luo, M.R.; Cui, G.; Rigg, B. The Development of the CIE 2000 colour-difference formula: CIEDE2000. Color. Res. Appl. 2001, 26, 340–350. [Google Scholar] [CrossRef]
- Bonizzoni, L.; Canevari, C.; Galli, A.; Gargano, M.; Ludwig, N.; Malagodi, M.; Rovetta, T. A multidisciplinary materials characterization of a Joannes Marcus Viol (16th century). Herit. Sci. 2014, 2, 15. [Google Scholar] [CrossRef]
- Gargano, M.; Ludwig, N.; Poldi, G. A New methodology for comparing IR reflectographic systems. Infrared Phys. Technol. 2007, 49, 249–253. [Google Scholar] [CrossRef]
- Rovetta, T.; Invernizzi, C.; Fiocco, G.; Albano, M.; Licchelli, M.; Gulmini, M.; Alf, G.; Fabbri, D.; Rombolà, A.G.; Malagodi, M. The case of Antonio Stradivari 1718 Ex-San Lorenzo Violin: History, restorations and conservation perspectives. J. Archaeol. Sci. Rep. 2019, 23, 443–450. [Google Scholar] [CrossRef]
- Griffiths, P.R.; de Haseth, J.A. Fourier Transform. Infrared Spectrometry; John Wiley & Sons: Hoboken, NJ, USA, 2007. [Google Scholar]
- Invernizzi, C.; Fichera, G.V.; Licchelli, M.; Malagodi, M. A non-invasive stratigraphic study by reflection FT-IR spectroscopy and UV-induced fluorescence technique: The case of historical violins. Microchem. J. 2018, 138, 273–281. [Google Scholar] [CrossRef]
- Invernizzi, C.; Daveri, A.; Vagnini, M.; Malagodi, M. Non-invasive identification of organic materials in historical stringed musical instruments by reflection infrared spectroscopy: A methodological approach. Anal. Bioanal. Chem. 2017, 409, 3281–3288. [Google Scholar] [CrossRef] [PubMed]
- Erasmus, E. The influence of thermal treatment on properties of Kaolin. Hem. Ind. 2016, 70, 595–601. [Google Scholar] [CrossRef]

| Mock-Up Ref. Number | Cleaning Systems | Components | Cleaning Agent | Delivery System |
|---|---|---|---|---|
| I | Synthetic saliva | Ammonium and Sodium Citrate tribasic solution + mucin (protein, surfactant)—CTS restauro | chelating molecules, mucin (surfactant) | Korean paper |
| Chelating solution | Ammonium and Sodium Citrate tribasic solution of synthetic saliva—CTS restauro | chelating molecules, water | agarose gel | |
| II | Water in oil (w/o) emulsion | Surfactant: BrijTM 30 (polyethylene glycol dodecyl ether)—Sigma-Aldrich; oil: isooctane [85% isooctane, 10% dH2O, 5% surfactants] | water micelles | oil |
| Oil in water (o/w) emulsion [21] | main surfactant: SDS (sodium dodecyl sulphate) co-surfactant: 1-pentanol; oil: isooctane [85% dH2O, 5% isooctane, 10% surfactants] | oil micelles | water/agarose gel | |
| III | Non-ionic surfactant 2% v/v water solution | TweenTM 20 (polyethylene glycol sorbitan monolaurate)—Sigma-Aldrich | water micelles | agarose gel and Korean paper |
| IV | Anionic surfactant 2% v/v water solution | Vulpex 1 (Potassium Methyl-cyclohexyl oleate and Methyl Cyclohexanol.)—Picreator Enterprises Ltd. | water micelles | agarose gel and Korean paper |
| Wavenumber | Band Shape | Assignment | Material Attribution |
|---|---|---|---|
| 2900–2800 cm−1 (m) | Absorption | ν C–H | Organic |
| 1035 (s) | Inverted | νas Si–O | Kaolin |
| 1006 (s) | Inverted | νas Si–O | Kaolin |
| 540 (m) | Inverted | δ Al–O–Si | Kaolin |
| 468 (m) | Inverted | in-plane δ Si–O | Kaolin |
| 396 (m) | Inverted | in-plane δ Si–O–Si | Kaolin |
