Green Coating Polymers in Meat Preservation
Abstract
:1. Introduction
2. Green Coating Polymers as Potential Matrices for Meat Preservation
2.1. Polymers Isolated from Biomass
2.1.1. Polysaccharides
2.1.2. Proteins
2.1.3. Lipids
2.2. Chemically Synthesized Polymers from Bio-Based Monomers
2.3. Polymers from Genetically Engineered Microorganisms
3. Brief Overview on the Preparation Techniques of Green Coating and Film Polymers
4. More Specific Applications of Green Coating Polymers in Meat Preservation
4.1. Enhancement of Mechanical Properties and Barrier Properties in Active Polymer Packages
4.2. Protection against Lipid Peroxidation and Discoloration
4.3. Antimicrobial Activity
5. Brief Overview of the Role of Functional Polymers in Smart Packaging
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Toldrá, F. Muscle Foods: Water, Structure and Functionality. Food Sci. Technol. Int. 2003, 9, 173–177. [Google Scholar] [CrossRef]
- Gagaoua, M.; Picard, B. Current Advances in Meat Nutritional, Sensory and Physical Quality Improvement. Foods 2020, 9, 321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gennadios, A.; Hanna, M.A.; Kurth, L.B. Application of Edible Coatings on Meats, Poultry and Seafoods: A Review. LWT Food Sci. Technol. 1997, 30, 337–350. [Google Scholar] [CrossRef]
- Parry, A.; James, K.; LeRoux, S. Strategies To Achieve Economic And Environmental Gains By Reducing Food Waste; WRAP: London, UK, 2015. [Google Scholar]
- Gagaoua, M.; Boudechicha, H.-R. Ethnic Meat Products of the North African and Mediterranean Countries: An Overview. J. Ethn. Foods 2018, 5, 83–98. [Google Scholar] [CrossRef]
- Porta, R.; Sabbah, M.; Di Pierro, P. Biopolymers as Food Packaging Materials. Int. J. Mol. Sci. 2020, 21, 4942. [Google Scholar] [CrossRef]
- Bonilla, J.; Atarés, L.; Vargas, M.; Chiralt, A. Edible Films and Coatings to Prevent the Detrimental Effect of Oxygen on Food Quality: Possibilities and Limitations. J. Food Eng. 2012, 110, 208–213. [Google Scholar] [CrossRef]
- Sánchez-Ortega, I.; García-Almendárez, B.E.; Santos-López, E.M.; Amaro-Reyes, A.; Barboza-Corona, J.E.; Regalado, C. Antimicrobial Edible Films and Coatings for Meat and Meat Products Preservation. Sci. World J. 2014, 2014, 248935. [Google Scholar] [CrossRef]
- Ünalan, İ.U.; Korel, F.; Yemenicioğlu, A. Active Packaging of Ground Beef Patties by Edible Zein Films Incorporated with Partially Purified Lysozyme and Na2EDTA. Int. J. Food Sci. Technol. 2011, 46, 1289–1295. [Google Scholar] [CrossRef] [Green Version]
- Min, B.J.; Han, I.Y.; Dawson, P.L. Antimicrobial Gelatin Films Reduce Listeria Monocytogenes on Turkey Bologna1. Poult. Sci. 2010, 89, 1307–1314. [Google Scholar] [CrossRef]
- Bermúdez-Oria, A.; Rodríguez-Gutiérrez, G.; Rubio-Senent, F.; Fernández-Prior, Á.; Fernández-Bolaños, J. Effect of Edible Pectin-Fish Gelatin Films Containing the Olive Antioxidants Hydroxytyrosol and 3,4-Dihydroxyphenylglycol on Beef Meat during Refrigerated Storage. Meat Sci. 2019, 148, 213–218. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Rhim, J.W.; Weller, C.L.; Hamouz, F.; Cuppett, S.; Schnepf, M. Moisture Loss and Lipid Oxidation for Precooked Beef Patties Stored in Edible Coatings and Films. J. Food Sci. 2000, 65, 300–304. [Google Scholar] [CrossRef]
- Umaraw, P.; Verma, A.K. Comprehensive Review on Application of Edible Film on Meat and Meat Products: An Eco-Friendly Approach. Crit. Rev. Food Sci. Nutr. 2017, 57, 1270–1279. [Google Scholar] [CrossRef] [PubMed]
- Surendhiran, D.; Li, C.; Cui, H.; Lin, L. Fabrication of High Stability Active Nanofibers Encapsulated with Pomegranate Peel Extract using Chitosan/PEO for Meat Preservation. Food Packag. Shelf Life 2020, 23, 100439. [Google Scholar] [CrossRef]
