A Study on the Corrosion Resistance of Hydrophobic Coatings on 65Mn Steel
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Coating Preparation
2.2. Characterization of the Coatings
2.3. Electrochemical Measurements
3. Results and Discussion
3.1. Phase Composition and Morphology of the Coatings
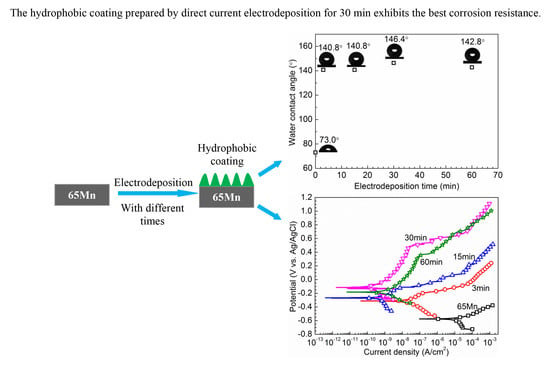
3.2. Hydrophobic Properties of the Coatings
3.3. Corrosion Protection of the Deposited Coatings
4. Conclusions
- (1)
- The deposition time obviously affect the morphology, wettability and thickness of the coatings. However, it has no influence on the phase composition which is calcium stearate.
- (2)
- The deposited coatings exhibit hydrophobicity thanks to the hierarchical micro/nanostructure of the coating surface and the low surface energy of calcium stearate.
- (3)
- The sample HC-30 has the best corrosion resistance due to a combination of superior hydrophobicity and thicker coating.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Toorani, M.; Aliofkhazraei, M.; Mahdavian, M.; Naderi, R. Superior corrosion protection and adhesion strength of epoxy coating applied on AZ31 magnesium alloy pre-treated by PEO/Silane with inorganic and organic corrosion inhibitors. Corros. Sci. 2021, 178, 109065. [Google Scholar] [CrossRef]
- Hu, C.; Li, Y.; Zhang, N.; Ding, Y. Synthesis and characterization of a poly(o-anisidine)–SiC composite and its application for corrosion protection of steel. RSC Adv. 2017, 7, 11732–11742. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Zhu, H.; He, J.; Liu, Y.; Zhao, G. Formation and mechanism of a super-hydrophobic surface with wear and salt spray resistance. RSC Adv. 2017, 7, 43181–43185. [Google Scholar] [CrossRef] [Green Version]
- Tong, J.; Liu, S.; Peng, R.; Sun, H.; Jiang, S. Development of a micro/nano composite sufper-hydrophobic silicon surface with nail-shaped texture/dual self-assembly monolayers and its wetting behavior. Appl. Surf. Sci. 2021, 544, 148803. [Google Scholar] [CrossRef]
- Zhao, G.; Li, J.; Huang, Y.; Yang, L.; Ye, Y.; Walsh, F.; Chen, J.; Wang, S. Robust Ni/WC superhydrophobic surfaces by electrodeposition. RSC Adv. 2017, 7, 44896–44903. [Google Scholar] [CrossRef] [Green Version]
- Guan, Y.; Yu, C.; Zhu, J.; Yang, R.; Li, X.; Wei, D.; Xu, X. Design and fabrication of vapor-induced superhydrophobic surfaces obtained from polyethylene wax and silica nanoparticles in hierarchical structures. RSC Adv. 2018, 8, 25150–25158. [Google Scholar] [CrossRef] [Green Version]
- Kuang, J.; Ba, Z.; Li, Z.; Wang, Z.; Qiu, J. The study on corrosion resistance of superhydrophobic coatings on magnesium. Appl. Surf. Sci. 2020, 501, 144137. [Google Scholar] [CrossRef]
- Feng, L.; Zhu, Y.; Wang, J.; Shi, X. One-step hydrothermal process to fabricate superhydrophobic surface on magnesium alloy with enhanced corrosion resistance and self-cleaning performance. Appl. Surf. Sci. 2017, 422, 566–573. [Google Scholar] [CrossRef]
- Ding, S.; Xiang, T.; Li, C.; Zheng, S.; Wang, J.; Zhang, M.; Dong, C.; Chan, W. Fabrication of self-cleaning super-hydrophobic nickel/graphene hybrid film with improved corrosion resistance on mild steel. Mater. Design 2017, 117, 280–288. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, T.; He, H.; Ouyang, L.; Yuan, S. A stearic Acid/CeO2 bilayer coating on AZ31B magnesium alloy with superhydrophobic and self-cleaning properties for corrosion inhibition. J. Alloys Compd. 2020, 834, 155210. [Google Scholar] [CrossRef]
