Abstract
A modern environmental safety approach requires the implementation of green or sustainable strategies, such as banning or significantly lowering the presence of harmful substances on the market or in the industrial environment. To date, the majority of highly performing solutions are still based on fluorine chemistry, even with a growing effort to lower its impact. Economic costs, but also persistence, long-term degradation, and transformation in the environment can raise issues about medium- and long-term effects on human health and wildlife. Coatings with high water and oil repellence are used worldwide in daily life and in industrial and research fields, such as self-cleansing, anti-icing, and anti-biofouling. The combination of a particular geometry or surface structure and low-energy materials results in unique properties related to a range of materials in natural or synthetic categories aiming to build, when possible, a fluorine-free world. This work revises recent and key literature to propose valid alternatives to fluoro compounds in terms of water and oil repellence, as well as stability and resistance to physico-chemical agents. In this paper, natural compounds like fatty acids and waxes are addressed together with more synthetic systems like silicon-based solutions, and polymeric and inorganic nanostructured coatings. Most of the revised papers deal with topics fulfilling environmental requirements but are mainly restricted to highly repellent water and aqueous systems. Nevertheless, new and sustainable strategies for providing suitable, highly oleophobic surfaces to lower fluorine presence have been reported from a small but growing body of literature.
1. Introduction
A green or sustainable approach to chemistry requires that unsafe products are banned or significantly lowered in their presence during chemical production and processes.
Most of the issues are not only related to economical costs, but are derived in terms of persistence, long-term degradation, and transformation in the environment with medium- to long term-effects on human health and wildlife [1].
High water and oil repellence has become a recommended feature in daily life, and industrial and research fields, such as self-cleansing, anti-icing, and anti-biofouling. Such specific surface properties are related to the presence of a particular geometry or surface structure combined with low-energy materials conferring their unique wettability features. These characteristics, in certain cases, can be easily manipulated or obtained through complex procedures, often involving both inorganic and organic chemicals according to the specific aim in a wide range of applications, such as biomedical, food, water treatment, solar panels, and fouling and corrosion in marine or harsh environments [2].
1.1. Surface Properties and General Principles of Wetting/Wettability
1.1.1. Interfacial Energy and Surface Tension
Interfacial energy is a measure of the energy required to create an interface or the force per unit length working along the interfaces at the triple line in equilibrium. Each system in an equilibrium state tries to reduce its energy, and these forces reflect this tendency. In general, the surface of a solid or liquid can be considered as a thin layer with different behaviours in respect to the bulk due to the high energy of surface atoms or molecules with respect to the interior atoms. At an interface, there are many molecules or atoms that have less binding with the nearby molecules or atoms than the atoms or molecules in the bulk, and the molecules at the interface have the potential and the necessity to make new bonds. Depending on their material composition, the interfaces exhibit different behaviours; high energy of an interface causes wetting of the solid surface and spreads the liquid on it while low energy produces non-wetting interfaces.
This excess of energy caused by the higher energy is called surface tension or free surface energy, identified by γ. The surface tension in the SI system is measured in N∙m−1 and interfacial energy is measured in J∙m−2. Thermodynamically, the concepts of surface tension and surface free energy are different.
Surface tension is the energy required to create a new unit area of the surface while surface free energy considers the breakage of intermolecular bonds that occurs when a surface is created. If it is possible to assume that the temperature and the pressure is constant, and that there is no adsorption at the interfaces, the two concepts are numerically equivalent to each other.
1.1.2. Polar and Non-Polar Substances and Intermolecular Forces
Some molecules, due to the composition or the geometry of the atoms, present a partial electrical charge; one side is positive, and the other side is negative. If a molecule shows such a configuration, it is called a polar molecule because it has two electrical poles. If the geometry and the composition do not allow the formation of poles, the molecule is nonpolar. An important consequence of this is that, in general, oil is a non-polar liquid [3] and is the main contributor to the surface tension London dispersion force. The situation for water (and for other molecules) is unique because it can form Hydrogen bonds. This is important because when water is in contact with a non-polar liquid/solid or air, the Gibbs free surface energy, ΔG = ΔH−TΔS, depends on the missing hydrogen bonds (excess enthalpy ΔH) and the entropic “hydrophobic effect” (excess entropy ΔS) due to the necessity of reorientation of hydrogen bonds. Because the entropic effect is stronger than the enthalpic effect, the water has a high surface tension of γ = 72.8 mN/m that is anomalous compared to other polar liquids [4].
1.1.3. Wettability Criteria
Adhesion is a term that describes the tendency of different particles or surfaces to stick to each other. This phenomenon depends on different types of attractive forces, such as van der Waals force, electrostatic force, chemical bonding, and the capillary force. Adhesion is an effect that is significant for micro- or nano-systems that have contacting surfaces. Adhesion force is important for the friction, mechanical contact, and tribological performance; for antifouling purposes, it is desirable to produce non-adhesive surfaces [5,6]
1.1.4. Contact Angle
The first feature used to characterise the surface wettability is the static contact angle (CA), which is the angle at vapour–liquid–solid interfaces. It depends on different factors, such as surface energy and surface roughness. Nowadays, the contact angle is usually measured using automatic software assisted by a goniometer that allows the spreading dynamics to be carefully followed. If the liquid has a static contact angle between 0° and 90°, it wets the surface (if the liquid is water the surface is called hydrophilic); if the liquid does not wet the surface (if it is water the surface is hydrophobic), the contact angle is between 90° and 180°, and the extreme state of water repellence can be observed with θ > 150° (Figure 1). This important thermodynamic quantity is applicable not only to solid–liquid interfaces but also to the interface between two different liquids.
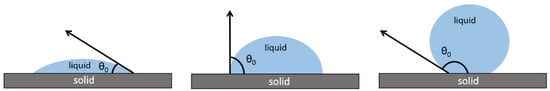
Figure 1.
Contact angle of a water drop on a hydrophilic, a hydrophobic, and superhydrophobic surface.
For non-polar substances, such as oils and organic liquids, we talk about oleophobicity as the behaviour of a surface repelling these kinds of liquids. In particular, the extreme situation in which a surface can repel, in addition to water, non-polar liquids, such as oils and organic liquids, is called superamphiphobicity. This behaviour is important for applications in non-aqueous environments such as polluted environments and in marine applications where oils or immiscible systems are present.
In nature, many surfaces are superhydrophobic or more oleophobic (oil CA > 90°) but no natural surface exhibits superamphiphobic behaviour [7,8]. A superamphiphobic surface shows a CA > 150° with both water and oils. The creation of superamphiphobic surfaces is more difficult because of the preparation required; it is not sufficient to combinate rough surface energy with low surface energy, but it is also necessary that the micro-nano structure has an overhanging structure [9]. The proper roughness can create a capillary force that prevents liquid from entering the grooves on the surface. From study [10], it emerged that the capillary force increases with the increasing of the interacting particles radius. This means that the liquid droplets cannot easily penetrate or diffuse into a small gap (<2 nm) on a liquid-repellent surface. In conclusion, it is necessary that a surface with low surface energy has dual scale roughness with a very small distance between protrusions, i.e., a surface with pores < 100 nm [11].
1.1.5. Contact Angle Hysteresis
The concept of the static contact angle is valid for an ideal and homogeneous surface. In a real situation, the measure of the contact angle is more complex because the system is not ideal due to the heterogeneity and roughness of the surface. Contact angle hysteresis is an important characteristic of a solid–liquid interface that considers roughness and heterogeneity.
The CAH is a measurement of the energy dissipation during the flow of a droplet on a surface. In the literature it is reported that, if the surface has a controlled roughness at the nanoscale level, the CAH is lower than 5° [12], which implies that the liquid has an almost negligible friction with the surface. In general, the hysteresis is impossible to eliminate but, by suitably controlling the surface pattern, it can be almost completely reduced. This feature plays a key role in fields like nano/microfluidics and self-cleaning materials.
1.1.6. Wenzel Model
A basic component of the Wenzel models is the interface area that, in respect to a smooth surface, increases. In the Wenzel state, the liquid is in contact with the entire solid surface and completely penetrates cavities
According to the Wenzel model, when r is greater than 1, a hydrophobic surface (CA > 90°) becomes more hydrophobic when roughness increases, and for a hydrophilic surface (CA < 90°), this means hydrophilicity increases [12,13]. This model can only describe surfaces with CA up to 120° such as Teflon.
1.1.7. Cassie–Baxter Model
The Cassie–Baxter model can describe the superhydrophobic state, i.e., CA with water larger than 150°, and to obtain a surface with this feature, many approaches have been used. This feature is important in all applications that need to avoid contact with water. As reported, this behaviour can be achieved combining a micro patterned roughness with hydrophobic coatings on a surface [14]. This result can be achieved in two ways:
- -
- making a rough surface from a low surface energy material.
- -
- modifying a rough surface with a low surface energy material.
The combination of dual scale roughness and low surface energy material minimizes the adhesion of water molecules on superhydrophobic surfaces.
The importance of a superhydrophobic surface particularly correlates with the self-cleaning effects [15].
1.1.8. Cassie–Wenzel Transition
In particular conditions, the superhydrophobic Cassie state could be destabilized turning into a Wenzel state. The transition from Cassie state to Wenzel state could be an irreversible event because the Cassie air trapping wetting regime corresponds to a higher energetic state compared to the Wenzel state, where the droplet is more energy favourable [12]. The transition from Cassie to Wenzel state could be a spontaneous or induced event. The transitions can occur in many ways, for example, by applying an external pressure or force on the droplet, vibrating the substrate, or by applying external stimuli.
1.2. Trends towards a Fluorine-Free, Eco-Friendly Transition
There are a wide range of materials and techniques involved in the development and customization of a specific surface feature. However, safety requirements related to human health put more and more restrictions on their use. The first materials to be banned are seemingly the long-chained fluorine compounds that play a primary role in building up highly water- and oil-repellent coatings due to the unique properties given to the surface by their low energy [16].
The risks related to the use of fluoro derivatives are at least twofold. First, chemical synthetic paths cannot be clearly classified as environmentally friendly because their by-products of degrade and accumulate, polluting natural resources like fresh water and endangering the food chain [17,18].
The primary risks are their potential to release perfluorooctanoic acid (PFOA) or perfluorooctanesulfonic acid (PFOS) as by-products of thermal treatment, which are still under investigation as harmful compounds when combined, with an even shorter chain. Bioaccumulation and permanence evidences [19] have forced government institutions like EPA in the USA or EU commissions [20,21] to impose serious restrictions toward investigations and innovative solutions for a more sustainable and health friendly fluorocarbon chemicals. The aim is to reduce side effects in humans and nature trying to maintain their features in terms of liquid repellence [22].
Nevertheless, we must also underline that the lack of sustainability sometimes goes beyond the ban of fluoro derivatives, because some complex procedures already require high energy methods, such as lithography, anodization, and chemical etching. These processes cannot be reasonably considered as sustainable [23].
In the following subsections, two main directions towards sustainable materials for liquid repellent coatings are addressed.
Fostered by new insights on lower toxicity in metabolic properties, C6-PFCs have shown faster excretion without accumulating in biological fluids and are more eco-friendly and sustainable for human health [16].
