Effects of Additives Containing Cyanopyridine on Electrodeposition of Bright Al Coatings from AlCl3-EMIC Ionic Liquids
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of Ionic Liquid Electrolyte
2.3. Pretreatment
2.4. Electrodeposition of Al Coatings
2.5. Characterization
3. Results and Discussion
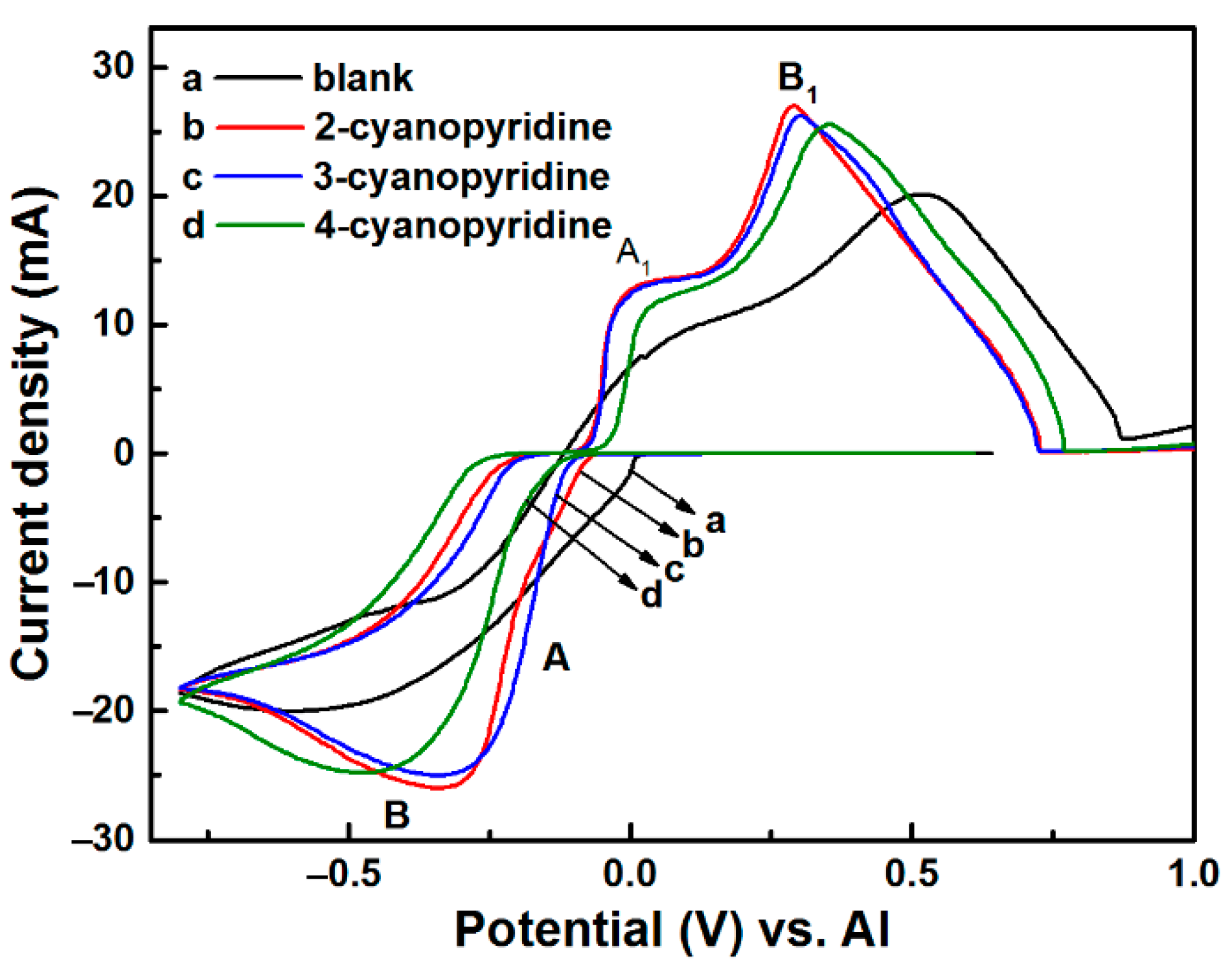
3.1. Voltammogram Measurements
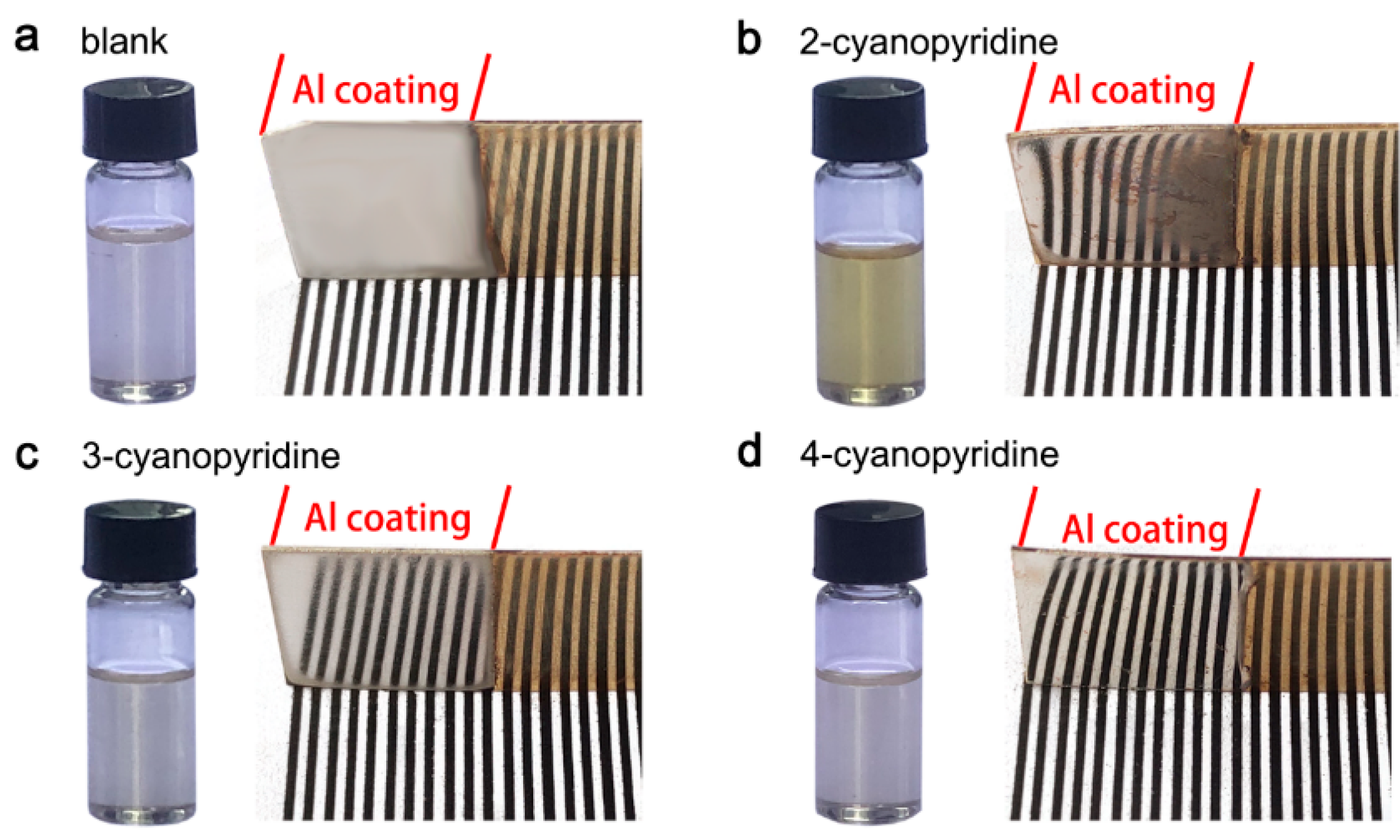
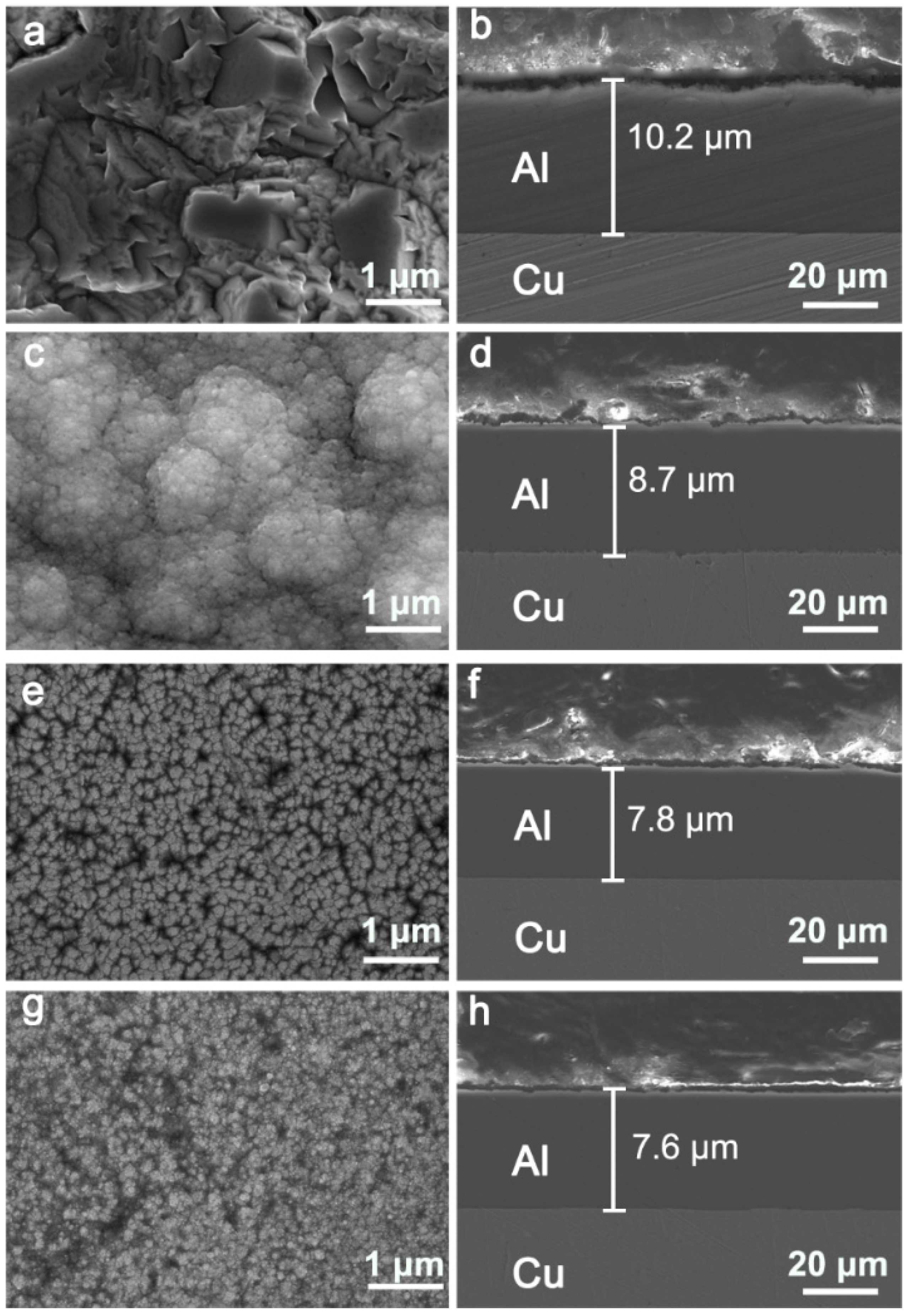
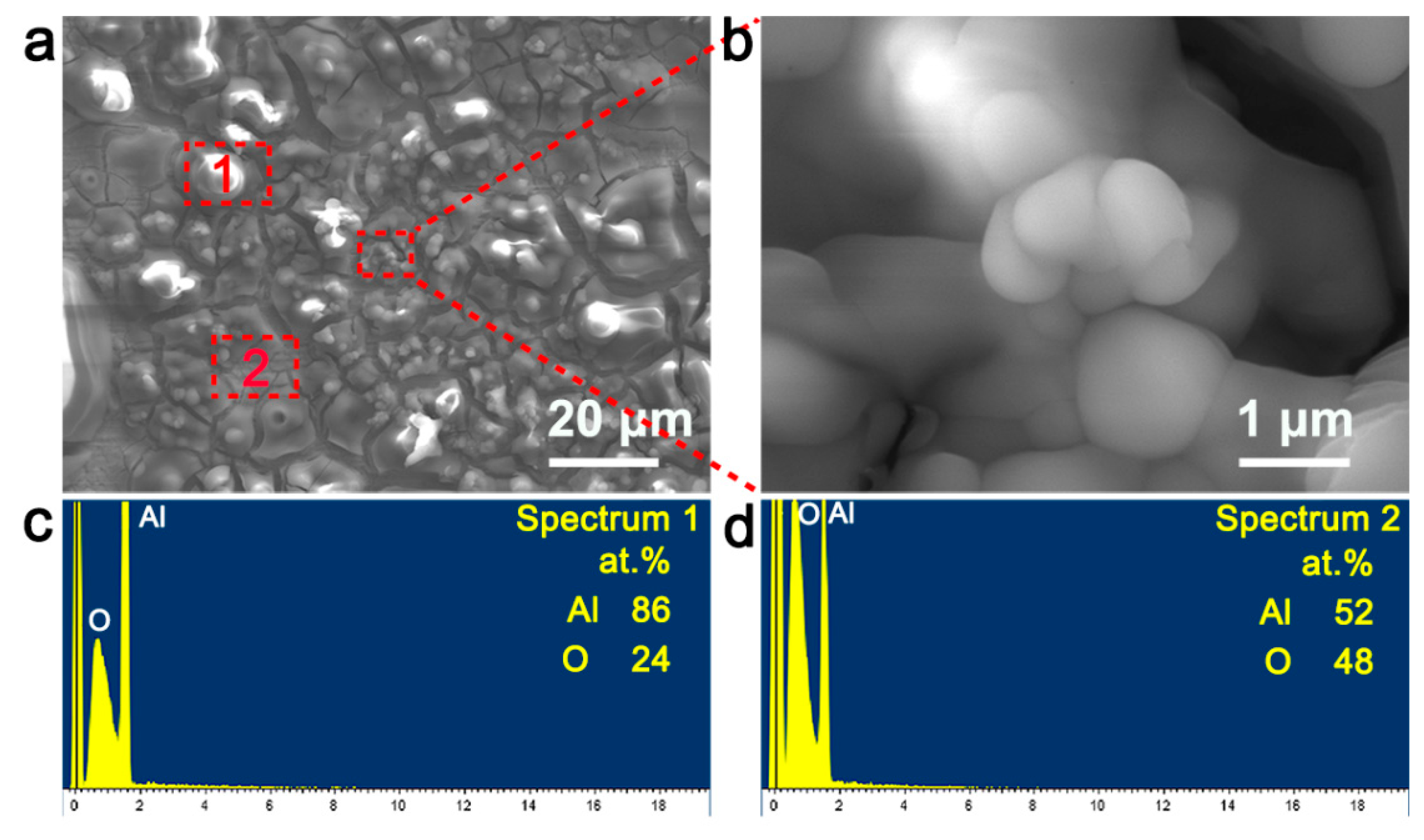
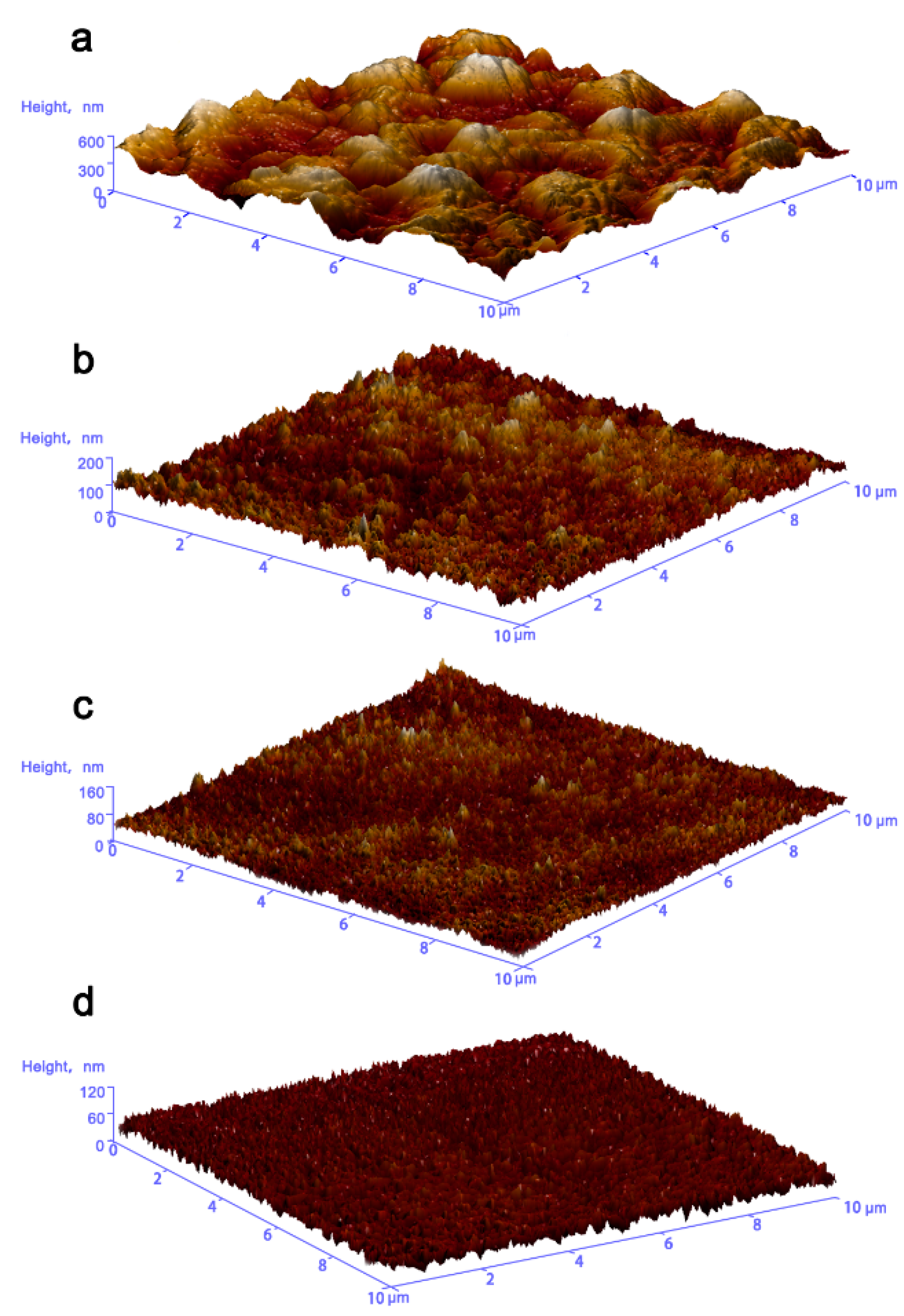
3.2. Electrodeposition and Microstructures of Al Coatings
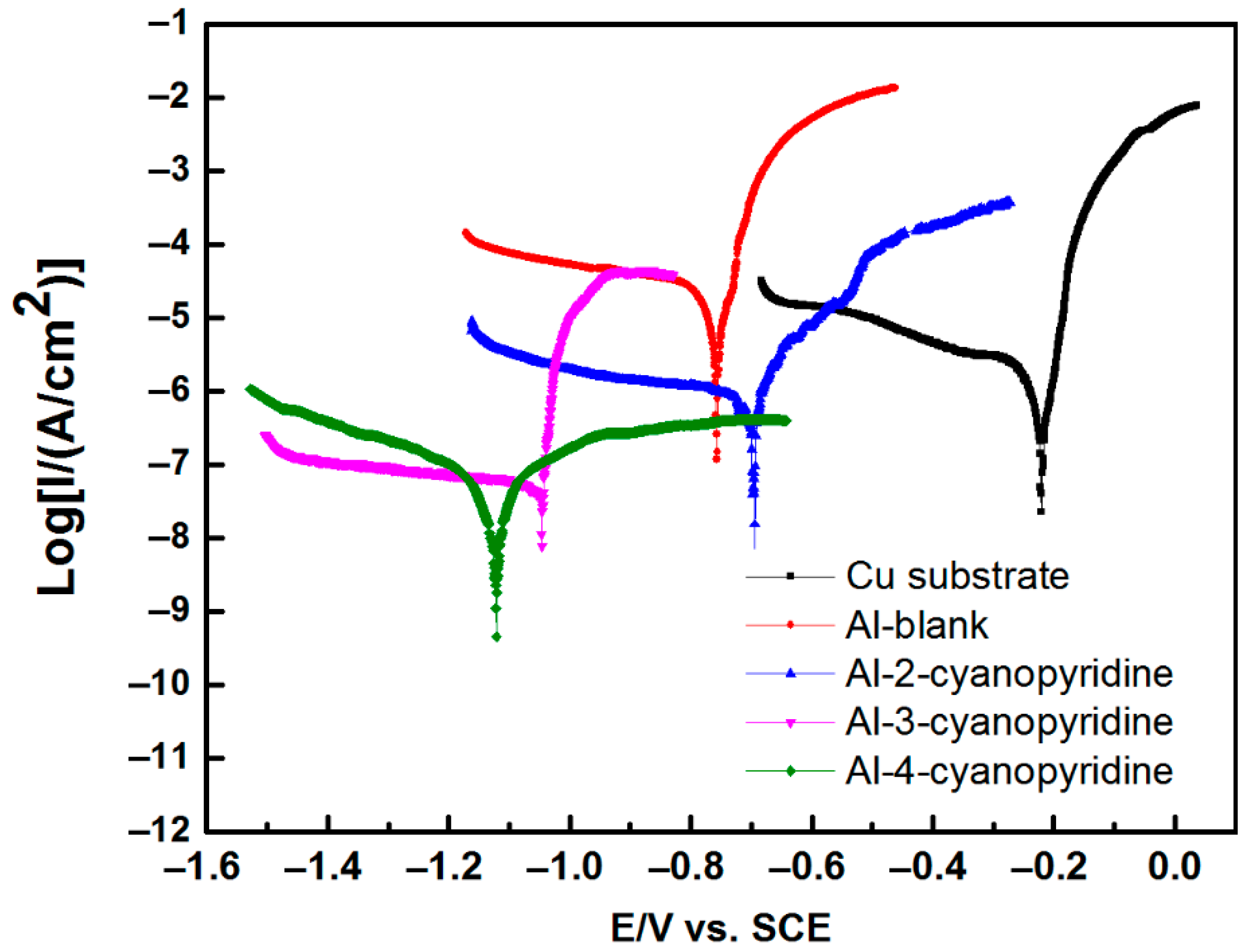
3.3. Corrosion Properties
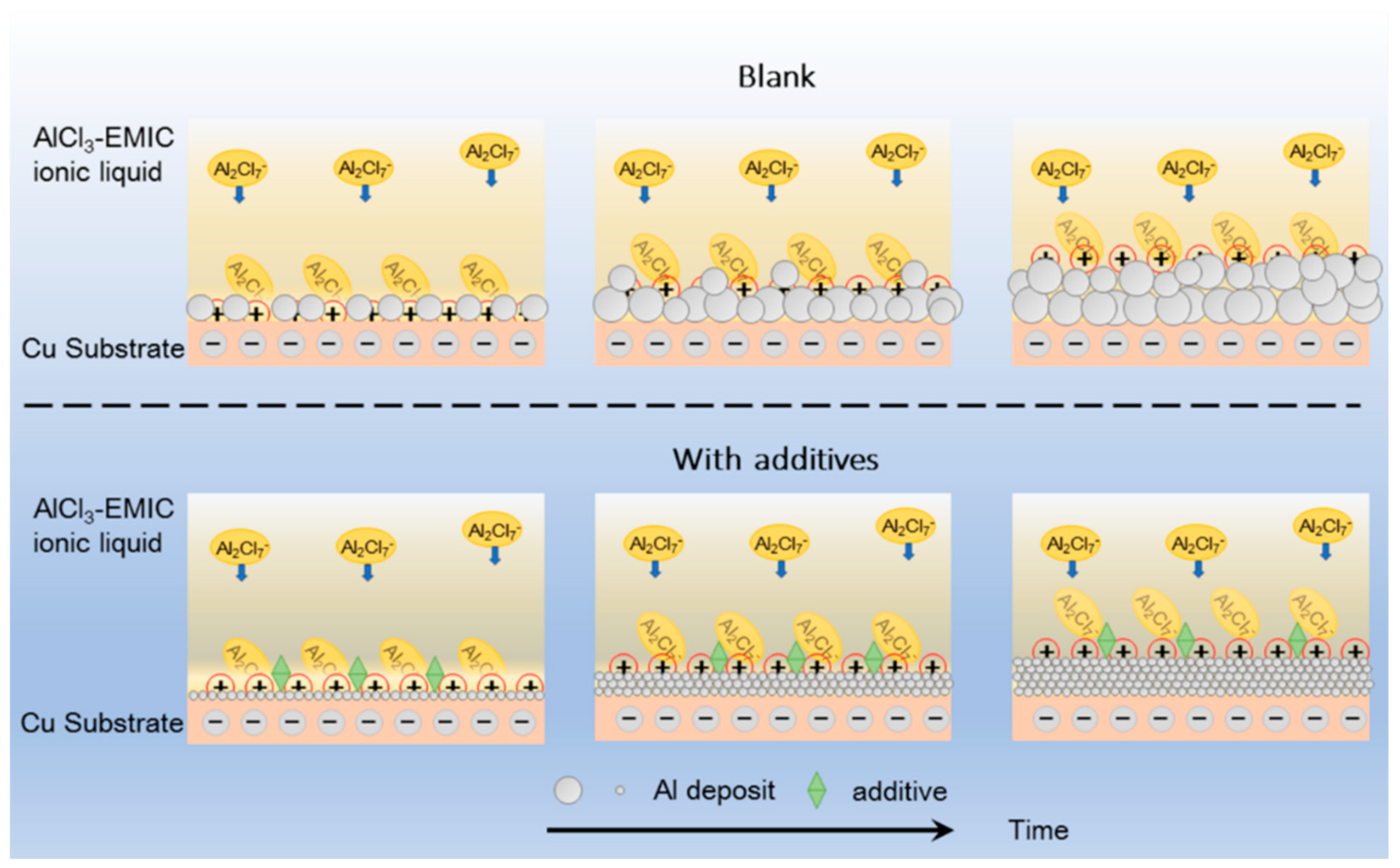
3.4. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Böttcher, R.; Mai, S.; Ispas, A.; Bund, A. Aluminum deposition and dissolution in [emim]Cl-based ionic liquids–kinetics of charge–transfer and the rate–determining step. J. Electrochem. Soc. 2020, 167, 102516. [Google Scholar] [CrossRef]
- Abbott, A.P.; Frisch, G.; Ryder, K.S. Electroplating using ionic liquids. Annu. Rev. Mater. Res. 2013, 43, 335–358. [Google Scholar] [CrossRef]
- El Abedin, S.Z.; Endres, F. Electrodeposition of metals and semiconductors in air- and water-stable ionic liquids. Chemphyschem 2006, 7, 58–61. [Google Scholar] [CrossRef]