| Analysis | Mode | Observation |
|---|---|---|
| Stereomicroscopy | Direct and grazing light | Preliminary surface check, granulometry of dirt and surface morphology |
| Energy Dispersive X-ray fluorescence spectroscopy (EDXRF) | Punctual and mapping mode |
|
| Reflection Fourier transformed infrared reflectance (FTIR) spectroscopy | Punctual mode |
|
| Multispectral imaging—color comparisons were both qualitative by image observation and quantitative by colorimetric extracted data | Visible imaging (VIS) | Surface color turns towards original varnish one |
| Ultraviolet-induced fluorescence imaging (UVIFL) |
| |
| Near-infrared imaging (NIR) | Removal of carbon black |
| - | EDXRF | FTIR | VIS Imaging Colorimetry | UVIFL Imaging | NIR Imaging | Notes | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cleaning Treatment | Delivery System | Si | S | K | Ca | Fe | Leftovers | Kaolin | Organic | Reappearing of fl. | Change in fl. Color | |||
| Synthetic saliva | A | ✓✓ | ✓✓ | ✓✓ | ✓✓ | - | X | ✓ | ✓ | ✓✓ | orange | ✓ | Efficacy grows with time of application in the removal of silicate. | |
| K | ✓✓ | ✓✓ | ✓✓ | ✓✓ | X | K leftovers for 5-h treatment | ✓✓ | ✓✓ | X | X | - | X | Efficacy grows with time of application in the removal of silicate. | |
| Emulsion | A (o/w) | ✓✓ | X | ✓ | ✓✓✓ | ✓✓ | S leftovers | ✓✓ | ✓✓✓ | ✓✓✓ | ✓✓✓ | not observed | ✓✓ | - |
| E (w/o) | ✓✓ | X | ✓ | ✓✓ | ✓✓✓ | - | ✓ | ✓✓ | ✓✓✓ | ✓✓✓ | not observed | ✓✓✓ | Efficacy grows with time of application in the removal of silicate. | |
| Tween 20 2% v/v solution | A | ✓✓ | X | X | ✓✓ | ✓✓ | - | X | X | ✓✓ | ✓✓ | not observed | ✓✓ | Efficacy grows with time of application. |
| K | ✓✓ | X | ✓ | ✓ | ✓ | K leftovers for treatments longer than 1 min | X | ✓✓ | ✓ | ✓ | not observed | ✓ | - | |
| Vulpex 2% v/v solution | A | X | ✓✓ | X | ✓✓ | X | K leftovers for 5-h treatment | ✓ | X | X | X | - | X | A slight cleaning result is observed after the long-time treatment. |
| K | ✓✓ | X | X | ✓ | X | - | X | ✓ | ✓ | X | - | X | - | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cazzaniga, I.; Gargano, M.; Invernizzi, C.; Ludwig, N.G.; Malagodi, M.; Canevari, C.; Rovetta, T. A Multi-Analytical Non-Invasive Approach to Aqueous Cleaning Systems in Treatments on Bowed String Musical Instruments. Coatings 2021, 11, 150. https://doi.org/10.3390/coatings11020150
Cazzaniga I, Gargano M, Invernizzi C, Ludwig NG, Malagodi M, Canevari C, Rovetta T. A Multi-Analytical Non-Invasive Approach to Aqueous Cleaning Systems in Treatments on Bowed String Musical Instruments. Coatings. 2021; 11(2):150. https://doi.org/10.3390/coatings11020150
Chicago/Turabian StyleCazzaniga, Ilaria, Marco Gargano, Claudia Invernizzi, Nicola G. Ludwig, Marco Malagodi, Claudio Canevari, and Tommaso Rovetta. 2021. "A Multi-Analytical Non-Invasive Approach to Aqueous Cleaning Systems in Treatments on Bowed String Musical Instruments" Coatings 11, no. 2: 150. https://doi.org/10.3390/coatings11020150
APA StyleCazzaniga, I., Gargano, M., Invernizzi, C., Ludwig, N. G., Malagodi, M., Canevari, C., & Rovetta, T. (2021). A Multi-Analytical Non-Invasive Approach to Aqueous Cleaning Systems in Treatments on Bowed String Musical Instruments. Coatings, 11(2), 150. https://doi.org/10.3390/coatings11020150











