Green Synthesis of Carbon-Encapsulated Magnetic Fe3O4 Nanoparticles Using Hydrothermal Carbonization from Rattan Holocelluloses
Abstract
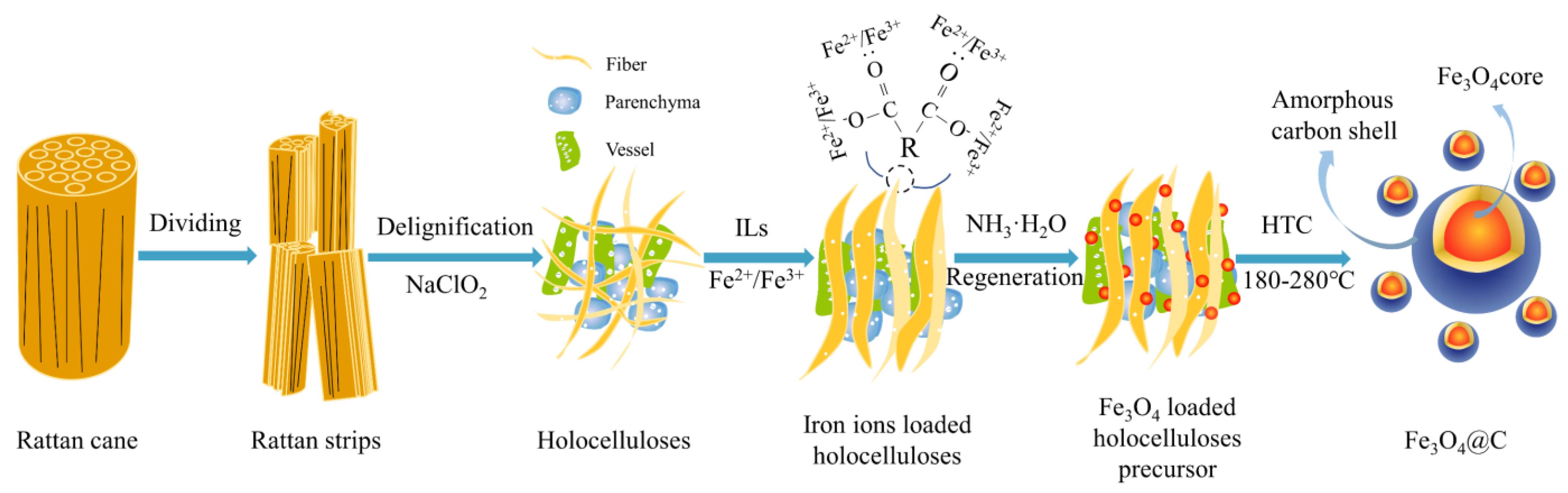
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. One-Pot Synthesis of Fe3O4@C Nanoparticles
2.3. Characterizations
3. Results and Discussion
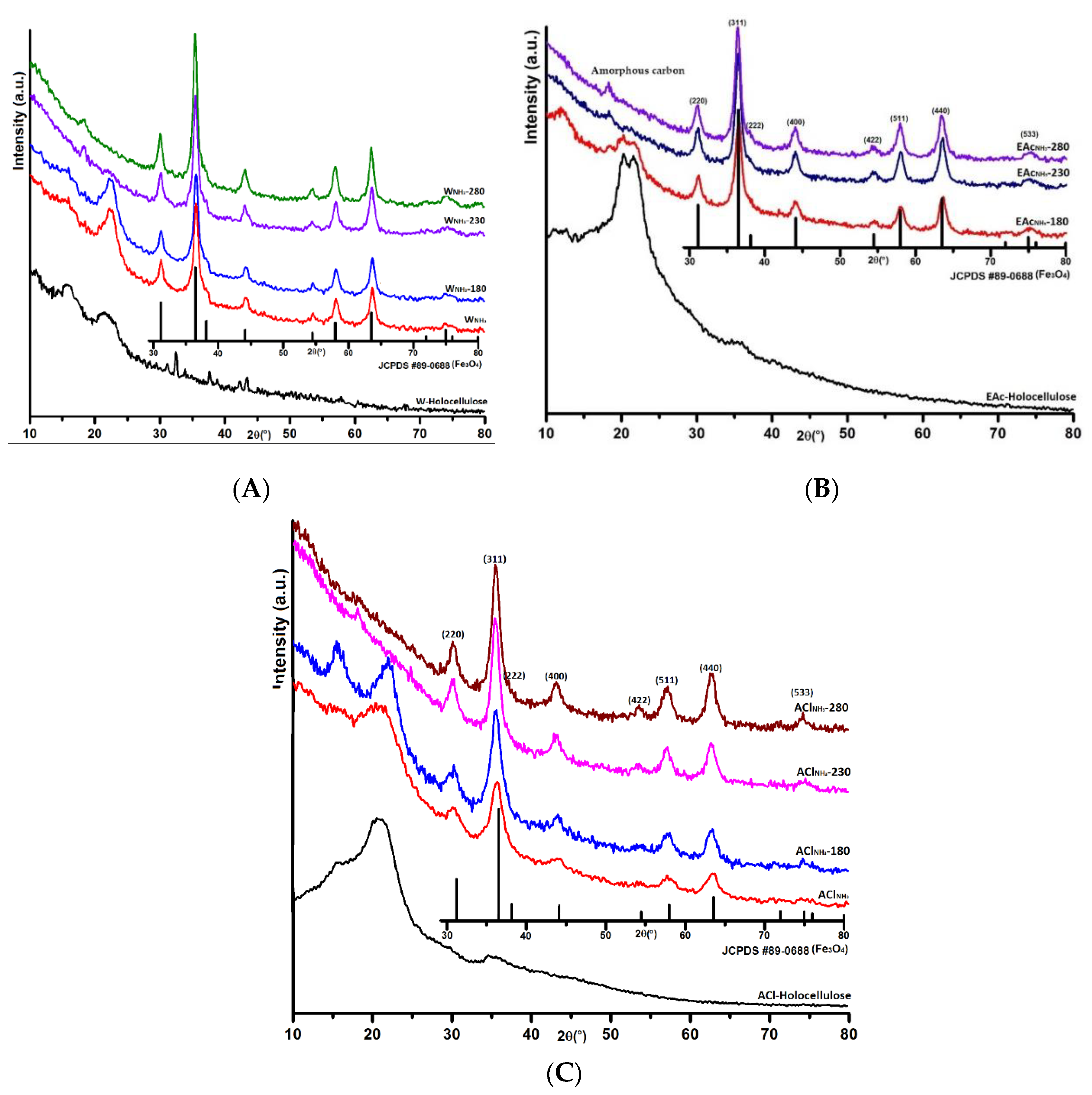
3.1. XRD
3.2. Raman and XPS
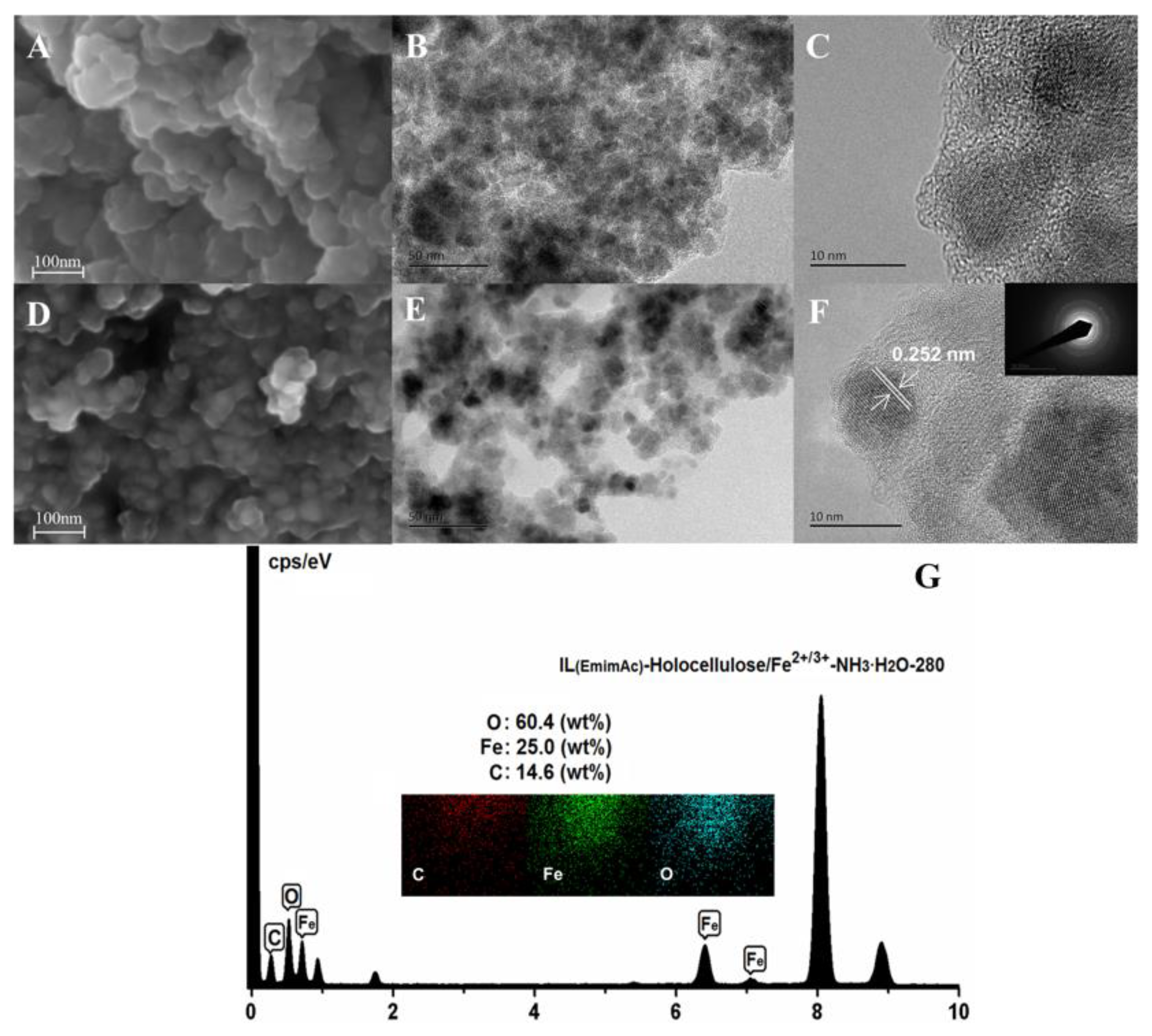
3.3. SEM and TEM
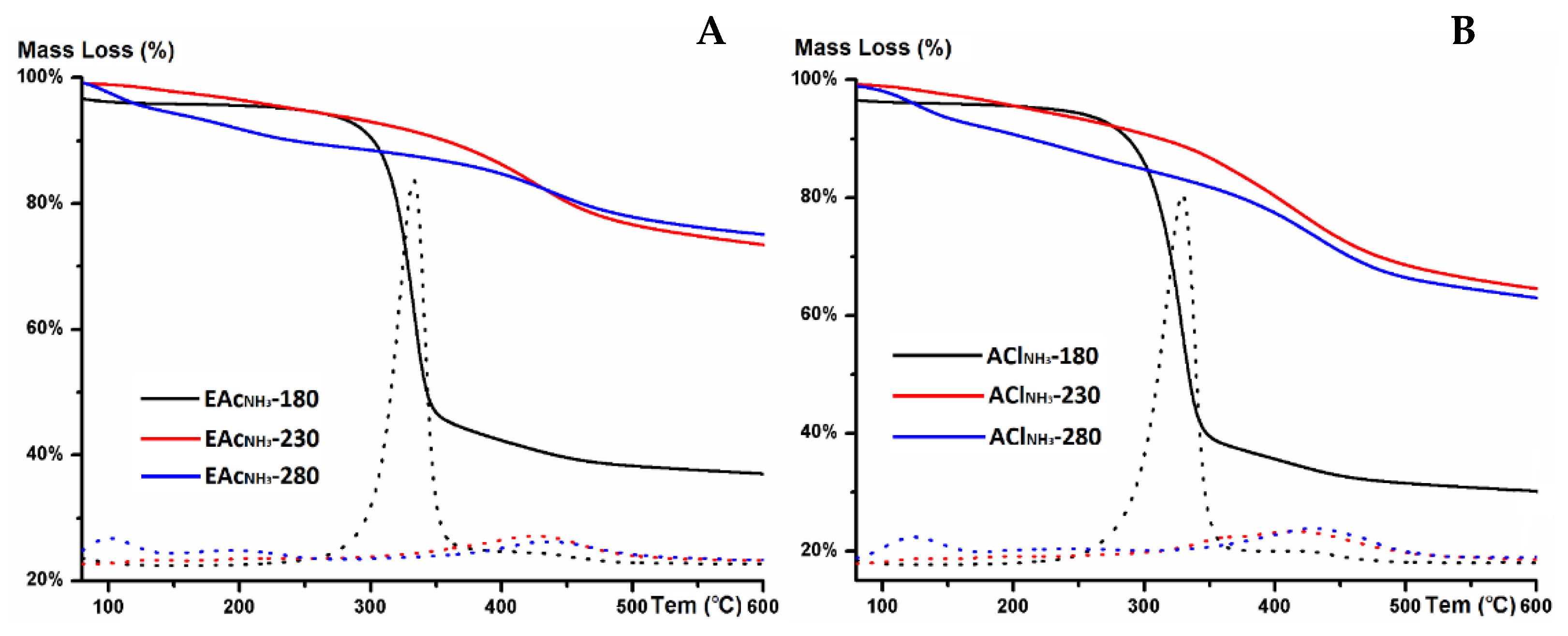
3.4. TGA
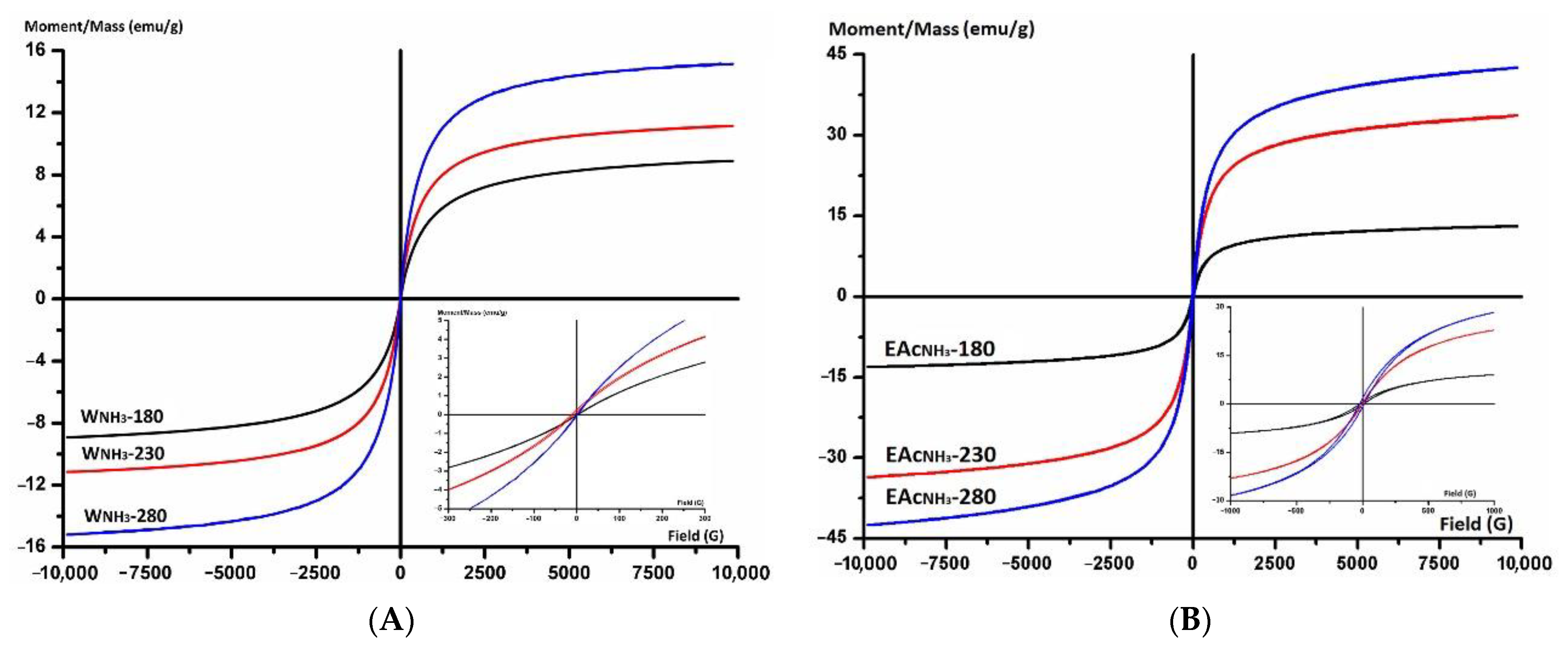
3.5. VSM
3.6. Proposed Growth Mechanism of Series Fe3O4@C Nanoparticles
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sayğılı, H. Hydrothermal synthesis of magnetic nanocomposite from biowaste matrix by a green and one-step route: Characterization and pollutant removal ability. Bioresour. Technol. 2019, 278, 242–247. [Google Scholar] [CrossRef]
- Lee, S.Y.; Lee, J.H.; Kim, Y.H.; Rhee, K.Y.; Park, S.J. Roles of london dispersive and polar components of nano-metal-coated activated carbons for improving carbon dioxide uptake. Coatings 2021, 11, 691. [Google Scholar] [CrossRef]
- Radwan, A.; Jin, H.; Liu, B.; Chen, Z.; Wu, Q.; Zhao, X.; He, D. 3D-ZIF scaffold derived carbon encapsulated iron nitride as a synergistic catalyst for ORR and zinc-air battery cathodes. Carbon 2021, 171, 368–375. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhu, Z.; Zhang, H.; Fang, W.; Jiang, W.; Lin, S.; Yang, Y. Polyurethane sponge-derived nitrogen-doped carbon-encapsulation composite for enhanced lithium-ion battery performances. Appl. Surf. Sci. 2020, 534, 147631. [Google Scholar] [CrossRef]
