Suitability of a Progenitor Cell-Enriching Device for In Vitro Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation of Primary Human Fibroblasts
2.2. Cell Culture Techniques
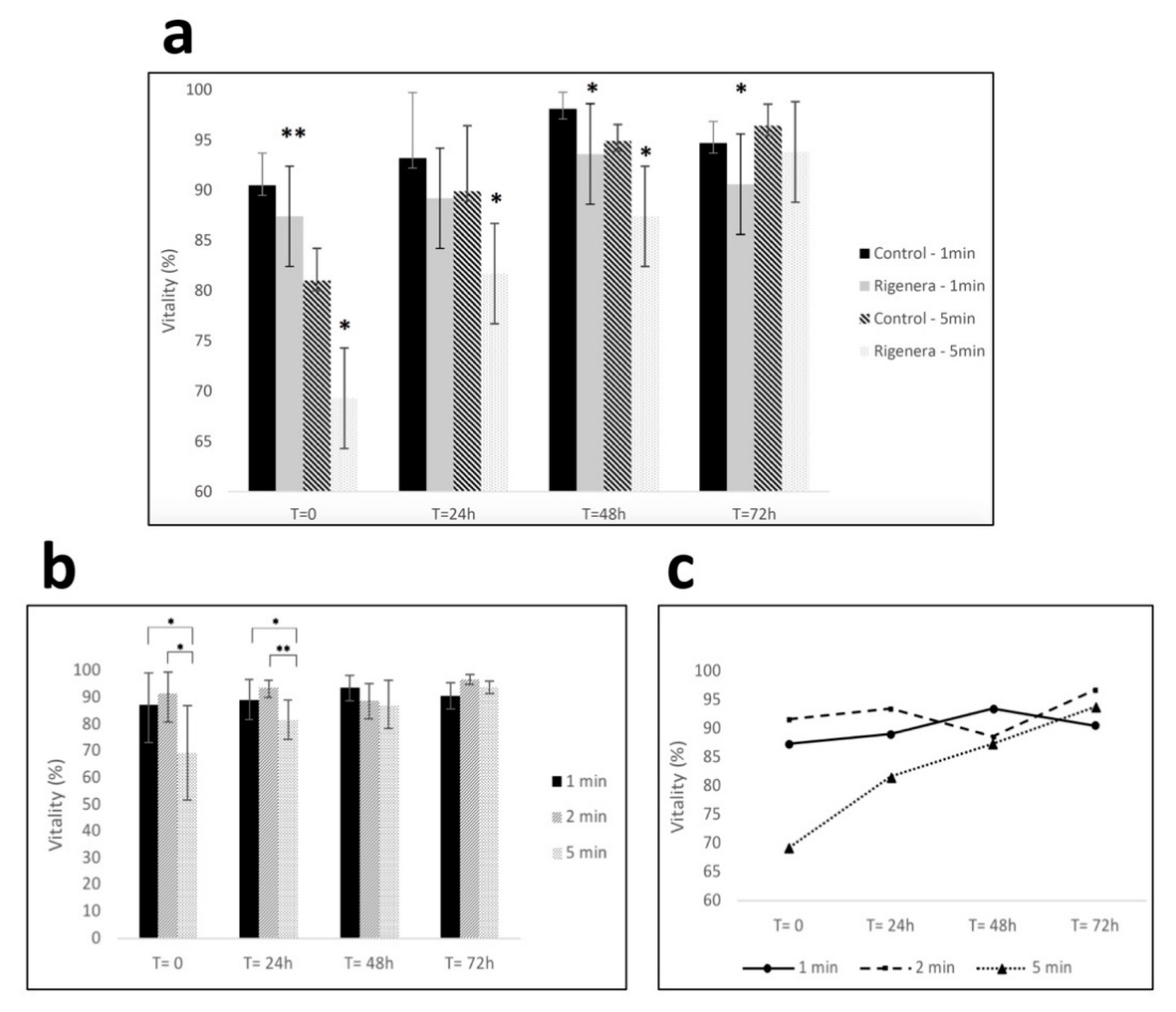
2.3. Effect of Rigenera® Processing on Human Fibroblast Viability In Vitro
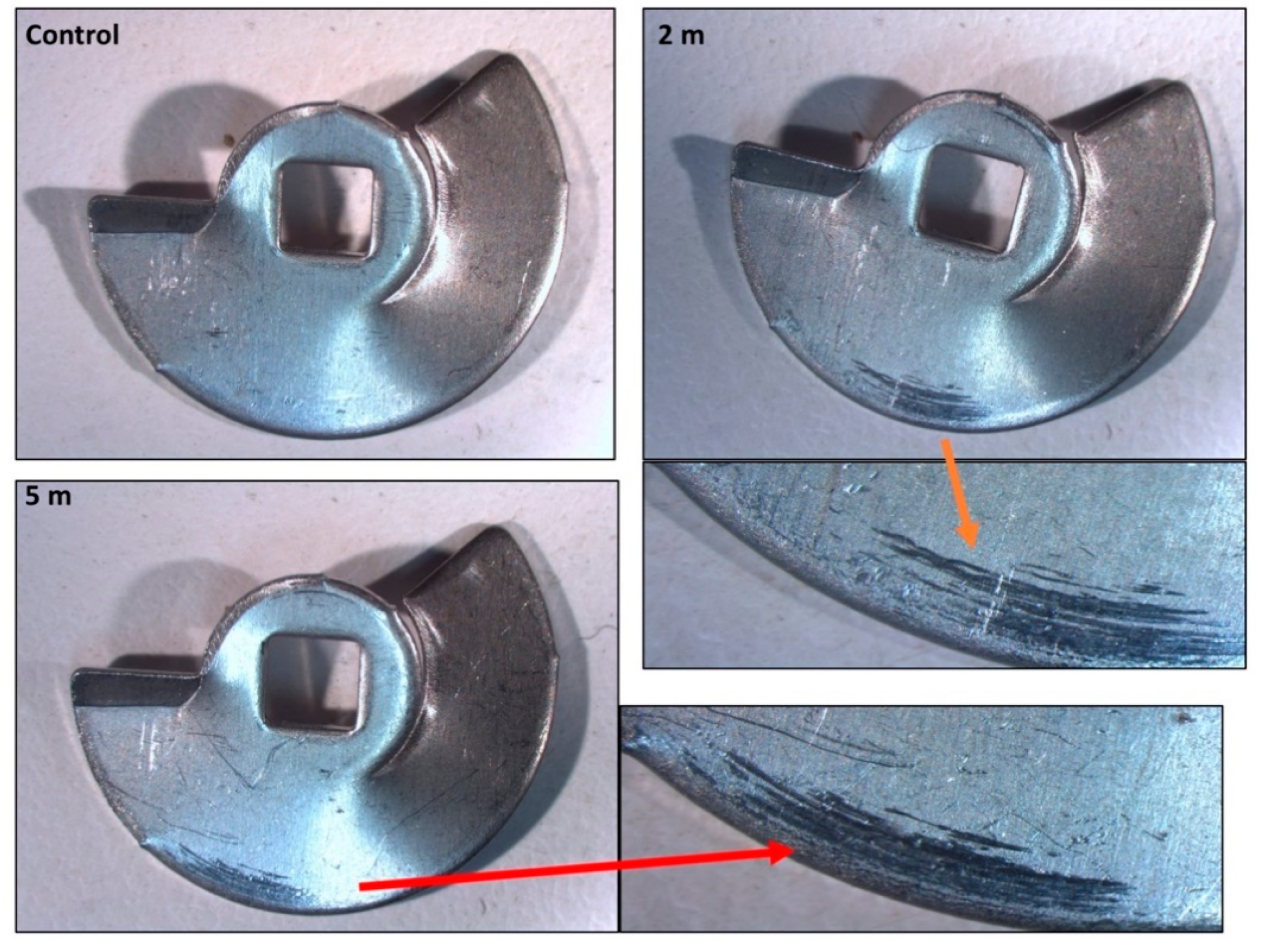
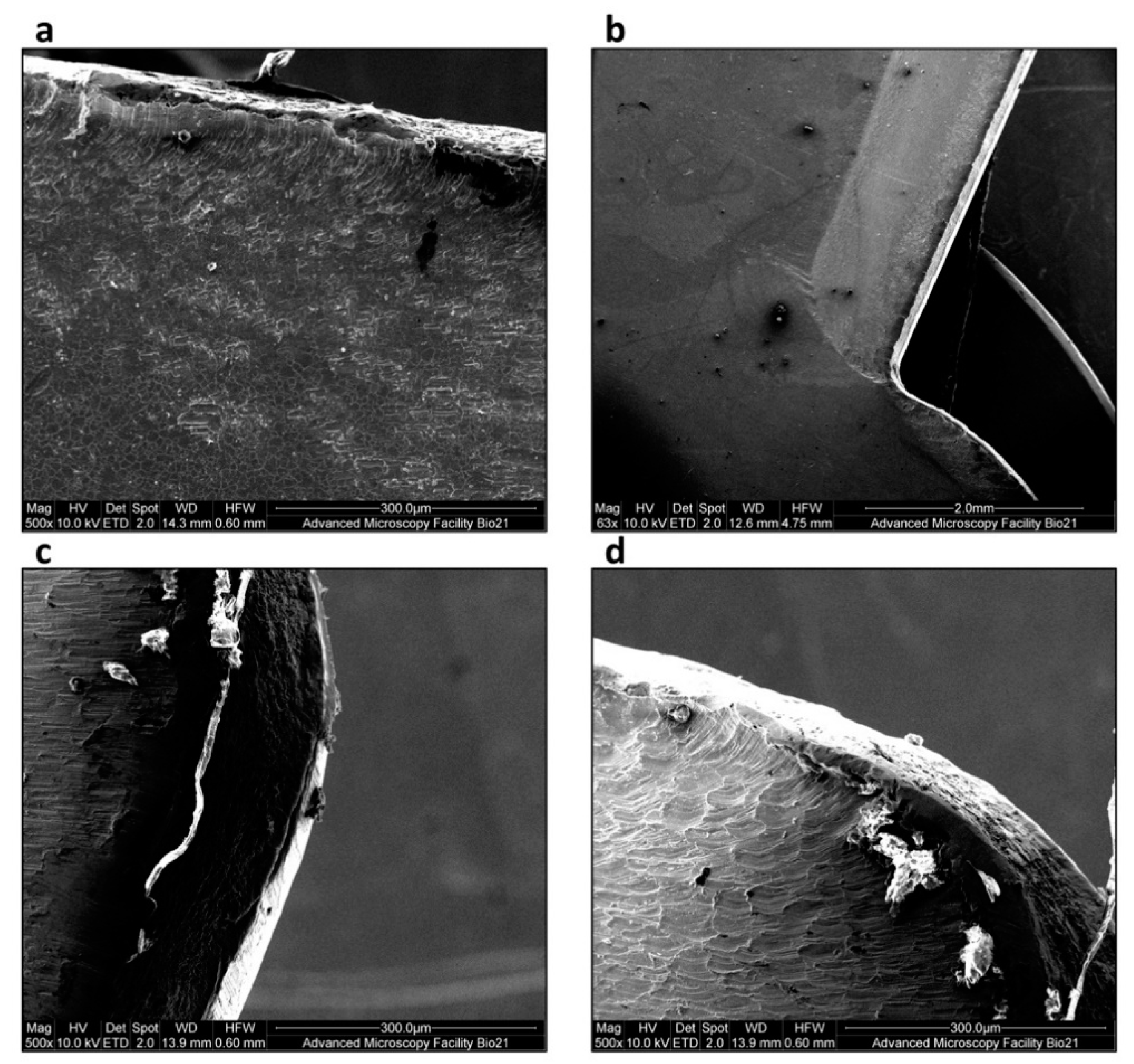
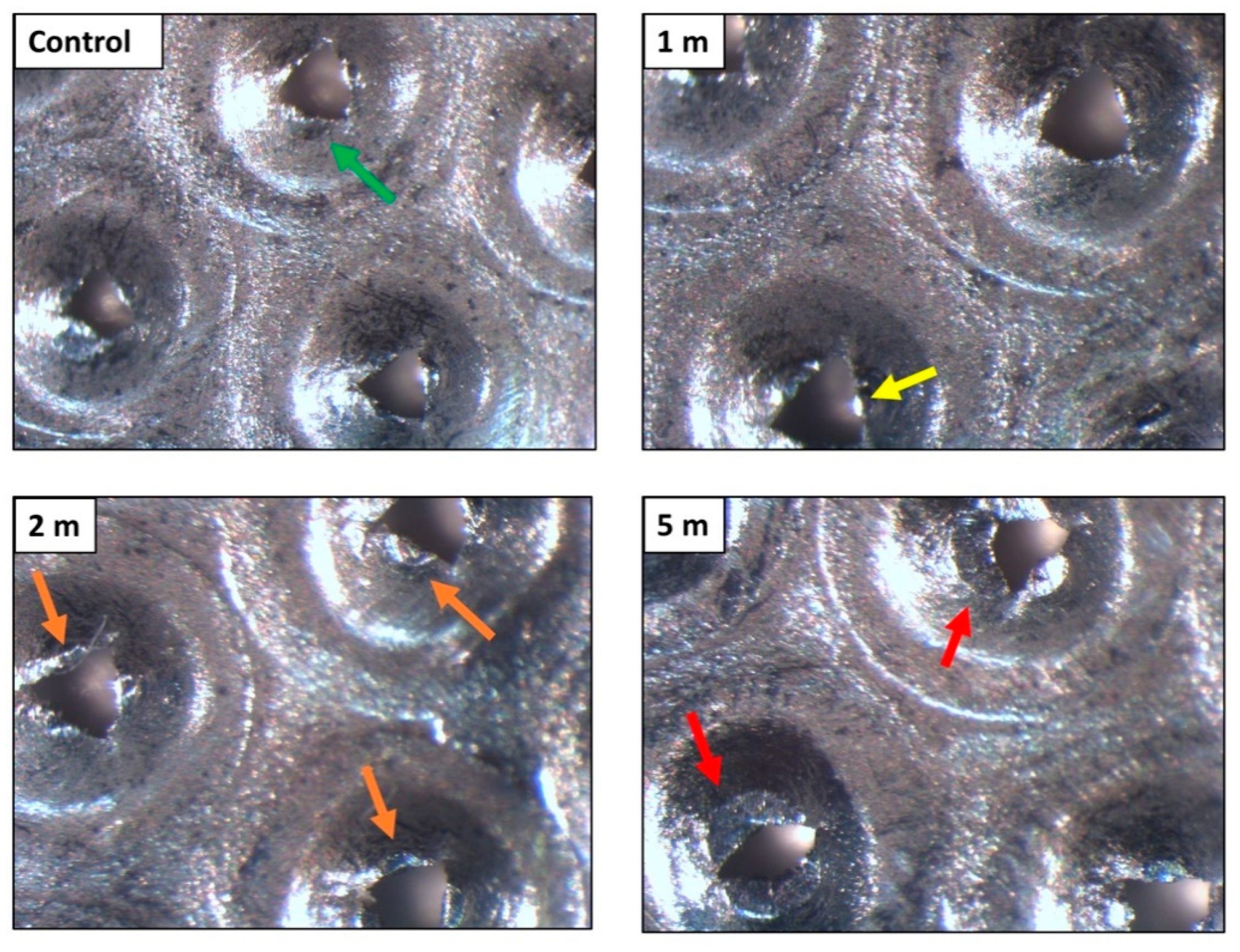
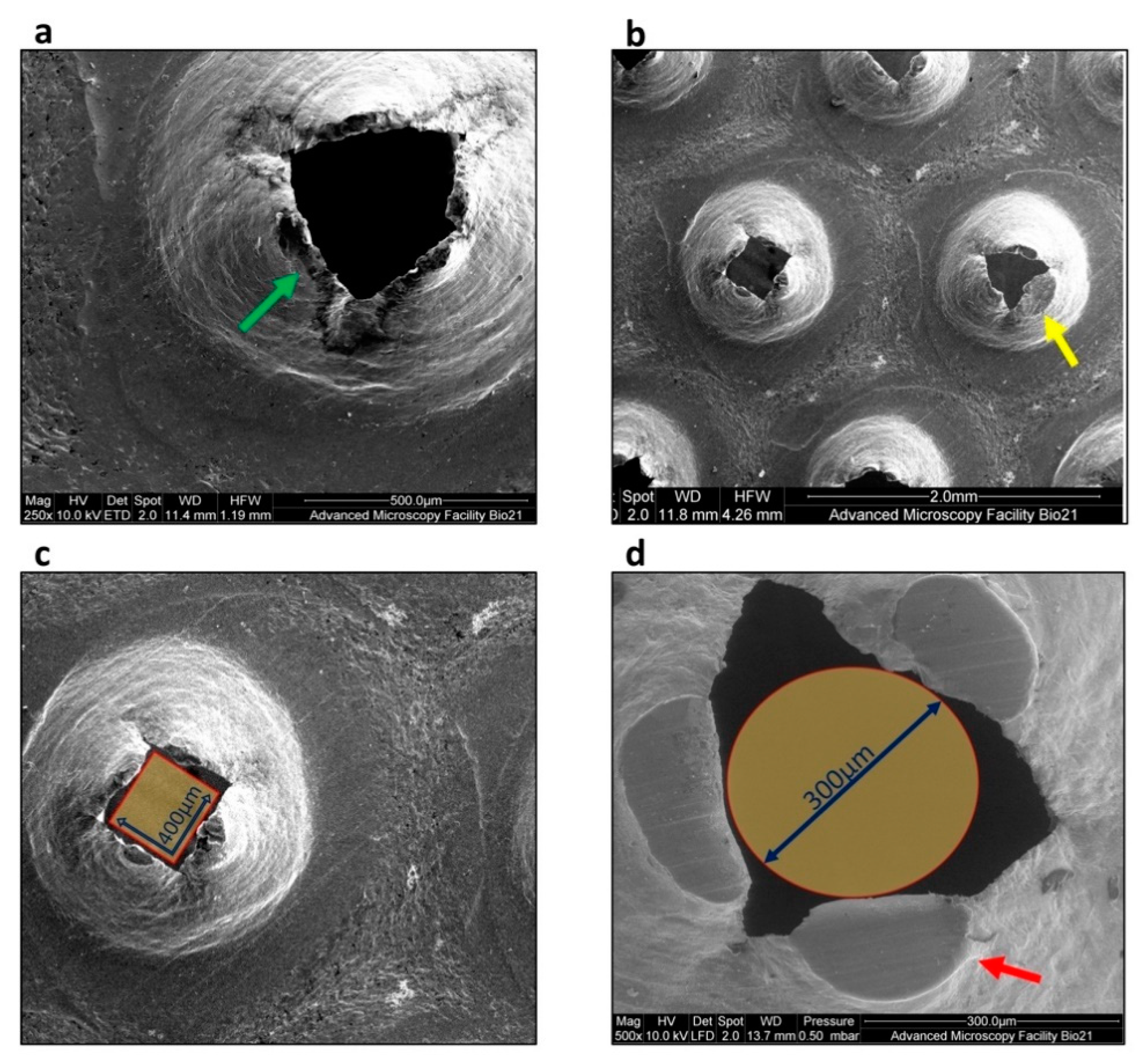
2.4. Characterisation of the Degree of Wear of Internal Components of Rigeneracons®
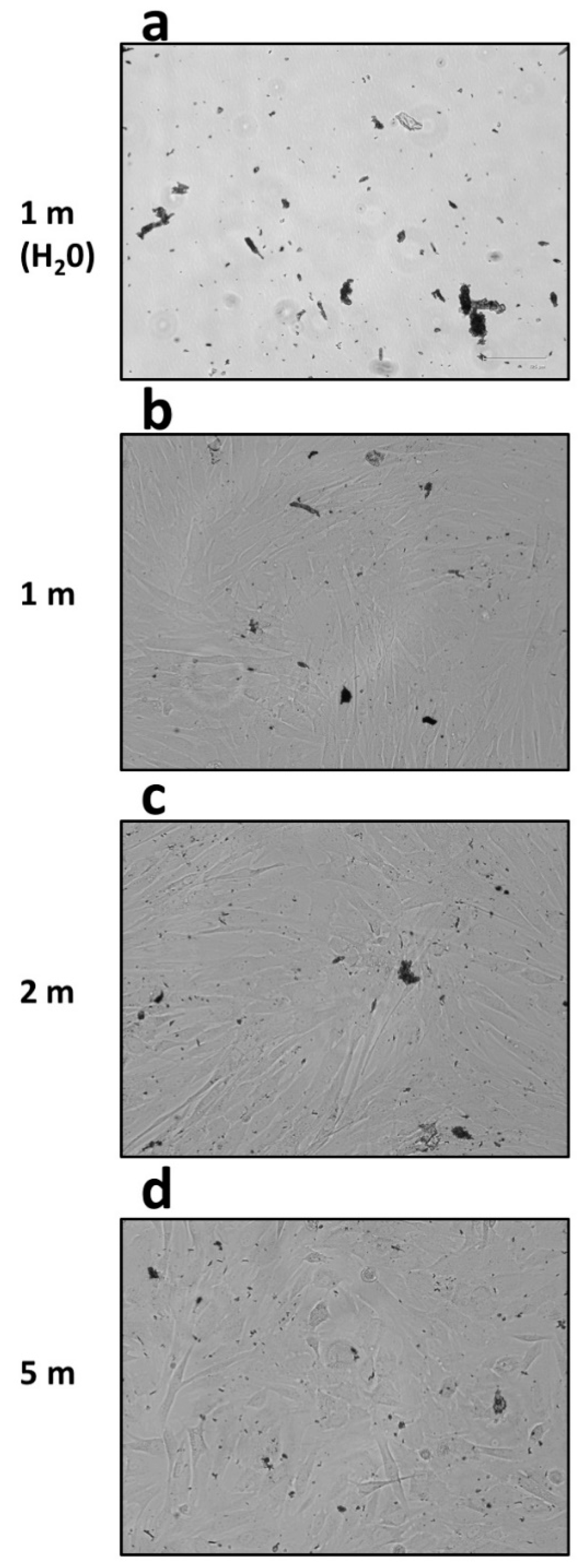
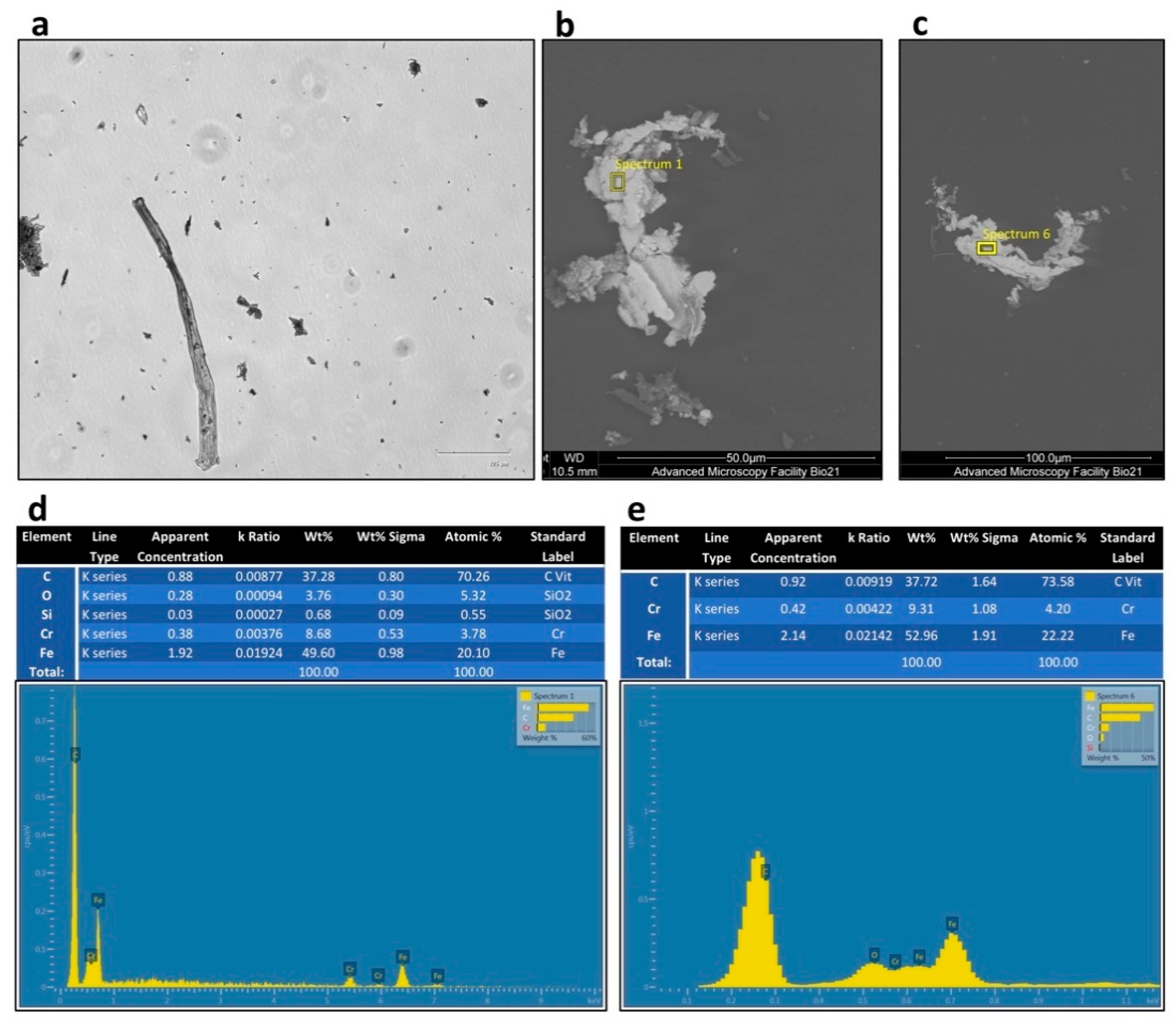
2.5. Characterisation of the Debris Particles from Rigenera® Protocol
2.6. Statistical Analysis
3. Results
3.1. Rigenera® Processing Affects Human Primary Fibroblast Viability In Vitro
3.2. Rigenera® Protocol is Associated with the Release of Metal Particles
3.3. Rigeneracons® Internal Components Show Marked Degree of Wear after Use
3.4. Assessment of Pore Size Range
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Calenic, B.; Greabu, M.; Caruntu, C.; Tanase, C.; Battino, M. Oral keratinocyte stem/progenitor cells: Specific markers, molecular signaling pathways and potential uses. Periodontol. 2000 2015, 69, 68–82. [Google Scholar] [CrossRef] [PubMed]
- Barrandon, Y.; Green, H. Cell size as a determinant of the clone-forming ability of human keratinocytes. Proc. Natl. Acad. Sci. USA 1985, 82, 5390–5394. [Google Scholar] [CrossRef] [PubMed]
