Corrosion Behavior of Chromium Coated Zy-4 Cladding under CANDU Primary Circuit Conditions
Abstract
:1. Introduction
- (a)
- Enhancing of both chemical composition and technology fabrication processes for Zr alloys leading to advanced materials such as E635, ZIRLO, M5, MDA, HiFi, X5A, etc. [8].
- (b)
- Development of advanced alloys suitable for use in special event The ATF new candidate materials, for replacements of current Zr include refractory metals, advanced steels or SiCf/SiC [9].
- (c)
- -
- Low neutron penalty and unmodified mechanical behavior if coatings are thinner than 20 μm;
- -
- Important decreases in corrosion kinetics for metallic and ceramic coatings;
- -
- a significant decrease and hydrogen embrittlement for such coatings due to reduced hydrogen pickup [17].
2. Materials and Methods
2.1. Coating Material
2.2. Coating Method
2.3. Coating Characterization
2.3.1. Morphological and Structural Surface Analysis
2.3.2. Electrochemical Tests
3. Results and Discussion
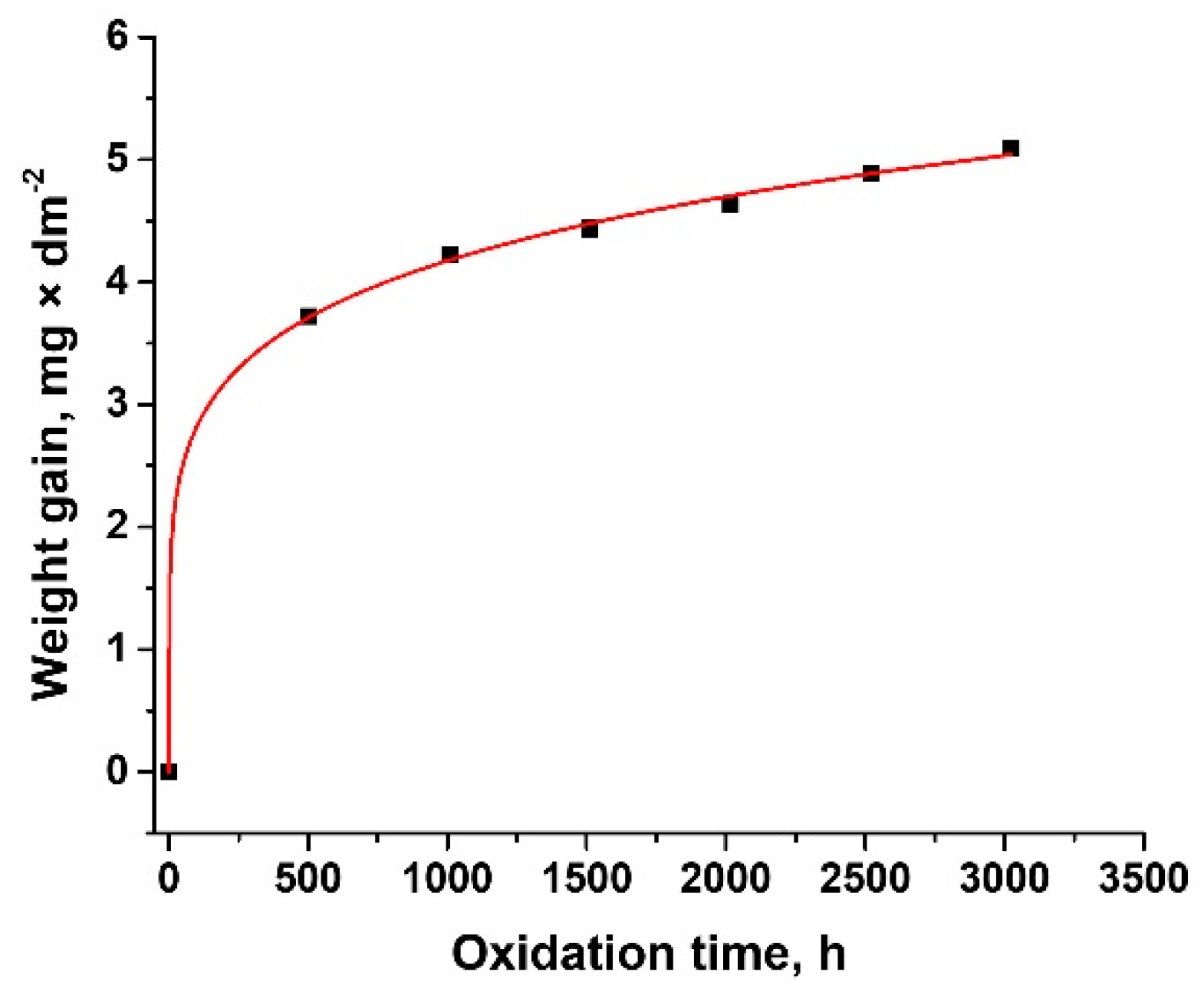
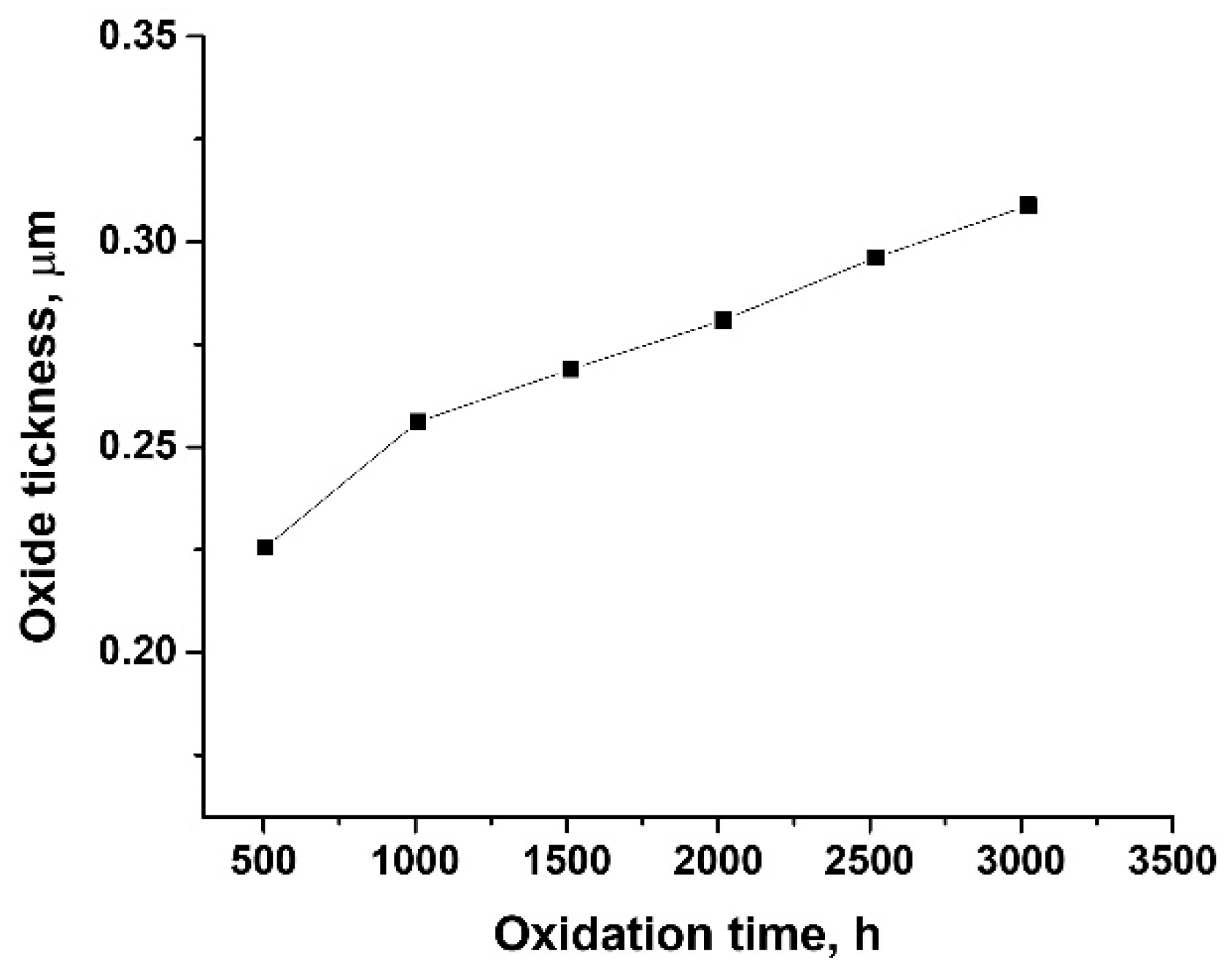
3.1. Oxidation Kinetics
