Fabrication and Physico-Chemical Properties of Antifungal Samarium Doped Hydroxyapatite Thin Films
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
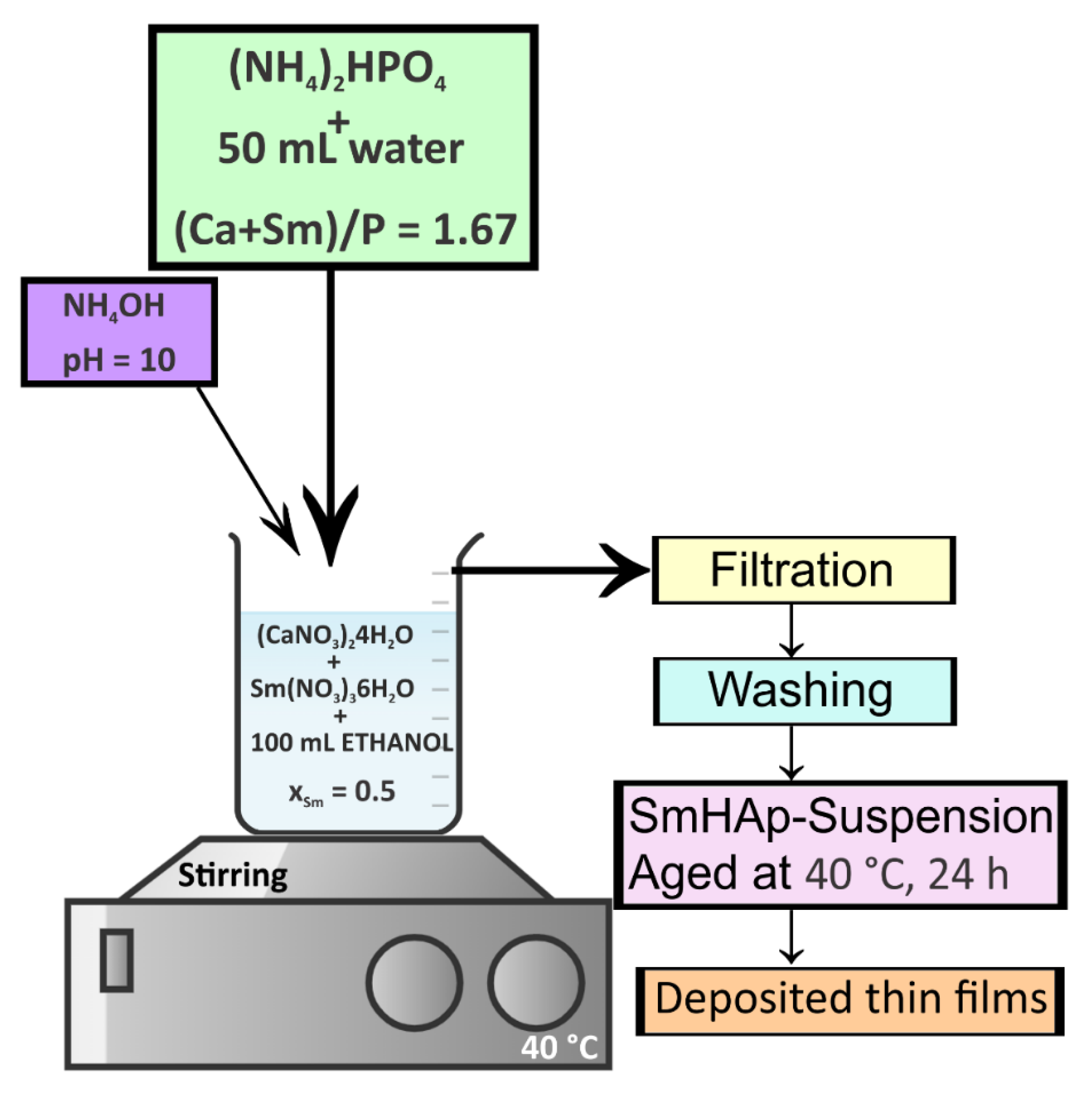
2.2. Samarium Doped Hydroxyapatite Thin Films Using Spin-Coating Technique
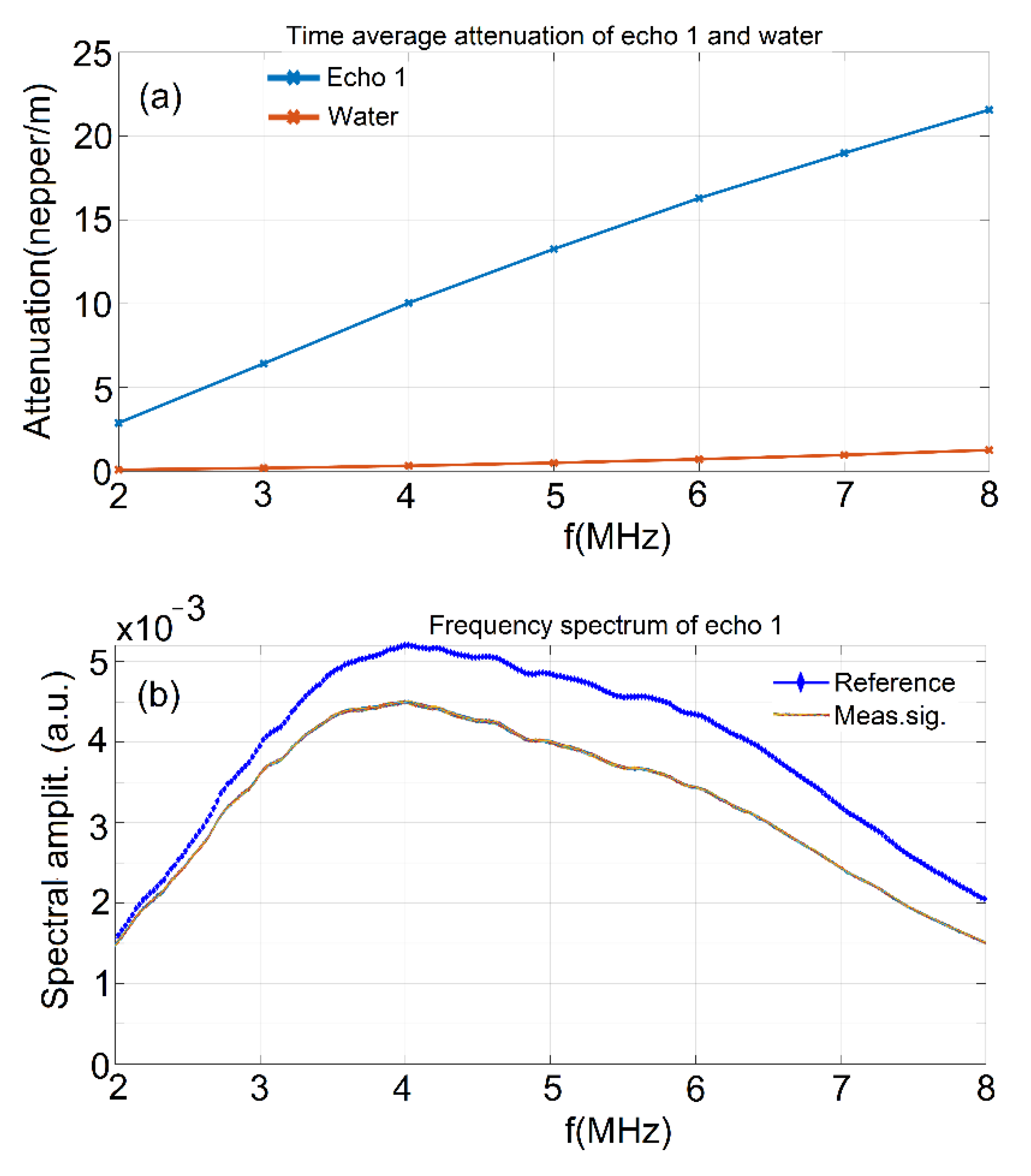
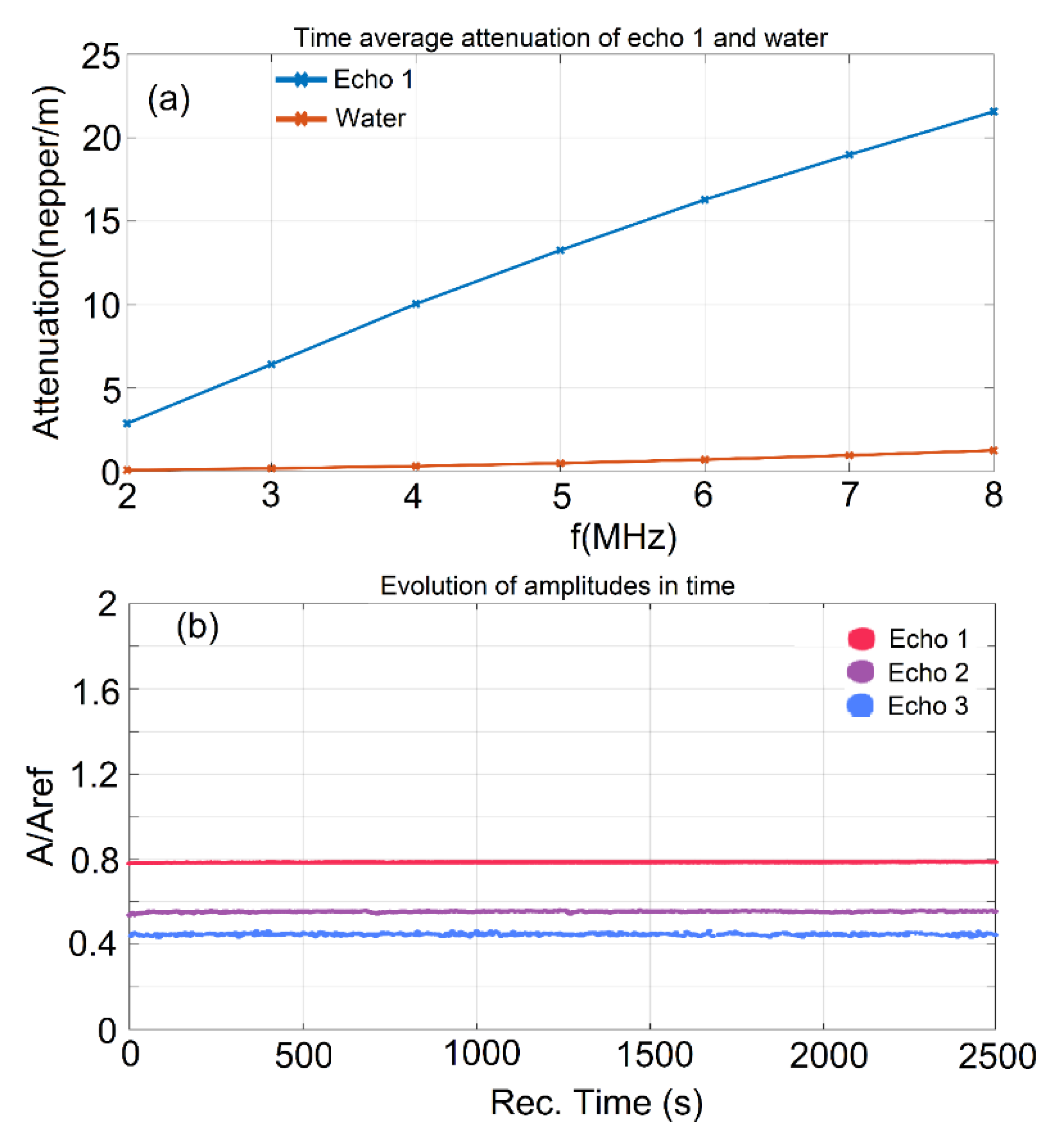
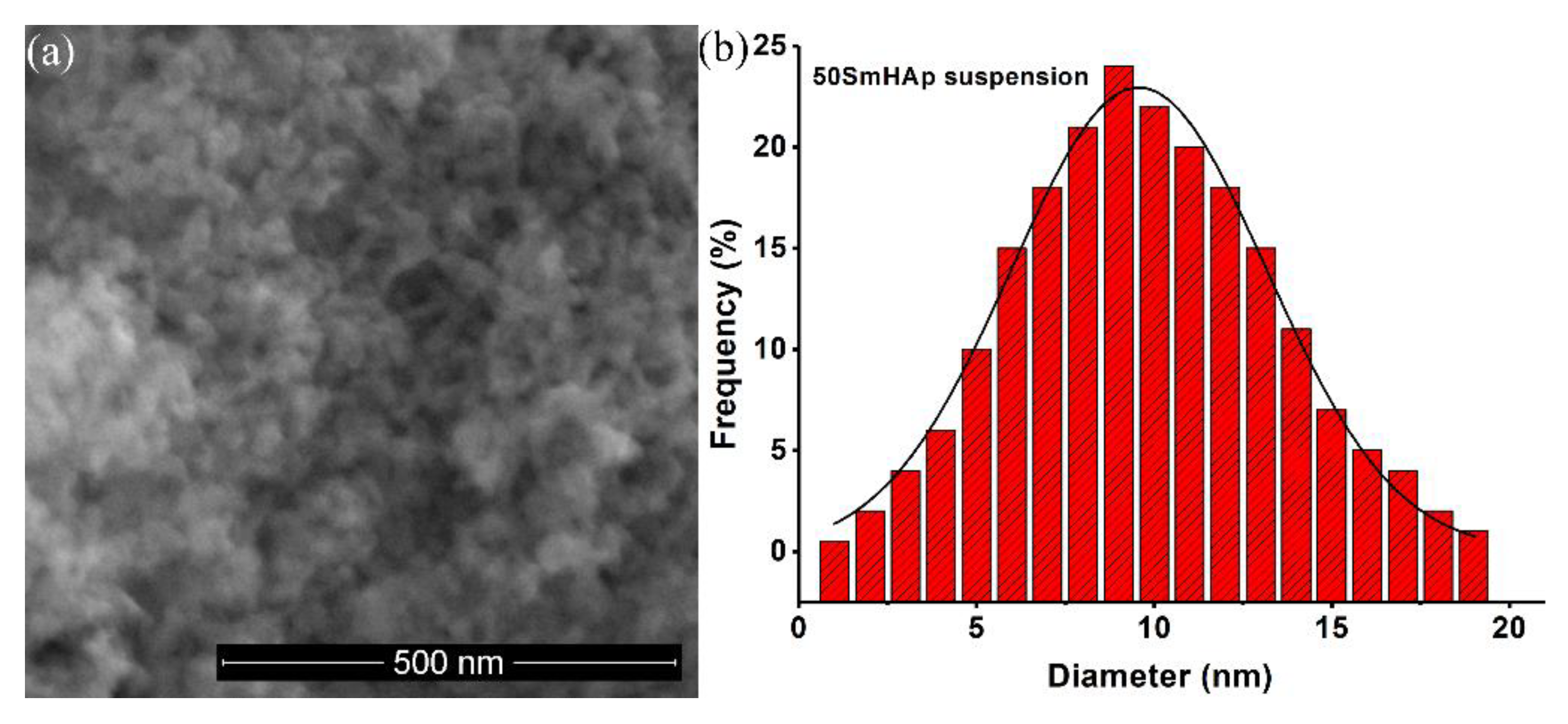
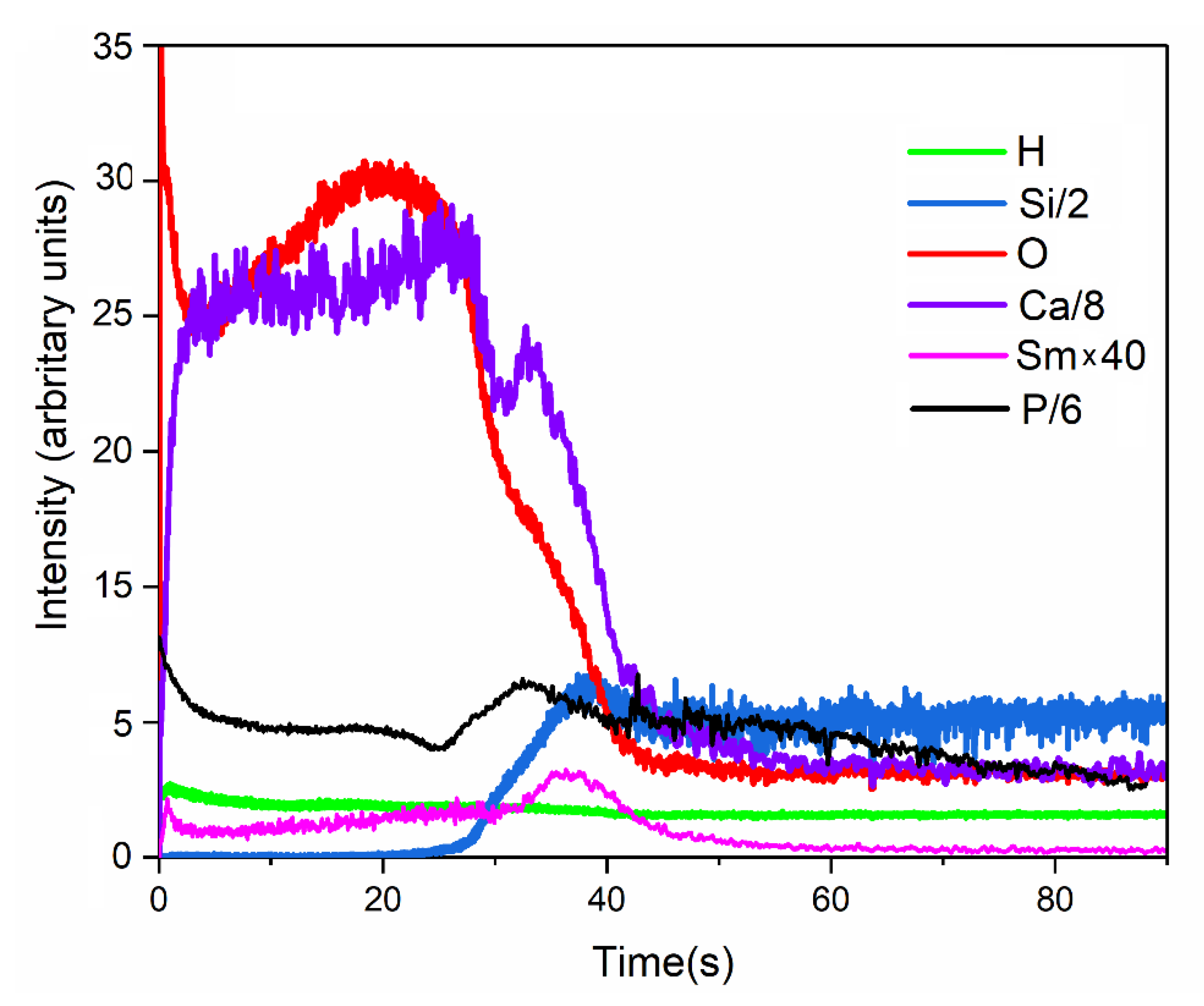
2.3. Characterization Methods
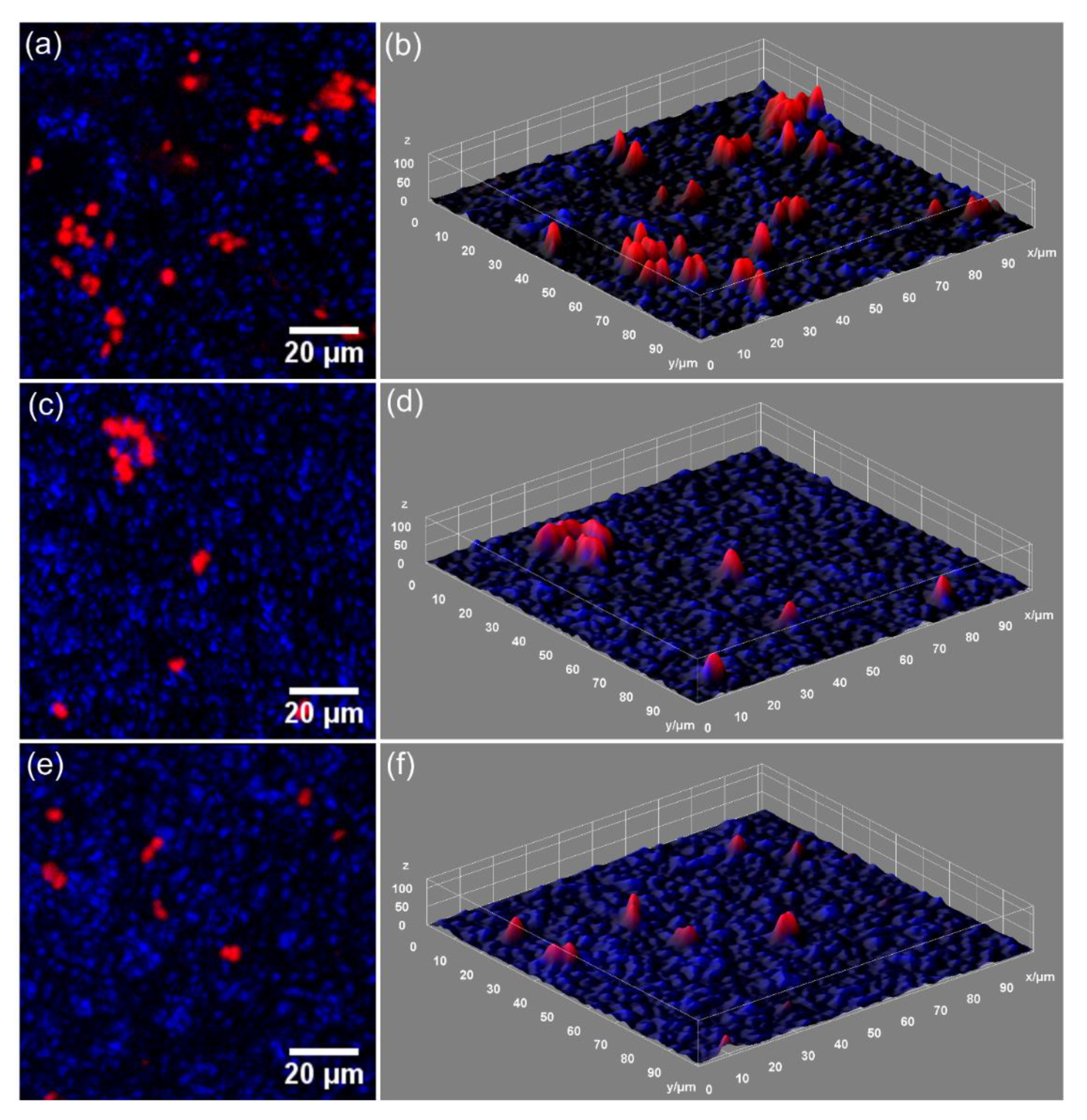
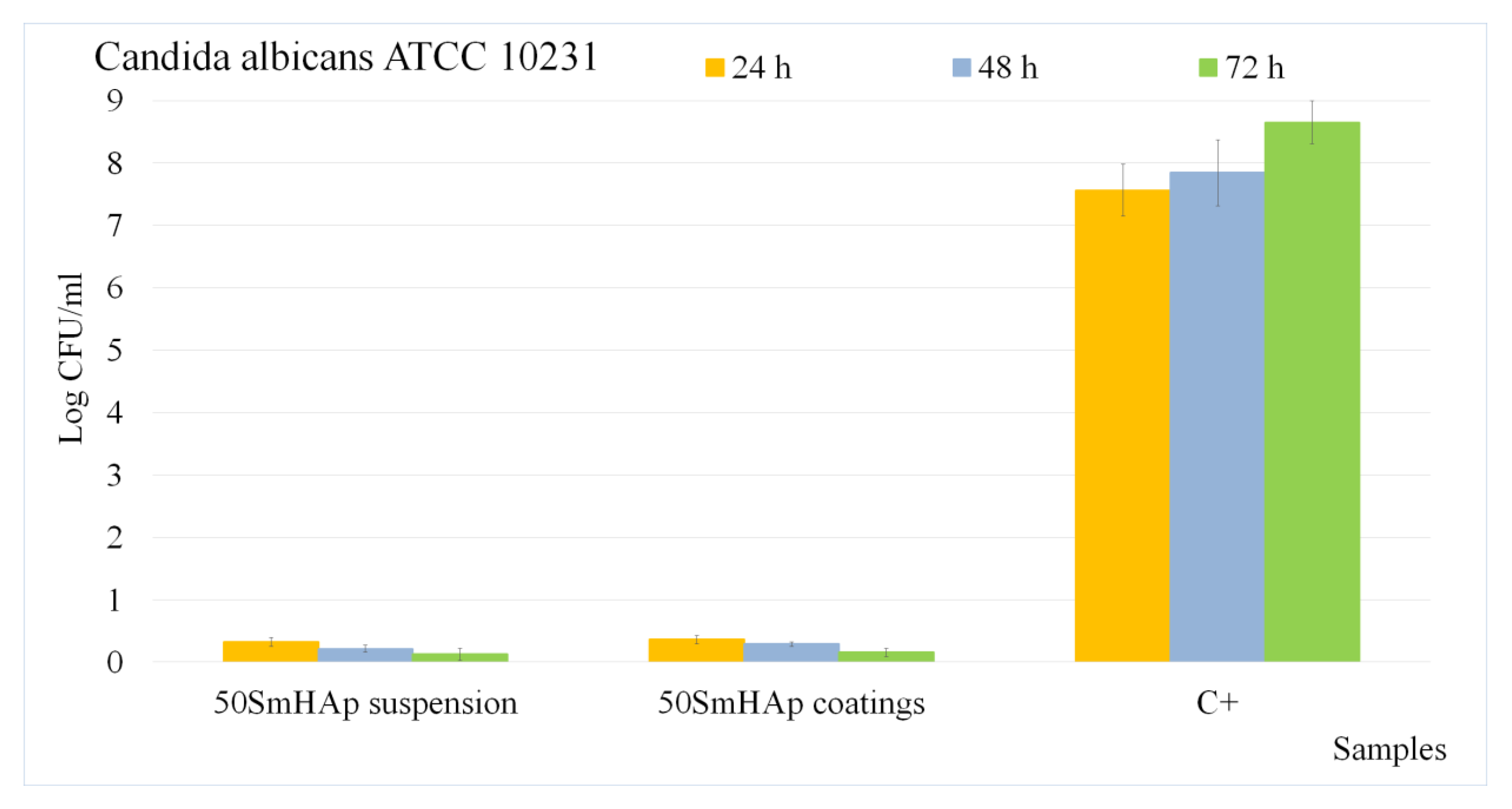
2.4. In Vitro Antifungal Assays
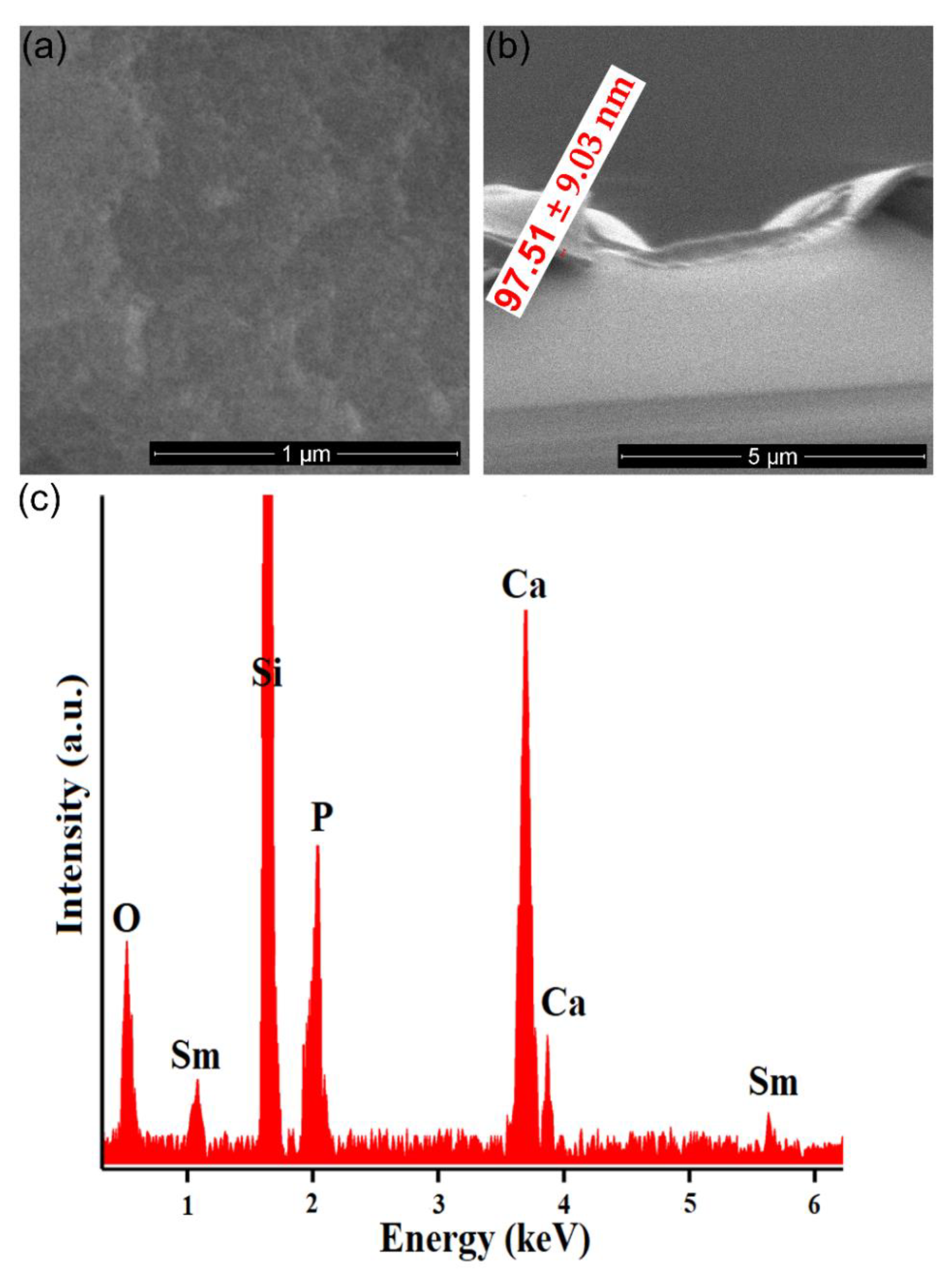
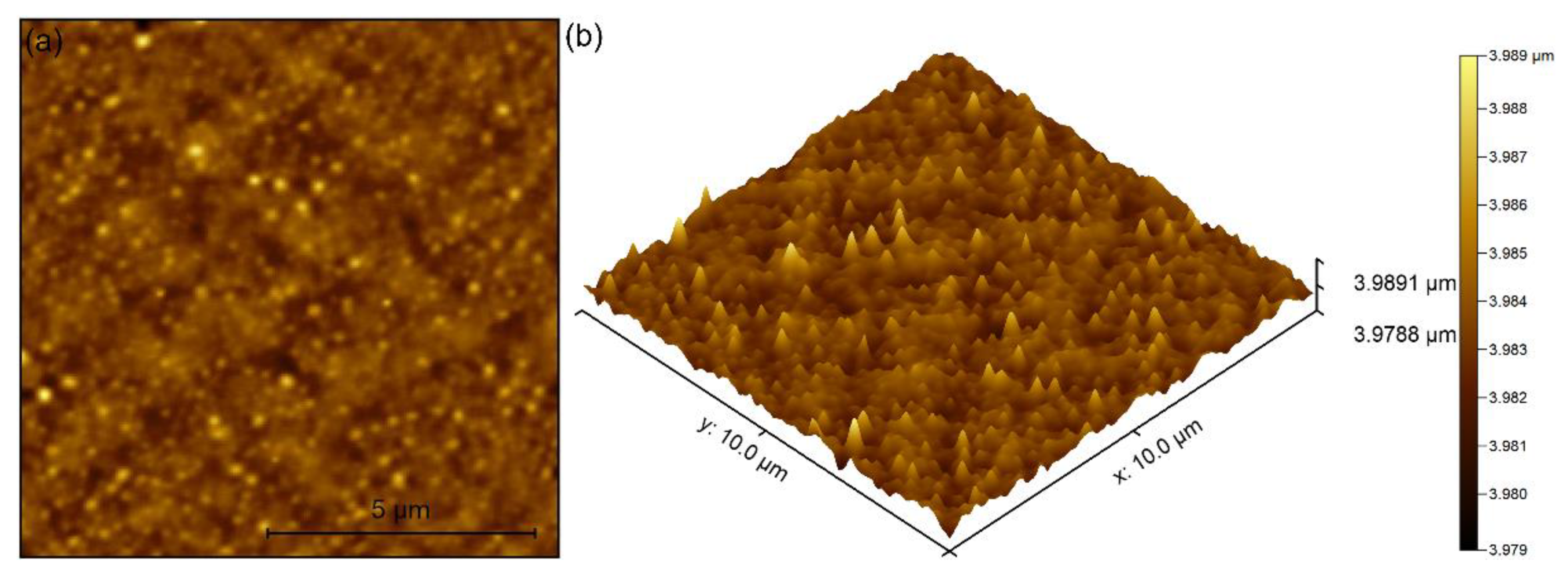
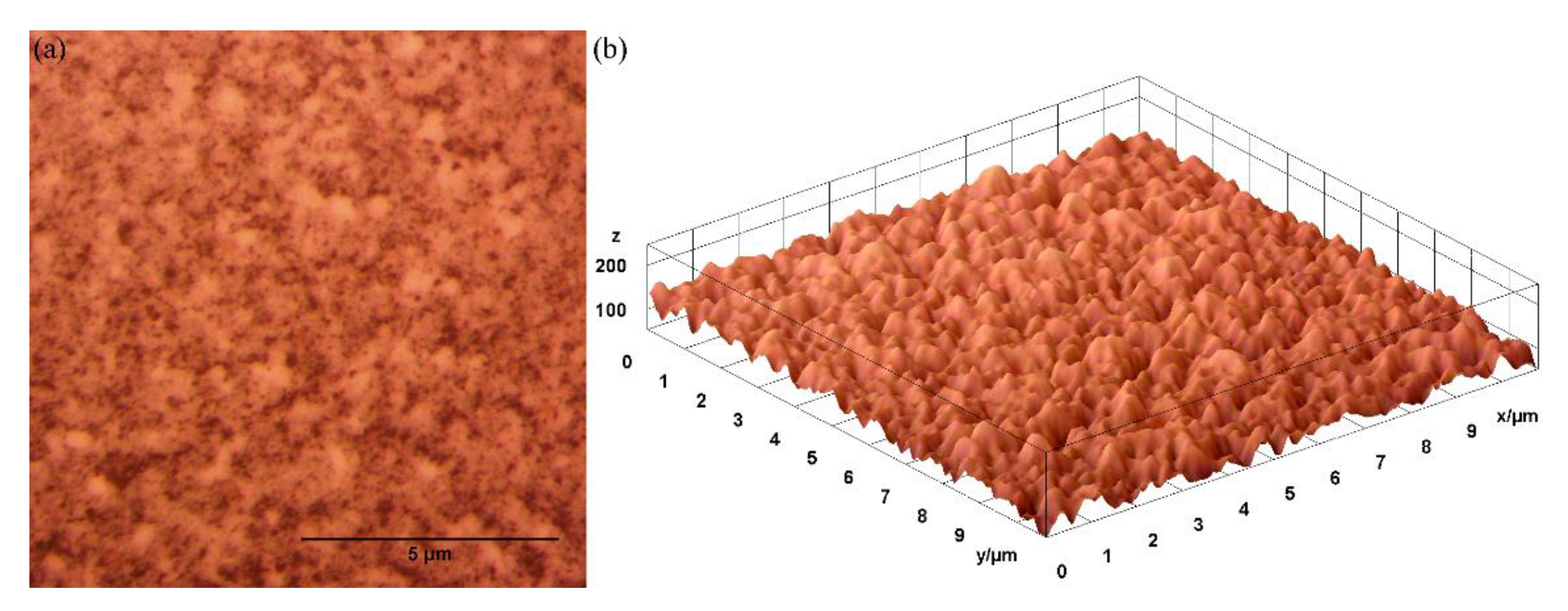
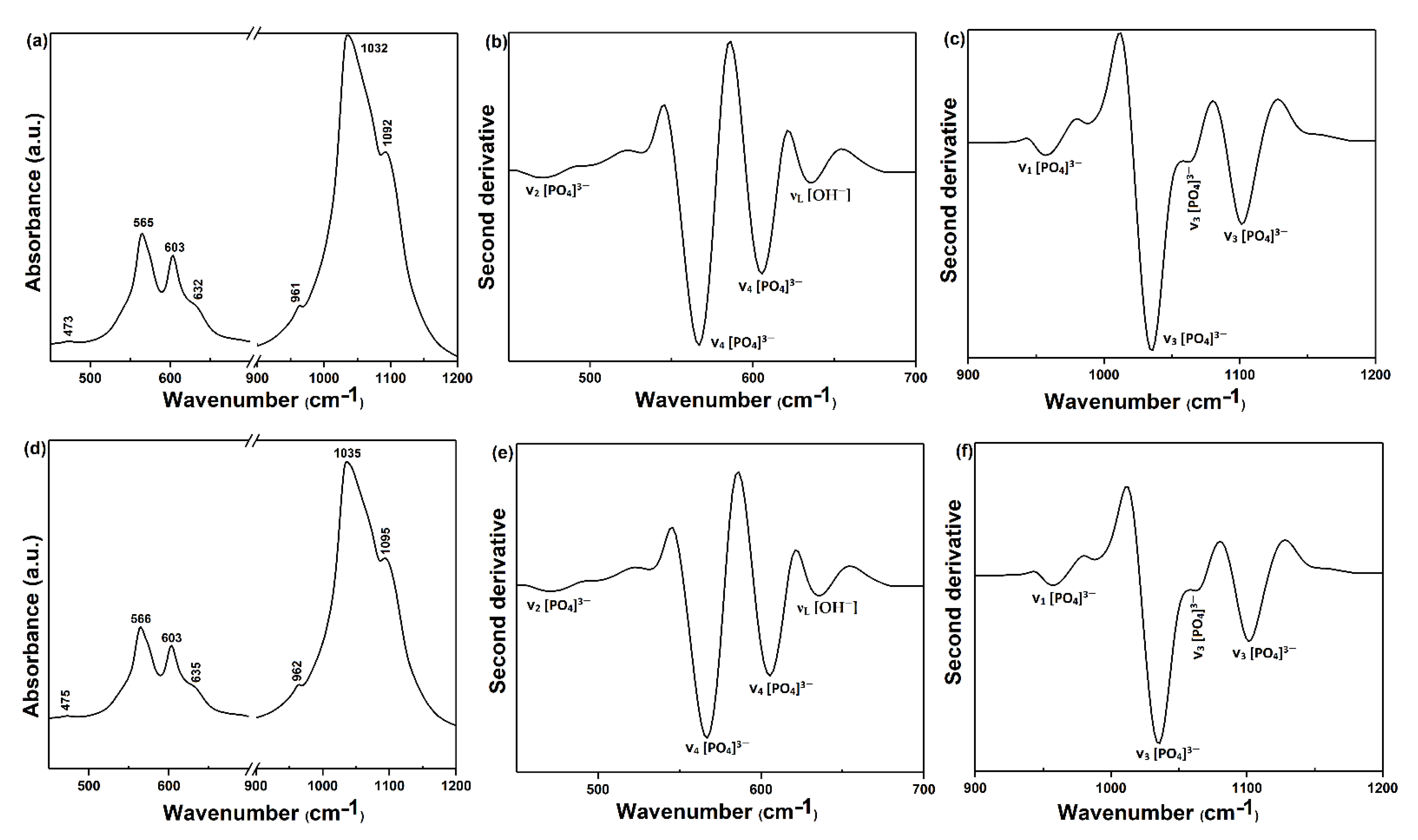
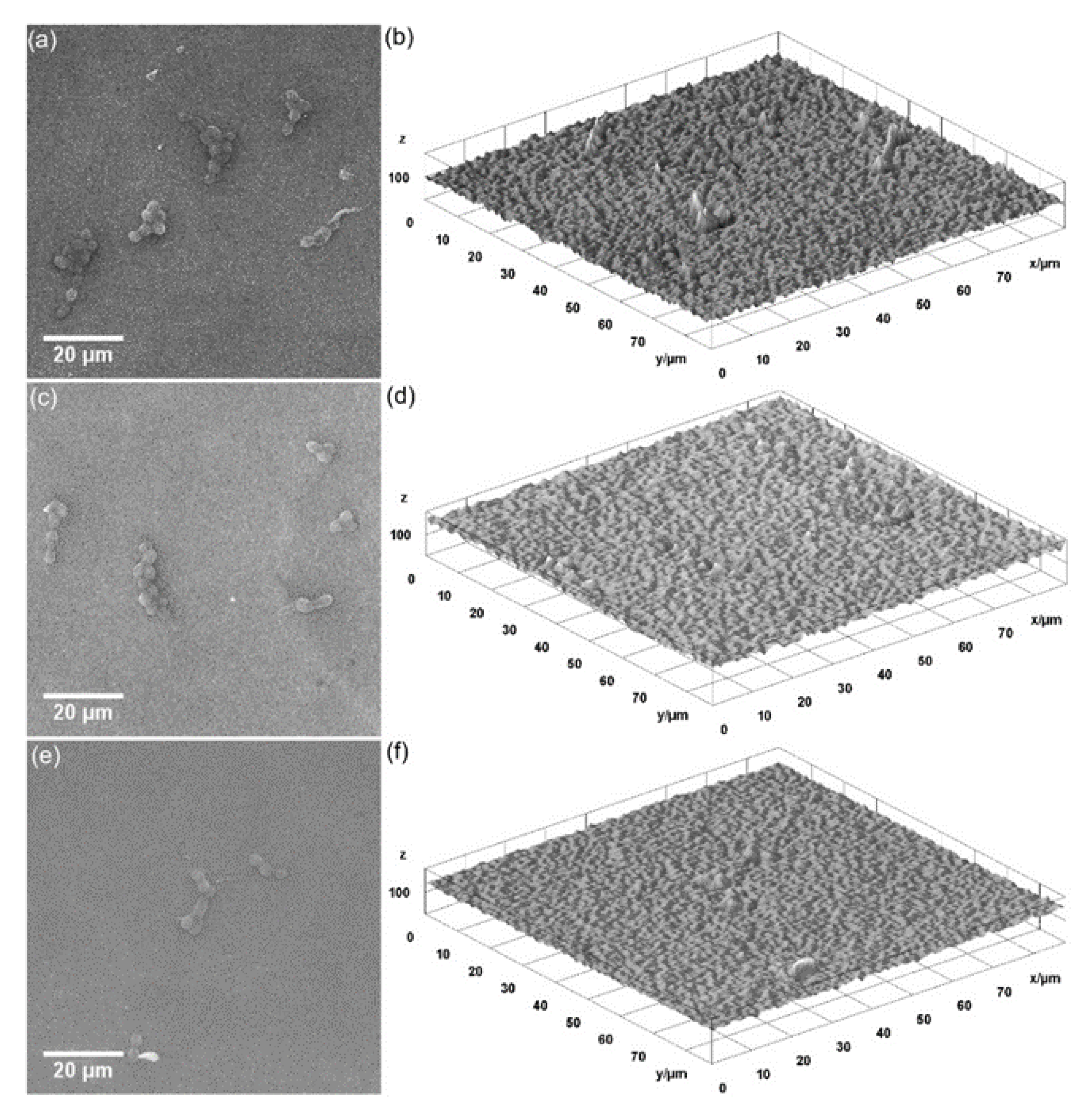
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Santos, M.H.; Valerio, P.; Goes, A.M.; Leite, M.F.; Heneine, L.G.; Mansur, H.S. Biocompatibility evaluation of hydroxyapatite/collagen nanocomposites doped with Zn+2. Biomed. Mater. 2007, 2, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.; Chang, J. Structure and properties of hydroxyapatite for biomedical applications. In Hydroxyapatite (HAp) for Biomedical Applications; Mucalo, M., Ed.; Woodhead Publishing: Sawston, UK, 2015; pp. 3–19. [Google Scholar]