- Lamri, M.; Bhattacharya, T.; Boukid, F.; Chentir, I.; Dib, A.L.; Das, D.; Djenane, D.; Gagaoua, M. Nanotechnology as a Processing and Packaging Tool to Improve Meat Quality and Safety. Foods 2021, 10, 2633. [Google Scholar] [CrossRef]
- Vroman, I.; Tighzert, L. Biodegradable Polymers. Materials 2009, 2, 307–344. [Google Scholar] [CrossRef]
- Ghanbarzadeh, B.; Almasi, H. Biodegradable Polymers. In Biodegrad.-Life Science; IntechOpen: London, UK, 2013; pp. 141–185. [Google Scholar] [CrossRef] [Green Version]
- Do Nascimento Alves, R.; Lorranne Santos Lima, T.; da Silva Chaves, K.; de Albuquerque Meireles, B.R.L. Biodegradable Films with Brassica Oleracea Capitata Extract as a Quality Indicator in Sheep Meat. J. Food Process. Preserv. 2021, 45, e14997. [Google Scholar] [CrossRef]
- Gupta, A.; Pal, A.K.; Patwa, R.; Dhar, P.; Katiyar, V. Green Composites with Excellent Barrier Properties. In Advanced Green Composites; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2018; p. 321. [Google Scholar]
- Bhattacharya, T.; Bandyopadhyay, N. Green Technology Based Nanostarch Films: Water Vapour, Thermal Properties and Micro structural Properties Detection. Int. J. Sci. Technol. Res. 2019, 8, 3058–3062. [Google Scholar]
- Fox, D.M.; Lee, J.; Citro, C.J.; Novy, M. Flame Retarded Poly(Lactic Acid) using POSS-Modified Cellulose. 1. Thermal and Combustion Properties of Intumescing Composites. Polym. Degrad. Stab. 2013, 98, 590–596. [Google Scholar] [CrossRef]
- Manohar, C.M.; Prabhawathi, V.; Sivakumar, P.M.; Doble, M. Design of a Papain Immobilized Antimicrobial Food Package with Curcumin as a Crosslinker. PLoS ONE 2015, 10, e0121665. [Google Scholar] [CrossRef] [Green Version]
- Singh, P.; Baisthakur, P.; Yemul, O.S. Synthesis, Characterization and Application of Crosslinked Alginate as Green Packaging Material. Heliyon 2020, 6, e03026. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharya, T.; Bandyopadhyay, N.; Ray, D.; Bhattacharyya, D.; Das, K. Preparation and Characterizations of Soya Protein Isolate Films coated with Palm Stearin and Modified with Antimicrobial Agent Chitosan. Int. J. Adv. Res. Technol. 2013, 2, 75–82. [Google Scholar]
- Fitch-Vargas, P.R.; Aguilar-Palazuelos, E.; de Jesús Zazueta-Morales, J.; Vega-García, M.O.; Valdez-Morales, J.E.; Martínez-Bustos, F.; Jacobo-Valenzuela, N. Physicochemical and Microstructural Characterization of Corn Starch Edible Films Obtained by a Combination of Extrusion Technology and Casting Technique. J. Food Sci. 2016, 81, E2224–E2232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salama, H.E.; Abdel Aziz, M.S. Optimized Carboxymethyl Cellulose and Guanidinylated Chitosan Enriched with Titanium Oxide Nanoparticles of Improved UV-Barrier Properties for the Active Packaging of Green Bell Pepper. Int. J. Biol. Macromol. 2020, 165, 1187–1197. [Google Scholar] [CrossRef] [PubMed]
- Flores-Jerónimo, G.; Silva-Mendoza, J.; Morales-San Claudio, P.C.; Toxqui-Terán, A.; Aguilar-Martínez, J.A.; Chávez-Guerrero, L. Chemical and Mechanical Properties of Films Made of Cellulose Nanoplatelets and Cellulose Fibers Obtained from Banana Pseudostem. Waste Biomass Valorization 2021, 12, 5715–5723. [Google Scholar] [CrossRef]
- Wu, J.; Du, X.; Yin, Z.; Xu, S.; Xu, S.; Zhang, Y. Preparation and Characterization of Cellulose Nanofibrils from Coconut Coir Fibers and Their Reinforcements in Biodegradable Composite Films. Carbohydr. Polym. 2019, 211, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Dixit, S.; Mishra, G.; Yadav, V.L. Optimization of Novel Bio-Composite Packaging Film based on Alkali-Treated Hemp Fiber/Polyethylene/Polypropylene Using Response Surface Methodology Approach. Polym. Bull. 2021, 1–25. [Google Scholar] [CrossRef]
