Study the Passivation Characteristics of Microwave Annealing Applied to APALD Deposited Al2O3 Thin Film
Abstract
:1. Introduction
2. Materials and Methods
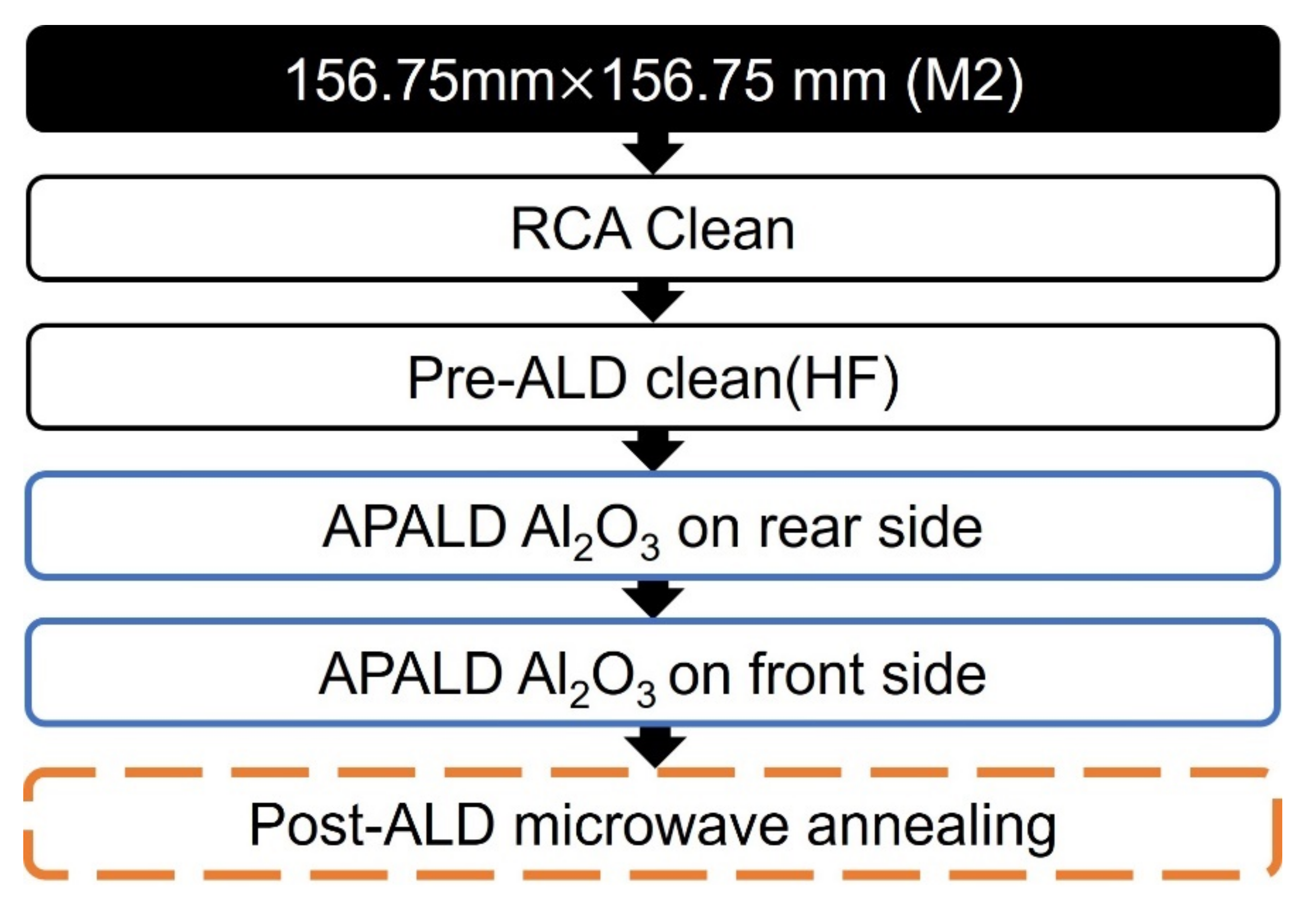
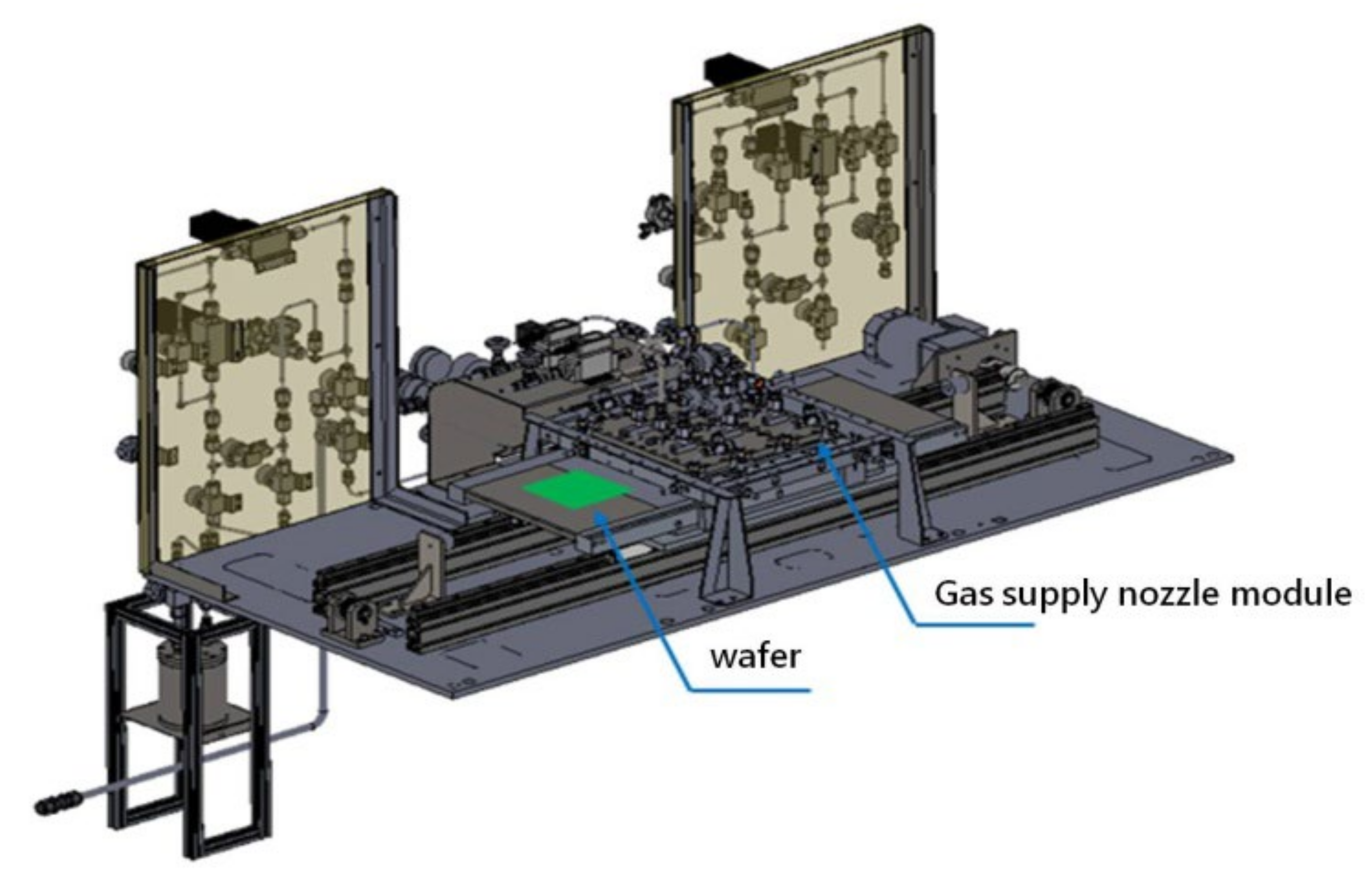
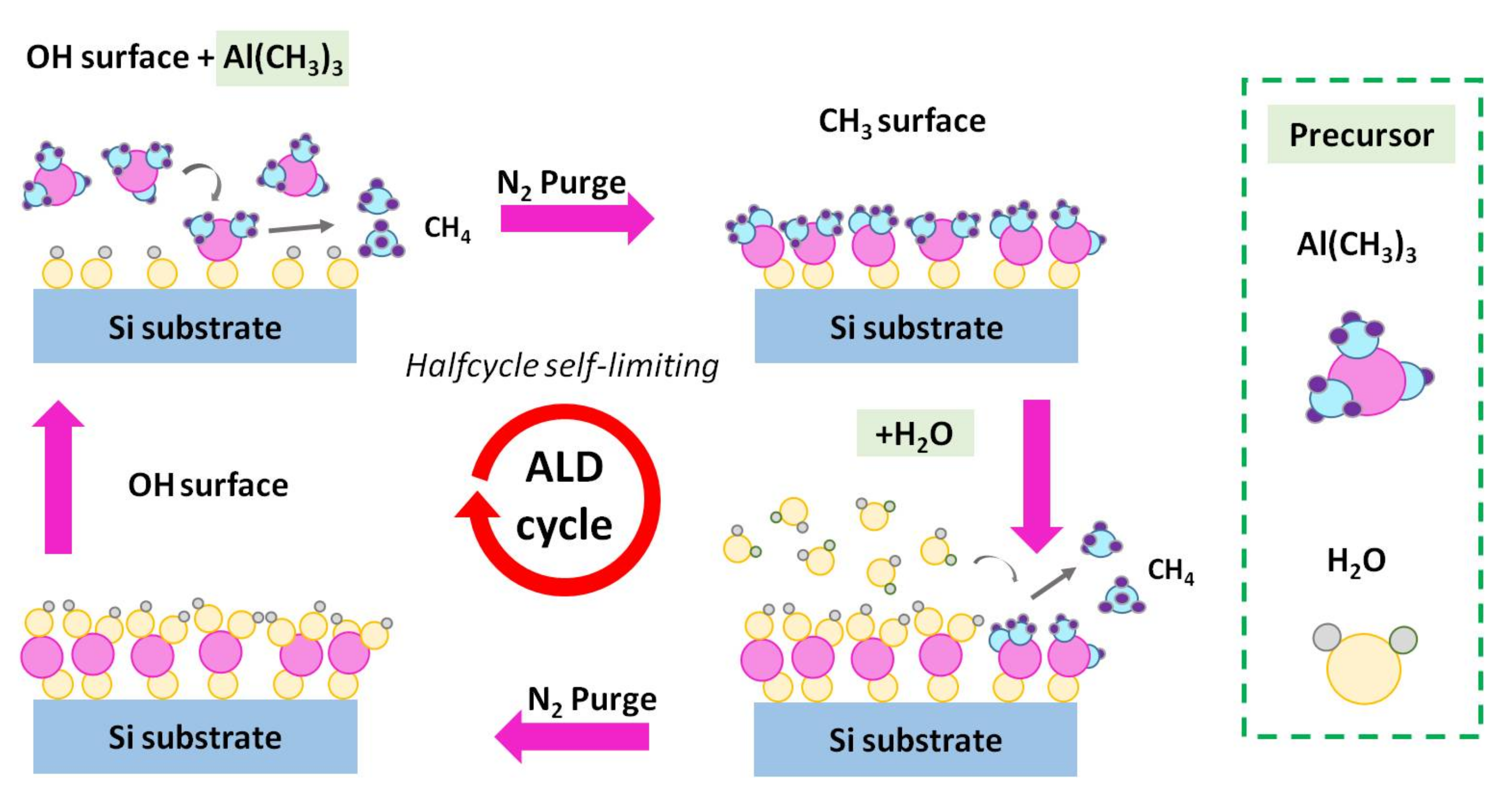
2.1. Al2O3 Film Growth via APALD of TMA and H2O
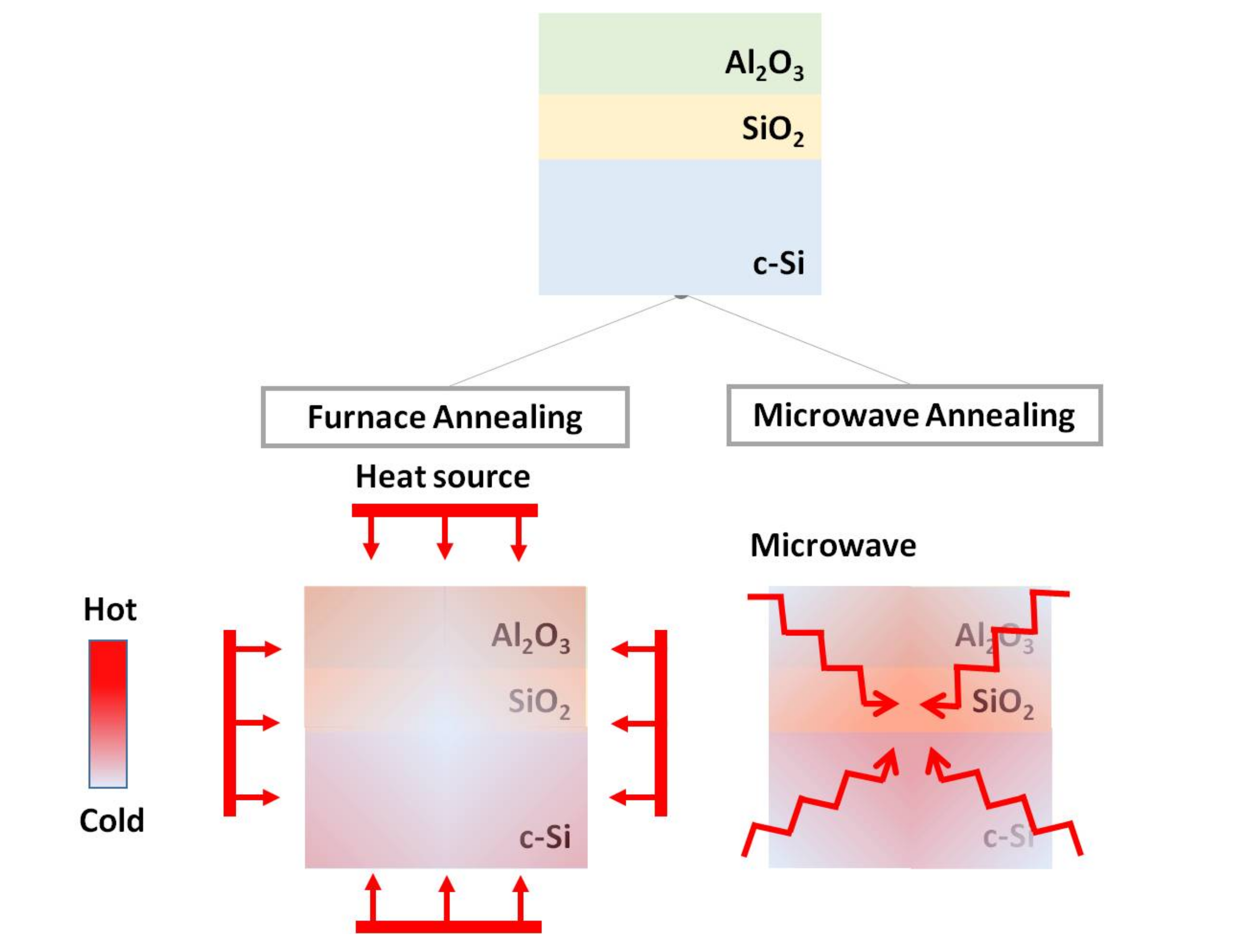
2.2. Microwave Annealing Process
3. Results and Discussion
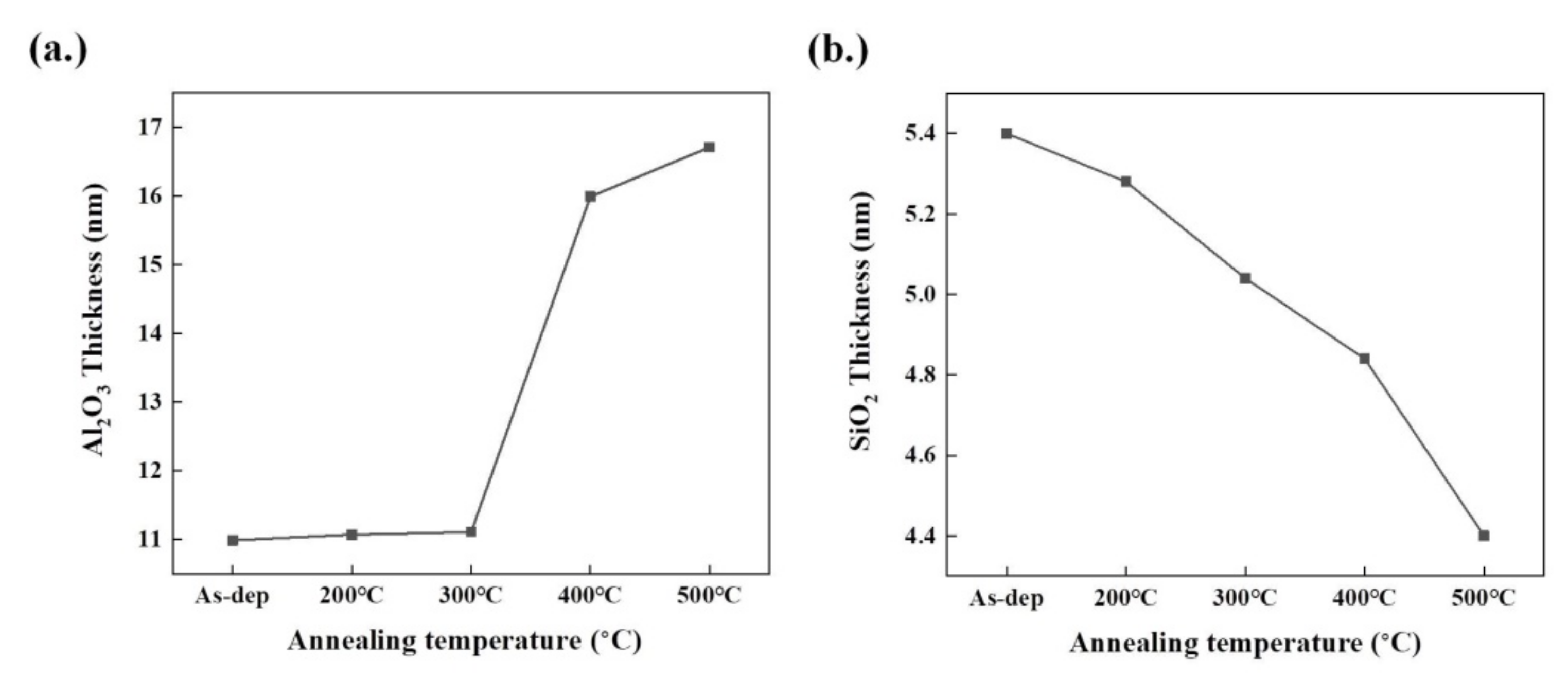
3.1. Transmission Electron Microscope (TEM) Image
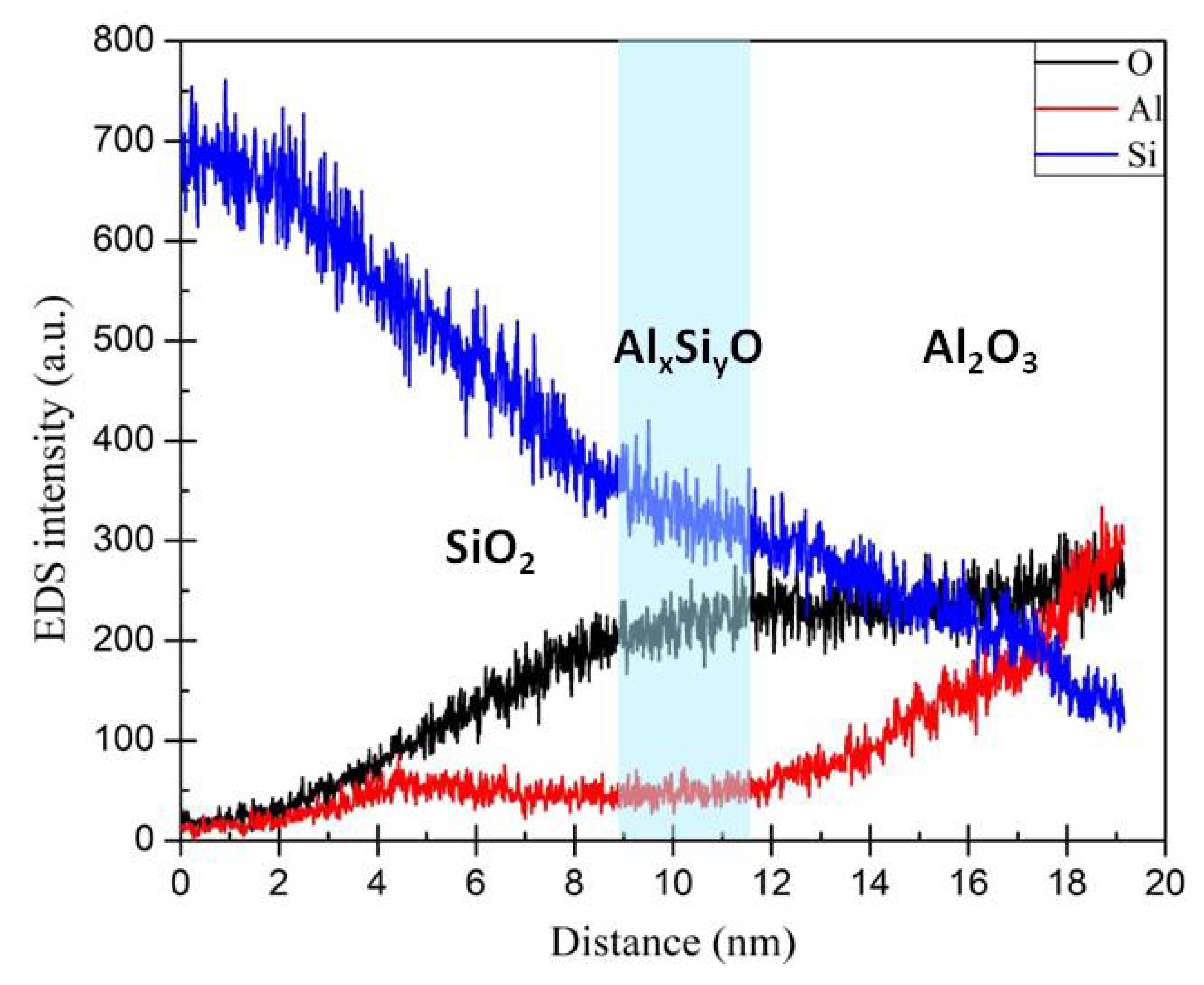
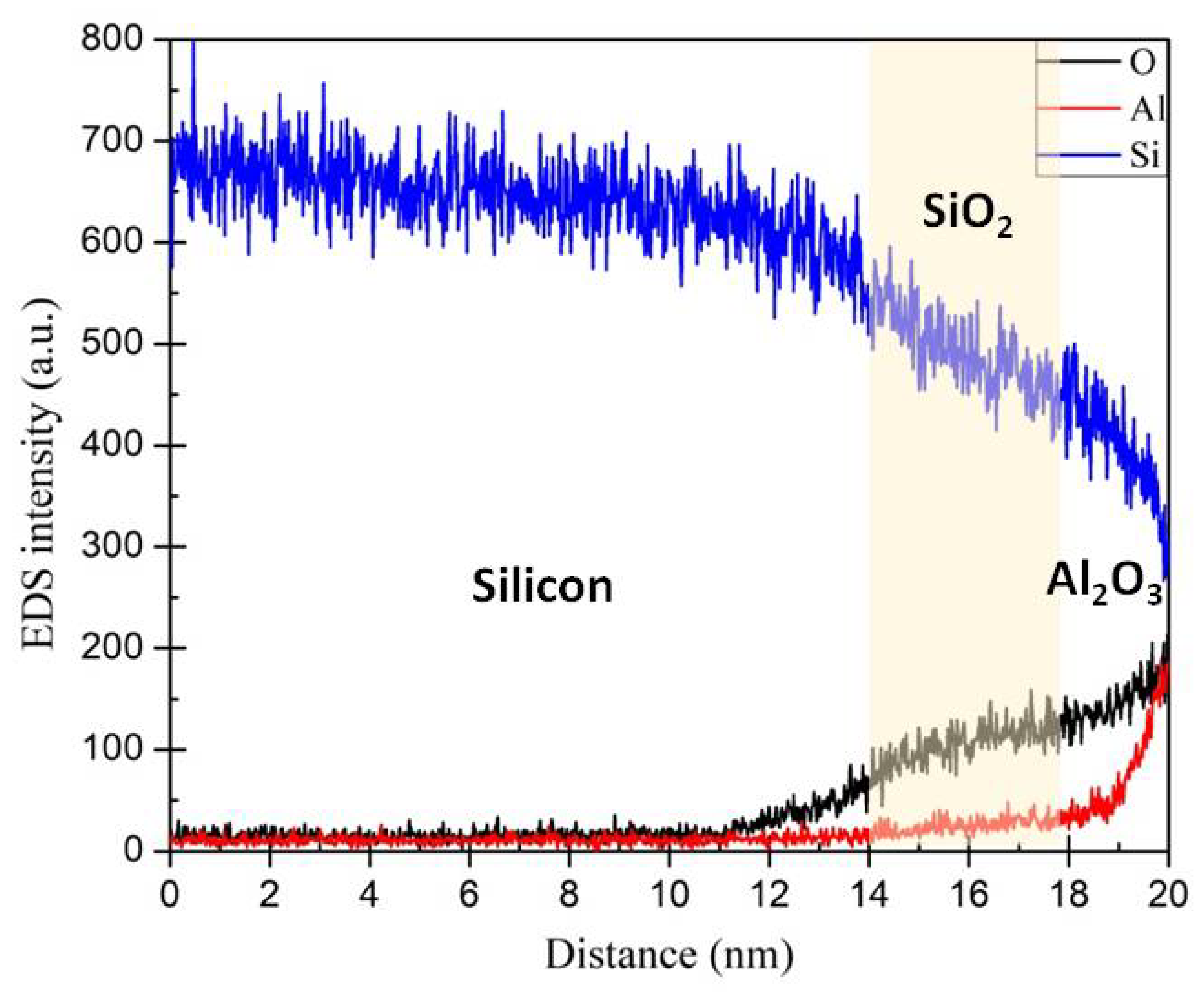
3.2. Energy-Dispersive Spectroscopy (EDS) Analysis
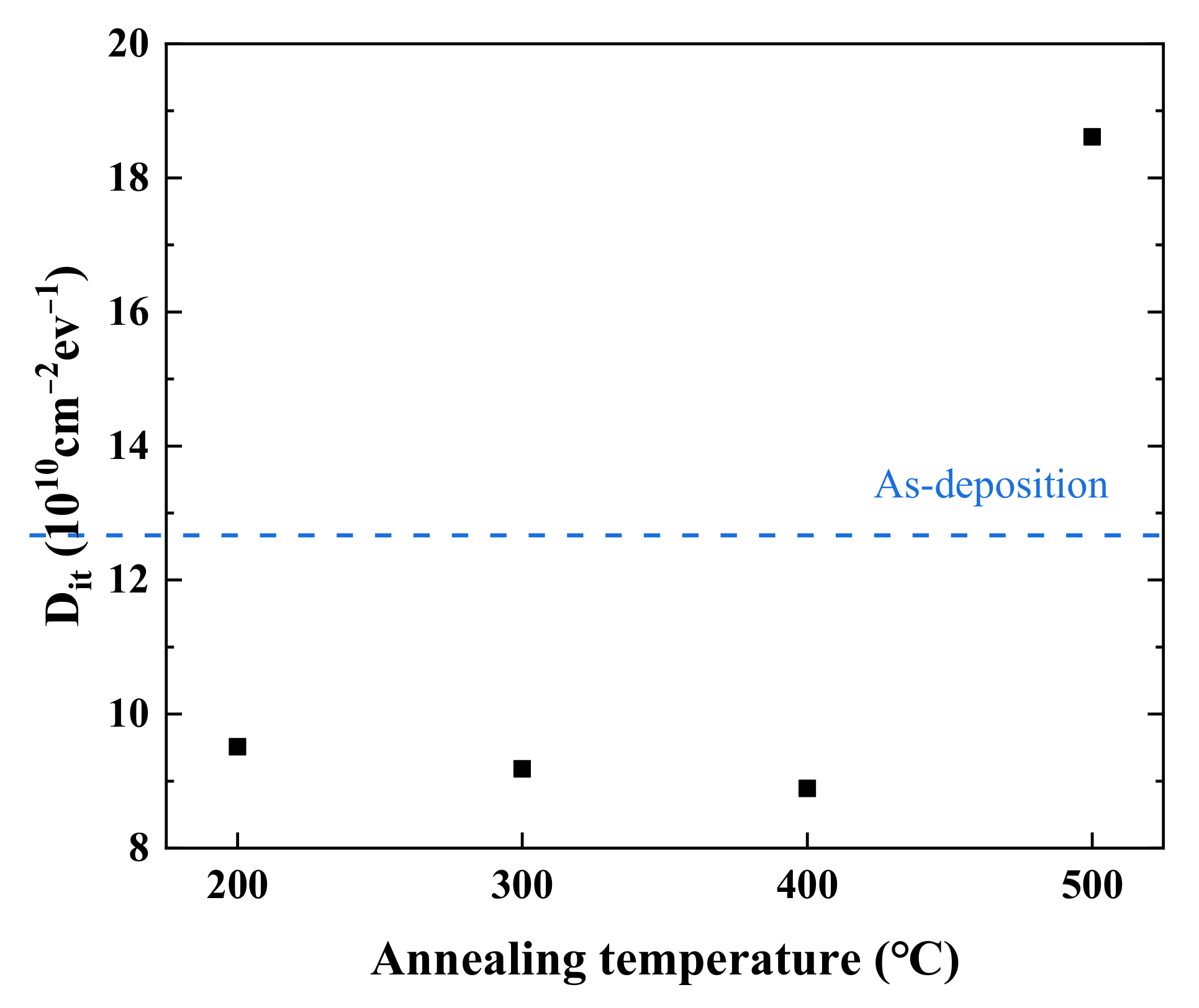
3.3. Capacitance-Voltage (C-V) Measurement
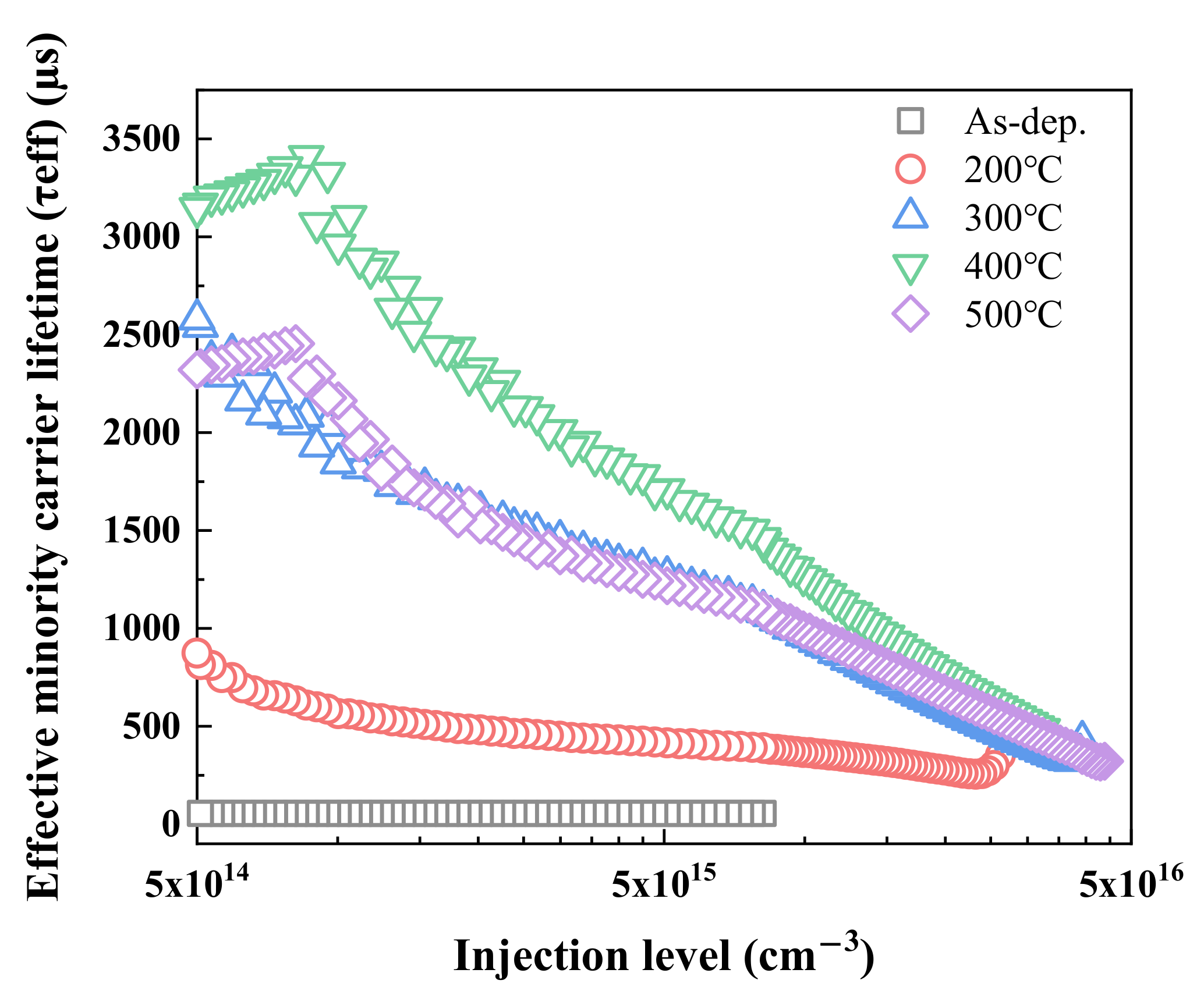
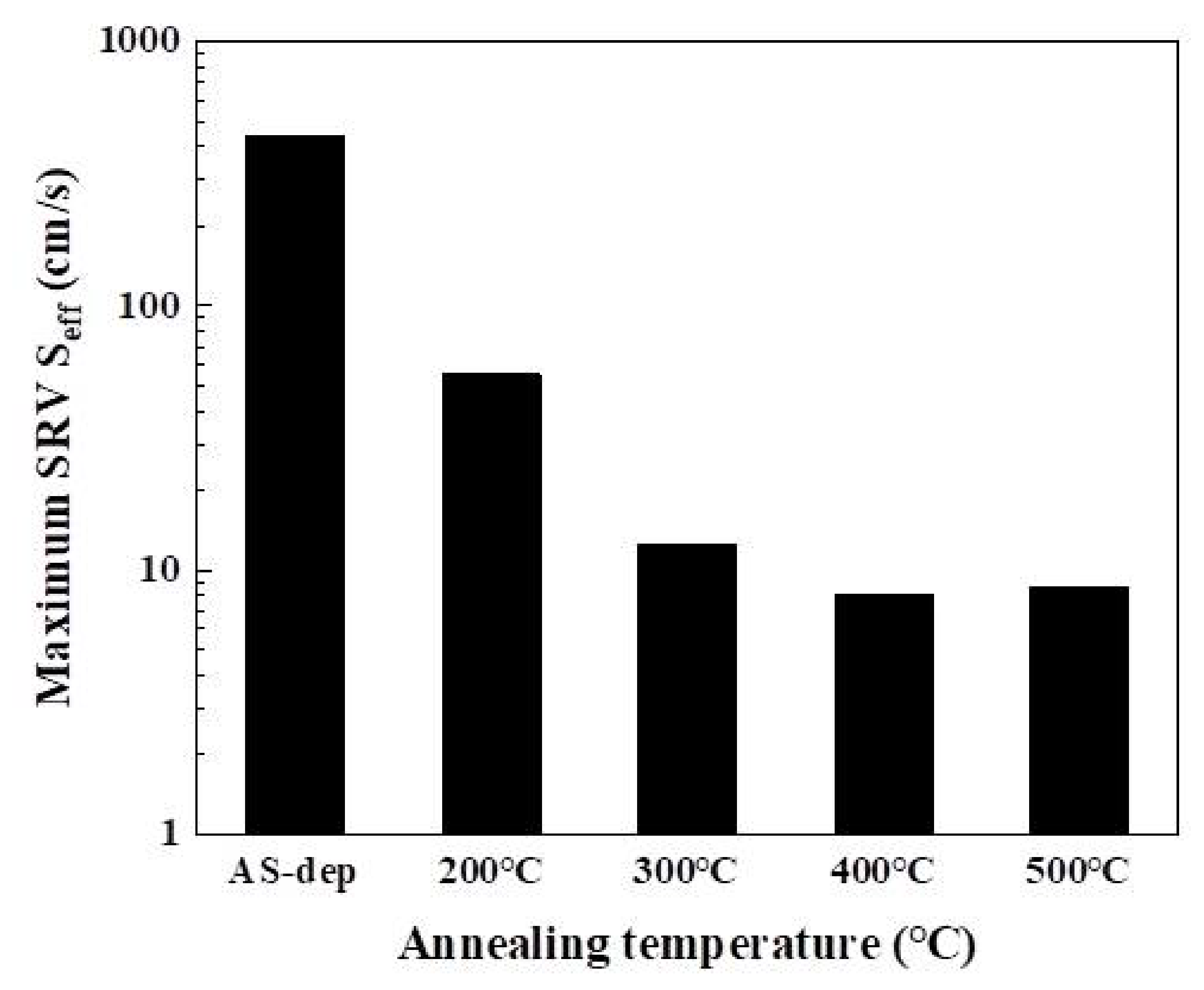
3.4. Quasi-Steady-State Photoconductance (QSSPC) Method
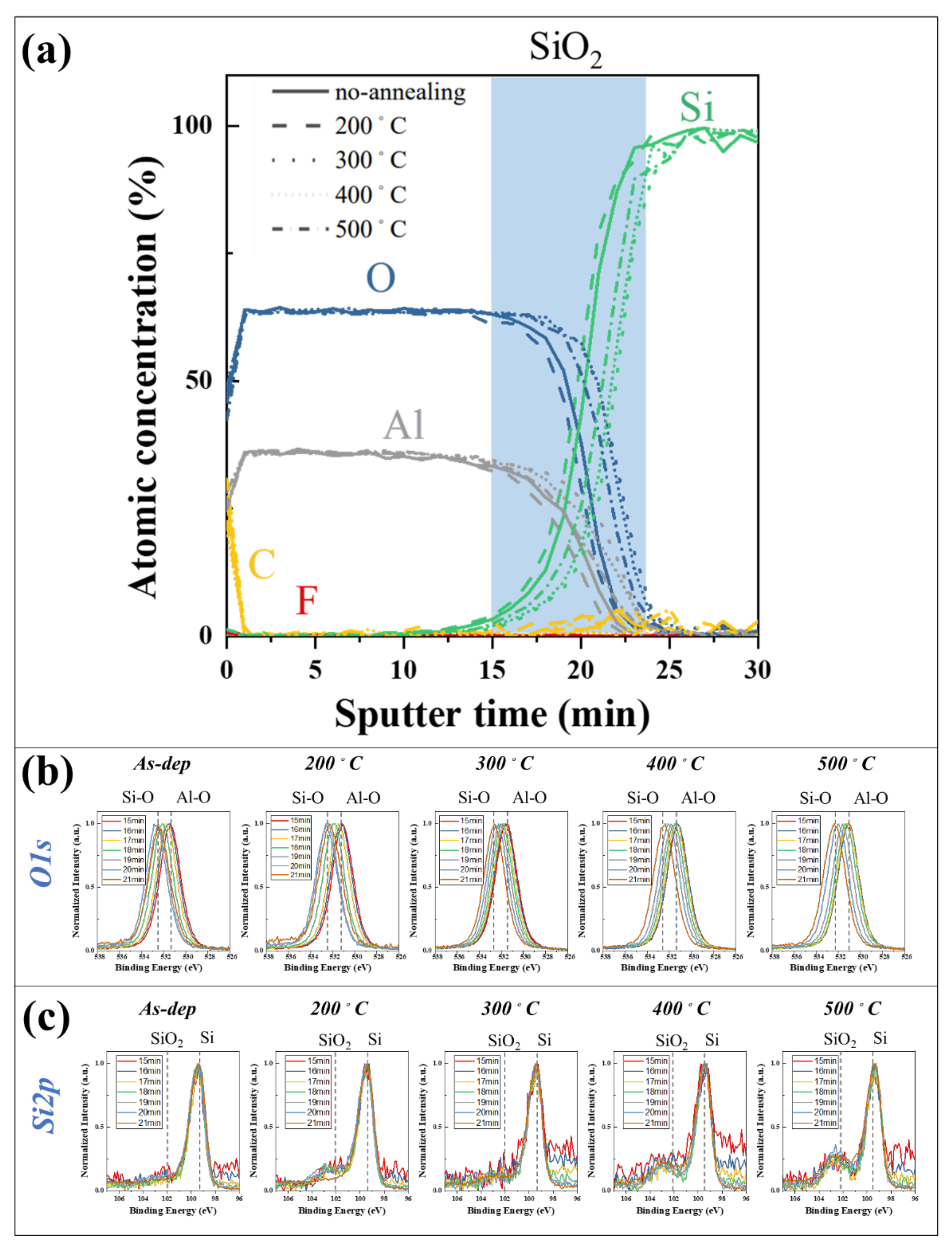
3.5. Surface Chemical Analysis (X-ray Photoelectron Spectroscopy)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Blakers, A. Development of the PERC Solar Cell. IEEE J. Photovolt. 2019, 9, 629–635. [Google Scholar] [CrossRef]
- Barar, A.; Manaila-Maximean, D. Ruthenium-based DSSC efficiency optimization by grapheme quantum dot doping. Univ. Politeh. Buchar. Sci. Bull. Ser. A Appl. Math. Phys. 2021, 2, 309–316. [Google Scholar]
- Barar, A.; Manaila-Maximean, D.; Danila, O.; Vladescu, M. Parameter extraction of an organic solar cell using asymptotic estimation and Lambert W function. In Advanced Topics in Optoelectronics, Microelectronics, and Nanotechnologies VIII; SPIE—International Society for Optics and Photonics: Constanta, Romania, 2016; Volume 10010, p. 1001034. [Google Scholar]