- Wu, T.; Liu, Y.; Zeng, X.; Cui, T.; Zhao, Y.; Li, Y.; Wang, S. Facile hydrothermal synthesis of Fe3O4/C core–shell nanorings for efficient low-frequency microwave absorption. ACS Appl. Mater. Interfaces 2016, 8, 7370–7380. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liu, S.; Tian, X.; Wang, J.; Xu, P.; Xiao, F.; Wang, S. Facile synthesis of N-doped porous carbon encapsulated bimetallic PdCo as a highly active and durable electrocatalyst for oxygen reduction and ethanol oxidation. J. Mater. Chem. A 2017, 5, 10876–10884. [Google Scholar] [CrossRef]
- Huang, S.; Meng, Y.; He, S.; Goswami, A.; Wu, Q.; Li, J.; Tong, S.; Asefa, T.; Wu, M. N-, O-, and S-tridoped carbon-encapsulated Co9S8 nanomaterials: Efficient bifunctional electrocatalysts for overall water splitting. Adv. Funct. Mater. 2017, 27, 1606585. [Google Scholar] [CrossRef]
- Gupta, A.K.; Naregalkar, R.R.; Vaidya, V.D.; Gupta, M. Recent advances on surface engineering of magnetic iron oxide nanoparticles and their biomedical applications. Nanomedicine 2007, 2, 23–29. [Google Scholar] [CrossRef]
- Dos Reis, G.S.; Larsson, S.H.; Thyrel, M.; Pham, T.N.; Claudio Lima, E.; de Oliveira, H.P.; Dotto, G.L. Preparation and application of efficient biobased carbon adsorbents prepared from spruce bark residues for efficient removal of reactive dyes and colors from synthetic effluents. Coatings 2021, 11, 772. [Google Scholar] [CrossRef]
- He, X.; Zhu, J.; Wang, H.; Zhou, M.; Zhang, S. Surface functionalization of activated carbon with phosphonium ionic liquid for CO2 adsorption. Coatings 2019, 9, 590. [Google Scholar] [CrossRef] [Green Version]