- Yadav, A.; Mangaraj, S.; Singh, R.; Kumar, N.; Arora, S. Biopolymers as Packaging Material In Food And Allied Industry. Chem. Int. J. Chem. Stud. 2018, 6, 2411–2418. [Google Scholar]
- Ludwicka, K.; Kaczmarek, M.; Białkowska, A. Bacterial Nanocellulose—A Biobased Polymer for Active and Intelligent Food Packaging Applications: Recent Advances and Developments. Polymers 2020, 12, 2209. [Google Scholar] [CrossRef]
- Siracusa, V.; Blanco, I. Bio-Polyethylene (Bio-PE), Bio-Polypropylene (Bio-PP) and Bio-Poly(ethylene terephthalate) (Bio-PET): Recent Developments in Bio-Based Polymers Analogous to Petroleum-Derived Ones for Packaging and Engineering Applications. Polymers 2020, 12, 1641. [Google Scholar] [CrossRef]
- Rahman, R.; Sood, M.; Gupta, N.; Bandral, J.D.; Hameed, F.; Ashraf, S. Bioplastics for Food Packaging: A Review. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 2311–2321. [Google Scholar] [CrossRef]
- Manikandan, N.A.; Pakshirajan, K.; Pugazhenthi, G. Preparation and Characterization Of Environmentally Safe And Highly Biodegradable Microbial Polyhydroxybutyrate (PHB) Based Graphene Nanocomposites For Potential Food Packaging Applications. Int. J. Biol. Macromol. 2020, 154, 866–877. [Google Scholar] [CrossRef]
- Rabnawaz, M.; Wyman, I.; Auras, R.; Cheng, S. A Roadmap Towards Green Packaging: The Current Status And Future Outlook For Polyesters In The Packaging Industry. Green Chem. 2017, 19, 4737–4753. [Google Scholar] [CrossRef]
- Mohamed, S.A.A.; El-Sakhawy, M.; El-Sakhawy, M.A.-M. Polysaccharides, Protein and Lipid -Based Natural Edible Films in Food Packaging: A Review. Carbohydr. Polym. 2020, 238, 116178. [Google Scholar] [CrossRef] [PubMed]
- Pérez Rodrigo, C.; Ruiz Vadillo, V. Wheat, Bread And Pasta In Mediterranean Diets. Arch. Lat. Nutr. 2004, 54, 52–58. [Google Scholar]
- Tosati, J.V.; Messias, V.C.; Carvalho, P.I.N.; Rodrigues Pollonio, M.A.; Meireles, M.A.A.; Monteiro, A.R. Antimicrobial Effect of Edible Coating Blend Based on Turmeric Starch Residue and Gelatin Applied onto Fresh Frankfurter Sausage. Food Bioprocess Technol. 2017, 10, 2165–2175. [Google Scholar] [CrossRef]
- Bel Haaj, S.; Thielemans, W.; Magnin, A.; Boufi, S. Starch Nanocrystals And Starch Nanoparticles From Waxy Maize As Nanoreinforcement: A Comparative Study. Carbohydr. Polym. 2016, 143, 310–317. [Google Scholar] [CrossRef]
- Dufresne, A.; Cavaillé, J.-Y.; Helbert, W. New Nanocomposite Materials: Microcrystalline Starch Reinforced Thermoplastic. Macromolecules 1996, 29, 7624–7626. [Google Scholar] [CrossRef]
- Kim, H.-Y.; Park, S.S.; Lim, S.-T. Preparation, Characterization And Utilization Of Starch Nanoparticles. Colloids Surf. B Biointerfaces 2015, 126, 607–620. [Google Scholar] [CrossRef]
- Xu, Y.; Rehmani, N.; Alsubaie, L.; Kim, C.; Sismour, E.; Scales, A. Tapioca Starch Active Nanocomposite Films And Their Antimicrobial Effectiveness On Ready-To-Eat Chicken Meat. Food Packag. Shelf Life 2018, 16, 86–91. [Google Scholar] [CrossRef]
- Chen, D.; Lawton, D.; Thompson, M.R.; Liu, Q. Biocomposites Reinforced With Cellulose Nanocrystals Derived From Potato Peel Waste. Carbohydr. Polym. 2012, 90, 709–716. [Google Scholar] [CrossRef] [Green Version]
- Ramanjaneyulu, B.; Venkatachalapathi, N.; Prasanthi, G. Mechanical Properties And Morphology Of Poly (Lactic Acid)(PLA) With Different Flexible Copolymers. Int. J. Mech. Eng. Technol. 2019, 10, 745–754. [Google Scholar]
- Aung, S.P.S.; Shein, H.H.H.; Aye, K.N.; Nwe, N. Environment-Friendly Biopolymers for Food Packaging: Starch, Protein, and Poly-lactic Acid (PLA). In Bio-Based Materials for Food Packaging: Green and Sustainable Advanced Packaging Materials; Ahmed, S., Ed.; Springer: Singapore, 2018; pp. 173–195. [Google Scholar] [CrossRef]