- Sathishkumar, S.; Louis, K.; Shinyjoy, E.; Gopi, D. Tailoring the Sm/Gd-substituted hydroxyapatite coating on biomedical AISI 316L SS: Exploration of corrosion resistance, protein profiling, osteocompatibility, and osteogenic differentiation for orthopedic implant applications. Ind. Eng. Chem. Res. 2016, 55, 6331–6344. [Google Scholar] [CrossRef]
- Kaygili, O.; Vural, G.; Keser, S.; Yahia, I.S.; Bulut, N.; Ates, T.; Koytepe, S.; Temuz, M.M.; Ercan, F.; İnce, T. Ce/Sm co-doped hydroxyapatites: Synthesis, characterization, and band structure calculation. J. Aust. Ceram. Soc. 2020, 57, 305–317. [Google Scholar] [CrossRef]
- Zantye, P.; Fernandes, F.; Ramanan, S.R.; Kowshik, M. Rare earth doped hydroxyapatite nanoparticles for in vitro bioimaging applications. Curr. Phys. Chem. 2019, 9, 94–109. [Google Scholar] [CrossRef]
- Dubnika, A.; Loca, D.; Rudovica, V.; Parekh, M.B.; Berzina-Cimdina, L. Functionalized silver doped hydroxyapatite scaffolds for controlled simultaneous silver ion and drug delivery. Ceram. Int. 2017, 43, 3698–3705. [Google Scholar] [CrossRef]
- Hidalgo-Robatto, B.M.; López-Álvarez, M.; Azevedo, A.S.; Dorado, J.; Serra, J.; Azevedo, N.F.; González, P. Pulsed laser deposition of copper and zinc doped hydroxyapatite coatings for biomedical applications. Surf. Coat. Technol. 2018, 333, 168–177. [Google Scholar] [CrossRef]
- Ciobanu, C.S.; Iconaru, S.L.; Popa, C.L.; Motelica-Heino, M.; Predoi, D. Evaluation of samarium doped hydroxyapatite, ceramics for medical application: Antimicrobial activity. J. Nanomater. 2015, 2015, 849216. [Google Scholar] [CrossRef] [Green Version]