- Rehman, A.; Park, M.; Park, S.J. Current progress on the surface chemical modification of carbonaceous materials. Coatings 2019, 9, 103. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Navarathna, C.M.; Leng, W.; Karunaratne, T.; Thirumalai, R.V.K.G.; Kim, Y.; Pittman, C.U., Jr.; Mlsna, T.; Cai, Z.; Zhang, J. Lignin-based few-layered graphene-encapsulated iron nanoparticles for water remediation. Chem. Eng. J. 2021, 417, 129199. [Google Scholar] [CrossRef]
- Zhang, Z.; Wen, G. Synthesis and characterization of carbon-encapsulated magnetite, martensite and iron nanoparticles by high-energy ball milling method. Mater. Charact. 2020, 167, 110502. [Google Scholar] [CrossRef]
- Zhao, N.; Wu, S.; He, C.; Wang, Z.; Shi, C.; Liu, E.; Li, J. One-pot synthesis of uniform Fe3O4 nanocrystals encapsulated in interconnected carbon nanospheres for superior lithium storage capability. Carbon 2013, 57, 130–138. [Google Scholar] [CrossRef]
- Suma, D.; Deng, D. Facile synthesis of Fe3O4@g-C nanorods for reversible adsorption of molecules and absorption of ions. ACS Sustain. Chem. Eng. 2015, 3, 133–139. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, H.; Duan, X.; Ang, H.M.; Tadé, M.O.; Wang, S. A new magnetic nano zero-valent iron encapsulated in carbon spheres for oxidative degradation of phenol. Appl. Catal. B Environ. 2015, 172, 73–81. [Google Scholar] [CrossRef]