- Attaran, S.A.; Hassan, A.; Wahit, M.U. Materials for Food Packaging Applications Based On Bio-Based Polymer Nanocomposites: A Review. J. Thermoplast. Compos. Mater. 2017, 30, 143–173. [Google Scholar] [CrossRef]
- Bher, A.; Uysal Unalan, I.; Auras, R.; Rubino, M.; Schvezov, C.E. Toughening of Poly(lactic acid) and Thermoplastic Cassava Starch Reactive Blends Using Graphene Nanoplatelets. Polymers 2018, 10, 95. [Google Scholar] [CrossRef] [Green Version]
- Bher, A.; Unalan, I.U.; Auras, R.; Rubino, M.; Schvezov, C.E. Graphene modifies the Biodegradation Of Poly(Lactic Acid)-Thermoplastic Cassava Starch Reactive Blend Films. Polym. Degrad. Stab. 2019, 164, 187–197. [Google Scholar] [CrossRef]
- Mitura, K.A.; Zarzycki, P.K. Chapter 3—Biocompatibility and Toxicity of Allotropic Forms of Carbon in Food Packaging. In Role of Materials Science in Food Bioengineering; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 73–107. [Google Scholar] [CrossRef]
- Darie-Niță, R.N.; Râpă, M.; Sivertsvik, M.; Rosnes, J.T.; Popa, E.E.; Dumitriu, R.P.; Marincaș, O.; Matei, E.; Predescu, C.; Vasile, C. PLA-Based Materials Containing Bio-Plasticizers and Chitosan Modified with Rosehip Seed Oil for Ecological Packaging. Polymers 2021, 13, 1610. [Google Scholar] [CrossRef] [PubMed]
- Petersen, K.; Nielsen, P.V.; Olsen, M.B. Physical and Mechanical Properties of Biobased Materials Starch, Polylactate and Polyhydroxybutyrate. Starch-Stärke 2001, 53, 356–361. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, Y.; Li, Y.; Luo, C.; Yang, C.; Shi, W.; Li, L. Covalent Immobilization of Polypeptides on Polylactic Acid Films and Their Application to Fresh Beef Preservation. J. Agric. Food Chem. 2020, 68, 10532–10541. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Thakur, M.; Bhattacharya, M.; Mandal, T.; Goswami, S. Commercial Application of Cellulose Nano-Composites—A Review. Biotechnol. Rep. 2019, 21, e00316. [Google Scholar] [CrossRef]
- Eichhorn, S.J. Cellulose Nanowhiskers: Promising Materials For Advanced Applications. Soft Matter 2011, 7, 303–315. [Google Scholar] [CrossRef]
- Habibi, Y.; Lucia, L.A.; Rojas, O.J. Cellulose Nanocrystals: Chemistry, Self-Assembly, and Applications. Chem. Rev. 2010, 110, 3479–3500. [Google Scholar] [CrossRef]
- Tsouko, E.; Kourmentza, C.; Ladakis, D.; Kopsahelis, N.; Mandala, I.; Papanikolaou, S.; Paloukis, F.; Alves, V.; Koutinas, A. Bacterial Cellulose Production from Industrial Waste and by-Product Streams. Int. J. Mol. Sci. 2015, 16, 14832–14849. [Google Scholar] [CrossRef] [PubMed]
- Hivechi, A.; Bahrami, S.H. A New Cellulose Purification Approach For Higher Degree Of Polymerization: Modeling, Optimization And Characterization. Carbohydr. Polym. 2016, 152, 280–286. [Google Scholar] [CrossRef]
- Pirsa, S.; Shamusi, T. Intelligent and Active Packaging Of Chicken Thigh Meat By Conducting Nano Structure Cellulose-Polypyrrole-ZnO Film. Mater. Sci. Eng. C 2019, 102, 798–809. [Google Scholar] [CrossRef]
- Rasouli, Y.; Moradi, M.; Tajik, H.; Molaei, R. Fabrication of Anti-Listeria Film Based On Bacterial Cellulose And Lactobacillus Sakei-Derived Bioactive Metabolites; Application In Meat Packaging. Food Biosci. 2021, 42, 101218. [Google Scholar] [CrossRef]
- Valdespino-León, M.; Calderón-Domínguez, G.; De La Paz Salgado-Cruz, M.; Rentería-Ortega, M.; Farrera-Rebollo, R.R.; Morales-Sánchez, E.; Gaona-Sánchez, V.A.; Terrazas-Valencia, F. Biodegradable Electrosprayed Pectin Films: An Alternative to Valorize Coffee Mucilage. Waste Biomass Valorization 2021, 12, 2477–2494. [Google Scholar] [CrossRef]