- Takahashi, S.; Koura, N.; Kohara, S.; Saboungi, M.L.; Curtiss, L.A. Technological and scientific issues of room-temperature molten salts. Plasmas Ions 1999, 2, 91–105. [Google Scholar] [CrossRef]
- Xu, B.; Zhang, M.; Ling, G. Electrolytic etching of AZ91D Mg alloy in AlCl3-EMIC ionic liquid for the electrodeposition of adhesive Al coating. Surf. Coat. Technol. 2014, 239, 1–6. [Google Scholar] [CrossRef]
- Zhang, M.M.; Kamavaram, V.; Reddy, R.G. New electrolytes for aluminum production: Ionic liquids. Jom-J. Miner. Met. Mater. Soc. 2003, 55, 54–57. [Google Scholar] [CrossRef]
- Simka, W.; Puszczyk, D.; Nawrat, G. Electrodeposition of metals from non-aqueous solutions. Electrochim. Acta 2009, 54, 5307–5319. [Google Scholar] [CrossRef]
- Chang, J.-K.; Chen, S.-Y.; Tsai, W.-T.; Deng, M.-J.; Sun, I.W. Electrodeposition of aluminum on magnesium alloy in aluminum chloride (AlCl3)-1-ethyl-3-methylimidazolium chloride (EMIC) ionic liquid and its corrosion behavior. Electrochem. Commun. 2007, 9, 1602–1606. [Google Scholar] [CrossRef]
- Yue, G.; Zhang, S.; Zhu, Y.; Lu, X.; Li, S.; Li, Z. A promising method for electrodeposition of aluminium on stainless steel in ionic liquid. Aiche J. 2009, 55, 783–796. [Google Scholar] [CrossRef]