- Liu, J.; Xie, L.; Wang, Z.; Mao, S.; Gong, Y.; Wang, Y. Biomass-derived ordered mesoporous carbon nano-ellipsoid encapsulated metal nanoparticles inside: Ideal nanoreactors for shape-selective catalysis. Chem. Commun. 2020, 56, 229–232. [Google Scholar] [CrossRef] [PubMed]
- Barin, G.B.; de Fátima Gimenez, I.; da Costa, L.P.; Souza Filho, A.G.; Barreto, L.S. Hollow carbon nanostructures obtained from hydrothermal carbonization of lignocellulosic biomass. J. Mater. Sci. 2014, 49, 665–672. [Google Scholar] [CrossRef]
- Sevilla, M.; Fuertes, A.B. The production of carbon materials by hydrothermal carbonization of cellulose. Carbon 2009, 47, 2281–2289. [Google Scholar] [CrossRef] [Green Version]
- Ahsan, M.A.; Puente Santiago, A.R.; Rodriguez, A.; Maturano-Rojas, V.; Alvarado-Tenorio, B.; Ber-nal, R.; Noveron, J.C. Biomass-derived ultrathin carbon-shell coated iron nanoparticles as high-performance tri-functional HER, ORR and Fenton-like catalysts. J. Clean. Prod. 2020, 275, 124141. [Google Scholar] [CrossRef]
- Liu, Y.; Li, X.; Haridas, A.K.; Sun, Y.; Heo, J.; Ahn, J.-H.; Lee, Y. Biomass-derived graphitic carbon encapsulated Fe/Fe3C composite as an anode material for high-performance lithium ion batteries. Energies 2020, 13, 827. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Liang, X.; Qiao, W.; Liu, C.; Liu, X.; Zhan, L.; Ling, L. Preparation of polystyrene-based activated carbon spheres with high surface area and their adsorption to dibenzothiophene. Fuel Process. Technol. 2009, 90, 381–387. [Google Scholar] [CrossRef]
- Cuesta, A.; Dhamelincourt, P.; Laureyns, J.; Martinez-Alonso, A.; Tascón, J.D. Raman microprobe studies on carbon materials. Carbon 1994, 32, 1523–1532. [Google Scholar] [CrossRef]