- Haniffa, M.A.; Collin, M.P.; Buckley, C.D.; Dazzi, F. Mesenchymal stem cells: The fibroblasts’ new clothes? Haematologica 2009, 9, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Potdar, P.D.; Jethmalani, Y.D. Human dental pulp stem cells: Applications in future regenerative medicine. World J. Stem Cells 2015, 7, 839–851. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Li, H.; Guo, Z. Mesenchymal stem cell-like properties in fibroblasts. Cell. Physiol. Biochem. 2014, 34, 703–714. [Google Scholar] [CrossRef]
- Dixit, P.; Katare, R. Challenges in identifying the best source of stem cells for cardiac regeneration therapy. Stem Cell Res. Ther. 2015, 6, 26. [Google Scholar] [CrossRef]
- Trovato, L.; Monti, M.; Del Fante, C.; Cervio, M.; Lampinen, M.; Ambrosio, L.; Redi, C.A.; Perotti, C.; Kankuri, E.; Ambrosio, G.; et al. A new medical device rigeneracons allows to obtain viable micro-grafts from mechanical disaggregation of human tissues. J. Cell Physiol. 2015, 230, 2299–2303. [Google Scholar] [CrossRef]
- Zanzottera, F.; Lavezzari, E.; Trovato, L.; Icardi, A.; Graziano, A. Adipose derived stem cells and growth factors applied on hair transplantation. follow-up of clinical outcome. J. Cosmet. Dermatol. Sci. Appl. 2014, 4, 268–274. [Google Scholar] [CrossRef]
- Rodriguez y Baena, R.; D’Aquino, R.; Graziano, A.; Trovato, L.; Aloise, A.C.; Ceccarelli, G.; Pelegrine, A.A.; Lupi, S.M. Autologous Periosteum-Derived Micrografts and PLGA/HA enhance the bone formation in sinus lift augmentation. Front Cell Dev. Biol. 2017, 5, 87. [Google Scholar] [CrossRef]