3.2. Morphological and Structural Characterization
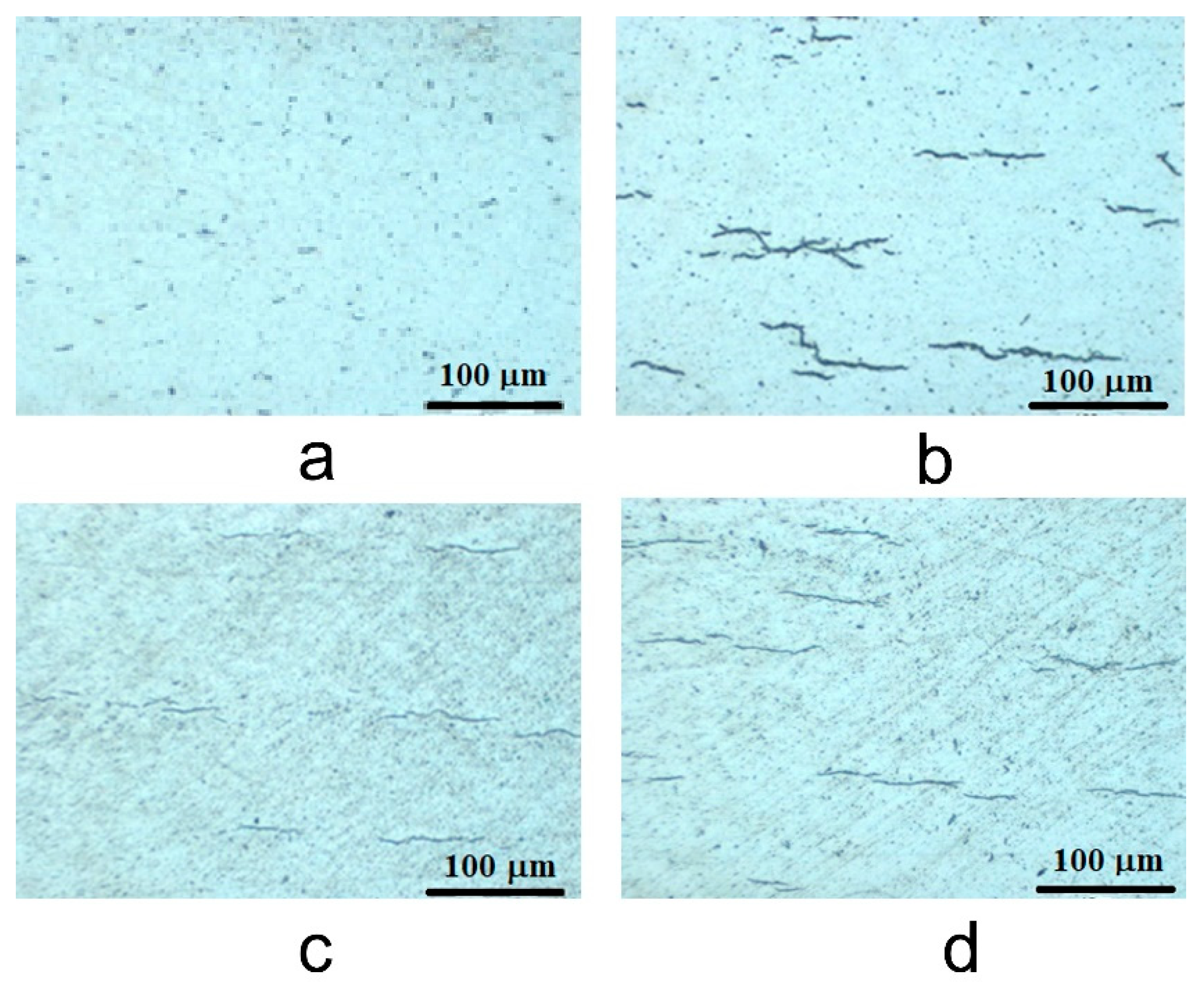
3.2.1. Metallographic Analysis (Optical Microscopy)
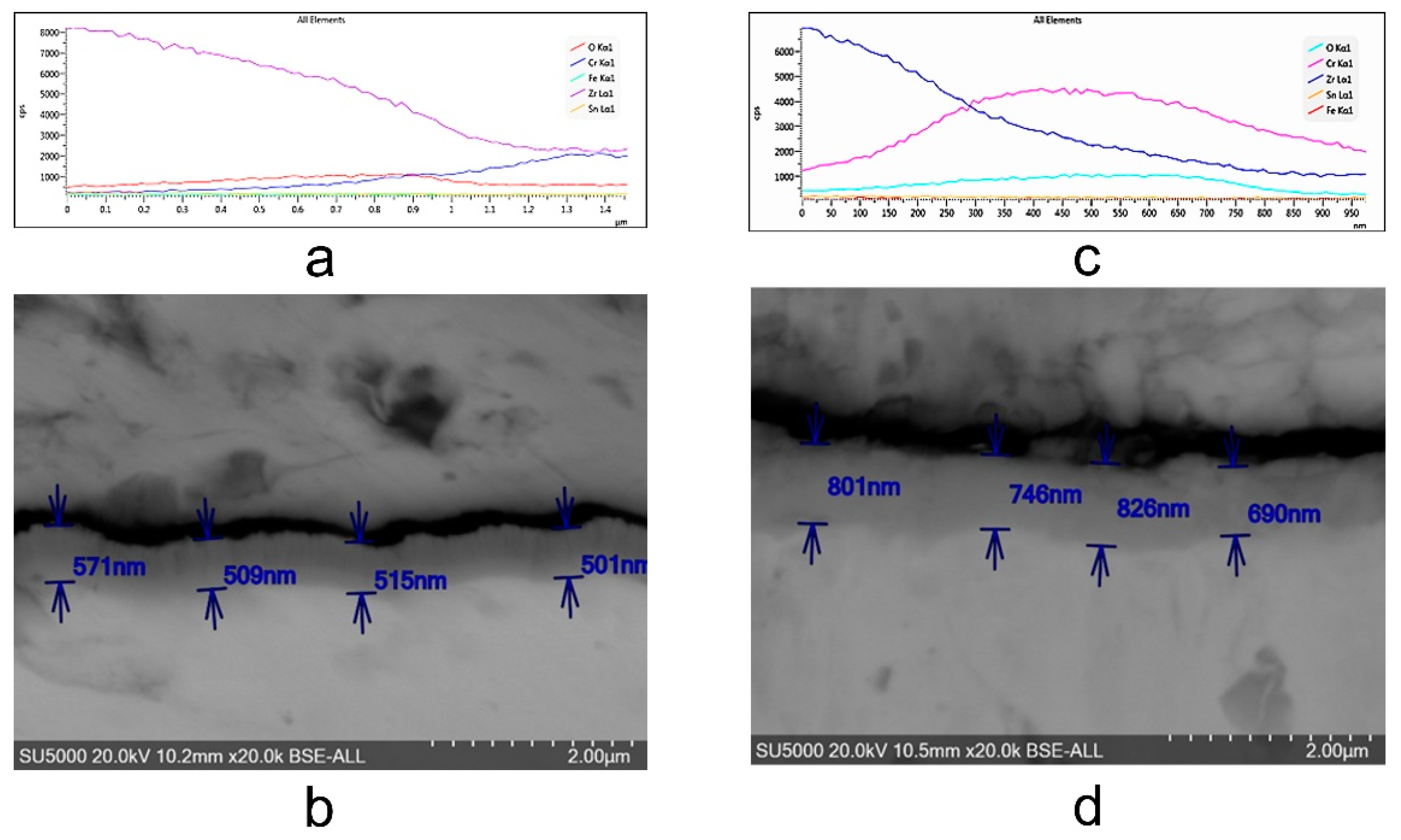
3.2.2. Scanning Electron Microscopy (SEM) Measurements
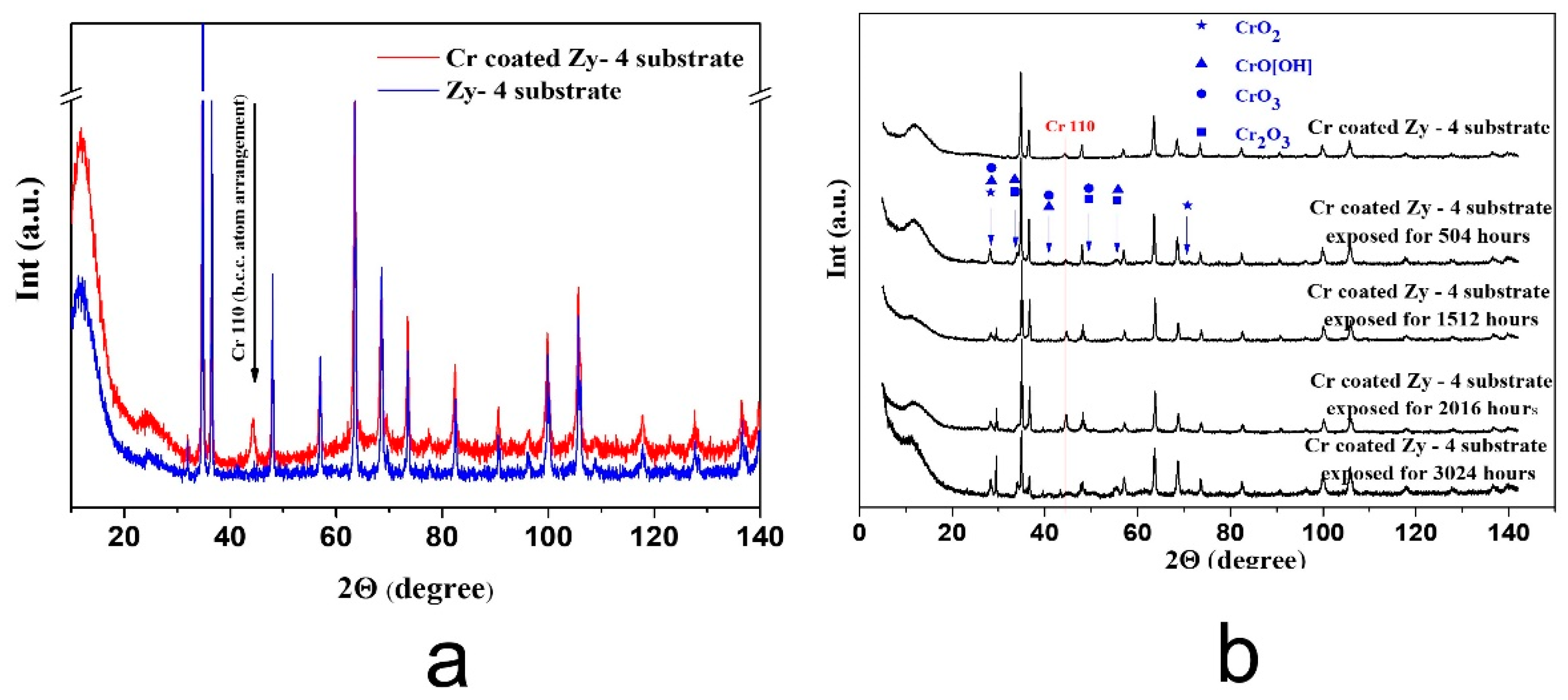
3.2.3. XRD Measurements
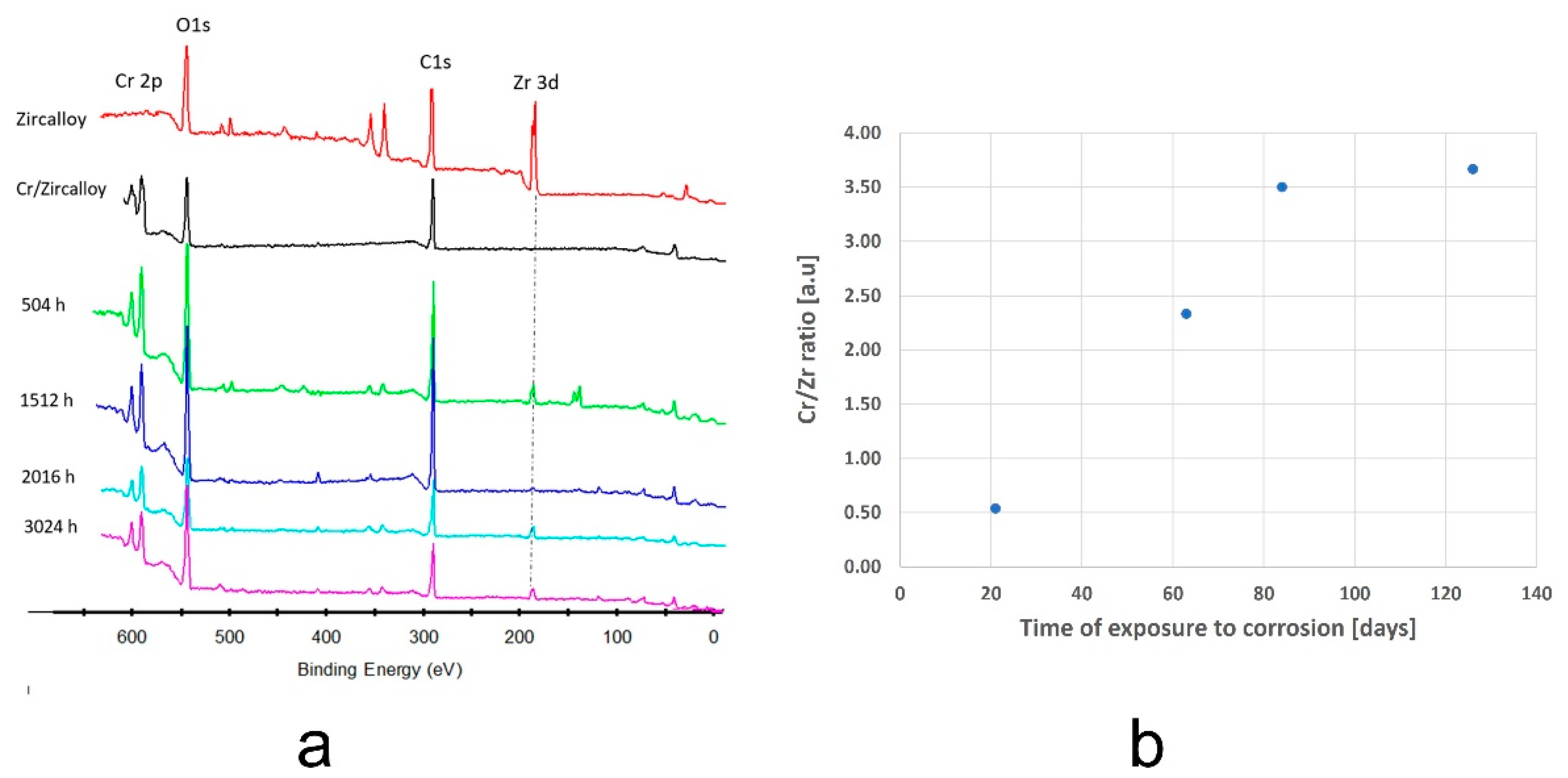
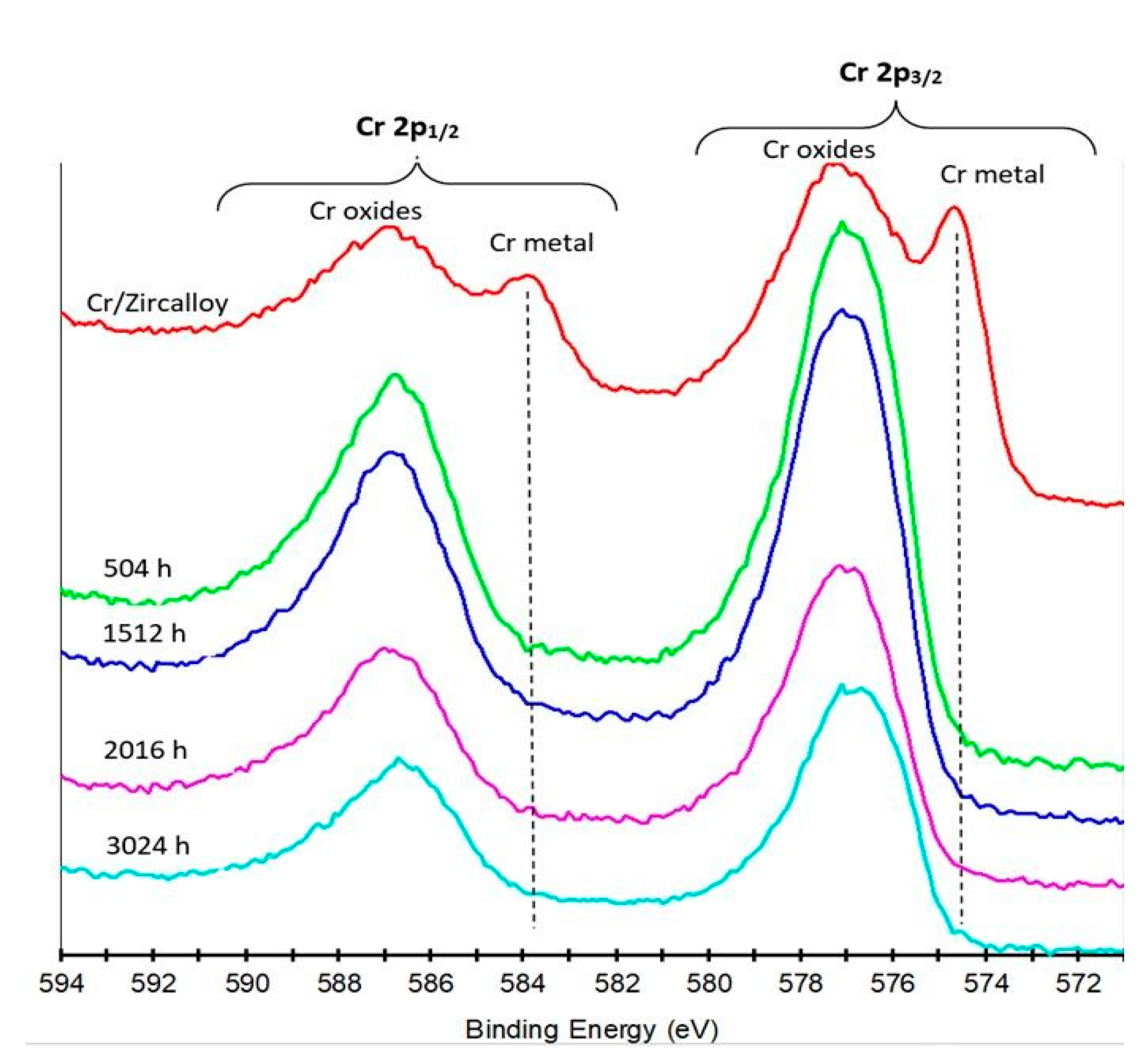
3.2.4. XPS Measurements
3.3. Electrochemichal Characterization
3.3.1. Open Circuit Potential Measurements
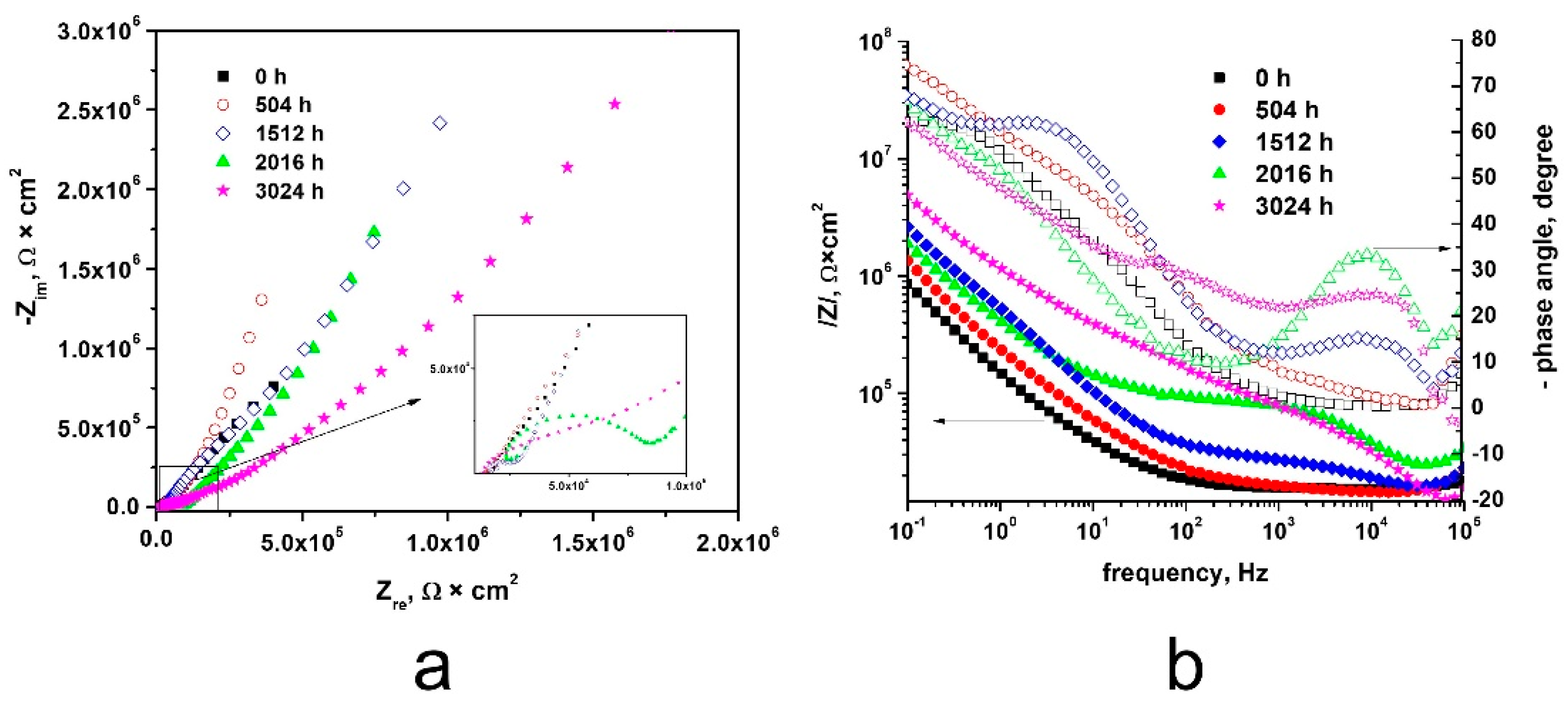