- Hannebauer, H.; Dullweber, T.; Baumann, U.; Falcon, T.; Brendel, R. 21.2%-efficient fineline-printed PERC solar cell with 5 busbar front grid. Phys. Status Solidi Rapid Res. Lett. 2014, 8, 675–679. [Google Scholar] [CrossRef]
- Ye, F.; Deng, W.; Guo, W.; Liu, R.; Chen, D.; Chen, Y.; Yang, Y.; Yuan, N.; Ding, J.; Feng, Z.; et al. 22.13% Efficient Industrial p-Type Mono PERC Solar Cell. In Proceedings of the IEEE 43rd Photovoltaic Specialists Conference, Portland, OR, USA, 5–10 June 2016; IEEE: New York, NY, USA. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, H.C.; Liu, C.; Dong, J.W.; Chow, P. Annealing of Al2O3 thin films prepared by atomic layer deposition. J. Phys. D 2007, 40, 3707–3713. [Google Scholar] [CrossRef]
- Naumann, V.; Otto, M.; Wehrspohn, R.B.; Hagendorf, C. Chemical and structural study of electrically passivating Al2O3/Si interfaces prepared by atomic layer deposition. J. Vac. Sci. Technol. B 2012, 30, 04D106-1–04D106-6. [Google Scholar] [CrossRef]
- Lei, D.; Yu, X.; Song, L.; Gu, X.; Li, G.; Yang, D. Modulation of atomic-layer-deposited Al2O3 film passivation of silicon surface by rapid thermal processing. Appl. Phys. Lett. 2011, 99, 052103. [Google Scholar] [CrossRef]
- Cast, P.S.; Heo, Y.H.; Billot, E.; Olwal, P.; Hofmann, M.; Rentsch, J.; Glunz, S.W.; Preu, R. Variation of the layer thickness to study the electrical property of PECVD Al2O3 / c-Si interface. Energy Procedia 2011, 8, 642–647. [Google Scholar] [CrossRef]