- Svolacchia, F.; De Francesco, F.; Trovato, L.; Graziano, A.; Ferraro, G.A. An innovative regenerative treatment of scars with dermal micrografts. J. Cosmet. Dermatol. 2016, 15, 245–253. [Google Scholar] [CrossRef]
- Giaccone, M.; Brunetti, M.; Camadona, M.; Trovato, L.; Graziano, M. A new medical device, based on Rigenera Protocol, in the management of complex wounds. J. Stem Cells Res. Rev. Rep. 2014, 1, 1013. [Google Scholar]
- Graziano, A.; Carinci, F.; Scolaro, S. Periodontal tissue generation using autologous dental ligament micro-grafts: Case report with 6 months follow-up. Ann. Oral Maxillofac. Surg. 2013, 1, 20. [Google Scholar] [CrossRef]
- Marcarelli, M.; Trovato, L.; Novarese, E.; Riccio, M.; Graziano, A. Rigenera protocol in the treatment of surgical wound dehiscence. Int. Wound J. 2017, 14, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Carinci, F.; Motroni, A.; Graziano, A.; Zollino, I.; Brunelli, G.; D′Aquino, R. Sinus lift tissue engineering using autologous pulp micro-grafts: A case report of bone density evaluation. J. Indian Soc. Periodontol. 2013, 17, 644–647. [Google Scholar] [CrossRef] [PubMed]
- Purpura, V.; Bondioli, E.; Graziano, A.; Trovato, L.; Melandri, D.; Ghetti, M.; Marchesini, A.; De Angelis, M.G.C.; Benedetti, L.; Ceccarelli, G.; et al. Tissue characterization after a new disaggregation method for skin micro-grafts generation. J. Vis. Exp. 2016. [Google Scholar] [CrossRef]