- Craig, B.; Schoetz, T.; Cruden, A.; Ponce de Leon, C. Review of current progress in non-aqueous aluminium batteries. Renew. Sustain. Energy Rev. 2020, 133, 110100. [Google Scholar] [CrossRef]
- Maniam, K.K.; Paul, S. A Review on the Electrodeposition of Aluminum and Aluminum Alloys in Ionic Liquids. Coatings 2021, 11, 80. [Google Scholar] [CrossRef]
- Karuppasamy, R.; Barik, D. Production methods of aluminium foam: A brief review. Mater. Today Proc. 2021, 37, 1584–1587. [Google Scholar] [CrossRef]
- Karimzadeh, A.; Aliofkhazraei, M.; Walsh, F.C. A review of electrodeposited Ni-Co alloy and composite coatings: Microstructure, properties and applications. Surf. Coat. Technol. 2019, 372, 463–498. [Google Scholar] [CrossRef]
- Torabinejad, V.; Aliofkhazraei, M.; Assareh, S.; Allahyarzadeh, M.H.; Rouhaghdam, A.S. Electrodeposition of Ni-Fe alloys, composites, and nano coatings–A review. J. Alloy. Compd. 2017, 691, 841–859. [Google Scholar] [CrossRef]
- Allahyarzadeh, M.H.; Aliofkhazraei, M.; Rezvanian, A.R.; Torabinejad, V.; Sabour Rouhaghdam, A.R. Ni-W electrodeposited coatings: Characterization, properties and applications. Surf. Coat. Technol. 2016, 307, 978–1010. [Google Scholar] [CrossRef]
- Yue, G.; Lu, X.; Zhu, Y.; Zhang, X.; Zhang, S. Surface morphology, crystal structure and orientation of aluminium coatings electrodeposited on mild steel in ionic liquid. Chem. Eng. J. 2009, 147, 79–86. [Google Scholar] [CrossRef]