- Saint-Cast, P.; Kania, D.; Heller, R.; Kuehnhold, S.; Hofmann, M.; Rentsch, J.; Preu, R. High-temperature stability of c-Si surface passivation by thick PECVD Al2O3 with and without hydrogenated capping layers. Appl. Surf. Sci. 2012, 258, 8371–8376. [Google Scholar] [CrossRef]
- Schmidt, J.; Wernera, F.; Veith, B.; Zielke, D.; Steingrube, S.; Altermatt, P.P.; Gatz, S.; Dullweber, T.; Brendel, R. Advances in the Surface Passivation of Silicon Solar Cells. Energy Procedia 2012, 15, 30–39. [Google Scholar] [CrossRef] [Green Version]
- Nowicki, R.S. Properties of rfsputtered Al2O3 films deposited by planar magnetron. J. Vac. Sci. Technol. 1977, 14, 127–133. [Google Scholar] [CrossRef]
- Edlmayr, V.; Moser, M.; Walter, C.; Mitterer, C. Thermal stability of sputtered Al2O3 coatings. Surf. Coat. Technol. 2010, 204, 1576–1581. [Google Scholar] [CrossRef]
- Hu, B.; Yao, M.; Xiao, R.; Chen, J.; Yao, X. Optical properties of amorphous Al2O3 thin films prepared by a sol-gel process. Ceram. Int. 2014, 40, 14133–14139. [Google Scholar] [CrossRef]
- Cibert, C.; Hidalgo, H.; Champeaux, C.; Tristant, P.; Tixier, C.; Desmaison, J.; Catherinot, A. Properties of aluminum oxide thin films deposited by pulsed laser deposition and plasma enhanced chemical vapor deposition. Thin Solid Films 2008, 516, 1290–1296. [Google Scholar] [CrossRef]
- Jakschik, S.; Avellan, A.; Schroeder, U.; Bartha, J.W. Influence of Al2O3 Dielectrics on the Trap-Depth Profiles in MOS Devices Investigated by the Charge-Pumping Method. IEEE Trans. Electron. Devices 2004, 51, 2252–2255. [Google Scholar] [CrossRef]
- Thomas, S.; Nalini, S.; Kumar, K.R. Microwave plasma-assisted ALD of Al2O3 thin films: A study on the substrate temperature dependence of various parameters of interest. Appl. Phys. A 2017, 123, 185. [Google Scholar] [CrossRef]