- Lorevice, M.V.; Otoni, C.G.; Moura, M.R.d.; Mattoso, L.H.C. Chitosan Nanoparticles On The Improvement Of Thermal, Barrier, And Mechanical Properties Of High- And Low-Methyl Pectin Films. Food Hydrocoll. 2016, 52, 732–740. [Google Scholar] [CrossRef] [Green Version]
- Xiong, Y.; Li, S.; Warner, R.D.; Fang, Z. Effect of Oregano Essential Oil And Resveratrol Nanoemulsion Loaded Pectin Edible Coating On The Preservation Of Pork Loin In Modified Atmosphere Packaging. Food Control 2020, 114, 107226. [Google Scholar] [CrossRef]
- Fasseas, M.K.; Mountzouris, K.C.; Tarantilis, P.A.; Polissiou, M.; Zervas, G. Antioxidant Activity In Meat Treated With Oregano And Sage Essential Oils. Food Chem. 2008, 106, 1188–1194. [Google Scholar] [CrossRef]
- Bekhit, A.E.D.; Geesink, G.H.; Ilian, M.A.; Morton, J.D.; Bickerstaffe, R. The Effects Of Natural Antioxidants On Oxidative Processes And Metmyoglobin Reducing Activity In Beef Patties. Food Chem. 2003, 81, 175–187. [Google Scholar] [CrossRef]
- Hasnain, M.S.; Jameel, E.; Mohanta, B.; Dhara, A.K.; Alkahtani, S.; Nayak, A.K. Chapter 1—Alginates: Sources, structure, and properties. In Alginates in Drug Delivery; Nayak, A.K., Hasnain, M.S., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 1–17. [Google Scholar] [CrossRef]
- Aarstad, O.A.; Stanisci, A.; Sætrom, G.I.; Tøndervik, A.; Sletta, H.; Aachmann, F.L.; Skjåk-Bræk, G. Biosynthesis and Function of Long Guluronic Acid-Blocks in Alginate Produced by Azotobacter vinelandii. Biomacromolecules 2019, 20, 1613–1622. [Google Scholar] [CrossRef] [Green Version]
- Lourenço, S.C.; Fraqueza, M.J.; Fernandes, M.H.; Moldão-Martins, M.; Alves, V.D. Application of Edible Alginate Films with Pineapple Peel Active Compounds on Beef Meat Preservation. Antioxidants 2020, 9, 667. [Google Scholar] [CrossRef] [PubMed]
- Alves, D.; Marques, A.; Milho, C.; Costa, M.J.; Pastrana, L.M.; Cerqueira, M.A.; Sillankorva, S.M. Bacteriophage ϕIBB-PF7A Loaded On Sodium Alginate-Based Films To Prevent Microbial Meat Spoilage. Int. J. Food Microbiol. 2019, 291, 121–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nanda, P.K.; Das, A.K.; Dandapat, P.; Dhar, P.; Bandyopadhyay, S.; Dib, A.L.; Lorenzo, J.M.; Gagaoua, M. Nutritional Aspects, Flavour Profile And Health Benefits Of Crab Meat Based Novel Food Products And Valorisation Of Processing Waste To Wealth: A Review. Trends Food Sci. Technol. 2021, 112, 252–267. [Google Scholar] [CrossRef]
- Kou, S.; Peters, L.M.; Mucalo, M.R. Chitosan: A Review Of Sources And Preparation Methods. Int. J. Biol. Macromol. 2021, 169, 85–94. [Google Scholar] [CrossRef]
- Joseph, S.M.; Krishnamoorthy, S.; Paranthaman, R.; Moses, J.A.; Anandharamakrishnan, C. A review On Source-Specific Chemistry, Functionality, And Applications Of Chitin And Chitosan. Carbohydr. Polym. Technol. Appl. 2021, 2, 100036. [Google Scholar] [CrossRef]
- Duran, A.; Kahve, H.I. The Effect Of Chitosan Coating And Vacuum Packaging On The Microbiological And Chemical Properties Of Beef. Meat Sci. 2020, 162, 107961. [Google Scholar] [CrossRef]
- Arkoun, M.; Daigle, F.; Holley, R.A.; Heuzey, M.C.; Ajji, A. Chitosan-Based Nanofibers As Bioactive Meat Packaging Materials. Packag. Technol. Sci. 2018, 31, 185–195. [Google Scholar] [CrossRef]
- Alirezalu, K.; Pirouzi, S.; Yaghoubi, M.; Karimi-Dehkordi, M.; Jafarzadeh, S.; Mousavi Khaneghah, A. Packaging of Beef Fillet With Active Chitosan Film Incorporated With Ɛ-Polylysine: An Assessment Of Quality Indices And Shelf Life. Meat Sci. 2021, 176, 108475. [Google Scholar] [CrossRef]
- Fang, Z.; Lin, D.; Warner, R.D.; Ha, M. Effect Of Gallic Acid/Chitosan Coating On Fresh Pork Quality In Modified Atmosphere Packaging. Food Chem. 2018, 260, 90–96. [Google Scholar] [CrossRef]