- Izumi, K.; Tobita, T.; Feinberg, S.E. Isolation of human oral keratinocyte progenitor/stem cells. J. Dent. Res. 2007, 86, 341–346. [Google Scholar] [CrossRef]
- Graziano, A.; D’Aquino, R. From the research to the clinical practice: The micro-grafts challenge. In Proceedings of the 4th World Congress on Cell Science & Stem Cell Research, Valencia, Spain, 24–26 June 2014. [Google Scholar]
- D’Aquino, R.; Trovato, L.; Graziano, A.; Ceccarelli, G.; de Angelis, G.C.; Nisio, A.; Galli, M.; Pasi, M.; Finotti, M.; Rizzo, S.; et al. Periosteum-derived micro-grafts for tissue regeneration of human maxillary bone. J. Transl. Sci. 2016, 2, 125–129. [Google Scholar] [CrossRef]
- De Francesco, F.; Graziano, A.; Trovato, L.; Ceccarelli, G.; Romano, M.; de Angelis, G.C.; Marcarelli, M.; Cillo, U.; Riccio, M.; Ferrao, G.A. A regenerative approach with dermal micrografts in the treatment of chronic ulcers. Stem Cell Rev. 2017, 13, 139–148. [Google Scholar] [CrossRef]
- Ibrahim, S.F.; Engh, G.V.D. High-speed cell sorting: Fundamentals and recent advances. Curr. Opin. Biotechnol. 2003, 14, 5–12. [Google Scholar] [CrossRef]
- Zhu, B.; Murthy, S.K. Stem cell separation technologies. Curr. Opin. Chem. Eng. 2013, 2, 3–7. [Google Scholar] [CrossRef]
- Rock, K.L.; Kono, H. The inflammatory response to cell death. Annu. Rev. Pathol. 2008, 3, 99–126. [Google Scholar] [CrossRef] [PubMed]
- Majno, G.; La Gattuta, M.; Thompson, T.E. Cellular death and necrosis: Chemical, physical and morphologic changes in rat liver. Virchows Arch. Pathol. Anat. Physiol. Klin. Med. 1960, 333, 421–465. [Google Scholar] [CrossRef] [PubMed]