- CvetkoviĆ, V.S.; VukiĆEviĆ, N.M.; JoviĆEviĆ, N.; StevanoviĆ, J.S.; JoviĆEviĆ, J.N. Aluminium electrodeposition under novel conditions from AlCl3–urea deep eutectic solvent at room temperature. Trans. Nonferrous Met. Soc. China 2020, 30, 823–834. [Google Scholar] [CrossRef]
- Arowolo, M.O.; Adekunle, A.A.; Odejobi, O.A. Parameters affecting qualitative and efficient electrodeposition of metals in an electrolytic cell: A review. Fudma J. Sci. 2020, 4, 584–589. [Google Scholar] [CrossRef]
- Peng, D.; Zhang, J.; Zhang, M.; Cong, D.; Chen, H.; Song, K.; Li, Z. Research progress of additives for electrodeposition of aluminum from ionic liquid. Surf. Technol. 2021, 50, 149–157. [Google Scholar]
- Endres, F.; Bukowski, M.; Hempelmann, R.; Natter, H. Electrodeposition of nanocrystalline metals and alloys from ionic liquids. Angew. Chem. Int. Edit. 2003, 42, 3428–3430. [Google Scholar] [CrossRef]
- Barchi, L.; Bardi, U.; Caporali, S.; Fantini, M.; Scrivani, A.; Scrivani, A. Electroplated bright alminium coatings for anticorrosion and decorative purposes. Prog. Org. Coat. 2010, 67, 146–151. [Google Scholar] [CrossRef]
- Liu, L.; Lu, X.; Cai, Y.; Zheng, Y.; Zhang, S. Influence of additives on the speciation, morphology, and nanocrystallinity of aluminium electrodeposition. Aust. J. Chem. 2012, 65, 1523–1528. [Google Scholar] [CrossRef]