- Chen, P.; Wang, F.; Qiao, Y.; Zhang, Z. Luminescence of samarium doped hydroxyapatite containing strontium: Effects of doping concentration. J. Rare Earths 2021, in press. [Google Scholar] [CrossRef]
- Iconaru, S.L.; Stanciu, G.A.; Hristu, R.; Ghita, R.V. Properties of Samarium doped hydroxyapatite thin films deposited by evaporation. Rom. Rep. Phys. 2017, 69, 508. [Google Scholar]
- Ciobanu, S.C.; Iconaru, S.L.; Predoi, D.; Prodan, A.M.; Predoi, M.V. Physico-chemical properties and in vitro antifungal evaluation of samarium doped hydroxyapatite coatings. Coatings 2020, 10, 827. [Google Scholar] [CrossRef]
- Iconaru, S.L.; Groza, A.; Gaiaschi, S.; Rokosz, K.; Raaen, S.; Ciobanu, S.C.; Chapon, P.; Predoi, D. Antimicrobial properties of samarium doped hydroxyapatite suspensions and coatings. Coatings 2020, 10, 1124. [Google Scholar] [CrossRef]
- Nica, I.C.; Popa, M.; Marutescu, L.; Dinischiotu, A.; Iconaru, S.L.; Ciobanu, S.C.; Predoi, D. Biocompatibility and antibiofilm properties of samarium doped hydroxyapatite coatings: An in vitro study. Coatings 2021, 11, 1185. [Google Scholar] [CrossRef]
- Morais, D.S.; Coelho, J.; Ferraz, M.P.; Gomes, P.S.; Fernandes, M.H.; Hussain, N.S.; Santos, J.D.; Lopes, M.A. Samarium doped glass-reinforced hydroxyapatite with enhanced osteoblastic performance and antibacterial properties for bone tissue regeneration. J. Mater. Chem. B 2014, 2, 5872–5881. [Google Scholar] [CrossRef] [PubMed]