- Suárez-López Del Amo, F.; Garaicoa-Pazmiño, C.; Fretwurst, T.; Castilho, R.M.; Squarize, C.H. Dental implants-associated release of titanium particles: A systematic review. Clin. Oral Implants Res. 2018, 29, 1085–1100. [Google Scholar] [CrossRef] [PubMed]
- Bio Rad. TC10™ Automated Cell Counter with Printer #1450009. Available online: http://www.bio-rad.com/en-au/sku/1450009-tc10-automated-cell-counter-with-printer?ID=1450009 (accessed on 15 January 2018).
- Winter, G.D. Tissue reaction to metallic wear and corrosion products in human patients. J. Biomed. Mater. Res. 1974, 8, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Brennan, F.R.; Jacobs, J.J.; Urban, R.M.; Ragasa, D.R.; Glant, T.T. Human monocyte/macrophage response to cobalt-chromium corrosion products and titanium particles in patients with total joint replacements. J. Orthop. Res. 1997, 15, 40–49. [Google Scholar] [CrossRef]
- Haynes, D.R.; Rogers, S.D.; Hay, S.; Pearcy, M.J.; Howie, D.W. The differences in toxicity and release of bone-resorbing mediators induced by titanium and cobalt-chromium-alloy wear particles. J. Bone Jt. Surg. Am. Vol. 1993, 75, 825–834. [Google Scholar] [CrossRef]
- Dayan, A.D.; Paine, A.J. Mechanisms of chromium toxicity, carcinogenicity and allergenicity: Review of the literature from 1985 to 2000. Hum. Exp. Toxicol. 2001, 20, 439–451. [Google Scholar] [CrossRef]