- Abbott, A.P.; Qiu, F.; Abood, H.M.A.; Ali, M.R.; Ryder, K.S. Double layer, diluent and anode effects upon the electrodeposition of aluminium from chloroaluminate based ionic liquids. Phys. Chem. Chem. Phys. 2010, 12, 1862–1872. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Fan, C.; Chen, Y.; Lou, J.; Yan, L. Pulse current electrodeposition of Al from an AlCl3-EMIC ionic liquid. Electrochim. Acta 2011, 56, 5478–5482. [Google Scholar] [CrossRef]
- Abbott, A.P.; McKenzie, K.J. Application of ionic liquids to the electrodeposition of metals. Phys. Chem. Chem. Phys. 2006, 8, 4265–4279. [Google Scholar] [CrossRef]
- Liao, Q.; Hussey, C.L. Densities, viscosities, and conductivities of mixtures of benzene with the lewis acidic aluminum chloride + 1-methyl-3-ethylimidazolium chloride molten salt. J. Chem. Eng. Data 1996, 41, 1126–1130. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, Q.; Zhang, S.; Lu, X. Effect of nicotinamide on electrodeposition of Al from aluminium chloride (AlCl3)-1-butyl-3-methylimidazolium chloride (Bmim Cl) ionic liquids. J. Solid State Electrochem. 2014, 18, 257–267. [Google Scholar] [CrossRef]