- Nabeel, A.I. Samarium enriches antitumor activity of ZnO nanoparticles via downregulation of CXCR4 receptor and cytochrome P450. Tumor Biol. 2020, 42, 1010428320909999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kannan, S.; Nallaiyan, R. Anticancer activity of Samarium-coated magnesium implants for immunocompromised patients. ACS Appl. Bio Mater. 2020, 3, 4408–4416. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, Y.; Cao, C.; Yang, Y.; Wu, Y.; Ju, D.; Li, F. Long-term biodistribution in vivo and toxicity of radioactive/magnetic hydroxyapatite nanorods. Biomaterials 2014, 35, 3348–3355. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Wu, J.; Qin, C.; Xu, A.; Zhang, Z.; Lin, S.; Ren, X.; Zhang, P. Spin-coating synthesis and characterization of Zn-doped hydroxyapatite/polylactic acid composite coatings. Surf. Coat. Technol. 2016, 307, 461–469. [Google Scholar] [CrossRef]
- Zavestovskaya, I.N.; Grigorieva, M.; Petrunya, D.; Grigoriev, A.; Deyev, S.M.; Prasad, P.N.; Kabashin, A.V. Novel advanced nanotechnologies for nuclear medicine. J. Phys. Conf. Ser. 2021, 2058, 012035. [Google Scholar] [CrossRef]
- Popova-Kuznetsova, E.; Tikhonowski, G.; Popov, A.A.; Duflot, V.; Deyev, S.; Klimentov, S.; Zavestovskaya, I.; Prasad, P.N.; Kabashin, A.V. Laser-ablative synthesis of isotope-enriched samarium oxide nanoparticles for nuclear nanomedicine. Nanomaterials 2020, 10, 69. [Google Scholar] [CrossRef] [Green Version]