- Gaggelli, E.; Berti, F.; D’Amelio, N.; Gaggelli, N.; Valensin, G.; Bovalini, L.; Paffetti, A.; Trabalzini, L. Metabolic pathways of carcinogenic chromium. Environ. Health Perspect. 2002, 110 (Suppl. 5), 733–738. [Google Scholar] [CrossRef]
- Rycroft, R.J.G.; Menn, T.; Frosch, P.J. Textbook of Contact Dermatitis, 3rd ed.; Springer: Berlin, Germany; New York, NY, USA, 2001. [Google Scholar]
- Daley, B.; Doherty, A.T.; Fairman, B.; Case, C.P. Wear debris from hip or knee replacements causes chromosomal damage in human cells in tissue culture. J. Bone Jt. Surgery. Br. 2004, 86, 598–606. [Google Scholar] [CrossRef]
- Messer, R.L.; Bishop, S.; Lucas, L.C. Effects of metallic ion toxicity on human gingival fibroblasts morphology. Biomaterials 1999, 20, 1647–1657. [Google Scholar] [CrossRef]
- Bhowmick, N.A.; Neilson, E.G.; Moses, H.L. Stromal fibroblasts in cancer initiation and progression. Nature 2004, 432, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Pietras, K.; Ostman, A. Hallmarks of cancer: Interactions with the tumor stroma. Exp. Cell Res. 2010, 316, 1324–1331. [Google Scholar] [CrossRef] [PubMed]
- Hallab, N.J.; Anderson, S.; Caicedo, M.; Brasher, A.; Mikecz, K.; Jacobs, J.J. Effects of soluble metals on human peri-implant cells. J. Biomed. Mater. Res. Part A 2005, 74, 124–140. [Google Scholar] [CrossRef] [PubMed]
- Hosman, A.H.; van der Mei, H.C.; Bulstra, S.K.; Busscher, H.J.; Neut, D. Effects of metal-on-metal wear on the host immune system and infection in hip arthroplasty. Acta Orthop. 2010, 81, 526–534. [Google Scholar] [CrossRef] [PubMed]