The literature related to this topic is, to date, not that extensive compared to its role in technology and research fields. There are a wide range of techniques and findings, including combination of surface geometry, such as nanofilaments, or re-entrant structures with chemical compositions, such as oxidized silicone. Moreover, it is a common character in the use of perfluorinated compounds (PFCs) that have raised several health and environmental issues [24]. The higher sustainability requirements of coating components has fostered research on suitable alternatives in surface chemistry that avoid the presence of long-chained, fluorinated compounds but keep the properties of oleorepellence. The presence of fluorine is often accompanied by a compromise between composition and performance. In fact, despite the risk assessment of long-chained fluorocarbon chemicals, finding steady and durable oleophobicity still requires more investigation to completely overcome their side effects. A combination with components able to create a suitable roughness and a limited use of low-energy compounds, even containing fluorine at lower degree of substitution, seems to be a good alternative strategy [24]. Higher costs and serious environmental issue foster research to find alternative ways to produce highly water- and oil-repellent materials that have definite sustainable features with superior industrial scalability and, importantly, cost-effectiveness.
Emerging studies demonstrate how extreme liquid repellence can be achieved without fluorinated compounds. It is worth mentioning that this final option covers recent research; still, a few basic rules must be studied over long-term applications. Their stability, the order of magnitude of surface energy, and the loss of the overall properties under certain conditions require alternative methods to be carefully checked, paving the pathway toward a fluorine-free world and restricting its use to a more limited group of applications in suitable waste management [25,26,27].
1.3. Natural or Synthetic: Stability and Sustainability
Recent literature proposes promising methods that can provide the highest performances in hydro- and, in a few cases, oleo-repellence using materials with environmental compatibility and biodegradability.
If, on one hand, the wide availability of waxes, fatty acids, or other natural compounds in nature promote the research of low-cost solutions and maintenance, then on the other hand, these categories still suffer from several issues related to their durability/robustness, chemical stability, and often low resistance to solvents or contact with acid and bases. Waxes, for example, are fairly soluble in organic solvents, such as chlorinated or hydrocarbons, and the low melting point, poor adhesion properties, and thermal stability make them unsuitable for applications requiring wearing resistance. Nevertheless, few of these drawbacks can be exploited for their dispersibility in water emulsions, alcohol solutions, or in combination with hydrophobic polymers. Moreover, they could be used for roughness control and filling porous substrates [28].
A more interesting category in view of its increased stability and less sensitivity to external factors, fatty acids represent an alternative choice. Their hydrophobic character, according to the carbon chain length, make them very popular and adaptable for different purposes because they are less soluble in organic chemicals, including alcohols. Even sharing with waxes, the low thermal and mechanical stability, they are widely employed for thin films with high adhesion and can undergo to electrochemical treatments as well as molecular modification holding to improved properties in terms of robustness and compatibility with inorganic ions or nanosized components [29,30,31].
Among the wide variety of solutions recently found to substitute fluorinated compounds but keep their unique performance, the combination of inorganic and organic materials is frequent choice. These mixed systems usually include the use of nanoparticles, mainly oxides (TiO2, SiO2, ZnO), but also recyclable polymers and an organic matrix of a low-energy, fundamental component for reducing adhesion, long alkyl chain thiols, natural waxes, polysiloxanes and silicones [28]. In these works [32,33,34], the authors used branched or ring-shaped organosilane monolayers, ultrathin layers of low-molecular-weight polydimethylsiloxane (PDMS), oligomeric layers from alkylmethyldichlorosilanes, and alkylsilane-derived sol–gel hybrid films that successfully controlled dynamic de-wetting behaviour and obtained highly comparable oleophobic properties (lowest hysteresis) with flexible and liquid-like behaviours.
The present review presents and analyses recent progress in the fabrication of non-fluorinated superhydrophobic and oleophobic coatings made by natural, biodegradable, and nontoxic, food-grade materials using techniques that do not involve the toxic solvents or economically expensive steps. In this context, most of the research is based on the use of waxes, fatty acids, silanes, and silicon-based compounds, but there are also works using other materials such as polyolefins, carbon nanotubes, and petroleum-derived polymers.
2. Superhydrophobic Coatings from Fatty Acids
Fatty acids, fundamental components of lipids, are molecules made of a long chain of hydrocarbon atoms (C > 13), aliphatic chain, terminating with a carboxyl group (—COOH). The aliphatic chain that constitutes them tends to be linear, and only in rare cases does it have a branched or cyclic form. The length and the degree of saturation of the chain is extremely important because it influences the physico-chemical properties of the fatty acid. For example, as it gets longer, the solubility in water decreases and, as a consequence, the melting point increases (higher consistency). Fatty acids and their derivatives are the natural substances most employed to replace fluorocarbon molecules to produce superhydrophobic surfaces. In particular, stearic acid (STA) and octadecanoic acid, are the most widely used due to their low cost.
Immersion is one of the most employed methods to obtain SHS using STA as a surface modification. Song et al. [31] prepared SH magnesium alloy with two immersion steps. In the first step, the substrate was treated with aqueous CuSO4 solution to obtain hierarchical micro-nano structures through the formation of alkali copper sulphate. The second immersion step was in STA/ethanol solution to lower the surface energy. The modified Mg alloy resulted in a superhydrophobic with CA > 151.3° and maintained its behaviour in the air for at least 8 months.
Following the same method, Si et al. [35] prepared a micro/nano sheet of Mg(OH)2 (MH) hydrophobized by immersion in STA/ethanol solution. The STA-MH SH powder was used to obtain SHS by spreading it on an epoxy coated substrate until a homogeneous layer was obtained. The epoxy resin @ STA-MH glass was rough and white, and had a CA of about 160° and low CAH. To assess the mechanical and chemical durability, different tests were conducted, such as a test with water at different pH levels (1–13), thermal treatment up to 120 °C, and irradiation with an artificial light source. A corrosion test in NaCl solution and an anti-icing test were also performed. The SHS maintained its behaviour for all performed tests; it delayed ice formation (in particular when epoxy resin @ STA-MH was applied on copper mesh) and has flame retardant capabilities (Figure 2).
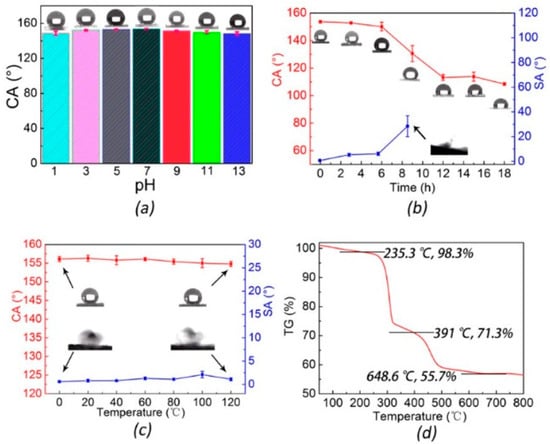
Figure 2.
(a) Column chart of CAs of acidic and basic aqueous solutions with pH values from 1 to 13 on coated glass slides. (b) Broken line graph of the CAs and SAs of coated glass slide sample after 0 to 18 h of strong UV-light exposure. Inset: corresponding optical images of CA and SA. Inset: optical images of CA and SA of the 0 and 120 °C samples. (c) Broken line graph of the CAs and SAs of coated glass slide sample stored in different temperatures from 0 to 120 °C for 2 h. (d) The TG of STA–MH powders to 800 °C. Reprinted with permission from [35], 2006, ACS Publications.
Recently, Kong [36] fabricated an SH “pipe” employing STA as a green energy surface reducer to improve the liquid transportation process. Phosphor copper mesh was used to create the pipe and, after cleaning, was immersed in aqueous stearate solution at room temperature. These conditions were necessary to allow the formation of nano-scale, petal-like structures with superhydrophobic behaviour (CA = 162.8°). It was observed that shorter immersion times did not allow the growth of dense and homogenous petal-like nano structures. Drop impact and immersion tests in distilled water showed low durability. The author finally tested the ability of drop loss free transportation, observing that the SH pipe did not stain, creating a very low material loss.
SH fabrics created by the two-step immersion method were produced by Dong et al. [37]. In the first step, using dopamine and CuSO4/H2O2 solution, the structure with micro/nano roughness was fabricated. Second, after drying, the roughened fabrics was immersed in hot stearic acid water emulsion at 75 °C. The obtained fabrics exhibited SH behaviour with low CAH, good mechanical/chemical stability, laundering durability, and UV shielding properties. In particular, the coating remained SH after 24 h of immersion in acid and alkali solution, maintaining CA > 150° for up to 5 laundering cycles, and after 1000 abrasion cycles.
Electrodeposition is a well-assessed method with wide application and in Wang et al. [38]. It has been used to prepare SH beryllium copper alloys for corrosion protection application. A water solution of STA, CuCl2, and HCl was prepared at 80 °C. When it was homogeneous, the Be–Cu alloy was used as a cathode and a graphite sheet as an anode were immersed. The electrodeposition process was carried out for different times to study the effect on roughness and wettability. The authors found that the surface roughness increased linearly with time but the maximum repellence, CA = 163°, was reached when the deposition time was 10 min, avoiding the undesirable distribution change of STA on the surface. Corrosion resistance was studied by potentiodynamic polarization curves and electrochemical impedance spectroscopy in NaCl solution. From these tests, it was observed that the modified SH alloy was 25 times more resistant to corrosion than the unmodified alloy.
Wen et al. [39] prepared SHS using eggshells from waste and ZnO combined with STA. They choose these raw materials to use only low-cost substances and to be more sustainable. Eggshell is a source of CaCO3, a compound widely used to prepare sustainable SHS. The authors dissolved STA in ethanol, and then eggshell powder and ZnO were added in different amount. To improve particle adhesion to the substrate, carboxy methyl cellulose (CMC) was added to the solution as adhesion agent. Once applied, the coating showed different properties depending on ZnO amount, as it contributes more to the coating roughness. Wettability was found to be dependent on the ZnO concentration (highest water CA 152.5°). To test environmental application, the STA SHS was UV irradiated with an artificial UV light source remaining SH up to 24 h. Also, anti-icing properties were tested, recording that the ice over time of SH glass was 237% longer than uncoated glass. Furthermore, the coated glass was resistant to a knife-scratch test without losing superhydrophobicity.
Xu et al. [40] fabricated an SHS via spin coating and functionalization to improve Al corrosion resistance. First, Al substrate was coated with epoxy resin. After that, a copper mesh, with a dendrite-like structure obtained by immersion in AgNO3 solution, was pressurized on resin. After this step, the mesh was peeled off and the Al surface was immersed into STA solution, obtaining a surface with CA = 154° without hysteresis. The prepared SHS seems to be sensitive in the extreme ranges of acidity and alkalinity with SH behaviour disappearing when pH > 12. Corrosion protection of epoxy/STA coating was evaluated by polarization curves conducted in NaCl solution, evidencing lower corrosion current density and higher polarization resistance with respect to pristine Al substrate.
Also, Alexander et al. [41], in their study on sustainable SHS, replaced fluorocarbons (FCs) with STA and isostearyl acids, comparing performances of both with traditional FCs SHS. Alumina NPs (avg. particle size of 13 nm) were refluxed overnight in toluene and carboxylic acids, fatty or FCs, and after centrifugation re-dispersed in 2-propanol. The NP dispersion was sprayed or spin coated onto glass substrate at 80 °C and then characterized. The two deposition methods and chemicals equally create surfaces with similar nanometric roughness and similar CA, particularly for spray-coated, branched fatty acids, CA was about 153°, comparable with CA of FCs prepared SHS (157°).
Semi-transparent and superhydrophobic film was obtained from Geissler et al. [42] using a fatty acid ester: cellulose stearoyl ester (CSE) derived from stearoyl chloride. CSE nano microparticles, with an average diameter of 102 nm, were obtained from nanoprecipitation of CSE dichloromethane solution in acetone or ethyl acetate and via spray coating, spin coating, or solvent casting, were deposited on a glass slide showing water CA = 158° and CAH = 5°. Due to this behaviour, a jet of water was completely repelled by the surface. The SHS was stable in air more than 2 months and under water for at least 96 h.