- Huang, Y.-C.; Chuang, R.W. Study on Annealing Process of Aluminum Oxide Passivation Layer for PERC Solar Cells. Coatings 2021, 11, 1052. [Google Scholar] [CrossRef]
- Vahdatifar, S.; Mortazavi, Y.; Khodadadi, A.A. Atmospheric pressure atomic layer deposition of iron oxide nanolayer on the Al2O3/SiO2/Si substrate for mmtall vertically aligned CNTs growth. J. Mater. Sci. 2020, 55, 13634–13657. [Google Scholar] [CrossRef]
- Hoye, R.L.Z.; Muñoz-Rojas, D.; Nelson, S.F.; Illiberi, A.; Poodt, P.; Roozeboom, F.; MacManus-Driscoll, J.L. Research Update: Atmospheric pressure spatial atomic layer deposition of ZnO thin films: Reactors, doping, and devices. APL Mater. 2015, 3, 040701. [Google Scholar] [CrossRef]
- Levy, D.H.; Nelson, S.F.; Freeman, D. Oxide Electronics by Spatial Atomic Layer. J. Display Technol. 2009, 5, 484–494. [Google Scholar] [CrossRef]
- Guo, J.; Wang, D.; Xu, Y.; Zhu, X.; Wen, K.; Miao, G.; Cao, W.; Si, J.; Lu, M.; Guo, H. Secondary electron emission characteristics of Al2O3 coatings prepared by atomic layer deposition. AIP Adv. 2019, 9, 095303. [Google Scholar] [CrossRef]
- Zhu, B.; Wu, X.; Liu, W.-J.; Ding, S.-J.; Zhang, D.W.; Fan, Z. Dielectric Enhancement of Atomic Layer-Deposited Al2O3/ZrO2/Al2O3 MIM Capacitors by Microwave Annealing. Nano Lett. 2019, 14, 53. [Google Scholar] [CrossRef]
- Bhattacharya, M.; Basak, T. A review on the susceptor assisted microwave processing of materials. Energy 2016, 97, 306–338. [Google Scholar] [CrossRef]