- Trujillo-Benítez, D.; Ferro-Flores, G.; Morales-Avila, E.; Jiménez-Mancilla, N.; Ancira-Cortez, A.; Ocampo-García, B.; Santos-Cuevas, C.; Escudero-Castellanos, A.; Luna-Gutiérrez, M.; Azorín-Vega, E. Synthesis and biochemical evaluation of samarium-153 oxide nanoparticles functionalized with iPSMA-bombesin heterodimeric peptide. J. Biomed. NanoTechnol. 2020, 16, 689–701. [Google Scholar] [CrossRef]
- Eressa, L.A.; Rao, P.V.B. Electrical properties of praseodymium and samarium co-doped ceria electrolyte for low-temperature solid oxide fuel cell application. Bull. Mater. Sci. 2021, 44, 255. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhu, J.; Song, H.; Huang, J.; Lu, Z.; Pan, G. Samarium doping improves luminescence efficiency of Cs3Bi2Br9 perovskite quantum dots enabling efficient white light-emitting diodes. J. Rare Earths 2021, 39, 374–379. [Google Scholar] [CrossRef]
- Leote, R.J.B.; Matei, E.; Apostol, N.G.; Enculescu, M.; Enculescu, I.; Diculescu, V.C. Monodispersed nanoplatelets of samarium oxides for biosensing applications in biological fluids. Electrochim. Acta 2021, 402, 139532. [Google Scholar] [CrossRef]
- Rao, N.S.; Rajesh, M.; Prasad, K.; Reddy, G.R.; Raju, B.D.P.; Dhanapandian, S. Study of trivalent samarium ion embedded lithium-based borate glass for high-density optical memory devices. Luminescence 2020, 35, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Sygnatowicz, M.; Tiwari, A. Controlled synthesis of hydroxyapatite-based coatings for biomedical application. Mater. Sci. Eng. C 2009, 29, 1071–1076. [Google Scholar] [CrossRef]