Biobased and biodegradable SH coating on cotton fabric, which could be used as O/W separation material, was fabricated by spray coating/immersion by Cheng et al. [43] using STA as a hydrophobic agent. The authors prepared and deposited an adhesive solution using only renewable and biodegradable materials such as epoxidized soyabean oil (ESO), sebacic acid (SA), and a green catalyst. Subsequently, ZnO NPs dispersion were prepared and sprayed on the adhesive layer. The coated cotton was dried and cured at 150° and then immersed in an STA/ethanol solution. The STA-coated fabric showed water CA = 155°. It was able to repel some liquid food and its behaviour remained unchanged after immersion in water and silicone oil for as long as 10 days with low and constant CA decreasing. Furthermore, it resisted acid and alkali drops in the pH range 1–13 and can efficiently separate various oil/water mixtures with a separation efficiency of more than 97%.
Oleic acid (OA) and octadec-9-enoic acid, mono-insatured fatty acids with an alkyl chain composed of 18 carbon atoms, are also used to produce SHS and are often employed to functionalize oxide or carbonated particles to obtain low wettable surfaces. Sabry et al., [44] using a hydrothermal method, synthesized ZnO nanostructures (30 nm) that were then functionalised by an OA/ethanol solution. When the solution was homogeneous, it was drop casted on a glass substrate and dried, reaching superhydrophobicity when the OA weight in 10 mL of solution was >4 mg, yielding full coverage of ZnO particles producing nanorods and nanotubes (Figure 3).
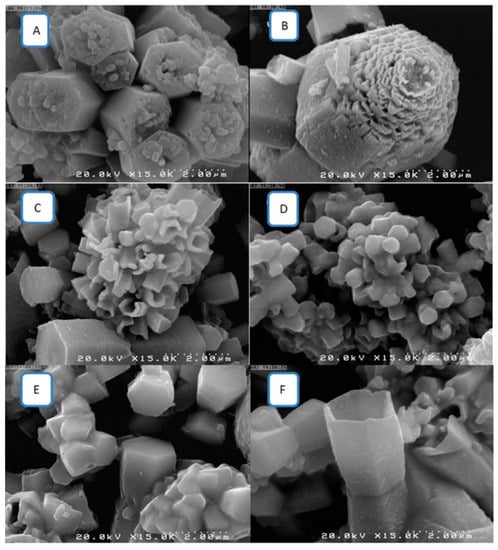
Figure 3.
FESEM images of ZnO surface morphologies (A) before, and modification using oleic acid with different weight ratios (B) 2 mg, (C) 4 mg, (D) 6 mg, (E) 8 mg, (F) 10 mg [44].
Oleic acid modified CaCO3 particles were used by Zheng et al. [45] and Hu et al. [46] to prepare SHS. The first ones achieved the result via precipitation in hot water an oleic insoluble calcium salt on carbonate that was then mixed with PDMS, to improve adhesion, and used as a solution for dip coating. The prepared SHS exhibits water CA = 167° only when the ratio between CaCO3/PDMS was 40/60; other combinations cannot provide appropriate surface characteristics for superhydrophobicity. In the second paper, precipitated calcium carbonate (PCC) was dispersed in hot water where OA was added and stirred to form an insoluble calcium salt on PCC surfaces. The SHS was created by spreading dry AO @ CaCO3 powder on glass covered with adhesive tape, but the hydrophobicity was dependent on the OA content, reaching the maximum value of CA = 164°, for 2.51 wt% OA, after which CA started to decrease.
Fatty acids modified CaCO3 nanoparticles used to create by spray SHS were prepared by Atta et al. [47]. In this work STA, OA, linoleic (LA) and linolenic (LNA) acid were used as a hydrophobic capping agent, and epoxidized oleic acid (EOA) was used to study its effect on CaCO3 wettability. Some differently modified CaCO3 NPs were produced, and it was observed that the capping agent had influence on morphology and final CaCO3 wettability. To coat the substrate, CaCO3 was dispersed in different amounts in epoxy resin and then sprayed. From CA measurements was observed that CA > 150° only for epoxy resin with 5 wt% CaCO3/EOA. For other CaCO3 combinations, the superhydrophobicity was not reached.
In the following section, there are reported papers where fatty acids other than stearic and oleic are used.
To avoid metal corrosion, Xiang et al. [48] developed a method that, through electroplating and surface modification with myristic acid, has resulted in a Zn–Ni–Co SHS. The fabrication method followed different steps, not always cheap, to create the right surface texture necessary to obtain superhydrophobicity before metal sample immersion in myristic acid/ethanol solution. After chemical modification, the metal substrate goes from strongly hydrophilic to superhydrophobic, maintaining its wettability after immersion in a salted solution and in pH range of 1–13. Electrochemical quantity measurements demonstrate the highest corrosion potential and lowest corrosion density of SH samples compared to the uncoated one.
An environmentally friendly method to produce SHS using fatty acid and green materials has been developed by Razavi et al. [49]. Sepiolite nanoparticles and cinnamic and myristic acid were used to obtain a functionalized material able to reduce the surface energy of the coated material. The capped NPs were dispersed in water based on perfluoroalkyl methacrylic copolymer (carbon atoms < 6) to obtain a stable dispersion in which it was possible to coat, by dip coating, different materials. The SH layer was thick (300 nm) and showed CA at about 160°. SH behaviour was maintained during thermal treatment, and immersion in acid and alkaline solution decreased the pH extremes range (Figure 4). An abrasion test with sandpaper showed that the coating immediately loses the superhydrophobicity but keeps the high hydrophobicity up to 30 abrasion cycles. Furthermore, the coating antibiofouling behaviour was tested with Gram-negative and Gram-positive bacteria, showing an inhibition of bacterial attachment rate of less than 10%.
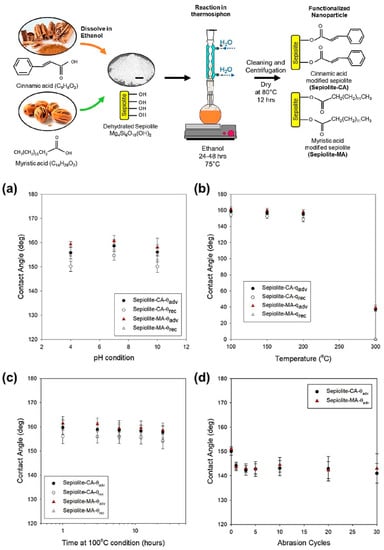
Figure 4.
Functionalization procedure of the sepiolite nanoparticles with CA or MA. Step (1) dehydration of the sepiolite particles, (2) acid solution preparation with ethanol, (3) functionalization reaction in a thermosiphon, and (4) cleaning and separation of nanoparticles from the reacted solution. Scale bar in the dehydrated sepiolite image is 2 mm. Durability of the coating as characterized by the apparent advancing and receding contact angles (a) after full immersion in different pH conditions (pH 4, pH 7, and pH 10), (b) at elevated temperature conditions up to 300 °C, (c) as a function of time during exposure to 100 °C in atmospheric conditions for up to 24 h, and (d) after repeated mechanical abrasion cycles. Measurements were done on 5 independent spatial locations for each sample. Error bars represent standard deviation of the 5 independent measurements. Reprinted with permission from [49] 2019 ACS Publication.
Bai et al. [50], by using the one-step dip coating method, were able to obtain different SH materials using hydrogenated castor oil (HCO). Porous structures formed by clusters of spherulites were obtained by dip coating the substrate in HCO ethanol solution. The HCO/ethanol concentration and the kind of substrate depended on the final CA. All covered substrates reached CA > 150° and generally a decrease of the contact angle value was observed at a certain concentration and temperature for each substrate.
Castor oil (CO) derived superhydrophobic cotton fabric and was fabricated by Shang et al. [51] by spray coating and UV photoinitiated thiol-ene chemistry. Firstly, CO-based thiolated oligomer (CO–SH) was synthesized by a two-step reaction and was successively mixed with octavinyl polyhedron oligomeric sesquiloxane (Octavinyl-POSS) as an ene group acceptor, and hydrophobic SiO2 NPs to construct the hierarchical micro/nano structure, and 2,2-dimethoxy-2-phenylacetophenone (DMPA) as photo initiator, obtaining a thiol-ene matrix. This matrix was sprayed on fabrics and then UV cured to obtain a tunable superhydrophobicity as a function of NPs concentration. SH behaviour and mechanical stability was tested with different methods, such as sandpaper abrasion cycles, ultrasound, and laundering cycles. Furthermore, for a possible application in water/oil separation process, separation efficiency was tested with oil/water emulsion and mixtures observing ultrahigh efficiency higher than 99.990%.
Palmitic acid (PA) was used in Agrawal et al. [52] as a surface energy lowering agent. PA/ZnO NPs precipitated in ethanol solution and, after washing, were deposited on appropriate substrates obtaining water CA close to 161°. The thermal test up to 130° showed the limited resistance of the coating because of the loss of PA from the ZnO surface due to the beginning of PA decomposition.
Lauric acid in Ayra et al. [53] was reported for the preparation of SHS in a water harvesting process. Flower-like SH microstructure was obtained on Al substrate by electrodeposition conducted in an electrolytic bath with lauric acid and MgCl2 in an ethanol solution, forming magnesium laureate. The authors observed that the SH behaviour was maintained after UV exposure and sandpaper abrasion cycles, despite having a slightly increased CAH but remaining <10°. The water collection rate increased for both SH samples and was more pronounced at a 90° position instead of references substrates unable to collect water.
Finally, the work of Heale et al. [54] reports that the authors studied the effects of fatty acid alkyl chain length on wettability of coated hydrophobic SiO2. They used octanoic, decanoic, dodecanoic, hexadecenoic, and octadecanoic acids that were separately mixed in ethanol with SiO2 particles (0.5–1.0 mm diameter) and hydrophobic surfaces were obtained for drop cast or spray coating on a glass substrate. In general, it was observed that the drop cast method produced more hydrophobic surface with respect to spray coating, except for octanoic acid and CA which increased with the length of the alkyl chain. All prepared hydrophobic surfaces were preserved after an oil immersion test and after six months of storage at room conditions.
As can be seen from numerous publications, the use of fatty acids for the production of SHS is widespread, in particular for functionalization via stearic and oleic acids. The use of fatty acids involves some disadvantages:
- Low thermal resistance and poor mechanical stability unless substances that increase adhesion to the substrate are used (CMC, epoxy resin);
- Low resistance at high pH range;
- Extensive use on metals requiring treatment to create suitable micro-nano roughness (chemical corrosion, electrochemical process);
- Temperature higher than melting point (T > room temperature) to obtain dispersion or solution.
- However, the use of fatty acids presents several advantages:
- They are easily soluble or dispersible in alcohol (ethanol and isopropanol) and hot water;
- Fatty acids can chemically bind to surface and oxide nanoparticles;
- They can be applied via easy, cheap, and scalable methods (immersion and spray coating).
3. Superhydrophobic Coatings from Waxes
Waxes are lipids in which there is an ester bond given by the union of long-chain carboxylic acids (C14 to C36) and long-chain alcohols (C16 to C30); the length of the chain, the degree of unsaturation and the presence of branching determine their chemical and physical properties. The ester bond leads to the formation of a molecule in which the hydrophobic part is preponderant over the small hydrophilic head, so the waxes are insoluble in water but soluble in apolar solvents and are water-repellent. They are harder and less greasy than fats and are resistant to moisture, oxidation and microbial degradation. In nature for these properties, they play a protective role against water by coating some fruits and leaves, lubricating and waterproofing feathers and skins of some vertebrates.