- Hsu, C.H.; Huang, C.W.; Cho, Y.S.; Wu, W.Y.; Wuu, D.S.; Zhang, X.Y.; Zhu, W.Z.; Lien, S.Y.; Ye, C.S. Efficiency improvement of PERC solar cell using an aluminum oxide passivation layer prepared via spatial atomic layer deposition and post annealing. Surf. Coat. Technol. 2019, 358, 968–975. [Google Scholar] [CrossRef]
- Lee, Y.-J.; Chuang, S.-S.; Hsueh, F.-K.; Lin, H.-M.; Wu, S.-C.; Wu, C.-Y.; Tseng, T.-Y. Dopant Activation in Single-Crystalline Germanium by Low-Temperature Microwave Annealing. IEEE Electron. Device Lett. 2011, 32, 194–196. [Google Scholar] [CrossRef]
- Song, K.; Koo, C.Y.; Jun, T.; Lee, D.; Jeong, Y.; Moon, J. Low-temperature soluble InZnO thin film transistors by microwave annealing. J. Cryst. Growth 2011, 326, 23–27. [Google Scholar] [CrossRef]
- Fu, C.; Wang, Y.; Xu, P.; Yue, L.; Sun, F.; Zhang, D.W.; Zhang, S.-L.; Luo, J.; Zhao, C.; Wu, D. Understanding the microwave annealing of silicon. AIP Adv. 2017, 7, 035214. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.-J.; Cho, T.-C.; Chuang, S.-S.; Hsueh, F.-K.; Lu, Y.-L.; Sung, P.-J.; Chen, H.-C.; Current, M.I.; Tseng, T.-Y.; Chao, T.-S.; et al. Low-Temperature Microwave Annealing Processes for Future IC Fabrication—A Review. IEEE Trans. Electron Devices 2014, 61, 651–665. [Google Scholar] [CrossRef]
- Ziegler, J.; Otto, M.; Sprafke, A.N.; Wehrspohn, R.B. Activation of Al2O3 passivation layers on silicon by microwave annealing. Appl. Phys. A 2013, 113, 285–290. [Google Scholar] [CrossRef]
- Yue, L.; Fu, C.-C.; Sun, F.; Qiu, Z.-J.; Zhang, S.-L.; Wu, D. Microwave Annealing as a Low Thermal Budget Technique for ZnO Thin-Film Transistors Fabricated Using Atomic Layer Deposition. IEEE Electron. Device Lett. 2017, 38, 1390–1393. [Google Scholar] [CrossRef]