- Hoa, V.-B.; Song, D.-H.; Seol, K.-H.; Kang, S.-M.; Kim, H.-W.; Kim, Y.-S.; Kim, J.-H.; Cho, S.-H. Coating with chitosan Containing Lauric Acid (C12:0) Significantly Extends The Shelf-Life Of Aerobically—Packaged Beef Steaks During Refrigerated Storage. Meat Sci. 2021, 184, 108696. [Google Scholar] [CrossRef]
- Montaño-Sánchez, E.; Torres-Martínez, B.d.M.; Vargas-Sánchez, R.D.; Huerta-Leidenz, N.; Sánchez-Escalante, A.; Beriain, M.J.; Torrescano-Urrutia, G.R. Effects of Chitosan Coating with Green Tea Aqueous Extract on Lipid Oxidation and Microbial Growth in Pork Chops during Chilled Storage. Foods 2020, 9, 766. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; He, P.; Kang, H.; Li, X. Antioxidant and Antimicrobial Effects Of Edible Coating Based On Chitosan And Bamboo Vinegar In Ready To Cook Pork Chops. LWT 2018, 93, 470–476. [Google Scholar] [CrossRef]
- Quesada, J.; Sendra, E.; Navarro, C.; Sayas-Barberá, E. Antimicrobial Active Packaging including Chitosan Films with Thymus vulgaris L. Essential Oil for Ready-to-Eat Meat. Foods 2016, 5, 57. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Mukherjee, A.; Dutta, J. Chitosan Based Nanocomposite Films And Coatings: Emerging Antimicrobial Food Packaging Alternatives. Trends Food Sci. Technol. 2020, 97, 196–209. [Google Scholar] [CrossRef]
- Liu, H.; Du, Y.; Wang, X.; Sun, L. Chitosan Kills Bacteria Through Cell Membrane Damage. Int. J. Food Microbiol. 2004, 95, 147–155. [Google Scholar] [CrossRef]
- Farris, S.; Unalan, I.U.; Introzzi, L.; Fuentes-Alventosa, J.M.; Cozzolino, C.A. Pullulan-Based Films And Coatings For Food Packaging: Present Applications, Emerging Opportunities, And Future Challenges. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef] [Green Version]
- Uysal Unalan, I.; Boyacı, D.; Trabattoni, S.; Tavazzi, S.; Farris, S. Transparent Pullulan/Mica Nanocomposite Coatings with Outstanding Oxygen Barrier Properties. Nanomaterials 2017, 7, 281. [Google Scholar] [CrossRef] [PubMed]
- Cozzolino, C.A.; Campanella, G.; Türe, H.; Olsson, R.T.; Farris, S. Microfibrillated Cellulose And Borax As Mechanical, O2-Barrier, And Surface-Modulating Agents Of Pullulan Biocomposite Coatings On BOPP. Carbohydr. Polym. 2016, 143, 179–187. [Google Scholar] [CrossRef]
- Uysal Unalan, I.; Boyacı, D.; Ghaani, M.; Trabattoni, S.; Farris, S. Graphene Oxide Bionanocomposite Coatings with High Oxygen Barrier Properties. Nanomaterials 2016, 6, 244. [Google Scholar] [CrossRef] [Green Version]
- Hassan, A.H.A.; Cutter, C.N. Development and Evaluation Of Pullulan-Based Composite Antimicrobial Films (CAF) Incorporated With Nisin, Thymol And Lauric Arginate To Reduce Foodborne Pathogens Associated With Muscle Foods. Int. J. Food Microbiol. 2020, 320, 108519. [Google Scholar] [CrossRef]
- Silva, N.H.C.S.; Vilela, C.; Almeida, A.; Marrucho, I.M.; Freire, C.S.R. Pullulan-Based Nanocomposite Films For Functional Food Packaging: Exploiting Lysozyme Nanofibers As Antibacterial And Antioxidant Reinforcing Additives. Food Hydrocoll. 2018, 77, 921–930. [Google Scholar] [CrossRef]
- Chen, H.; Wang, J.; Cheng, Y.; Wang, C.; Liu, H.; Bian, H.; Pan, Y.; Sun, J.; Han, W. Application of Protein-Based Films and Coatings for Food Packaging: A Review. Polymers 2019, 11, 2039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tkaczewska, J. Peptides and Protein Hydrolysates As Food Preservatives And Bioactive Components Of Edible Films And Coatings—A Review. Trends Food Sci. Technol. 2020, 106, 298–311. [Google Scholar] [CrossRef]
- Umaraw, P.; Munekata, P.E.S.; Verma, A.K.; Barba, F.J.; Singh, V.P.; Kumar, P.; Lorenzo, J.M. Edible Films/Coating With Tailored Properties For Active Packaging Of Meat, Fish And Derived Products. Trends Food Sci. Technol. 2020, 98, 10–24. [Google Scholar] [CrossRef]