- Pawlyta, M.; Rouzaud, J.N.; Duber, S. Raman microspectroscopy characterization of carbon blacks: Spectral analysis and structural information. Carbon 2015, 84, 479–490. [Google Scholar] [CrossRef]
- Wang, Y.; Alsmeyer, D.C.; McCreery, R.L. Raman spectroscopy of carbon materials: Structural basis of observed spectra. Chem. Mater. 1990, 2, 557–563. [Google Scholar] [CrossRef]
- Laszlo, K.; Tombacz, E.; Josepovits, K. Effect of activation on the surface chemistry of carbons from polymer precursors. Carbon 2001, 39, 1217–1228. [Google Scholar] [CrossRef]
- Pittman, C.U., Jr.; Jiang, W.; Yue, Z.R.; Gardner, S.; Wang, L.; Toghiani, H.; y Leon, C.L. Surface properties of electrochemically oxidized carbon fibers. Carbon 1999, 37, 1797–1807. [Google Scholar] [CrossRef]
- Enterría, M.; Martín-Jimeno, F.J.; Suárez-García, F.; Paredes, J.I.; Pereira, M.F.R.; Martins, J.I.; Martínez-Alonso, A.; Tascón, J.M.D.; Figueiredo, J.L. Effect of nanostructure on the supercapacitor performance of activated carbon xerogels obtained from hydrothermally carbonized glucose-graphene oxide hybrids. Carbon 2016, 105, 474–483. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Dai, F.; Xiao, Q.; Yang, L.; Shen, J.; Zhang, C.; Cai, M. Nitrogen-doped activated carbon for a high energy hybrid supercapacitor. Energy Environ. Sci. 2016, 9, 102–106. [Google Scholar] [CrossRef]