- Shi, S.; Qian, S.; Hou, X.; Mu, J.; He, J.; Chou, X. Structural and Optical Properties of Amorphous Al2O3 Thin Film Deposited by Atomic Layer Deposition. Adv. Condens. Matter Phys. 2018, 2018, 7598978. [Google Scholar] [CrossRef] [Green Version]
- Djebaili, K.; Mekhalif, Z.; Boumaza, A.; Djelloul, A. XPS, FTIR, EDX, and XRD Analysis of Al2O3 Scales Grown on PM2000 Alloy. J. Spectrosc. 2015, 2015, 868109. [Google Scholar] [CrossRef] [Green Version]
- Glunz, S.W.; Preu, R.; Biro, D. Crystalline Silicon Solar Cells. In Comprehensive Renewable Energy; Sayigh, A., Ed.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 353–387. [Google Scholar] [CrossRef]
- Lale, A.; Scheid, E.; Cristiano, F.; Datas, L.; Reig, B.; Launay, J.; Temple-Boyer, P. Study of aluminium oxide thin films deposited by plasmaenhanced atomic layer deposition from tri-methyl-aluminium and dioxygen precursors: Investigation of interfacial and structural properties. Thin Solid Films 2018, 666, 20–27. [Google Scholar] [CrossRef] [Green Version]
- Sinton, R.A. Contactless determination of current–voltage characteristics and minority-carrier lifetimes in semiconductors from quasi-steady-state photoconductance data. Appl. Phys. Lett. 1996, 69, 2510. [Google Scholar] [CrossRef]
- Demaurex, B.; Seif, J.P.; Smit, S.; Macco, B.; Kessels, W.M.M.; Geissbühler, J.S.; Wolf, D.; Ballif, C. Atomic Layer Deposited Transparent Electrodes for Silicon Heterojunction Solar Cells. IEEE J. Photovolt. 2014, 4, 1387–1396. [Google Scholar] [CrossRef]