- Montenero, A.; Gnappi, G.; Ferrari, F.; Cesari, M.; Salvioli, E.; Mattogno, L.; Kaciulis, S.; Fini, M. Sol-gel derived hydroxyapatite coatings on titanium substrate. J. Mater. Sci. 2000, 35, 2791–2797. [Google Scholar] [CrossRef]
- Huang, L.Y.; Xu, K.W.; Lu, J. A study of the process and kinetics of electrochemical deposition and the hydrothermal synthesis of hydroxyapatite coatings. J. Mater. Sci. Mater. Med. 2000, 11, 667–673. [Google Scholar] [CrossRef]
- Münch, A.; Please, C.P.; Wagner, B. Spin coating of an evaporating polymer solution. Phys. Fluids 2011, 23, 102101. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Calcium orthophosphate coatings, films and layers. Prog. Biomater. 2012, 1, 1–40. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Tang, T. Surface treatment strategies to combat implant-related infection from the beginning. J. Orthop. Transl. 2019, 17, 42–54. [Google Scholar] [CrossRef]
- Eguia, A.; Arakistain, A.; De-la-Pinta, I.; López-Vicente, J.; Sevillano, E.; Quindós, G.; Eraso, E. Candida albicans biofilms on different materials for manufacturing implant abutments and prostheses. Med. Oral Patol. Oral Cir. Bucal 2020, 25, e13–e20. [Google Scholar] [CrossRef]
- Predoi, D.; Iconaru, S.L.; Predoi, M.V.; Stan, G.E.; Buton, N. Synthesis, characterization, and antimicrobial activity of magnesium-doped hydroxyapatite suspensions. Nanomaterials 2019, 9, 1295. [Google Scholar] [CrossRef] [Green Version]