- Guinea, E.; Salicio-Paz, A.; Iriarte, A.; Grande, H.-J.; Medina, E.; García-Lecina, E. Robust Aluminum Electrodeposition from Iionic Liquid Electrolytes Containing Light Aromatic Naphta as Additive. ChemistryOpen 2019, 8, 1094–1099. [Google Scholar] [CrossRef] [Green Version]
- Ueda, M.; Hariyama, S.; Ohtsuka, T. Al electroplating on the AZ121 Mg alloy in an EMIC-AlCl3 ionic liquid containing ethylene glycol. J. Solid State Electr. 2012, 16, 3423–3427. [Google Scholar] [CrossRef]
- Sheng, P.F.; Chen, B.; Shao, H.B.; Wang, J.M.; Zhang, J.Q.; Cao, C.N. Electrodeposition and corrosion behavior of nanocrystalline aluminum from a chloroaluminate ionic liquid. Mater. Corros. 2015, 66, 1338–1343. [Google Scholar] [CrossRef]
- Xue, D.; Xu, B.; Chen, Y.; Zhang, Q.; Ling, G. Electrodeposition of bright Al and Al-Mn coatings from AlCl3-EMIC ionic liquid. CIESC J. 2015, 66, 282–286. [Google Scholar]
- Leng, M.; Chen, S.; Zhang, J.; Lang, H.; Kang, Y. Effects of organic additives containing carbonyl group on electrodeposition of al from AlCl3-[emim]Cl ionic liquid. Acta Chim. Sinica 2015, 73, 403–408. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Zhang, Q.; Chen, B.; Lu, X.; Zhang, S. Electrodeposition of bright al coatings from 1-butyl-3-methylimidazolium chloroaluminate ionic liquids with specific additives. J. Electrochem. Soc. 2015, 162, D320–D324. [Google Scholar] [CrossRef]