3.3.2. Electrochemical Impedance Spectroscopy
3.3.3. Potentiodynamic Polarization Tests
4. Conclusions
- a.
- The thickness of samples determined from SEM images before autoclaving is around 500 nm; after autoclaving the period layers increase, the kinetic of oxides growth being a logarithmic one compared to the kinetics of the uncoated sample which is a parabolic one. The evolution of ratio Cr/Zr a.u. in time based on XPS experimental data sustain this type of increase.
- b.
- The XRD determinations indicated the appearance of a crystalline phase of Chromium with a 110 preferred orientation. In the case of autoclaved samples, patterns characteristic to chromium oxides and hydroxides appear in the collected spectra can be observed.
- c.
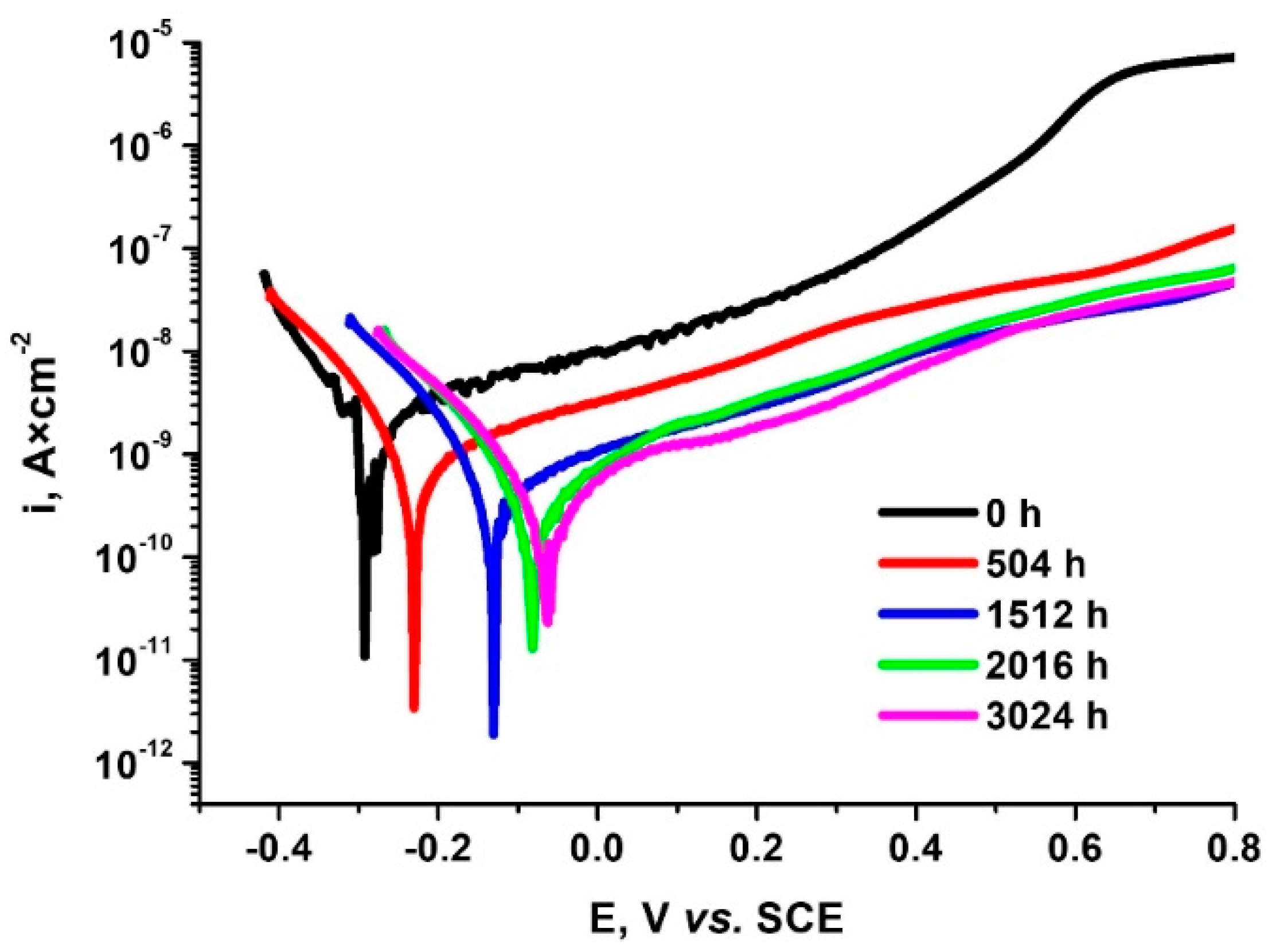
- Decrease in corrosion current density values simultaneously with the increase of the time spent in autoclave reaching the best value for 3024 h A shift to more positive values of corrosion potential and was identified at the same time. For 3024 h autoclaving the protection coating efficiency reached the highest value, being 91.76%
- d.