- Descostes, M.; Mercier, F.; Thromat, N.; Beaucaire, C.; Gautier-Soyer, M. Use of XPS in the determination of chemical environment and oxidation state of iron and sulfur samples: Constitution of a data basis in binding energies for Fe and S reference compounds and applications to the evidence of surface species of an oxidized pyrite in a carbonate medium. Appl. Surf. Sci. 2000, 165, 288–302. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Quan, C.; Gao, N.; Song, Q. Pyrolysis of biomass components in a TGA and a fixed-bed reactor: Thermochemical behaviors, kinetics, and product characterization. J. Anal. Appl. Pyrol. 2016, 121, 84–92. [Google Scholar] [CrossRef]
- Kumar, A.; Kostikov, Y.; Orberger, B.; Nessim, G.D.; Mariotto, G. Natural laterite as a catalyst source for the growth of carbon nanotubes and nanospheres. ACS Appl. Nano Mater. 2018, 1, 6046–6054. [Google Scholar] [CrossRef]
- Kang, S.; Li, X.; Fan, J.; Chang, J. Characterization of hydrochars produced by hydrothermal carbonization of lignin, cellulose, D-xylose, and wood meal. Ind. Eng. Chem. Res. 2012, 51, 9023–9031. [Google Scholar] [CrossRef]
- Hu, X.; Xu, J.; Wu, M.; Xing, J.; Bi, W.; Wang, K.; Ma, J. Effects of biomass pre-pyrolysis and pyrolysis temperature on magnetic biochar properties. J. Anal. Appl. Pyrol. 2017, 127, 196–202. [Google Scholar] [CrossRef]
- Clough, M.T.; Geyer, K.; Hunt, P.A.; Son, S.; Vagt, U.; Welton, T. Ionic liquids: Not always innocent solvents for cellulose. Green Chem. 2015, 17, 231–243. [Google Scholar] [CrossRef] [Green Version]
- Raj, T.; Gaur, R.; Dixit, P.; Gupta, R.P.; Kagdiyal, V.; Kumar, R.; Tuli, D.K. Ionic liquid pretreatment of biomass for sugars production: Driving factors with a plausible mechanism for higher enzymatic digestibility. Carbohyd. Polym. 2016, 149, 369–381. [Google Scholar] [CrossRef]