Some of the most important plant waxes include: candelilla, carnauba, castor, ceresin and ozokerite waxes, Japan wax, and palm and soy waxes.
In animals, waxes are produced by special glands and are generally found in the covering tissues. They may have a protective function, such as the wax secreted by the steatopygium gland of birds making feathers impermeable to water; a plastic function, as in bees; or a floating function, such as the spermaceti found in the head of the sperm whale.
There are also synthetic waxes mostly obtained by the processing of mineral oil, in particular from the different stages of lubricating oil distillation. Petroleum waxes are hydrocarbons, mixtures of alkanes usually in a homologous series and are classified on the basis of their average molecular weight. Millions of tons of paraffin waxes are produced annually. They are used in foods (such as chewing gum and cheese wrapping), in candles and cosmetics, as non-stick and waterproofing coatings and in polishes.
Plant-based components were employed by Morrissette et al. [28] to prepare different superhydrophobic surface by spray coating without the use of fluorinated compounds combining different water based polymeric dispersion (polyolefin dispersion, alkyl ketene dimer emulsion, carnauba wax and beeswax suspension) to decrease the surface energy of the substrate and natural filler material (microcrystalline cellulose MCC and lycopodium spores L) to obtain the adequate surface roughness. All tested combinations have shown static CA > 150° but the best result was obtained for lycopodium-laden coatings with a natural wax binder (carnauba wax or beeswax) while for some the CAH was larger obtaining coatings displaying water adhesion.
Also Wang et al. [55] fabricated superhydrophobic coatings employing carnauba wax and beeswax by spray coating wax-in-acetone emulsion on glass substrate. The surface became SH with good CAH when the coating surface density was >0.55 mg cm−2 (Figure 5). To mimic edible liquids with different surface tension the authors employed water with various concentrations of sodium dodecyl sulphate (SDS), they found that the SHS maintained its behaviour for liquids with surface tension >45 mN m–1. The coating was tested using 3T3-J2 murine embryonic fibroblasts as a standard method for assessing any potential material toxicity [56,57] and it was observed that SHS are nontoxic to cells at coating concentrations up to 4 orders of magnitude higher than the maximum expected coating concentration due to leaching.
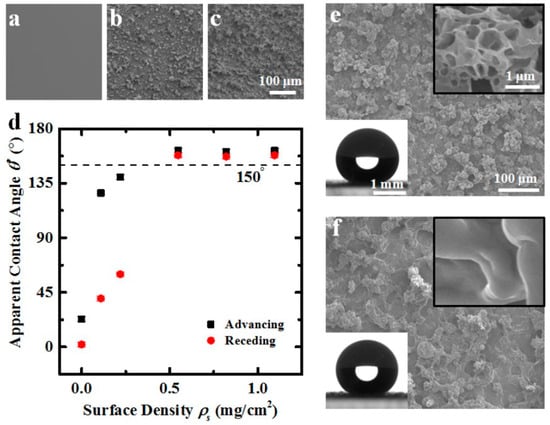
Figure 5.
(a–c) Glass slide coated with 0, 0.2, and 0.55 mg cm–2 emulsion, respectively. (d) Advancing and receding apparent contact angles of water on a glass slide coated with varying ρs of carnauba wax. The surface becomes superhydrophobic when ρs ≳ 0.55 mg cm–2. (e,f) Scanning electron microscope (SEM) images of glass slides coated with 0.55 mg cm–2 of carnauba wax and 1.1 mg cm–2 of beeswax, respectively. The upper right inset of each frame shows a higher magnification image of the microscale structures and the lower left inset shows θ* > 150° for both coating materials. Reprinted with permission from [55] 2016 ACS Publications.
A combination of natural and synthetic solutions can be found in Torun et al. [58] and Celik et al. [59] using carnauba wax with PDMS. In the first paper, it is co-dispersed in ethanol and applied by spray to obtain a homogeneous and textured coating.
In the second paper, the authors prepared a biocompatible and eco-friendly SHS by applying a colloidal wax solution by spray coating on a PDMS film (tens of microns) with a microscale textured surface obtained by transferring the surface structure of paper foil. The wax-coated PDMS achieved superhydrophobicity by rubbing the surface under a load against an Al foil. In this condition, the wax-coated PDMS exhibited CA = 169° and SA = 3°. Resistance and durability were studied against water impact and stream as well as whole blood impact, observing that the surface maintains its extreme non-wettability under different tests. In this paper, the authors use only biocompatible materials and cheap and scalable techniques to obtain robust and durable SHS.
The use of the spray, given its simplicity and cost-effectiveness, is also used by Liu et al. [60] to prepare SHS with edible waxes, candelilla. and rice bran on polypropylene (PP) substrate for food packaging purposes. The SH behaviour increase was found to be dependent on the surface density of the wax. Edible SH coating showed repellence to various food liquids and the worst wettability is observed for non-Newtonian fluids like yogurt, when CA = 150.1°. The coated PP maintained its superhydrophobicity when exposed to hot water, after finger touch, multiple abrasions with sandpaper, and repeated bending.
Zhao et al. [61] report the preparation of superhydrophobic coating using different waxes like paraffin wax, beeswax, microcrystalline wax, and carnauba wax. To enhance mechanical durability of the coating, it was necessary to anneal the coating at 40 °C. The paper reports the CA of each wax SHS but the mechanical and durability tests were reported only for the paraffin wax coatings of a particular performance after test. A few liquid foods were tested on paraffin coating, and all have shown CA > 150° with some difference in the sliding angle.
Superhydrophobic coating from natural waxes was also produced by Niemietz et al. [62] from Tropeaoleum majus leaves by thermal evaporation at 140 °C. This was constituted exclusively by aliphatic compounds. Superhydrophobicity of the prepared samples was only found after they had been stored at 50 °C for a minimum of 15 h (CA = 154.7°). This phenomenon has been explained by the fact that over time, temperature induces the recrystallization of nonacosanol (secondary) alcohol predominant in the T. majus wax tubules. As the annealing time increases, the structures grow, resulting in surfaces with higher CA.
Similar to the previous work, Yang et al. [63] used a thermal treatment, in this case at ambient pressure, to obtain superhydrophobic material. The authors have employed different food-grade starting materials such as lard, beeswax, and food-grade paraffin obtaining SH coatings. Considering that the work is aimed at producing coatings for use in the food industry, numerous tests have been carried out to evaluate their durability. The coatings can maintain their SH behaviour after air exposure and immersion, even if water impact (>1 ms−1) mechanically damaged the surface and most of the tested liquid foods (γ > 61.62 mN m−1) were repelled by the coatings, leaving almost no residue when the liquids flowed over the coated surfaces.
Pechook et al. [64], working on different n-paraffin waxes and their combination, demonstrated the importance of the formation of hierarchical roughness by subsequent deposition of wax layers. The formation of SHS was achieved by a sequential process of thermal evaporation of different C2nH2n+2 waxes, finding the best repellence with CA = 171° obtained for the combination of C36H74 + C50H102. When increasing wax molecular weight and successive spontaneous recrystallization at room temperature, lower Mw waxes grew larger micro crystals, and higher Mw waxes produced nano roughness due to low-particle mobility at mild temperature conditions.
With the aim to produce nano roughness from micrometre spherical structures, the annealing process after wax coating deposition was also used by Zhang et al. [65]. In this case, double scale roughness was achieved by a phase separation process in which annealing was conducted at a temperature such that one of the wax blend components remained solid while the beeswax melted. If the carnauba wax content was too high (>70%), the formation of the sub-micrometre texture in the wax mixture beads did not occur. The prepared coating on paper substrate maintained its behaviour for at least 6six months in atmosphere conditions.
Regarding matching microscale crystal growth and inorganic nanoparticles, Guan et al. [66] reported a superhydrophobic coating preparation method by dip-coating substrate in a suspension of silica (SiO2), hydroxyl acrylic resin, cross-linking agent, and polyethylene wax (PEW), controlling the cooling and dry process. After 24 h, the PEW whiskers grew and uniform carpet-like hierarchical structures were obtained, which were able to repel some liquids (orange juice and vinegar), and was resistant to cyclohexane contamination and sandpaper cycle abrasion under loading.
A similar mixed organic–inorganic approach is found in the work of Atta et al. [67] where the authors used paraffin wax (n-alkanes with 18–38 carbon atoms) not as coating on a surface but to create capsules of hydrophobic silica nanoparticles as coatings for desert sand using an emulsion technique for developing superhydrophobic sand for desert water storage and transportation. Different types of silica nanoparticles were made and, when coated with paraffin wax, the authors obtained modified desert sand with water CA = 165°, a good water-holding capacity, and high thermal stability.
Natural and synthetic waxes, like fatty acids, are widely used to prepare sustainable SHS and the articles reviewed in this paper show that the most commonly used waxes are carnauba and beeswax. Some issues regarding the use of waxes for SHS preparation are in the following list:
- Waxes have low melting point and produce coatings are not suitable for high-thermal application;
- Slight changes in temperature can modify wax texture and morphology;
- Wax coating does not have high adhesion nor mechanical and abrasion resistance.
- Nevertheless, the use of waxes for the production of SHS has several advantages:
- Waxes, due to their low melting point, can be easily emulsified with water and dispersed in ethanol;
- Waxes can be applied by spray coating (emulsion, dispersion) but also by thermal evaporation and dip coating without the employ of further solvent;
- The micro nano roughness necessary for superhydrophobicity can be achieved directly from wax crystallization (as it occurs in nature) without the use of nanoparticles;
- Liquid wax can impregnate porous substrate.
4. Superhydrophobic Silicon-Based Coatings
In the biocompatible preparation of highly water-repellent surfaces, numerous Si-based compounds such as silanes (tetraethoxysilane—TEOS, octadecyltrichlorosilane—OTS, methyltrimethoxysilane—MTMS, etc.) and polysiloxanes (PDMS, etc.) are used. Silicon-based compounds owe their wide use to their low surface energy, resistance to high temperatures and the possibility of application through various techniques (chemical vapor deposition, spray coating, and spin coating) [68]. Silicon-based compounds are also biocompatible and are therefore often used in the medical and cosmetic fields. In this section, only some works regarding the preparation of silicon-based SHS focusing on those with more sustainable materials and methods are reported.
The immersion process was employed by Lyu et al. [69] to prepare glass SH coating with CA larger than 160°, including coating a glass substrate by epoxy resin and the preparation of octyltrimethoxysilane-modified hydrophilic silica particles. Epoxy-coated glass was immersed in warm water and then modified silica particles were added to the water and stirred to form a uniform layer via a self-assembly process based on hydrophobic interactions. Prepared SHS underwent a wide range of tests to assess its employ for outdoor applications. The surface proved to be SH after the impacts of sand and water jet were applied beyond the annual precipitation in the tropical areas and after immersion in H2SO4 5M to simulate acid rain and also after exposure to UV illumination.
Fu et al. [70], in their work, presented the fabrication of SH aluminium alloy employing an ethanol solution of decyltriethoxysilane (DTS) as a surface energy lowering agent. Before chemical modification, Al alloy was roughened with metallographic abrasive papers and etched in an aqueous solution of Cu(NO3)2/HNO3. Without using the abrasive papers, the CA was 146.8° due to a smoother surface with respect to the SH one and the concentration of the etching solution plays a key role in hydrophobization. The SHS Al alloy maintained its behaviour, with little variations of CA, after five months of storage in the air, demonstrating long-term stability in ambient condition.