- Raja, T.; Jeyasubramanian, K. Tuning the superhydrophobicity of magnesium stearate decorated ZnO porous structures for self-cleaning urinary coatings. Appl. Surf. Sci. 2017, 423, 293–304. [Google Scholar] [CrossRef]
- Zhang, J.; Gu, C.; Tu, J. Robust slippery coating with superior corrosion resistance and anti-icing performance for AZ31B Mg alloy protection. ACS Appl. Mater. Interfaces 2017, 9, 11247–11257. [Google Scholar] [CrossRef]
- Zuo, Z.; Liao, R.; Guo, C.; Yuan, Y.; Zhao, X.; Zhuang, A.; Zhang, Y. Fabrication and anti-icing property of coral-like superhydrophobic aluminum surface. Appl. Surf. Sci. 2015, 331, 132–139. [Google Scholar] [CrossRef]
- Bhushan, B.; Jung, Y. Natural and biomimetic artificial surfaces for superhydrophobicity, self-cleaning, low adhesion, and drag reduction. Prog. Mater. Sci. 2011, 56, 1–108. [Google Scholar] [CrossRef] [Green Version]
- Cai, Y.; Zhao, Q.; Quan, X.; Feng, W.; Wang, Q. Fluorine-free and hydrophobic hexadecyltrimethoxysilane-TiO2 coated mesh for gravity-driven oil/water separation. Colloids Surf. A 2020, 586, 124189. [Google Scholar] [CrossRef]
- Singh, A.; Singh, J. An efficient use of waste PE for hydrophobic surface coating and its application on cotton fibers for oil-water separator. Prog. Org. Coat. 2019, 131, 301–310. [Google Scholar] [CrossRef]
- Cai, Y.; Li, S.; Cheng, Z.; Xu, G.; Quan, X.; Zhou, Y. Facile fabrication of super-hydrophobic FAS modified electroless Ni-P coating meshes for rapid water-oil separation. Colloids Surf. A 2018, 540, 224–232. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhou, X.; Xing, Z.; Tu, T. Fabrication of a superhydrophobic surface with underwater air-retaining properties by electrostatic flocking. RSC Adv. 2018, 8, 10719–10726. [Google Scholar] [CrossRef] [Green Version]
- Khalili, E.; Sarafbidabad, M. Combination of laser patterning and nano PTFE sputtering for the creation a super-hydrophobic surface on 304 stainless steel in medical applications. Surf. Interfaces 2017, 8, 219–224. [Google Scholar] [CrossRef]
- Xue, Y.; Wang, S.; Zhao, G.; Taleb, A.; Jin, Y. Fabrication of Ni Co coating by electrochemical deposition with high super-hydrophobic properties for corrosion protection. Surf. Coat. Technol. 2019, 363, 352–361. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, T. Influence of duty cycle on properties of the superhydrophobic coating on an anodized magnesium alloy fabricated by pulse electrodeposition. Colloids Surf. A 2019, 568, 43–50. [Google Scholar] [CrossRef]
- Cai, Y.; Zhao, Q.; Quan, X.; Zhu, J.; Zhou, C. Corrosion-resistant hydrophobic MFI-Type zeolite-coated mesh for continuous oil–water separation. Ind. Eng. Chem. Res. 2020, 59, 3498–3510. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, J.; Lei, Y.; Wang, Y.; Zhou, G.; Xu, C.; Rao, Y.; Wang, K. Preparation of intricate nanostructures on 304 stainless steel surface by SiO2-assisted HF etching for high superhydrophobicity. Colloids Surf. A 2020, 586, 124287. [Google Scholar] [CrossRef]
- Gao, P.; Gao, M.; Wu, A.; Wu, X.; Liu, C.; Zhang, Y.; Zhou, H.; Peng, X.; Xie, Z. Electrochemical characteristics of electroplating and impregnation Ni-P/SiC/PTFE composite coating on 316L stainless steel. J. Cent. South Univ. 2020, 27, 3615–3624. [Google Scholar] [CrossRef]
- Jena, G.; Thinaharan, C.; George, R.; Philip, J. Robust nickel-reduced graphene oxide-myristic acid superhydrophobic coating on carbon steel using electrochemical codeposition and its corrosion resistance. Surf. Coat. Technol. 2020, 397, 125942. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, S.; Hu, J.; Lin, T. Formation mechanism and corrosion resistance of the hydrophobic coating on anodized magnesium. Corros. Sci. 2016, 111, 334–343. [Google Scholar] [CrossRef]