- Ciobanu, C.S.; Iconaru, S.L.; Massuyeau, F.; Constantin, L.V.; Costescu, A.; Predoi, D. Synthesis, structure, and luminescent properties of europium-doped hydroxyapatite nanocrystalline powders. J. Nanomater. 2012, 2012, 942801. [Google Scholar] [CrossRef] [Green Version]
- Predoi, D.; Iconaru, S.L.; Predoi, M.V.; Motelica-Heino, M.; Guegan, R.; Buton, N. Evaluation of antibacterial activity of zinc-doped hydroxyapatite colloids and dispersion stability using ultrasounds. Nanomaterials 2019, 9, 515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Predoi, D.; Iconaru, S.L.; Predoi, M.V.; Motelica-Heino, M. Removal and oxidation of As(III) from water using iron oxide coated CTAB as adsorbent. Polymers 2020, 12, 1687. [Google Scholar] [CrossRef] [PubMed]
- Predoi, D.; Iconaru, S.L.; Ciobanu, S.C.; Predoi, S.-A.; Buton, N.; Megier, C.; Beuran, M. Development of iron-doped hydroxyapatite coatings. Coatings 2021, 11, 186. [Google Scholar] [CrossRef]
- Predoi, S.-A.; Ciobanu, C.S.; Motelica-Heino, M.; Chifiriuc, M.C.; Badea, M.L.; Iconaru, S.L. Preparation of porous hydroxyapatite using cetyl trimethyl ammonium bromide as surfactant for the removal of lead ions from aquatic solutions. Polymers 2021, 13, 1617. [Google Scholar] [CrossRef]
- Gwyddion. Available online: http://gwyddion.net/ (accessed on 20 June 2021).
- ImageJ. Available online: http://imagej.nih.gov/ij (accessed on 20 June 2021).