Wang et al. [71], working on the surface roughness modification, obtained SH coating with high transmittance. In particular, the work was focused on the solvent effect on the dispersion of fumed silica NPs so that the aggregation and degree of clustering could be controlled. SiO2 NPs, added to the chosen solvent, were mixed with MTMS and HCl, and then the sol-gel solution was spun coated on glass substrate. The authors found that the best transmittance was obtained using polar solvents, i.e., alcohols, while CA > 150° was reached only using hexane, heptane, and ethanol. Considering the same silica size (12 nm), the highest hydrophobicity was obtained using hexane, CA = 160°, but a slight CA increase was observed for silica/ethanol dispersion with a silica size of 40 nm. This study demonstrated the importance of surface roughness on wettability and transparency, as well as obtaining sustainable SHS.
Similarly, a transparent SH coating by green route was fabricated by Li et al. [72] using N-Boroxine-PDMS and SiO2 NPs. N-Boroxine-PDMS was synthesized from aminopropyl-terminated PDMS (NH2-PDMS-NH2) and 2-formylphenylboronic acid (PBA) dissolved in ethanol with a long, multi-step procedure. The obtained N-Boroxine-PDMS dissolved in ethanol was then mixed with SiO2 NPs, and after stirring the mixture was manually sprayed on hot substrate to obtain an SHS. After 10 spray cycles, the surface showed CA = 160.8° and transmittance > 95%, increasing the cycles and making the coating become less transparent and slightly more SH. An important feature of this coating was its self-healing ability when it was exposed to H2O2 or O2 plasma at room temperature because the conditions allowed the oligomers of N-Boroxine-PDMS readily migrated to the damaged surface.
Wu et al. [73] prepared a silicon-based superhydrophobic coating with hierarchical micro/nanostructures by electrodeposition and immersion in a silanizing agent (dodecyltrimethoxysilane (DTMS)/water solution). The CA increases from about 97° on bare substrate to over 150° on treated substrate after seconds of immersion. Chemical resistance was tested by immersion in solutions with pH from 2 to 13 for 24 h and the coating remained superhydrophobic.
We will now present some works using silicon-based compounds as raw materials, but with some drawbacks regarding sustainability due to either the methods or the solvents employed.
Liu et al. [74] presented the fabrication of a PVA/SiO2 polymer SH coating on wooden substrate. SiO2 particles were prepared following the Stöber method, using TEOS as a precursor and then mixed with PVA/water solution. Wood was coated by drop casting, becoming superhydrophobic after the OTS/hexane functionalization, with good mechanical stability in an abrasion test (Figure 6). It was observed that abrasion test influenced CAH but not CA (always > 150°), making the surface stickier due to the change in microstructure orientation. In this procedure, a slight problem related to sustainability is constituted by using hexane, which is not considered a green solvent.
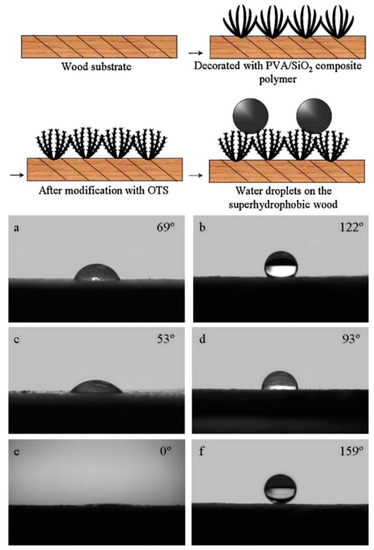
Figure 6.
A schematic diagram of the forming process for superhydrophobic wood and images of water droplets on different surfaces: (a) the pristine poplar wood surface; (b) the wood surface modified by OTS reagent; (c) the wood surface covered with pure PVA; (d) the wood surface coated with pure PVA then modified by OTS reagent; (e) the wood surface coated with the PVA/SiO2 composite polymer; (f) the wood surface coated with the PVA/SiO2 composite polymer then followed by OTS modification. Reprinted with permission from [74] 2013 Elsevier.
A combination of silica NPs and silanes was also found in Wang et al. [75] where the authors prepared a SHS by dry-brushing SiO2 NPs functionalised with octadecyltrimethoxysilane (OTMS) dissolved in toluene on a previously prepared PDMS layer. Prepared SHS, when deposited on glass, showed transmittance of about 85% and corrosion resistance when applied on metallic substrate. Authors tested mechanical and thermal stability, observing that the low wettability was maintained for up to 10 cycles of sandpaper abrasion and after heating at 360 °C, but mechanical stability decreased at 320 °C due to the pyrolyzation of PDMS. Nevertheless, the authors claim the method to be environmentally friendly (fluorine free, easy deposition method) but the use of toluene and hexane (PDMS dissolution) as solvent means that the method cannot be considered entirely sustainable.
In the next papers, the limit to sustainability was due to the preparation method in which high temperatures were necessary to reach superhydrophobic condition.
A non-toxic method to obtain SH Al alloy sheets with anti-icing behaviour was developed by Li et al. [76]. First, cleaned Al alloy sheets were irradiated two times with a laser to create micro-cavities; then, the samples were immersed in a CuCl2·2H2O solution to create a cauliflower-like, microsphere complex structure. After these steps, chemical vapor deposition of PDMS was performed on substrates at different temperatures up to 220 °C, obtaining the highest water CA, 155°, at 180 °C. The authors investigated the effect of temperature on the coating, losing its SH features at 400 °C, but recovering them after air exposure for 120 days with increased CAH. Anti-icing properties were also tested, observing lowest ice adhesion strength and best de-icing process in the highest SH sample.
Tuvshindorj et al., in [77], prepared SH organically modified silica (ormosil) coating through the use of MTMS dissolved in methanol. To obtain SHS, ormosil colloids were spin-coated two times on glass and then cured at 450 °C for 1 h. Double-layer SH coatings show nanometric roughness, a water CA of 169°, and partial transparency with transmittance up to 85% at 800 nm. Furthermore, the authors in this work investigated the Cassie–Wenzel transition using different ormosil surfaces (roughness and wettability were the discriminating factors) demonstrating that the SH coating showed a Cassie to Wenzel transition under the external pressure of 1600 Pa with respect to 80 Pa of SH ormosil coating without the double layer porous structure.
Shen et al. [78] used silicon materials to create SHS in a different way than the previous works. They burned small pieces of PDMS in the flame of an alcohol lamp until the silicon was completely burned, and the combustion product had superhydrophobic behaviour when sprinkled randomly on a surface. The coating was obtained by hot-pressing the powder on the substrate, and it maintained its SH behaviour after 50 cycles of the sandpaper abrasion test.
Silicon-based SH coatings are the most widely used and studied materials due to their low surface energy after fluorinated compounds. In particular, as already stated, they are considered biocompatible and eco-friendly materials. The employ of such compounds presents some drawbacks:
- Chemical reactions that sometimes take many hours to complete;
- Silicon-based compounds often do not dissolve in green solvent;
- Thermal treatments at high temperatures are often necessary to obtain the desired properties.
However, silicon-based materials present many advantages, so they are applied in different fields:
- Covalent bonds within the coating matrix enhance film integrity;
- Strong adhesion to most substrates, and high chemical, thermal, and UV resistance.
- Possibility to obtain transparent and SH coating;
- Several application techniques.
5. Superhydrophobic Coatings from Other Substances
Many other substances can be used to achieve superhydrophobic properties and, at the same time, sustainable surfaces. These include carbon nanotubes and polymers of various natures. A common feature of these substances is that they can lower the surface energy of the system to which they are applied, thanks to an adequate roughness of the substrate.
Biocompatible poly(ethyl α-cyanoacyrylate) (PECA) was used by Li et al. [79] to obtain a superhydrophobic surface with a wetting gradient. This result was achieved by vapor phase polymerization using water droplets as a template and initiator. The reaction was carried out on a glass substrate covered by a monomer under the nozzle where the water vapour was blown out. After the reaction and drying process, microparticles crimpled, forming microballoons or microcups depending on polymer shell thickness. CA > 150° was obtained for a porous network of microballoons and, when the majority of the sample consisted of microcups, the surface was not rough enough to reach superhydrophobicity (CA = 120°).
Mates et al. [80] obtained sustainable and environment friendly SHS by using hydrophilic TiO2 nanoparticles and polyolefin copolymers through the spray coating technique. Both substances are considered safe and FDA approved, and the deposition method is one of the most sustainable. The authors have studied the influence of the TiO2 particle size and phase (anatase, rutile, or mix phase) on the final surface wettability, finding some important differences, such as surface adsorption kinetics of anatase with PE water with respect to rutile and mixed phase influencing the aggregates dimension. In conclusion, the best performance was obtained from the suspension of the smallest nanoparticle mixed phase with mass fraction between 0.6–0.75. It was stable for several weeks produced spatially uniform coating.
Self-healing superhydrophobic film was fabricated by Wu et al. [81] using layer by layer (LbL) assembly of poly(sodium 4-styrenesulfonate) (PSS)−1-octadecylamine (ODA) complexes (PSS-ODA) and poly(allylamine hydrochloride) (PAH)-sodium dodecyl sulfonate (SDS) (PAH-SDS) complexes. Aggregates with double-scale structure appear on the surface, obtaining SH behaviour dependent on the layers number. The (PSS-ODAx/PAH-SDS)*30 film durability was investigated using the sand abrasion test and by immersion in different solutions (aqueous HCl or NaOH solution, alcohol, acetone and dichlone) for some hours. After each test, the superhydrophobicity of the film remained unchanged. Furthermore, the SH film showed self-healing abilities after mechanical damage or O2 plasma etching in ambient conditions (Figure 7).
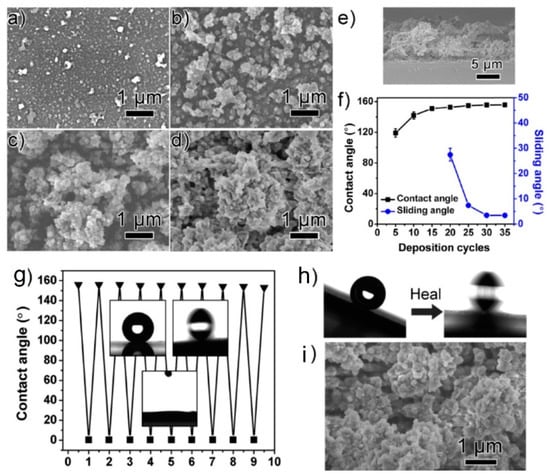
Figure 7.
Top-view SEM images of the (PSS−ODA1.5/PAH−SDS)∗n films, with n being 5 (a), 15 (b), 25 (c), and 30 (d). (e) Cross-sectional SEM image of the (PSS−ODA1.5/PAH−SDS)∗30 film. (f) Changes in water contact angles and sliding angles of the (PSS−ODA1.5/PAH−SDS)∗n films with different deposition cycles (g) changes in the water contact angle of the superhydrophobic (PSS−ODA1.5/PAH−SDS)∗30 film upon repeated O2 plasma etching (■) and self-healing (▼). Insets: shapes of the water droplets (4 μL) of the as-prepared (top left), O2-plasma-etched (bottom), and healed (top right) superhydrophobic films. (h) Shapes of the water droplets of the sand-impinged (PSS−ODA1.5/PAH−SDS)∗30 films before (left) and after (right) the self-healing process. (i) Top-view SEM image of the sand-impinged (PSS−ODA1.5/PAH−SDS)∗30 film. Reprinted with permission from [81] 2016 ACS Publications.