- Brodner, W.; Bitzan, P.; Meisinger, V.; Kaider, A.; Gottsauner-Wolf, F.; Kotz, R. Elevated serum cobalt with metal-on-metal articulating surfaces. J Bone Jt. Surg. Br. 1997, 79, 316–321. [Google Scholar] [CrossRef]
- Keegan, G.M.; Learmonth, I.D.; Case, C.P. Orthopaedic metals and their potential toxicity in the arthroplasty patient: A review of current knowledge and future strategies. J. Bone Jt. Surg. Br. 2007, 89, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Cobb, A.G.; Schmalzreid, T.P. The clinical significance of metal ion release from cobalt-chromium metal-on-metal hip joint arthroplasty. Proc. Inst. Mech. Eng. H 2006, 220, 385–398. [Google Scholar] [CrossRef]
- Ferreira, M.E.; de Lourdes Pereira, M.; Garcia e Costa, F.; Sousa, J.P.; de Carvalho, G.S. Comparative study of metallic biomaterials toxicity: A histochemical and immunohistochemical demonstration in mouse spleen. J. Trace Elem. Med. Biol. 2003, 17, 45–49. [Google Scholar] [CrossRef]
- Hasegawa, M.; Iino, T.; Sudo, A. Immune response in adverse reactions to metal debris following metal-on-metal total hip arthroplasty. BMC Musculoskelet. Disord. 2016, 17, 1–5. [Google Scholar] [CrossRef]
- Hallab, N.J.; Jacobs, J.J. Biologic effects of implant debris. Bull. NYU Hosp. Jt. Dis. 2009, 67, 182–188. [Google Scholar]
- Jomova, K.; Valko, M. Advances in metal-induced oxidative stress and human disease. Toxicology 2011, 283, 65–87. [Google Scholar] [CrossRef] [PubMed]