- Uehara, K.; Yamazaki, K.; Gunji, T.; Kaneko, S.; Tanabe, T.; Ohsaka, T.; Matsumoto, F. Evaluation of key factors for preparing high brightness surfaces of aluminum films electrodeposited from AlCl3-1-ethyl-3-methylimidazolium chloride-organic additive baths. Electrochim. Acta 2016, 215, 556–565. [Google Scholar] [CrossRef]
- Peng, D.; Cong, D.; Song, K.; Ding, X.; Wang, X.; Bai, Y.; Yang, X.; Yin, C.; Zhang, Y.; Rao, J.; et al. Mirror-like Bright Al-Mn Coatings Electrodeposition from 1-Ethyl-3 Methylimidazolium Chloride-AlCl3-MnCl2 Ionic Liquids with Pyridine Derivatives. Materials 2021, 14, 6226. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, T.; Nohira, T.; Ito, Y. Nucleation and surface morphology of aluminum–lanthanum alloy electrodepsited in a LaCl3-saturated AlCl3–EtMeImCl room temperature molten salt. Electrochim. Acta 2002, 47, 2817–2822. [Google Scholar] [CrossRef]
- Bakkar, A.; Neubert, V. Electrodeposition and corrosion characterisation of micro- and nano-crystalline aluminium from AlCl3/1-ethyl-3-methylimidazolium chloride ionic liquid. Electrochim. Acta 2013, 103, 211–218. [Google Scholar] [CrossRef]



| TC (hkl) | TC (111) | TC (200) | TC (220) | TC (311) |
|---|---|---|---|---|
| Al-blank | 0.99 | 1.36 | 0.57 | 0.61 |
| Al-2-cyanopyridine | 0.48 | 2.38 | 0.0 | 0.0 |