De Nicola et al. [82] prepared SH stainless steel with Salvinia effect (CA = 154° but no roll off angle) by growing of multi-walled carbon nanotube (MWCNT) by CVD of acetylene as a green component. This effect was due to the two-fold hierarchical morphology networks in which hydrophilic carbonaceous nanostructures are present on the top of hydrophobic carbon nano tubes. The result could be interesting, but the application is not very sustainable because of the high operational temperature (730 °C).
Another study, green and sustainable in terms of materials but less eco friendly in terms of the used technique, is presented by Barshilia et al. [83]. They reported the fabrication of a micro-nano, rough, zinc oxide (ZnO), superhydrophobic coating on TiAlN/TiAlON/Si3N4 solar-selective coating. The ZnO layer was deposited by sputtering a Zn target on the solar coating followed by oxidation and annealing at high temperature. The prepared ZnO layer showed superhydrophobicity when its thickness was greater than 1200 nm and transparency with transmittance of about 70%.
Kong et al. [84] easily prepared an SH coating, CA = 155.8°, on polyester fabric by the spray coating of commercial adhesive and ethanol suspension of hydrophobic SiO2 NPs (multiple layers). The authors tested the prepared SH fabric with various tests to demonstrate the excellent mechanical stability and to employ this coating in different textiles and applications. Furthermore, the SH coating remained dry and clean from slurry after drops fell from different heights.
A process is considered eco-friendly if it uses a substance that does not release pollutants in the environment and if it does not use any potential toxic chemicals or excess energy. For this reason, obtaining SHS from petroleum-derived polymers via a cheap method could be considered sustainable [17]. In this field is the work of Zhang et al. [85]. The authors obtained an SH surface through the lamination exfoliation method using polypropylene (PP) and high-density polyethylene (HDPE). The two polymers were blended so that reduced viscosity permits the polymer to diffuse at the interface. At room temperature, the matrix was unzipped, obtaining a highly textured surface with low wettability. The prepared surface showed resistance to drop-water impact at low and high velocities, and remained unaltered after the impact of silica powder.
Ferrari et al. [86] started using hydrophobic petroleum-derived polymers like polystyrene (PS), from commercial packaging, and polytetrafluoroethylene (PTFE) to obtain coating with superhydrophobic behaviour. The authors prepared a dispersion of polymers in ethyl acetate and, by spray coating at room temperature, they obtained an SHS on a different substrate, glass, and commercial aluminium alloy. The samples with different surface roughness (Sa) were tested in a real marine environment with immersion up to two months, observing that the sample with higher Sa was more resistant to fouling attach due to a stable air plastron maintaining its superhydrophobicity. Furthermore, the bare Al sample and SH Al samples were tested by a potentiodynamic scan after two months of immersion in natural seawater. The authors observed that the SH-produced coating was able to control corrosion phenomena, decreasing the corrosion current density of about two orders of magnitude with respect to the Al sample used as a control.
As previously reported, short fluorocarbon chain compounds (C < 7) are unique fluorocarbon molecules that can be used to lower the surface energy without problems of bioaccumulation in the environment and human body. Wang et al. [87] prepared hydrophobic nanofibrous membranes using 2,2,3,3,4,4,4-heptafluorobutyl acrylate (HFBA) by polymerization with polycaprolactone (PCL). In this way, the authors obtained a membrane with water, CA = 136°, which has hemocompatibility, anticoagulation, and anti-fouling properties.
In this section, works where coatings with high water repellence were prepared using different green and sustainable starting materials with respect to waxes, fatty acids, and silicon-based materials have been reported. In this case, it is not possible to talk about advantages and disadvantages in a general way but it is possible to observe that the coatings tested, in terms of durability and resistance, are reported as interesting and promising data, while in the other cases, there is no data to conclude anything about this important aspect.
6. Oleophobic Sustainable Coatings
Even though the preparation of superhydrophobic surfaces is currently well-assessed, at least at research level, superoleophobic surfaces are still more complex to fabricate even at lab scale. The liquid repellence requirements appear to be more restrictive in comparison with water, because the coating material should feature high de-wetting properties for nonpolar alkane liquids with low surface tensions (in particular, lower than 27 dyn cm−1), usually with small (few μL) droplets and low hysteresis [88].
Oleophobicity and superoleophobicity were reached only through the optimization of the surface texture and the final perfluorination, obtaining in this way a very low surface energy. As we already mentioned, the toxicity of long chain PFAS (C > 7) is well documented, and it is very important the to use non-toxic and sustainable material to reach oleophobic behaviour. Unlike superhydrophobic coatings, in this context it has not yet been possible to achieve a superoleophobic surface using sustainable molecules. Only in a few works was oleophobicity, or the dynamic one, obtained.
Indeed, in the literature we found only few papers where the authors achieve this result with the use of low-fluorinated or fluorine-free compounds. Cheng et al. [24] were first to introduce the concept of a dynamically oleophobic surface. In fact, in their work they prepared an oleophilic surface, n-Hexadecane CA = 32°, but with a tilt angle of 5° so that the oils could be repelled. The authors prepared the surface by grafting vinyl-terminated PDMS to the Si-H moieties of a 1,3,5,7-tetramethylcyclote- trasiloxane (D4H)-derived monomeric layer on oxidized Si (SiSiO 2) surfaces through Pt-catalysed hydrosilylation.
Park et al. [16] demonstrated the possibility to obtain an oleophobic surface without the use of prefluorinated compounds. They prepared a dynamically oleophobic coating by 3,3,3-trifluoro-propyltrimethoxysilane (FAS3) and tetramethoxysilane (TMOS). The surface was obtained by spin coating in a FAS3/TMOS mixture, obtained from sol-gel, after storage at room temperature for 24 h. Oleophobic behaviour was tested using n-Decane, n-Dodecane and n-Hexadecane. In all wettability tests, the prepared surface did not result in oleophobic (CA ≥ 90°) because CA was 31°, 37°, and 42°, respectively. However, oleo repellence was observed when the surface was tilted; for each alkane liquids the tilt angle was <10° and for n-Decane, it was about 5°, similar to the value obtained for perfluorinated superoleophobic surface.
As in the latter case, Cai et al. [89] obtained a surface hydrophobic and, in a wider sense, oleophobic with oil CA = 78.4° by the dip coating of a glass substrate in a mixture of base-catalysed TEOS sol with acid-catalysed MTES sol with the weight ratio of 3:7. The film maintained its behaviour after being rubbed 3000 times by an abrasion-resistance machine and after four months of outdoor exposition.
The repellence of oil from textiles is very important and has applications worldwide, but nowadays it is achieved only through the employ of long perfluorocarbon compounds and sustainable treatments for oil repellent fabric development have not yet been found [90]. Shabanian et al. [91] described, through a very careful and in-depth study, how it was possible to obtain oil repellent fabric through careful surface architecture design and surface chemical modification. Working on the wettability theory and its application for cylindrical fibres, they obtained oleophobic, PDMS-treated nylon jacket fabric. The fabric showed oil CA > 90° for castor, olive, and canola oil; for n-Hexadecane CA = 35°. Finally, the authors affirmed that a textile could be oleophobic without the use of perfluoro compounds but required accurate design of texture and chemical modification.
As observed, oleophobicity, or even less, superoleophobicity, was very difficult to obtain without the employ of fluorinated compounds. In the literature, there are fre articles with oleo repellence behaviour and in most cases, there is no real oleophobicity (CA < 90°), only a dynamic one. In view of the above fact, obtaining oleophobic/superoleophobic surfaces from green and sustainable materials is a goal that has not yet been achieved requires great effort.
7. Conclusions
In this work, we reviewed the available recent literature related to materials for innovative applications in view of a partial or complete substitution of fluorine compounds in highly hydro and oleophobic coatings. Superhydrophobicity is largely covered by most of the papers revised, with topics fulfilling environmental requirements, but still restricted to highly repellent water and aqueous systems. A small but growing body of literature can be found devoted to new and sustainable strategies for providing suitable, highly oleophobic surfaces to overcome fluorine presence.
Currently, restrictions in terms of human and environmental safety oblige markets and industries to foster their investigations toward fluorine-free domains avoiding bioaccumulation and persistence in living organisms.
New insights in this area already allow us to create promising pathways even though the extreme performances offered by fluorocarbon chemicals are still unparalleled. Nevertheless, fostering the use of natural products like waxes or fatty acids or more synthetic-like, silicon-based materials will be at least one way to remove fluorine derivatives from those products that more directly affect health, leaving them for applications that can undergo suitable waste management. Future perspectives must involve and cross the work of many different fields, from organic synthetic chemistry to biology to environmental sciences, in order to overcome the limitations still existing today.
Author Contributions
The manuscript was written through the contributions of all authors. M.F.: conceptualization and supervision. F.C. and M.F.: writing—original draft. F.C. and M.F.: validation, resources, investigation, writing—review and editing; F.C.: image processing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This work is performed under the umbrella of the European Space Agency Topical Team: Biofilms from an interdisciplinary perspective.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Prontera, C.T.; Sico, G.; Montanino, M.; Del Mauro, A.D.G.; Tassini, P.; Maglione, M.G.; Minarini, C.; Manini, P. Sustainable, fluorine-free, low cost and easily processable materials for hydrophobic coatings on flexible plastic substrates. Materials 2019, 12, 2234. [Google Scholar] [CrossRef] [Green Version]
- Ghasemlou, M.; Daver, F.; Ivanova, E.P.; Adhikari, B. Bio-inspired sustainable and durable superhydrophobic materials: From nature to market. J. Mater. Chem. A 2019, 7, 16643–16670. [Google Scholar] [CrossRef]
- Bormashenko, E. Why are the values of the surface tension of most organic liquids similar? Am. J. Phys. 2010, 78, 1309. [Google Scholar] [CrossRef]
- Israelachvili, J.N. Interactions Involving Polar Molecules. In Intermolecular and Surface Forces, 3rd ed.; Israelachvili, J.N., Ed.; Academic Press: San Diego, CA, USA, 2011; pp. 71–90. ISBN 978-0-12-375182-9. [Google Scholar]
- Bhushan, B. Measurement Techniques and Applications. In Nanotribology and Nanomechanics: An Introduction; Springer: Berlin/Heidelberg, Germany, 2008; pp. 1–34. ISBN 978-3-540-77608-6. [Google Scholar]
- Bhushan, B.; Jung, Y.C. Natural and biomimetic artificial surfaces for superhydrophobicity, self-cleaning, low adhesion, and drag reduction. Prog. Mater. Sci. 2011, 56, 1–108. [Google Scholar] [CrossRef] [Green Version]
- Barthlott, W.; Neinhuis, C. Purity of the sacred lotus, or escape from contamination in biological surfaces. Planta 1997, 202, 1–8. [Google Scholar] [CrossRef]
- Zhiguang, G.; Liu, W.; Su, B.-L. Superhydrophobic surfaces: From natural to biomimetic to functional. J. Colloid Interface Sci. 2011, 353, 335–355. [Google Scholar]
- Shirtcliffe, N.J.; McHale, G.; Atherton, S.; Newton, M.I. An introduction to superhydrophobicity. Adv. Colloid Interface Sci. 2010, 161, 124–138. [Google Scholar] [CrossRef] [Green Version]
- Butt, H.-J.; Roismann, I.; Brinkmann, M.; Papadopoulos, P.; Vollmer, D.; Semprebon, C. Characterization of super liquid-repellent surfaces. Curr. Opin. Colloid Interface Sci. 2014. [Google Scholar] [CrossRef] [Green Version]
- Jiang, W.; Grozea, C.M.; Shi, Z.; Liu, G. Fluorinated Raspberry-like Polymer Particles for Superamphiphobic Coatings. ACS Appl. Mater. Interfaces 2014, 6, 2629–2638. [Google Scholar] [CrossRef]
- Nosonovsky, M.; Bhushan, B. Energy transitions in superhydrophobicity: Low adhesion, easy flow, and bouncing. J. Phys. Condens. Matter. 2008, 20, 395005. [Google Scholar] [CrossRef]
- Nosonovsky, M.; Bhushan, B. Hierarchical roughness optimization for biomimetic superhydrophobic surfaces. Ultramicroscopy 2007, 107, 969–979. [Google Scholar] [CrossRef]
- Celia, E.; Darmanin, T.; Taffin de Givenchy, E.; Amigoni, S.; Guittard, F. Recent advances in designing superhydrophobic surfaces. J. Colloid Interface Sci. 2013, 402, 1–18. [Google Scholar] [CrossRef]
- Fürstner, R.; Barthlott, W.; Neinhuis, C.; Walzel, P. Wetting and self-cleaning properties of artificial superhydrophobic surfaces. Langmuir 2005, 21, 956–961. [Google Scholar] [CrossRef]
- Park, J.; Urata, C.; Masheder, B.; Cheng, D.F.; Hozumi, A. Long perfluoroalkyl chains are not required for dynamically oleophobic surfaces. Green Chem. 2013, 15, 100–104. [Google Scholar] [CrossRef]
- Nilsson, M.A.; Daniello, R.J.; Rothstein, J.P. A novel and inexpensive technique for creating superhydrophobic surfaces using Teflon and sandpaper. J. Phys. D Appl. Phys. 2010, 43, 045301. [Google Scholar] [CrossRef]
- Byrne, F.P.; Jin, S.; Paggiola, G.; Petchey, T.H.M.; Clark, J.H.; Farmer, T.J.; Hunt, A.J.; Robert McElroy, C.; Sherwood, J. Tools and techniques for solvent selection: Green solvent selection guides. Sustain. Chem. Process. 2016, 4, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Jing, P.; Rodgers, P.J.; Amemiya, S. High lipophilicity of perfluoroalkyl carboxylate and sulfonate: Implications for their membrane permeability. J. Am. Chem. Soc. 2009, 131, 2290–2296. [Google Scholar] [CrossRef] [Green Version]
- Environmental Protection Agency. Long-Chain Perfluoroalkyl Carboxylate and Perfluoroalkyl Sulfonate Chemical Substances; Significant New Use Rule; EPA: New York, NY, USA, 2015; Volume 80.