- Kim, W.; Ryu, J.-H.; Kim, Y. Application Of Yuba Films For Preserving Beef Patties. LWT 2020, 131, 109746. [Google Scholar] [CrossRef]
- Chollakup, R.; Pongburoos, S.; Boonsong, W.; Khanoonkon, N.; Kongsin, K.; Sothornvit, R.; Sukyai, P.; Sukatta, U.; Harnkarnsujarit, N. Antioxidant and Antibacterial Activities Of Cassava Starch And Whey Protein Blend Films Containing Rambutan Peel Extract And Cinnamon Oil For Active Packaging. LWT 2020, 130, 109573. [Google Scholar] [CrossRef]
- Akcan, T.; Estévez, M.; Serdaroğlu, M. Antioxidant Protection Of Cooked Meatballs During Frozen Storage By Whey Protein Edible Films With Phytochemicals From Laurus Nobilis L. And Salvia Officinalis. LWT 2017, 77, 323–331. [Google Scholar] [CrossRef]
- Hazlett, R.; Schmidmeier, C.; O’Mahony, J. Encyclopedia of Food Chemistry: Milk Proteins in Encyclopedia of Food Chemistry; Academic Press: Oxford, UK, 2019; pp. 138–147. [Google Scholar] [CrossRef]
- Chevalier, E.; Chaabani, A.; Assezat, G.; Prochazka, F.; Oulahal, N. Casein/Wax Blend Extrusion For Production Of Edible Films As Carriers Of Potassium Sorbate—A Comparative Study Of Waxes And Potassium Sorbate Effect. Food Packag. Shelf Life 2018, 16, 41–50. [Google Scholar] [CrossRef]
- Ucpinar Durmaz, B.; Aytac, A. Effects of Polyol-Based Plasticizer Types and Concentration on the Properties of Polyvinyl Alcohol and Casein Blend Films. J. Polym. Environ. 2021, 29, 313–322. [Google Scholar] [CrossRef]
- Sothornvit, R.; Krochta, J.M. Water Vapor Permeability and Solubility of Films from Hydrolyzed Whey Protein. J. Food Sci. 2000, 65, 700–703. [Google Scholar] [CrossRef]
- Trevisani, M.; Cecchini, M.; Siconolfi, D.; Mancusi, R.; Rosmini, R. Effects of Beeswax Coating on the Oxidative Stability of Long-Ripened Italian Salami. J. Food Qual. 2017, 2017, 8089135. [Google Scholar] [CrossRef]
- Vasile, C.; Tudorachi, N.; Zaharescu, T.; Darie-Nita, R.N.; Cheaburu-Yilmaz, C.N. Study on Thermal Behavior of Some Biocompatible and Biodegradable Materials Based on Plasticized PLA, Chitosan, and Rosemary Ethanolic Extract. Int. J. Polym. Sci. 2020, 2020, 4269792. [Google Scholar] [CrossRef]
- Sangeetha, V.H.; Deka, H.; Varghese, T.O.; Nayak, S.K. State of the Art And Future Prospectives Of Poly(Lactic Acid) Based Blends And Composites. Polym. Compos. 2018, 39, 81–101. [Google Scholar] [CrossRef]
- Madhavan Nampoothiri, K.; Nair, N.R.; John, R.P. An overview of the recent developments in polylactide (PLA) research. Bioresour. Technol. 2010, 101, 8493–8501. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, S.; Manna, S.; Roy, U.; Das, P. Manufacturing of biodegradable Poly Lactic Acid (PLA): Green alternatives to petroleum derived plastics. In Encyclopedia of Renewable and Sustainable Materials; Hashmi, S., Choudhury, I.A., Eds.; Elsevier: Oxford, UK, 2020; pp. 561–569. [Google Scholar]
- Sin, L.T.; Tueen, B.S. Polylactic Acid: A Practical Guide for the Processing, Manufacturing, and Applications of PLA; William Andrew: Oxford, UK, 2019. [Google Scholar]
- Raza, Z.A.; Aslam, M.; Azeem, A.; Maqsood, H.S. Development and characterization Of Nano-Crystalline Cellulose Incorporated Poly(Lactic Acid) Composite Films. Mater. Und Werkst. 2019, 50, 64–73. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, J.; Varshney, S.K. Polylactides—Chemistry, Properties and Green Packaging Technology: A Review. Int. J. Food Prop. 2011, 14, 37–58. [Google Scholar] [CrossRef]
- Pölöskei, K.; Csézi, G.; Hajba, S.; Tábi, T. Investigation of the thermoformability Of Various D-Lactide Content Poly(Lactic Acid) Films By Ball Burst Test. Polym. Eng. Sci. 2020, 60, 1266–1277. [Google Scholar] [CrossRef] [Green Version]