- Zhang, B.; Zhao, X.; Li, Y.; Hou, B. Fabrication of durable anticorrosion superhydrophobic surfaces on aluminum substrates via a facile one-step electrodeposition approach. RSC Adv. 2016, 6, 35455–35465. [Google Scholar] [CrossRef]
- Xu, N.; Sarkar, D.; Chen, X.; Tong, W. Corrosion performance of superhydrophobic nickel stearate/nickel hydroxide thin films on aluminum alloy by a simple one-step electrodeposition process. Surf. Coat. Technol. 2016, 302, 173–184. [Google Scholar] [CrossRef]
- Jiang, S.; Guo, Z.; Deng, Y.; Dong, H.; Li, X.; Liu, J. Effect of pulse frequency on the one-step preparation of superhydrophobic surface by pulse electrodeposition. Appl. Surf. Sci. 2018, 458, 603–611. [Google Scholar] [CrossRef]
- Zhong, Y.; Hu, J.; Zhang, Y.; Tang, S. The one-step electroposition of superhydrophobic surface on AZ31 magnesium alloy and its time-dependence corrosion resistance in NaCl solution. Appl. Surf. Sci. 2018, 427, 1193–1201. [Google Scholar] [CrossRef]
- Zheng, T.; Hu, Y.; Pan, F.; Zhang, Y.; Tang, A. Fabrication of corrosion-resistant superhydrophobic coating on magnesium alloy by one-step electrodeposition method. J. Magnes. Alloy. 2019, 7, 193–202. [Google Scholar] [CrossRef]
- Kang, Z.; Li, W. Facile and fast fabrication of superhydrophobic surface on magnesium alloy by one-step electrodeposition method. J. Ind. Eng. Chem. 2017, 50, 50–56. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, D.; Kang, Z. One-step electrodeposition process to fabricate corrosion-resistant superhydrophobic surface on magnesium alloy. ACS Appl. Mater. Interfaces 2015, 7, 1859–1867. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.; Ma, H.; Huang, M.; Gao, Z.; Cao, J.; Wang, C.; Dong, C.; Wang, Y.; Kunwar, A. Fabrication of cerium myristate coating for a mechanochemically robust modifier-free superwettability system to enhance the corrosion resistance on 316L steel by one-step electrodeposition. Surf. Coat. Technol. 2020, 398, 125970. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, X.; Hu, J. Corrosion protection of mild steel by one-step electrodeposition of superhydrophobic silica film. Corros. Sci. 2014, 85, 482–487. [Google Scholar] [CrossRef]
- Li, J.; Tong, J.; Hu, B.; Ma, Y. Biomimetic functional surface of reducing soil adhesion on 65Mn steel. Adv. Mech. Eng. 2019, 11, 1687814019889801. [Google Scholar] [CrossRef]
- García-Lecina, E.; García-Urrutia, I.; Díez, J.; Fornell, J.; Pellicer, E.; Sort, J. Codeposition of inorganic fullerene-like WS2 nanoparticles in an electrodeposited nickel matrix under the influence of ultrasonic agitation. Electrochim. Acta 2013, 114, 859–867. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, S.; Lin, T.; Liu, G.; Hu, J. Corrosion properties of calcium stearate-based hydrophobic coatings on anodized magnesium alloy. Acta Metall. Sin. Engl. 2019, 32, 1111–1121. [Google Scholar] [CrossRef] [Green Version]
- Karapanagiotis, I.; Grosu, D.; Aslanidou, D.; Aifantis, K. Facile method to prepare superhydrophobic and water repellent cellulosic paper. J. Nanomater. 2015, 16, 219013. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, X. A non-particle and fluorine-free superhydrophobic surface based on one-step electrodeposition of dodecyltrimethoxysilane on mild steel for corrosion protection. Corros. Sci. 2020, 163, 108284. [Google Scholar] [CrossRef]