- Falco, C.; Baccile, N.; Titirici, M.M. Morphological and structural differences between glucose, cellulose and lignocellulosic biomass derived hydrothermal carbons. Green Chem. 2011, 13, 3273–3281. [Google Scholar] [CrossRef] [Green Version]
- Kruse, A.; Funke, A.; Titirici, M.M. Hydrothermal conversion of biomass to fuels and energetic materials. Curr. Opin. Chem. Biol. 2013, 17, 515–521. [Google Scholar] [CrossRef]
- Titirici, M.M.; Thomas, A.; Yu, S.H.; Müller, J.O.; Antonietti, M. A direct synthesis of mesoporous carbons with bicontinuous pore morphology from crude plant material by hydrothermal carbonization. Chem. Mater. 2007, 19, 4205–4212. [Google Scholar] [CrossRef]


| Samples | Yield (%) a | Raman b | Magnetic Parameters | ||
|---|---|---|---|---|---|
| IG/ID | Ms (emu/g) | Mr (emu/g) | Hc (Oe) | ||
| WNH3-180 | 53.1 | 0.53 | 8.9 | 0.4 | 13.6 |
| WNH3-230 | 43.1 | 0.65 | 11.5 | 1.0 | 15.9 |
| WNH3-280 | 34.2 | 0.73 | 15.2 | 1.5 | 20.2 |
| EAcNH3-180 | 85.9 | 0.62 | 13.1 | 0.2 | 3.1 |
| EAcNH3-230 | 37.8 | 0.85 | 33.6 | 0.4 | 2.7 |
| EAcNH3-280 | 37.9 | 0.83 | 42.6 | 0.6 | 4.1 |
| EAcH2O-180 | 78.4 | 0.60 | 10.4 | 0.3 | 2.5 |
| EAcH2O-230 | 33.8 | 0.65 | 13.8 | 0.4 | 1.9 |
| EAcH2O-280 | 31.5 | 0.80 | 15.1 | 0.3 | 2.1 |
| AClNH3-180 | 65.5 | 0.74 | 14.2 | 0.1 | 1.5 |
| AClNH3-230 | 31.6 | 0.84 | 28.2 | 0.3 | 1.5 |
| AClNH3-280 | 23.6 | 0.89 | 33.0 | 0.6 | 0.9 |
| AClH2O-180 | 65.5 | 0.64 | - | - | - |
| AClH2O-230 | 32.8 | 0.72 | - | - | - |
| AClH2O-280 | 31.1 | 0.82 | - | - | - |
| Samples | C1 | C2 | C3 | C4 | C5 | O1 | O2 | O3 | O4 |