- High corrosion resistance demonstrated by Tafel plots is supported with highest impedance obtained in EIS experiments for 3024 h. Comparing the Nyquist diagrams for non-autoclaved sample, a single open capacitive appeared, while for all autoclaved Cr coated Zy-4 samples two capacitive semicircles are present, sustaining the interfaces created. Also, for all autoclaved samples, higher values of the capacitive semicircle diameter were recorded compared to the non-autoclaved Cr coated zircalloy.
- e.
- All surface investigations sustain electrochemical results and promote the Cr coating on Zircaloy-4 alloy autoclaved for 3024 h as the one with best corrosion resistance based on decrease in corrosion current density values simultaneously with the increase of the time spent in autoclave.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cantowine, P.; Rand, B. Irradiation performance: Light water reactor fuels. In Encyclopedia of Nuclear Energy; Elsevier: Amsterdam, The Netherlands, 2021; Volume 2, pp. 377–391. [Google Scholar]
- Kim, H.G.; Yang, J.H.; Kim, W.J.; Koo, Y.H. Development status of accident-tolerant fuel for light water reactors in Korea. Nucl. Eng. Technol. 2016, 48, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Hirano, M.; Yonomoto, T.; Ishigaki, M.; Watanabe, N.; Maruyama, Y.; Sibamoto, Y.; Watanabe, T.; Moriyama, K. Insights from review and analysis of the fukushima daiichi accident. J. Nucl. Sci. Technol. 2012, 49, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Goldner, F. Development Strategy for Advanced LWR Fuels with Enhanced Accident Tolerance; USDOE Office of Nuclear Energy: Washington, DC, USA, 2012. [Google Scholar]
- Development of Light Water Reactor Fuels with Enhanced Accident Tolerance—Report to Congress. April 2015. Available online: https://www.energy.gov/ne/downloads/development-light-water-reactor-fuels-enhanced-accident-tolerance-report-congress (accessed on 10 September 2021).
- Sowder, A.G. Challenges and opportunities for commercialization of enhanced accident tolerant fuel for light water reactors: A utility-in-formed perspectively. IAEA TECDOC Ser. 2016, 119–127. [Google Scholar]
- Cheng, B.; Kim, Y.J.; Chou, P. Improving accident tolerance of nuclear fuel with coated Mo-alloy cladding. Nucl. Eng. Technol. 2016, 48, 16–25. [Google Scholar] [CrossRef] [Green Version]
- Reback, R. Accident Tolerant Materials for Light Water Reactor Fuels; Elsevier: Amsterdam, The Netherlands, 2020; Chapter 2; pp. 15–41. [Google Scholar]
- Unocic, K.; Yukinori, Y.; Pint, B. Effect of Al and Cr content on air and steam oxidation of FeCrAl alloys and commercial APMT alloy. Oxid. Met. 2017, 87, 431–441. [Google Scholar] [CrossRef]
- Maier, B.; Yeom, H.; Johnson, G.; Dabney, T.; Walters, J.; Xu, P.; Romero, J.; Shah, H.; Sridharan, K. Development of cold spray chromium coatings for improved accident tolerant zirconium-alloy cladding. J. Nucl. Mater. 2019, 519, 247–254. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.G.; Kim, I.H.; Jung, Y.I.; Park, D.J.; Park, J.Y.; Koo, Y.H. Adhesion property and High-temperature oxidation behavior of Cr-coated Zircaloy-4 cladding tube prepared by 3D laser coating. J. Nucl. Mater. 2015, 465, 531–539. [Google Scholar] [CrossRef]
- Wei, T.; Zhang, R.; Yang, H.; Liu, H.; Qiu, S.; Wang, Y.; Du, P.; He, K.; Hu, X.; Dong, C. Microstructure, corrosion resistance and oxidation behavior of Cr-coatings on Zircaloy-4 prepared by vacuum arc plasma deposition. Corros. Sci. 2019, 158, 108077. [Google Scholar] [CrossRef]
- Park, J.H.; Kim, H.G.; Park, J.Y.; Jung, Y.I.; Park, D.J.; Koo, Y.H. High temperature steam-oxidation behavior of arc ion plated Cr coatings for accident tolerant fuel claddings. Surf. Coat. Technol. 2015, 280, 256–259. [Google Scholar] [CrossRef]
- Tang, C.; Stueber, M.; Seifert, H.J.; Steinbrueck, M. Protective coatings on zirconium-based alloys as accident-tolerant fuel (ATF) claddings. Corros. Rev. 2017, 35, 141–165. [Google Scholar] [CrossRef]
- Bischoff, J.; Vauglin, C.; Delafoy, C.; Barberis, P.; Perche, D.; Guerin, B.; Vassault, J.; Brachet, J. Development of Cr-coated zirconium alloy cladding for enhanced accident tolerance. In Proceedings of the Top Fuel 2016, Boise, ID, USA, 11–16 September 2016; pp. 1165–1171. [Google Scholar]
- Krejci, J.; Kabatova, J.; Manoch, F.; Koci, J.; Cvrcek, L.; Malek, J.; Krum, S.; Sutta, P.; Bublikova, P.; Halodova, P.; et al. Development and testing of multicomponent fuel cladding with enhanced accidental performance. Nucl. Eng. Technol. 2020, 52, 597–609. [Google Scholar] [CrossRef]
- Brachet, J.C.; Rouesne, E.; Guilbert, T.; Ribis, J.; Le Saux, M.; Nony, G.; Palancher, H.; David, A.; Bischoff, J.; Augereau, J. High temperature steam oxidation of chromium-coated zirconium-based alloys: Kinetics and process. Corros. Sci. 2020, 167, 108537. [Google Scholar] [CrossRef]
- Yang, J.; Stegmaier, U.; Tang, C.; Steinbruck, M.; Grobe, M.; Wang, S.; Seifert, H.J. High temperature Cr-Zr interaction of two types of Cr-coated Zr alloys in inert gas environment. J. Nucl. Mater. 2021, 547, 152806. [Google Scholar] [CrossRef]