- Yuan, X.; Yue, Z.; Chen, X.; Wen, S.; Li, L.; Feng, T. The protective and adhesion properties of silicone-epoxy hybrid coatings on 2024 Al-alloy with a silane film as pretreatment. Corros. Sci. 2016, 104, 84–97. [Google Scholar] [CrossRef]
- Nardi, J.; Strauss, J.; Fardo, F.; Ferreira, L.; Martini, E.; Horowitz, F. Wettability and anticorrosion of thin PTFE-like/alumina coatings on carbon steel. Prog. Org. Coat. 2020, 148, 105823. [Google Scholar] [CrossRef]
- Tang, S.; Zhang, Y.; San, H.; Hu, J. Hydrophobic surface contained Ca and/or Ce myristate fabricated on AZ31 by one-step electrodeposition for corrosion protection in NaCl. Appl. Surf. Sci. 2019, 496, 143627. [Google Scholar] [CrossRef]









| Element | C | Si | Mn | S | P | Cr | Ni | Cu | Fe |
|---|---|---|---|---|---|---|---|---|---|
| wt.% | 0.62–0.70 | 0.17–0.37 | 0.90–1.20 | ≤0.035 | ≤0.035 | ≤0.25 | ≤0.25 | ≤0.25 | Balance |
| Samples | Ecorr (V vs. Ag/AgCl) | icorr (A/cm2) | Ebd (V vs. Ag/AgCl) | βc (V/dec) | βa (V/dec) |
|---|---|---|---|---|---|
| 65Mn substrate | −0.58 ± 0.01 | (2.74 ± 0.12) × 10−5 | — | 0.99 ± 0.03 | 0.08 ± 0.01 |
| HC-3 | −0.31 ± 0.01 | (3.14 ± 0.38) × 10−8 | −0.20 ± 0.01 | 0.17 ± 0.01 | 0.16 ± 0.01 |
| HC-15 | −0.27 ± 0.01 | (1.58 ± 0.03) × 10−9 | −0.11 ± 0.01 | 0.66 ± 0.04 | 0.10 ± 0.01 |
| HC-30 | −0.11 ± 0.02 | (7.46 ± 0.60) × 10−10 | 0.50 ± 0.02 | 0.44 ± 0.02 | 0.23 ± 0.02 |
| HC-60 | −0.18 ± 0.01 | (1.67 ± 0.13) × 10−9 | 0.37 ± 0.02 | 0.18 ± 0.01 | 0.24 ± 0.01 |
| Sample | Time | Rs (Ω cm2) | Rcoat (Ω cm2) | CPEcoat | Rct (Ω cm2) | CPEdl | |||
|---|---|---|---|---|---|---|---|---|---|
| Y0 (Ω−1 cm−2 sn) | n | Y0 (Ω−1 cm−2 sn) | n | χ2 (×10−3) | |||||
| HC-15 | 1 h | 16.8 | 1.41 × 104 | 1.59 × 10−5 | 0.59 | 2.10 × 105 | 1.94 × 10−6 | 0.98 | 3.64 |
| 16 h | 21.1 | 1.04 × 104 | 3.21 × 10−5 | 0.60 | 6.45 × 104 | 1.09 × 10−5 | 0.95 | 2.22 | |
| 24 h | 31.3 | 1.19 × 104 | 4.30 × 10−5 | 0.56 | 2.76 × 104 | 1.13 × 10−5 | 0.94 | 1.29 | |
| HC-30 | 1 h | 38.5 | 4.33 × 106 | 6.36 × 10−7 | 0.60 | — | — | — | 5.14 |
| 12 h | 20.7 | 4.61 × 106 | 1.47 × 10−6 | 0.53 | — | — | — | 4.92 | |
| 16 h | 30.8 | 9.94 × 105 | 1.87 × 10−6 | 0.47 | 4.35 × 106 | 3.12 × 10−7 | 0.96 | 1.94 | |
| 24 h | 42.7 | 3.66 × 105 | 4.40 × 10−6 | 0.28 | 1.43 × 106 | 7.72 × 10−6 | 0.94 | 0.46 | |
| HC-60 | 1 h | 21.4 | 1.47 × 105 | 1.64 × 10−6 | 0.44 | 6.16 × 105 | 9.58 × 10−6 | 0.77 | 0.39 |
| 8 h | 43.1 | 3.93 × 104 | 6.45 × 10−6 | 0.41 | 3.41 × 105 | 1.15 × 10−5 | 0.90 | 1.43 | |
| 16 h | 39.3 | 2.04 × 104 | 1.65 × 10−5 | 0.29 | 1.98 × 105 | 1.47 × 10−5 | 0.80 | 0.94 | |
| 24 h | 15.7 | 1.38 × 104 | 1.57 × 10−5 | 0.17 | 7.30 × 104 | 3.65 × 10−5 | 0.74 | 0.47 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Du, Q.; Lin, T.; Tang, S.; Hu, J. A Study on the Corrosion Resistance of Hydrophobic Coatings on 65Mn Steel. Coatings 2021, 11, 1399. https://doi.org/10.3390/coatings11111399
Zhang Y, Du Q, Lin T, Tang S, Hu J. A Study on the Corrosion Resistance of Hydrophobic Coatings on 65Mn Steel. Coatings. 2021; 11(11):1399. https://doi.org/10.3390/coatings11111399
Chicago/Turabian StyleZhang, Yufen, Qingcheng Du, Tiegui Lin, Shawei Tang, and Jin Hu. 2021. "A Study on the Corrosion Resistance of Hydrophobic Coatings on 65Mn Steel" Coatings 11, no. 11: 1399. https://doi.org/10.3390/coatings11111399
APA StyleZhang, Y., Du, Q., Lin, T., Tang, S., & Hu, J. (2021). A Study on the Corrosion Resistance of Hydrophobic Coatings on 65Mn Steel. Coatings, 11(11), 1399. https://doi.org/10.3390/coatings11111399