- European Commission. Poly- and Perfluoroalkyl Substances (PFAS): Chemicals Strategy for Sustainability Towards a Toxic-Free Environment; Commission Staff Working Document; European Comission: Brussels, Belgium, 2020; pp. 1–22. [Google Scholar]
- Chi, H.; Xu, Z.; Ma, Y.; Tang, T.; Zhang, T.; Zhao, Y. Multifunctional Highly Oleophobic and Superhydrophilic Fabric Coatings Prepared by Facile Photopolymerization. Adv. Sustain. Syst. 2020, 4, 1–8. [Google Scholar] [CrossRef]
- Caldona, E.B.; Sibaen, J.W.; Tactay, C.B.; Mendiola, S.L.D.; Abance, C.B.; Añes, M.P.; Serrano, F.D.D.; De Guzman, M.M.S. Preparation of spray-coated surfaces from green-formulated superhydrophobic coatings. SN Appl. Sci. 2019, 1, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Cheng, D.F.; Urata, C.; Yagihashi, M.; Hozumi, A. A Statically Oleophilic but Dynamically Oleophobic Smooth Nonperfluorinated Surface. Angew. Chem. 2012, 124, 3010–3013. [Google Scholar] [CrossRef]
- Rohrbach, K.; Li, Y.; Zhu, H.; Liu, Z.; Dai, J.; Andreasen, J.; Hu, L. A cellulose based hydrophilic, oleophobic hydrated filter for water/oil separation. Chem. Commun. 2014, 50, 13296–13299. [Google Scholar] [CrossRef]
- Ivanković, A. Review of 12 Principles of Green Chemistry in Practice. Int. J. Sustain. Green Energy 2017, 6, 39. [Google Scholar] [CrossRef] [Green Version]
- Saifaldeen, Z.S.; Khedir, K.R.; Cansizoglu, M.F.; Demirkan, T.; Karabacak, T. Superamphiphobic aluminum alloy surfaces with micro and nanoscale hierarchical roughness produced by a simple and environmentally friendly technique. J. Mater. Sci. 2014, 49, 1839–1853. [Google Scholar] [CrossRef]
- Morrissette, J.M.; Carroll, P.J.; Bayer, I.S.; Qin, J.; Waldroup, D.; Megaridis, C.M. A methodology to produce eco-friendly superhydrophobic coatings produced from all-water-processed plant-based filler materials. Green Chem. 2018, 20, 5169–5178. [Google Scholar] [CrossRef]
- Wei, Z.; Jiang, D.; Chen, J.; Ren, S.; Li, L. Fabrication of mechanically robust superhydrophobic aluminum surface by acid etching and stearic acid modification. J. Adhes. Sci. Technol. 2017, 31, 2380–2397. [Google Scholar] [CrossRef]
- El-Maiss, J.; Darmanin, T.; Guittard, F. Branched versus linear perfluorocarbon chains in the formation of superhydrophobic electrodeposited films with low bioaccumulative potential. J. Mater. Sci. 2014, 49, 7760–7769. [Google Scholar] [CrossRef]
- Song, J.; Lu, Y.; Huang, S.; Liu, X.; Wu, L.; Xu, W. A simple immersion approach for fabricating superhydrophobic Mg alloy surfaces. Appl. Surf. Sci. 2013, 266, 445–450. [Google Scholar] [CrossRef]
- Fadeev, A.Y.; McCarthy, T.J. Binary monolayer mixtures: Modification of nanopores in silicon-supported tris(trimethylsiloxy)silyl monolayers. Langmuir 1999, 15, 7238–7243. [Google Scholar] [CrossRef]
- Fadeev, A.Y.; McCarthy, T.J. Trialkylsilane monolayers covalently attached to silicon surfaces: Wettability studies indicating that molecular topography contributes to contact angle hysteresis. Langmuir 1999, 15, 3759–3766. [Google Scholar] [CrossRef]
- Chen, W.; Fadeev, A.Y.; Hsieh, M.C.; Öner, D.; Youngblood, J.; McCarthy, T.J. Ultrahydrophobic and ultralyophobic surfaces: Some comments and examples. Langmuir 1999, 15, 3395–3399. [Google Scholar] [CrossRef]
- Si, Y.; Guo, Z.; Liu, W. A Robust Epoxy Resins @ Stearic Acid-Mg(OH)2 Micronanosheet Superhydrophobic Omnipotent Protective Coating for Real-Life Applications. ACS Appl. Mater. Interfaces 2016, 8, 16511–16520. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.H. An environmentally friendly method for fabrication of superhydrophobic “pipe” with loss-free liquid transportation properties. Surf. Coat. Technol. 2021, 407, 126777. [Google Scholar] [CrossRef]
- Dong, X.; Gao, S.; Huang, J.; Li, S.; Zhu, T.; Cheng, Y.; Zhao, Y.; Chen, Z.; Lai, Y. A self-roughened and biodegradable superhydrophobic coating with UV shielding, solar-induced self-healing and versatile oil-water separation ability. J. Mater. Chem. A 2019, 7, 2122–2128. [Google Scholar] [CrossRef]
- Wang, H.; Dong, S.; Wang, Z. One-step fabrication of superhydrophobic surface on beryllium copper alloys and corrosion protection application. Colloids Surf. A Physicochem. Eng. Asp. 2018, 556, 291–298. [Google Scholar] [CrossRef]
- Wen, G.; Huang, J.X.; Guo, Z.G. Energy-effective superhydrophobic nanocoating based on recycled eggshell. Colloids Surf. A Physicochem. Eng. Asp. 2019, 568, 20–28. [Google Scholar] [CrossRef]
- Xu, S.; Wang, Q.; Wang, N. Eco-friendly fabrication of superhydrophobic surface with anti-corrosion by transferring dendrite-like structures to aluminum substrate. Colloids Surf. A Physicochem. Eng. Asp. 2020, 595, 124719. [Google Scholar] [CrossRef]
- Alexander, S.; Eastoe, J.; Lord, A.M.; Guittard, F.; Barron, A.R. Branched Hydrocarbon Low Surface Energy Materials for Superhydrophobic Nanoparticle Derived Surfaces. ACS Appl. Mater. Interfaces 2016, 8, 660–666. [Google Scholar] [CrossRef] [Green Version]
- Geissler, A.; Chen, L.; Zhang, K.; Bonaccurso, E.; Biesalski, M. Superhydrophobic surfaces fabricated from nano- and microstructured cellulose stearoyl esters. Chem. Commun. 2013, 49, 4962–4964. [Google Scholar] [CrossRef]
- Cheng, Q.Y.; Liu, M.C.; Li, Y.D.; Zhu, J.; Du, A.K.; Zeng, J.B. Biobased super-hydrophobic coating on cotton fabric fabricated by spray-coating for efficient oil/water separation. Polym. Test. 2018, 66, 41–47. [Google Scholar] [CrossRef]
- Saadon Sabry, R. Fabrication of Superhydrophobic Surface of ZnO Thin Films by Using Oleic Acid. Am. J. Nanosci. 2018, 4, 7. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Qing, Y.; Hu, C.; Mo, Q.; He, Y. Preparation of superhydrophobic coating using modified CaCO3. Appl. Surf. Sci. 2013, 265, 532–536. [Google Scholar] [CrossRef]
- Hu, Z.; Deng, Y. Superhydrophobic surface fabricated from fatty acid-modified precipitated calcium carbonate. Ind. Eng. Chem. Res. 2010, 49, 5625–5630. [Google Scholar] [CrossRef]
- Atta, A.M.; Al-Lohedan, H.A.; Ezzat, A.O.; Al-Hussain, S.A. Characterization of superhydrophobic epoxy coatings embedded by modified calcium carbonate nanoparticles. Prog. Org. Coat. 2016, 101, 577–586. [Google Scholar] [CrossRef]
- Xiang, T.; Han, Y.; Guo, Z.; Wang, R.; Zheng, S.; Li, S.; Li, C.; Dai, X. Fabrication of Inherent Anticorrosion Superhydrophobic Surfaces on Metals. ACS Sustain. Chem. Eng. 2018, 6, 5598–5606. [Google Scholar] [CrossRef]
- Razavi, S.M.R.; Oh, J.; Haasch, R.T.; Kim, K.; Masoomi, M.; Bagheri, R.; Slauch, J.M.; Miljkovic, N. Environment-Friendly Antibiofouling Superhydrophobic Coatings. ACS Sustain. Chem. Eng. 2019, 7, 14509–14520. [Google Scholar] [CrossRef]
- Bai, H.; Zhang, L.; Gu, D. Micrometer-sized spherulites as building blocks for lotus leaf-like superhydrophobic coatings. Appl. Surf. Sci. 2018, 459, 54–62. [Google Scholar] [CrossRef]
- Shang, Q.; Liu, C.; Chen, J.; Yang, X.; Hu, Y.; Hu, L.; Zhou, Y.; Ren, X. Sustainable and Robust Superhydrophobic Cotton Fabrics Coated with Castor Oil-Based Nanocomposites for Effective Oil-Water Separation. ACS Sustain. Chem. Eng. 2020, 8, 7423–7435. [Google Scholar] [CrossRef]
- Agrawal, N.; Munjal, S.; Ansari, M.Z.; Khare, N. Superhydrophobic palmitic acid modified ZnO nanoparticles. Ceram. Int. 2017, 43, 14271–14276. [Google Scholar] [CrossRef]
- Rius-Ayra, O.; Fiestas-Paradela, S.; Llorca-Isern, N. Non-fluorinated, sustainable, and durable superhydrophobic microarrayed surface for water-harvesting. Coatings 2020, 10, 314. [Google Scholar] [CrossRef] [Green Version]
- Heale, F.L.; Page, K.; Wixey, J.S.; Taylor, P.; Parkin, I.P.; Carmalt, C.J. Inexpensive and non-toxic water repellent coatings comprising SiO2 nanoparticles and long chain fatty acids. RSC Adv. 2018, 8, 27064–27072. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Lockwood, K.; Boyd, L.M.; Davidson, M.D.; Movafaghi, S.; Vahabi, H.; Khetani, S.R.; Kota, A.K. Superhydrophobic Coatings with Edible Materials. ACS Appl. Mater. Interfaces 2016, 8, 18664–18668. [Google Scholar] [CrossRef]
- Verissimo, T.V.; Santos, N.T.; Silva, J.R.; Azevedo, R.B.; Gomes, A.J.; Lunardi, C.N. In vitro cytotoxicity and phototoxicity of surface-modified gold nanoparticles associated with neutral red as a potential drug delivery system in phototherapy. Mater. Sci. Eng. C 2016, 65, 199–204. [Google Scholar] [CrossRef]