- Ma, H.-B.; Yan, J.; Zhao, Y.-H.; Liu, T.; Ren, Q.-S.; Liao, Y.-H.; Zuo, J.-D.; Liu, G.; Yao, M.-Y. Oxidation behavior of Cr-coated zirconium alloy cladding in high-temperature steam above 1200 °C. Mater. Degrad. 2021, 5, 7. [Google Scholar] [CrossRef]
- Kashkarov, E.; Afornu, B.; Sidelev, D.; Krinitcyn, M.; Gouws, V.; Lider, A. Recent advances in protective coatings for accident tolerant Zr-based fuel claddings. Coatings 2021, 11, 557. [Google Scholar] [CrossRef]
- Kashkarov, E.; Sidelev, D.; Syrtanov, M.; Tang, C.; Steinbrück, M. Oxidation kinetics of Cr-coated zirconium alloy: Effect of coating thickness and microstructure. Corros. Sci. 2020, 175, 10888. [Google Scholar] [CrossRef]
- Han, X.C.; Xue, J.X.; Peng, S.M.; Zhang, H.B. An interesting oxidation phenomenon of Cr coatings on Zry-4 substrates in high temperature steam environment. Corros. Sci. 2019, 156, 117–124. [Google Scholar] [CrossRef]
- Sawarn, T.K.; Banerjee, S.; Samanta, A.; Rath, B.N.; Kumar, S. Study of oxide and α-Zr(O) growth kinetics from high temperature steam oxidationof Zircaloy-4 cladding. J. Nucl. Mater. 2015, 467, 820–831. [Google Scholar] [CrossRef]
- Zieliński, A.; Sobieszczyk, S. Hydrogen-enhanced degradation and oxide effects in zirconium alloys for nuclear applications. Int. J. Hydrog. Energy 2011, 36, 8619–8629. [Google Scholar] [CrossRef]
- Terrani, K.A. Accident tolerant fuel cladding development: Promise, status, and challenges. J. Nucl. Mater. 2018, 501, 13–30. [Google Scholar] [CrossRef]
- Musil, J.; Sklenka, J.; Cerstvý, R. Protection of brittle film against cracking. Appl. Surf. Sci. 2016, 370, 306–311. [Google Scholar] [CrossRef]
- Musil, J. Flexible hard nanocomposite coatings. RSC. Adv. 2015, 5, 60482–60495. [Google Scholar] [CrossRef]
- Brachet, J.-C.; Idarraga-Trujillo, I.; Le Flem, M.; Le Saux, M.; Vandenberghe, V.; Urvoy, S.; Rouesne, E.; Guilbert, T.; ToffolonMasclet, C.; Tupin, M. Early studies on Cr-Coated Zircaloy-4 as enhanced accident tolerant nuclear fuel claddings for light water reactors. J. Nucl. Mater. 2019, 517, 268–285. [Google Scholar] [CrossRef]
- Wang, X.; Guan, H.; Liao, Y.; Zhu, M.; Xu, C.; Jin, X.; Liao, B.; Xue, W.; Zhang, Y.; Bai, G. Enhancement of high temperature steam oxidation resistance of Zr–1Nb alloy with ZrO2/Cr bilayer coating. Corros. Sci. 2021, 187, 109494. [Google Scholar] [CrossRef]
- Kashkarov, E.; Sidelev, D.; Rombaeva, M.; Syrtanov, M.; Bleykher, G. Chromium coatings deposited by cooled and hot target magnetron sputtering for accident tolerant nuclear fuel claddings. Surf. Coat. Technol. 2020, 389, 125618. [Google Scholar] [CrossRef]
- Yeom, H.; Sridharan, K. Cold spray technology in nuclear energy applications: A review of recent advances. Ann. Nucl. Energy 2021, 150, 107835. [Google Scholar] [CrossRef]
- Li, G.; Liu, Y.; Zhang, Y.; Li, H.; Wang, X.; Zheng, M.; Li, Y. High Temperature Anti-Oxidation Behavior and Mechanical Property of Radio Frequency Magnetron Sputtered Cr Coating. Metals 2020, 10, 1509. [Google Scholar] [CrossRef]
- Sevecek, M.; Gurgen, A.; Seshadri, A.; Che, Y.; Wagih, M.; Phillips, B.; Champagne, V.; Shirvan, K. Development of Cr cold spray–coated fuel cladding with enhanced accident tolerance. Nucl. Eng. Technol. 2018, 50, 229–236. [Google Scholar] [CrossRef]
- Takiishi, H.; Duvaizen, J.H.; Sato, I.M.; Rossi, J.L.; Pereira, L.A.T.; Martinez, L.G. Recycling of Zircaloy machining chips by VAR remelting and powder metallurgy techniques. Mater. Sci. Forum 2012, 727, 356–361. [Google Scholar] [CrossRef]
- Surdu-Bob, C.; Mustata, I.; Iacob, C. General characteristics of the thermoionic vacuum arc plasma. J. Optoelectron. Adv. Mater. 2007, 9, 2932–2934. [Google Scholar]
- Surdu-Bob, C.; Vladoiu, R.; Badulescu, M.; Musa, G. Control over the sp2/sp3 ratio by tuning plasma parameters of the thermoionic vacuum arc. Diam. Relat. Mater. 2008, 17, 1625–1628. [Google Scholar] [CrossRef]
- Diniasi, D.; Golgovici, F.; Marin, A.; Negrea, A.D.; Fulger, M.; Demetrescu, I. Long-Term Corrosion Testing of Zy-4 in a LiOH solution under high pressure and temperature conditions. Materials 2021, 14, 4586. [Google Scholar] [CrossRef]
- Yagnik, S.; Garde, A. Zirconium alloys for LWR fuel cladding and core internals. In Structural Alloys for Nuclear Energy Applications; Odette, G.R., Zinkle, S.J., Eds.; Oak Ridge National Laboratory: Oak Ridge, TN, USA, 2019; pp. 247–291. [Google Scholar]
- Motta, A.T.; Chen, L.Q. Hydride formation in zirconium alloys. JOM 2012, 64, 1403–1408. [Google Scholar] [CrossRef]
- Yu, Q.; Reyes, M.; Shah, N.; Marian, J. Kinetic model of incipient hydride formation in Zr clad under dynamic oxide growth conditions. Materials 2020, 13, 1088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blackmur, M.S.; Robson, J.; Preuss, M.; Zanellato, O.; Cernik, R.; Shi, S.Q.; Ribeiro, F.; Andrieux, J. Zirconium hydride precipitation kinetics in Zircaloy-4 observed with synchrotron X-ray diffraction. J. Nucl. Mater. 2015, 464, 160–169. [Google Scholar] [CrossRef]