- Iconaru, S.L.; Motelica-Heino, M.; Predoi, D. Study on europium-doped hydroxyapatite nanoparticles by fourier transform infrared spectroscopy and their antimicrobial properties. J. Spectrosc. 2013, 2013, 284285. [Google Scholar] [CrossRef] [Green Version]
- Predoi, D.; Groza, A.; Iconaru, S.L.; Predoi, G.; Barbuceanu, F.; Guegan, R.; Motelica-Heino, M.S.; Cimpeanu, C. Properties of basil and lavender essential oils adsorbed on the surface of hydroxyapatite. Materials 2018, 11, 652. [Google Scholar] [CrossRef] [Green Version]
- Iconaru, S.L.; Prodan, A.M.; Turculet, C.S.; Beuran, M.; Ghita, R.V.; Costescu, A.; Groza, A.; Chifiriuc, M.C.; Chapon, P.; Gaiaschi, S.; et al. Enamel based composite layers deposited on titanium substrate with antifungal activity. J. Spectrosc. 2016, 2016. [Google Scholar] [CrossRef]
- Iconaru, S.L.; Groza, A.; Stan, G.E.; Predoi, D.; Gaiaschi, S.; Trusca, R.; Chifiriuc, C.M.; Marutescu, L.; Tite, T.; Stanciu, G.A.; et al. Preparations of silver/montmorillonite biocomposite multilayers and their antifungal activity. Coatings 2019, 9, 817. [Google Scholar] [CrossRef] [Green Version]
- Ciobanu, C.S.; Groza, A.; Iconaru, S.L.; Popa, C.L.; Chapon, P.; Chifiriuc, M.C.; Hristu, R.; Stanciu, G.A.; Negrila, C.C.; Ghita, R.V.; et al. Antimicrobial activity evaluation on silver doped hydroxyapatite/polydimethylsiloxane composite layer. BioMed Res. Int. 2015, 2015, 926513. [Google Scholar] [CrossRef] [Green Version]
- ASTM E2149-13a. Standard Test Method for Determining the Antimicrobial Activity of Antimicrobial Agents under Dynamic Contact Conditions; ASTM International: West Conshohocken, PA, USA, 2013. [Google Scholar]
- Fuchs, A.V.; Ritz, S.; Pütz, S.; Mailänder, V.; Landfester, K.; Ziener, U. Bioinspired phosphorylcholine containing polymer films with silver nanoparticles combining antifouling and antibacterial properties. Biomater. Sci. 2013, 1, 470–477. [Google Scholar] [CrossRef]
- Predoi, D.; Iconaru, S.L.; Predoi, M.V.; Buton, N.; Motelica-Heino, M. Zinc doped hydroxyapatite thin films prepared by sol–gel spin coating procedure. Coatings 2019, 9, 156. [Google Scholar] [CrossRef] [Green Version]
- Dukhin, A.S.; Goetz, P.J. Ultrasound for Characterizing Colloids; Elsevier: Amsterdam, The Netherlands, 2002. [Google Scholar]
- Shimizu, K.; Habazaki, H.; Skeldon, P.; Thompson, G.E. Radiofrequency GDOES: A powerful technique for depth profiling analysis of thin films. Surf. Interface Anal. 2003, 35, 564–574. [Google Scholar] [CrossRef]
- Reich, S.; Mazur, S.; Avakian, P.; Wilson, F.C. Sintering of silver interlayers in polyimide films. J. Appl. Phys. 1987, 62, 287–292. [Google Scholar] [CrossRef]
- Zheng, H.; Berry, D.; Ngo, K.D.; Lu, G.Q. Chip-bonding on copper by pressureless sintering of nanosilver paste under controlled atmosphere. IEEE Trans. Compon. Packag. Manuf. Technol. 2014, 4, 377–384. [Google Scholar] [CrossRef]
- Shimizu, K.; Payling, R.; Habazaki, H.; Skeldon, P.; Thompson, G.E. Rf-GDOES depth profiling analysis of a monolayer of thiourea adsorbed on copper. J. Anal. At. Spectrom. 2004, 19, 692–695. [Google Scholar] [CrossRef]
- Hultquist, G.; Seo, M.; Leitner, T.; Leygraf, C.; Sato, N. The dissolution behavior of iron, chromium, molybdenum and copper from pure metals and from ferritic stainless-steels. Corros. Sci. 1987, 27, 937–946. [Google Scholar] [CrossRef]
- Turculet, C.S.; Prodan, A.M.; Negoi, I.; Teleanu, G.; Popa, M.; Andronescu, E.; Beuran, M.; Stanciu, G.A.; Hristu, R.; Badea, M.L.; et al. Preliminary evaluation of the antifungal activity of samarium doped hydroxyapatite thin films. Rom. Biotechnol. Lett. 2018, 23, 13927–13932. [Google Scholar] [CrossRef]
- Jiang, W.U.; Zhang, G.; Jie, L.I.U.; Hongbing, G.A.O.; Chunxiang, S.O.N.G.; Haoran, D.U.; Zhang, L.; Zhongping, G.O.N.G.; Yuguang, L.Ü. Synthesis, characteristics, and antibacterial activity of a rare-earth samarium/silver/titanium dioxide inorganic nanomaterials. J. Rare Earths 2014, 32, 727–732. [Google Scholar] [CrossRef]
- Ain, Q.; Pandey, S.K.; Pandey, O.P.; Sengupta, S.K. Synthesis, spectroscopic, thermal and antimicrobial studies of neodymium(III) and samarium(III) complexes derived from tetradentate ligands containing N and S donor atoms. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 140, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Nandal, P.; Kumar, R.; Khatkar, A.; Khatkar, P.; Taxak, V.B. Synthesis, characterization, enhanced photoluminescence, antimicrobial and antioxidant activities of novel Sm(III) complexes containing 1-(2-hydroxy-4,6-dimethoxyphenyl)ethanone and nitrogen containing ancillary ligands. J. Mater. Sci. Mater. Electron. 2016, 27, 878–885. [Google Scholar] [CrossRef]
- Morais, D.S.; Rodrigues, M.A.; Lopes, M.A.; Coelho, M.J.; Maurıcio, A.C.; Gomes, R.; Amorim, I.; Ferraz, M.P.; Santos, J.D.; Botelho, C.M. Biological evaluation of alginate-based hydrogels, with antimicrobial features by Ce(III) incorporation, as vehicles for a bone substitute. J. Mater. Sci. Mater. Med. 2013, 24, 2145–2155. [Google Scholar] [CrossRef] [Green Version]
- Barry, J.N.; Cowley, A.; McNally, P.J.; Dowling, D.P. Influence of substrate metal alloy type on the properties of hydroxyapatite coatings deposited using a novel ambient temperature deposition technique. J. Biomed. Mater. Res. A 2014, 102, 871–879. [Google Scholar] [CrossRef] [PubMed]
- Ferraris, S.; Yamaguchi, S.; Barbani, N.; Cristallini, C.; Gautier di Confiengo, G.; Barberi, J.; Cazzola, M.; Miola, M.; Vernè, E.; Sprian, S. The mechanical and chemical stability of the interfaces in bioactive materials: The substrate-bioactive surface layer and hydroxyapatite bioactive surface layer interfaces. Mater. Sci. Eng. C 2020, 116, 111238. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciobanu, C.S.; Predoi, D.; Chapon, P.; Predoi, M.V.; Iconaru, S.L. Fabrication and Physico-Chemical Properties of Antifungal Samarium Doped Hydroxyapatite Thin Films. Coatings 2021, 11, 1466. https://doi.org/10.3390/coatings11121466
Ciobanu CS, Predoi D, Chapon P, Predoi MV, Iconaru SL. Fabrication and Physico-Chemical Properties of Antifungal Samarium Doped Hydroxyapatite Thin Films. Coatings. 2021; 11(12):1466. https://doi.org/10.3390/coatings11121466
Chicago/Turabian StyleCiobanu, Carmen Steluta, Daniela Predoi, Patrick Chapon, Mihai Valentin Predoi, and Simona Liliana Iconaru. 2021. "Fabrication and Physico-Chemical Properties of Antifungal Samarium Doped Hydroxyapatite Thin Films" Coatings 11, no. 12: 1466. https://doi.org/10.3390/coatings11121466
APA StyleCiobanu, C. S., Predoi, D., Chapon, P., Predoi, M. V., & Iconaru, S. L. (2021). Fabrication and Physico-Chemical Properties of Antifungal Samarium Doped Hydroxyapatite Thin Films. Coatings, 11(12), 1466. https://doi.org/10.3390/coatings11121466