| Al-3-cyanopyridine | 0.34 | 2.51 | 0.0 | 0.0 |
| Al-4-cyanopyridine | 0.0 | 2.84 | 0.0 | 0.0 |
| Materials | Ecorr (V) | Icorr (A/cm2) |
|---|---|---|
| Al-blank | −0.764 | 2.9 × 10−5 |
| Al-2-cyanopyridine | −0.703 | 1.02 × 10−6 |
| Al-3-cyanopyridine | −1.057 | 9.88 × 10−8 |
| Al-4-cyanopyridine | −1.123 | 6.34 × 10−8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Peng, D.; Peng, F.; Huang, A.; Song, K.; He, Q.; Yin, C.; Rao, J.; Zhang, Y.; Chen, H.; et al. Effects of Additives Containing Cyanopyridine on Electrodeposition of Bright Al Coatings from AlCl3-EMIC Ionic Liquids. Coatings 2021, 11, 1396. https://doi.org/10.3390/coatings11111396
Zhang M, Peng D, Peng F, Huang A, Song K, He Q, Yin C, Rao J, Zhang Y, Chen H, et al. Effects of Additives Containing Cyanopyridine on Electrodeposition of Bright Al Coatings from AlCl3-EMIC Ionic Liquids. Coatings. 2021; 11(11):1396. https://doi.org/10.3390/coatings11111396
Chicago/Turabian StyleZhang, Min, Dong Peng, Feifei Peng, Anwei Huang, Kaiqiang Song, Qingbing He, Changqing Yin, Jinsong Rao, Yuxin Zhang, Haitao Chen, and et al. 2021. "Effects of Additives Containing Cyanopyridine on Electrodeposition of Bright Al Coatings from AlCl3-EMIC Ionic Liquids" Coatings 11, no. 11: 1396. https://doi.org/10.3390/coatings11111396