- Marciulescu, O. Operating Manual—Primary Heat Transport System Chemistry Control; Cernavoda NPP: Cernavodă, Romania , 1997; pp. 25–40. [Google Scholar]
- Zotica, D.; Marciulescu, O. Primary Heat Transport System Chemical Control Review; IAEA: Vienna, Austria, 1999. [Google Scholar]
- Wang, Z.; Li, H.; Xu, L.; Liu, Q.; Zha, L.; Lin, S. Electrochemical corrosion study of Zircaloy-4 in a LiOH solution at high temperature and pressure. Int. J. Electrochem. Sci. 2018, 13, 12163–12171. [Google Scholar] [CrossRef]
- Wang, Z.; Garbe, U.; Li, H.; Harrison, R.P.; Kaestner, A.; Lehmann, E. Observations on the zirconium hydride precipitation and distribution in Zircalloy-4. Metall. Mater. Trans. B 2014, 45, 532–539. [Google Scholar] [CrossRef] [Green Version]
- Lucan, D. Behaviour of the steam generator tubing in water with different pH values. Nucl. Eng. Des. 2011, 241, 1172–1176. [Google Scholar] [CrossRef]
- Pirvan, I.; Radulescu, M.; Lucan, D.; Visan, T. The influence some anions and the oxygen dissolved in the medium have upon Zircaloy-4 and Zr-2.5% Nb alloys oxidation and hydration. Rev. Chim. 1998, 49, 713–719. [Google Scholar]
- Yang, S.W.; Chang, T.J. Corrosion and Protection of Materials; Harbin Engineering University Press: Harbin, China, 2003; pp. 102–108. [Google Scholar]
- Cicek, V. Corrosion Engineering; Wiley: Beverly, MA, USA, 2014. [Google Scholar]
- Khangholia, A.; Revillaa, R.; Lutza, A.; Loulidib, S.; Roggeb, E.; Van Asschec, G.; De Graevea, I. Electrochemical characterization of plasma coatings on printed circuit boards. Prog. Org. Coat. 2019, 137, 105256. [Google Scholar] [CrossRef]
- ASTM G102-89 e1. Standard Practice for Calculation of Corrosion Rates and Related Information from Electrochemical Measurements; ASTM: West Conshohocken, PA, USA, 2016; pp. 12–27. [Google Scholar]
- Waheed, A.F.; Kamel, A.N.; Hamed, H.M. Effect of acidic and alkaline solutions on electrochemical corrosion of Zircaloy-2. Int. J. Adv. Sci. Technol. 2018, 8, 1–9. [Google Scholar] [CrossRef]
- Tato, W.; Landolt, D. Electrochemical determination of the porosity of single and duplex PVD coatings of titanium and titanium nitride on brass. J. Electrochem. Soc. 1998, 145, 4173–4181. [Google Scholar] [CrossRef]
- Bensaada, S. Corrosion Omniscriptum; Publishing Group: Riga, Latvia, 2018. [Google Scholar]


| Alloying Elements, (wt.%) | ||||
|---|---|---|---|---|
| Sn | Fe | Cr | O | Zr |
| 1.32 | 0.29 | 0.14 | 0.12 | Balance |
| Kinetic Equation | kp | n | R2 |
|---|---|---|---|
| y = 1.286 × t0.17 | 1.286 | 0.17 | 0.999 |
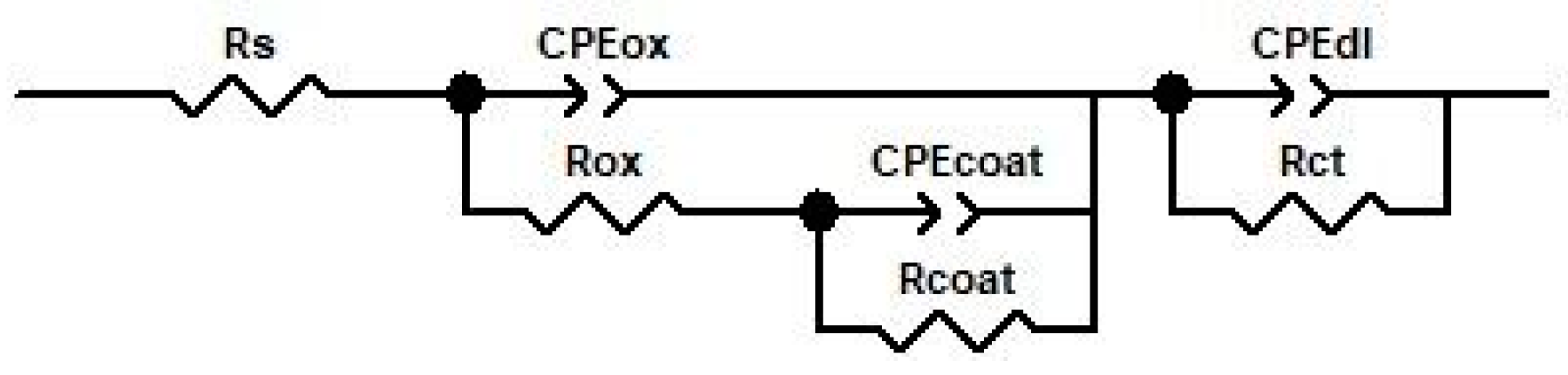
| Oxidation Period, h | Rs, Ω × cm2 | CPEox-T nF × cm−2 | CPEox-P | Rox KΩ × cm2 | CPEcoat-T μF × cm−2 | CPEcoat-P | Rcoat Ω × cm2 | CPEdl-T μF × cm−2 | CPEdl-P | Rct Ω × cm2 | Chi-Squared |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 158.5 | – | – | – | 1.51 | 0.89 | 8.62 × 105 | 1.97 | 0.76 | 26,974 | 2.7 × 10−3 |
| 504 | 158.7 | 0.161 | 0.98 | 1.25 | 1.93 | 0.63 | 1.97 × 105 | 1.81 | 0.94 | 2.89 × 107 | 4.1 × 10−3 |
| 1512 | 143.2 | 1.35 | 0.93 | 57.97 | 1.21 | 0.79 | 1.81 × 106 | 0.122 | 0.98 | 3.82 × 1010 | 1.1 × 10−3 |
| 2016 | 170.7 | 9.19 | 0.9 | 11.78 | 0.89 | 0.73 | 1.13 × 106 | 0.766 | 0.99 | 1.94 × 107 | 8.9 × 10−4 |
| 3024 | 152.3 | 3.56 | 0.88 | 46.06 | 0.94 | 0.78 | 56,051 | 0.295 | 0.69 | 4.9 × 1014 | 1.8 × 10−3 |
| Sample after Different Autoclaving Time, h | Ecorr, mV | icorr, nA × cm−2 | Vcorr nm × Year−1 | Rp MΩ × cm2 | Pi (%) | P (%) |
|---|---|---|---|---|---|---|
| 0 | −283 ± 0.02 | 1.19 ± 0.02 | 1.43 ± 0.02 | 5.7 ± 0.03 | – | – |
| 504 | −225 ± 0.02 | 0.492 ± 0.01 | 5.93 ± 0.02 | 7.2 ± 0.07 | 58.65 ± 0.01 | 0.0124 ± 0.01 |
| 1512 | −119 ± 0.01 | 0.147 ± 0.01 | 1.77 ± 0.02 | 24 ± 0.05 | 87.65 ± 0.01 | 1.25× 10−4 ± 0.01 |
| 2016 | −74 ± 0.01 | 0.107 ± 0.01 | 1.29 ± 0.02 | 33 ± 0.07 | 91.01 ± 0.01 | 2.16× 10−5 ± 0.01 |
| 3024 | −59 ± 0.01 | 0.0981 ± 0.01 | 1.1 ± 0.02 | 39 ± 0.07 | 91.76 ± 0.01 | 1.13× 10−5 ± 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diniasi, D.; Golgovici, F.; Anghel, A.; Fulger, M.; Surdu-Bob, C.C.; Demetrescu, I. Corrosion Behavior of Chromium Coated Zy-4 Cladding under CANDU Primary Circuit Conditions. Coatings 2021, 11, 1417. https://doi.org/10.3390/coatings11111417
Diniasi D, Golgovici F, Anghel A, Fulger M, Surdu-Bob CC, Demetrescu I. Corrosion Behavior of Chromium Coated Zy-4 Cladding under CANDU Primary Circuit Conditions. Coatings. 2021; 11(11):1417. https://doi.org/10.3390/coatings11111417
Chicago/Turabian StyleDiniasi, Diana, Florentina Golgovici, Alexandru Anghel, Manuela Fulger, Carmen Cristina Surdu-Bob, and Ioana Demetrescu. 2021. "Corrosion Behavior of Chromium Coated Zy-4 Cladding under CANDU Primary Circuit Conditions" Coatings 11, no. 11: 1417. https://doi.org/10.3390/coatings11111417
APA StyleDiniasi, D., Golgovici, F., Anghel, A., Fulger, M., Surdu-Bob, C. C., & Demetrescu, I. (2021). Corrosion Behavior of Chromium Coated Zy-4 Cladding under CANDU Primary Circuit Conditions. Coatings, 11(11), 1417. https://doi.org/10.3390/coatings11111417