- Morán, M.C.; Ruano, G.; Cirisano, F.; Ferrari, M. Mammalian cell viability on hydrophobic and superhydrophobic fabrics. Mater. Sci. Eng. C 2019, 99, 241–247. [Google Scholar] [CrossRef]
- Torun, I.; Ruzi, M.; Er, F.; Onses, M.S. Superhydrophobic coatings made from biocompatible polydimethylsiloxane and natural wax. Prog. Org. Coat. 2019, 136, 105279. [Google Scholar] [CrossRef]
- Celik, N.; Sahin, F.; Ruzi, M.; Yay, M.; Unal, E.; Onses, M.S. Blood repellent superhydrophobic surfaces constructed from nanoparticle-free and biocompatible materials. Colloids Surf. B Biointerfaces 2021, 205, 111864. [Google Scholar] [CrossRef]
- Liu, B.Y.; Xue, C.H.; An, Q.F.; Jia, S.T.; Xu, M.M. Fabrication of superhydrophobic coatings with edible materials for super-repelling non-Newtonian liquid foods. Chem. Eng. J. 2019, 371, 833–841. [Google Scholar] [CrossRef]
- Zhao, X.; Hu, T.; Zhang, J. Superhydrophobic coatings with high repellency to daily consumed liquid foods based on food grade waxes. J. Colloid Interface Sci. 2018, 515, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Niemietz, A.; Wandelt, K.; Barthlott, W.; Koch, K. Thermal evaporation of multi-component waxes and thermally activated formation of nanotubules for superhydrophobic surfaces. Prog. Org. Coat. 2009, 66, 221–227. [Google Scholar] [CrossRef]
- Yang, C.; Zhu, Y.; Wang, Y.; Fu, H.; Deng, X.; Yue, H.; Lu, H.; Jiang, W.; Liang, B. Preparation of edible superhydrophobic Fe foil with excellent stability and durability and its applications in food containers with little residue. New J. Chem. 2019, 43, 2908–2919. [Google Scholar] [CrossRef]
- Pechook, S.; Pokroy, B. Bioinspired hierarchical superhydrophobic structures formed by n-paraffin waxes of varying chain lengths. Soft Matter. 2013, 9, 5710–5715. [Google Scholar] [CrossRef]
- Zhang, W.; Lu, P.; Qian, L.; Xiao, H. Fabrication of superhydrophobic paper surface via wax mixture coating. Chem. Eng. J. 2014, 250, 431–436. [Google Scholar] [CrossRef]
- Guan, Y.; Yu, C.; Zhu, J.; Yang, R.; Li, X.; Wei, D.; Xu, X. Design and fabrication of vapor-induced superhydrophobic surfaces obtained from polyethylene wax and silica nanoparticles in hierarchical structures. RSC Adv. 2018, 8, 25150–25158. [Google Scholar] [CrossRef] [Green Version]
- Atta, A.M.; Abdullah, M.M.S.; Al-Lohedan, H.A.; Mohamed, N.H. Coating sand with new hydrophobic and superhydrophobic silica/paraffin wax nanocapsules for desert water storage and transportation. Coatings 2019, 9, 124. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Li, B.; Dong, J.; Zhang, J. Roles of silanes and silicones in forming superhydrophobic and superoleophobic materials. J. Mater. Chem. A 2016, 4, 13677–13725. [Google Scholar] [CrossRef]
- Lyu, J.; Wu, B.; Wu, N.; Peng, C.; Yang, J.; Meng, Y.; Xing, S. Green preparation of transparent superhydrophobic coatings with persistent dynamic impact resistance for outdoor applications. Chem. Eng. J. 2021, 404, 126456. [Google Scholar] [CrossRef]
- Fu, X.; He, X. Fabrication of super-hydrophobic surfaces on aluminum alloy substrates. Appl. Surf. Sci. 2008, 255, 1776–1781. [Google Scholar] [CrossRef]
- Wang, C.; Wu, A.H.F.; Lamb, R.N. Superhydrophobicity and Optical Transparency in Thin Films: Criteria for Coexistence. J. Phys. Chem. C 2014, 118, 5328–5335. [Google Scholar] [CrossRef]
- Li, X.; Li, B.; Li, Y.; Sun, J. Nonfluorinated, transparent, and spontaneous self-healing superhydrophobic coatings enabled by supramolecular polymers. Chem. Eng. J. 2021, 404, 126504. [Google Scholar] [CrossRef]
- Wu, L.-K.; Hu, J.-M.; Zhang, J.-Q.; Cao, C.-N. Superhydrophobic surface constructed on electrodeposited sol–gel silica film. Electrochem. Commun. 2013, 26, 85–88. [Google Scholar] [CrossRef]
- Zhang, F.L.M.; Ma, M.; Wang, C.; Li, J. Improvement of mechanical robustness of the superhydrophobicwood surface by coating PVA/SiO2 composite polymer. Appl. Surf. Sci. 2013, 280, 686–692. [Google Scholar]
- Wang, J.; Wang, H.; Wang, Y.; Wang, M.; Singh, V.; Men, X.; Zhang, Z. Rapid fabrication of a transparent superhydrophobic coating: Potential application with pollution-free under construction. Appl. Phys. A Mater. Sci. Process. 2020, 126, 1–10. [Google Scholar] [CrossRef]
- Li, X.; Wang, G.; Moita, A.S.; Zhang, C.; Wang, S.; Liu, Y. Fabrication of bio-inspired non-fluorinated superhydrophobic surfaces with anti-icing property and its wettability transformation analysis. Appl. Surf. Sci. 2020, 505, 144386. [Google Scholar] [CrossRef]
- Tuvshindorj, U.; Yildirim, A.; Ozturk, F.E.; Bayindir, M. Robust Cassie state of wetting in transparent superhydrophobic coatings. ACS Appl. Mater. Interfaces 2014, 6, 9680–9688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, L.; Qiu, W.; Liu, B.; Guo, Q. Stable superhydrophobic surface based on silicone combustion product. RSC Adv. 2014, 4, 56259–56262. [Google Scholar] [CrossRef]
- Li, X.; Dai, H.; Tan, S.; Zhang, X.; Liu, H.; Wang, Y.; Zhao, N.; Xu, J. Facile preparation of poly(ethyl α-cyanoacrylate) superhydrophobic and gradient wetting surfaces. J. Colloid Interface Sci. 2009, 340, 93–97. [Google Scholar] [CrossRef]
- Mates, J.E.; Ibrahim, R.; Vera, A.; Guggenheim, S.; Qin, J.; Calewarts, D.; Waldroup, D.E.; Megaridis, C.M. Environmentally-safe and transparent superhydrophobic coatings. Green Chem. 2016, 18, 2185–2192. [Google Scholar] [CrossRef]
- Wu, M.; An, N.; Li, Y.; Sun, J. Layer-by-Layer Assembly of Fluorine-Free Polyelectrolyte-Surfactant Complexes for the Fabrication of Self-Healing Superhydrophobic Films. Langmuir 2016, 32, 12361–12369. [Google Scholar] [CrossRef] [PubMed]
- De Nicola, F.; Castrucci, P.; Scarselli, M.; Nanni, F.; Cacciotti, I.; Crescenzi, M. De Super-hydrophobic multi-walled carbon nanotube coatings for stainless steel. Nanotechnology 2015, 26, 145701. [Google Scholar] [CrossRef] [PubMed]
- Barshilia, H.C.; John, S.; Mahajan, V. Nanometric multi-scale rough, transparent and anti-reflective ZnO superhydrophobic coatings on high temperature solar absorber surfaces. Sol. Energy Mater. Sol. Cells 2012, 107, 219–224. [Google Scholar] [CrossRef]
- Kong, X.; Zhu, C.; Lv, J.; Zhang, J.; Feng, J. Robust fluorine-free superhydrophobic coating on polyester fabrics by spraying commercial adhesive and hydrophobic fumed SiO2 nanoparticles. Prog. Org. Coat. 2020, 138, 105342. [Google Scholar] [CrossRef]
- Zhang, Z.-X.; Li, Y.; Ye, M.; Boonkerd, K.; Xin, Z.; Vollmer, D.; Kim, J.K.; Deng, X. Fabrication of superhydrophobic surface by a laminating exfoliation method. J. Mater. Chem. A 2014, 2, 1268. [Google Scholar] [CrossRef]
- Ferrari, M.; Benedetti, A.; Cirisano, F. Superhydrophobic coatings from recyclable materials for protection in a real sea environment. Coatings 2019, 9, 303. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Liu, Y.; Liu, M.; Qian, W.; Zhou, D.; Liu, T.; Luo, G.; Xing, M. Short fluorocarbon chains containing hydrophobic nanofibrous membranes with improved hemocompatibility, anticoagulation and anti-fouling performance. Colloids Surf. B Biointerfaces 2019, 180, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Ghaffari, S.; Aliofkhazraei, M.; Barati Darband, G.; Zakeri, A.; Ahmadi, E. Review of superoleophobic surfaces: Evaluation, fabrication methods, and industrial applications. Surf. Interfaces 2019, 17, 100340. [Google Scholar] [CrossRef]
- Cai, S.; Xue, Q.; Xia, B.; Yang, J.; Lv, H.B.; Yan, H.W.; Jiang, B. Hydrophobic-oleophobic antireflective film with excellent optical property prepared by a imple sol-gel route. Mater. Lett. 2015, 156, 14–16. [Google Scholar] [CrossRef]
- Schellenberger, S.; Hill, P.J.; Levenstam, O.; Gillgard, P.; Cousins, I.T.; Taylor, M.; Blackburn, R.S. Highly fluorinated chemicals in functional textiles can be replaced by re-evaluating liquid repellency and end-user requirements. J. Clean. Prod. 2019, 217, 134–143. [Google Scholar] [CrossRef]
- Shabanian, S.; Khatir, B.; Nisar, A.; Golovin, K. Rational design of perfluorocarbon-free oleophobic textiles. Nat. Sustain. 2020, 3, 1059–1066. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).