- Goodarzi, M.; Sinton, R.; Macdonald, D. Quasi-steady-state photoconductance bulk lifetime measurements on silicon ingots with deeper photogeneration. AIP Adv. 2019, 9, 015128. [Google Scholar] [CrossRef] [Green Version]
- Kontermann, S.; Wolf, A.; Reinwand, D.; Grohe, A.; Biro, D.; Preu, R. Optimizing annealing steps for crystalline silicon solar cells with screen printed front side metallization and an oxide-passivated rear surface with local contacts. Prog. Photovolt Res. Appl. 2009, 17, 554–566. [Google Scholar] [CrossRef]
- Dauwe, S.; Schmidt, J.; Hezel, R. Very low surface recombination velocities on p- and n-type silicon wafers passivated with hydrogenated amorphous silicon films. In Proceedings of the Twenty-Ninth IEEE Photovoltaic Specialists Conference, New Orleans, LA, USA, 19–24 May 2002; IEEE: New York, NY, USA. [Google Scholar] [CrossRef]
- Lelièvre, J.-F.; Fourmond, E.; Kaminski, A.; Palais, O.; Ballutaud, D.; Lemiti, M. Study of the composition of hydrogenated silicon nitride SiNx:H for efficient surface and bulk passivation of silicon. Sol. Energy Mater Sol. Cells 2009, 93, 1281–1289. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Juhl, M.; Abbott, M.; Hameiri, Z.; Yao, Y.; Lennon, A. Use of QSSPC and QSSPL to monitor recombination processes in p-type silicon solar cells. Energy Procedia 2014, 55, 169–178. [Google Scholar] [CrossRef] [Green Version]
- Werner, F.; Veith, B.; Zielke, D.; Kühnemund, L.; Tegenkamp, C.; Seibt, C.; Brendel, R.; Schmidt, J. Electronic and chemical properties of the c-Si/Al2O3 interface. J. Appl. Phys. 2011, 109, 113701. [Google Scholar] [CrossRef]