- Ghio, A.J.; Pavlisko, E.N.; Roggli, V.L. Iron and iron-related proteins in asbestosis. J. Environ. Pathol. Toxicol. Oncol. 2015, 34, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Hershko, C.; Link, G.; Cabantchik, I. Pathophysiology of iron overload. Ann. N. Y. Acad. Sci. 1998, 850, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Ryan, T.P.; Aust, S.D. The role of iron in oxygen-mediated toxicities. Crit. Rev. Toxicol. 1992, 22, 119–141. [Google Scholar] [CrossRef]
- Bhasin, G.; Kauser, H.; Athar, M. Iron augments stage-I and stage-II tumor promotion in murine skin. Cancer Lett. 2002, 183, 113–122. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Celentano, A.; Yap, T.; Pantaleo, G.; Paolini, R.; McCullough, M.; Cirillo, N. Suitability of a Progenitor Cell-Enriching Device for In Vitro Applications. Coatings 2021, 11, 146. https://doi.org/10.3390/coatings11020146
Celentano A, Yap T, Pantaleo G, Paolini R, McCullough M, Cirillo N. Suitability of a Progenitor Cell-Enriching Device for In Vitro Applications. Coatings. 2021; 11(2):146. https://doi.org/10.3390/coatings11020146
Chicago/Turabian StyleCelentano, Antonio, Tami Yap, Giuseppe Pantaleo, Rita Paolini, Michael McCullough, and Nicola Cirillo. 2021. "Suitability of a Progenitor Cell-Enriching Device for In Vitro Applications" Coatings 11, no. 2: 146. https://doi.org/10.3390/coatings11020146
APA StyleCelentano, A., Yap, T., Pantaleo, G., Paolini, R., McCullough, M., & Cirillo, N. (2021). Suitability of a Progenitor Cell-Enriching Device for In Vitro Applications. Coatings, 11(2), 146. https://doi.org/10.3390/coatings11020146