|---|---|---|---|---|---|---|---|---|---|
| Carbidic C | C–C | C–O | C=O | O=C–O | C=O | C–O–C | C–O | O–C=O | |
| EAcNH3-180 | 284.8/11.9% | 285.3/21.4% | 286.7/55.0% | 288.0/11.7% | 530.6/5.4% | - | - | 533.1/95.6% | |
| EAcNH3-230 | 284.2/26.6% | 284.8/38.6% | 285.3/22.4% | 286.3/12.4% | - | 530.2/15.1% | 531.9/63.5% | - | 533.3/21.4% |
| EAcNH3-280 | 284.2/21.2% | 284.8/34.3% | 285.3/32.3% | 286.3/12.2% | - | 530.2/23.0% | 531.2/26.9% | 532.2/28.4% | 533.2/21.7% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, L.; Jin, Z.; Liu, X.; Feng, L.; Ma, J.; Ling, Z. Green Synthesis of Carbon-Encapsulated Magnetic Fe3O4 Nanoparticles Using Hydrothermal Carbonization from Rattan Holocelluloses. Coatings 2021, 11, 1397. https://doi.org/10.3390/coatings11111397
Dai L, Jin Z, Liu X, Feng L, Ma J, Ling Z. Green Synthesis of Carbon-Encapsulated Magnetic Fe3O4 Nanoparticles Using Hydrothermal Carbonization from Rattan Holocelluloses. Coatings. 2021; 11(11):1397. https://doi.org/10.3390/coatings11111397
Chicago/Turabian StyleDai, Linxin, Zhi Jin, Xinge Liu, Long Feng, Jianfeng Ma, and Zhe Ling. 2021. "Green Synthesis of Carbon-Encapsulated Magnetic Fe3O4 Nanoparticles Using Hydrothermal Carbonization from Rattan Holocelluloses" Coatings 11, no. 11: 1397. https://doi.org/10.3390/coatings11111397
APA StyleDai, L., Jin, Z., Liu, X., Feng, L., Ma, J., & Ling, Z. (2021). Green Synthesis of Carbon-Encapsulated Magnetic Fe3O4 Nanoparticles Using Hydrothermal Carbonization from Rattan Holocelluloses. Coatings, 11(11), 1397. https://doi.org/10.3390/coatings11111397