- Cho, M.; Park, H.B.; Park, J.; Hwang, C.S.; Lee, J.-C.; Oh, S.-J.; Jeong, J.K.; Hyun, S.; Kang, H.-S.; Kim, Y.-W.; et al. Thermal annealing effects on the structural and electrical properties of HfO2 / Al2O3 gate dielectric stacks grown by atomic layer deposition on Si substrates. J. Appl. Phys. 2003, 94, 2563. [Google Scholar] [CrossRef]






| Parameters | Value |
|---|---|
| Substrate temperature (°C) | 140 |
| TMA temperature (°C) | 17.5 |
| H2O temperature (°C) | 45 |
| Pipe Temperature (°C) | 70 |
| TMA flow rate (sccm) | 300 |
| H2O flow rate (sccm) | 500 |
| RUN_Cycles | 110 |
| Thickness (nm) | 10 |
| Annealing Condition | Temperature (°C) |
|---|---|
| 150 W, 5 min | 200 |
| 500 W, 20 min | 300 |
| 600 W, 20 min | 400 |
| 800 W, 20 min | 500 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.-C.; Chuang, R.W.; Lin, K.-M.; Wu, T.-C. Study the Passivation Characteristics of Microwave Annealing Applied to APALD Deposited Al2O3 Thin Film. Coatings 2021, 11, 1450. https://doi.org/10.3390/coatings11121450
Huang Y-C, Chuang RW, Lin K-M, Wu T-C. Study the Passivation Characteristics of Microwave Annealing Applied to APALD Deposited Al2O3 Thin Film. Coatings. 2021; 11(12):1450. https://doi.org/10.3390/coatings11121450
Chicago/Turabian StyleHuang, Yu-Chun, Ricky Wenkuei Chuang, Keh-Moh Lin, and Tsung-Chieh Wu. 2021. "Study the Passivation Characteristics of Microwave Annealing Applied to APALD Deposited Al2O3 Thin Film" Coatings 11, no. 12: 1450. https://doi.org/10.3390/coatings11121450
APA StyleHuang, Y.-C., Chuang, R. W., Lin, K.-M., & Wu, T.-C. (2021). Study the Passivation Characteristics of Microwave Annealing Applied to APALD Deposited Al2O3 Thin Film. Coatings, 11(12), 1450. https://doi.org/10.3390/coatings11121450