- Arrieta, M.P.; Fortunati, E.; Dominici, F.; Rayón, E.; López, J.; Kenny, J.M. Multifunctional PLA–PHB/Cellulose Nanocrystal Films: Processing, Structural And Thermal Properties. Carbohydr. Polym. 2014, 107, 16–24. [Google Scholar] [CrossRef]
- Indriyati; Frecilla, N.; Nuryadin, B.W.; Irmawati, Y.; Srikandace, Y. Enhanced Hydrophobicity and Elasticity of Bacterial Cellulose Films by Addition of Beeswax. Macromol. Symp. 2020, 391, 1900174. [Google Scholar] [CrossRef]
- Pirsa, S.; Sharifi, K.A. A Review Of The Applications Of Bioproteins In The Preparation Of Biodegradable Films And Polymers. J. Chem. Lett. 2020, 1, 47–58. [Google Scholar] [CrossRef]
- Akin, O.; Tihminlioglu, F. Effects of Organo-Modified Clay Addition and Temperature on the Water Vapor Barrier Properties of Polyhydroxy Butyrate Homo and Copolymer Nanocomposite Films for Packaging Applications. J. Polym. Environ. 2018, 26, 1121–1132. [Google Scholar] [CrossRef]
- Anukiruthika, T.; Sethupathy, P.; Wilson, A.; Kashampur, K.; Moses, J.A.; Anandharamakrishnan, C. Multilayer packaging: Advances in Preparation Techniques And Emerging Food Applications. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1156–1186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azeredo, H.M.C.; Barud, H.; Farinas, C.S.; Vasconcellos, V.M.; Claro, A.M. Bacterial Cellulose as a Raw Material for Food and Food Packaging Applications. Front. Sustain. Food Syst. 2019, 3, 7. [Google Scholar] [CrossRef] [Green Version]
- Desvita, H.; Faisal, M.; Mahidin; Suhendrayatna. Preservation of Meatballs With Edible Coating Of Chitosan Dissolved In Rice Hull-Based Liquid Smoke. Heliyon 2020, 6, e05228. [Google Scholar] [CrossRef]
- Abbasi, Z.; Aminzare, M.; Hassanzad Azar, H.; Rostamizadeh, K. Effect of Corn Starch Coating Incorporated With Nanoemulsion Of Zataria Multiflora Essential Oil Fortified With Cinnamaldehyde On Microbial Quality Of Fresh Chicken Meat And Fate Of Inoculated Listeria Monocytogenes. J. Food Sci. Technol. 2021, 58, 2677–2687. [Google Scholar] [CrossRef]
- Liu, F.; Chang, W.; Chen, M.; Xu, F.; Ma, J.; Zhong, F. Tailoring Physicochemical Properties of Chitosan Films and Their Protective Effects on Meat by Varying Drying Temperature. Carbohydr. Polym. 2019, 212, 150–159. [Google Scholar] [CrossRef]
- Huang, Y.a.; Zeng, X.; Zhu, Q.; Lu, K.; Xu, Q.; Ye, C. Development of an active Packaging With Molecularly Imprinted Polymers For Beef Preservation. Packag. Technol. Sci. 2018, 31, 213–220. [Google Scholar] [CrossRef]
- Khumkomgool, A.; Saneluksana, T.; Harnkarnsujarit, N. Active Meat Packaging From Thermoplastic Cassava Starch Containing Sappan And Cinnamon Herbal Extracts Via LLDPE Blown-Film Extrusion. Food Packag. Shelf Life 2020, 26, 100557. [Google Scholar] [CrossRef]
- Garavito, J.; Moncayo-Martínez, D.; Castellanos, D.A. Evaluation of Antimicrobial Coatings on Preservation and Shelf Life of Fresh Chicken Breast Fillets Under Cold Storage. Foods 2020, 9, 1203. [Google Scholar] [CrossRef]
- Faisal, M.; Gani, A.; Mulana, F. Preliminary Assessment Of The Utilization Of Durian Peel Liquid Smoke As A Natural Preservative For Mackerel. F1000Res 2019, 8, 240. [Google Scholar] [CrossRef]
- Negm, N.A.; Hefni, H.H.H.; Abd-Elaal, A.A.A.; Badr, E.A.; Abou Kana, M.T.H. Advancement on Modification Of Chitosan Biopolymer And Its Potential Applications. Int. J. Biol. Macromol. 2020, 152, 681–702. [Google Scholar] [CrossRef] [PubMed]
- Adilah, A.N.; Jamilah, B.; Noranizan, M.A.; Hanani, Z.A.N. Utilization of Mango Peel Extracts On The Biodegradable Films For Active Packaging. Food Packag. Shelf Life 2018, 16, 1–7. [Google Scholar] [CrossRef]
- Scartazzini, L.; Tosati, J.V.; Cortez, D.H.C.; Rossi, M.J.; Flôres, S.H.; Hubinger, M.D.; Di Luccio, M.; Monteiro, A.R. Gelatin Edible Coatings With Mint Essential Oil (Mentha Arvensis): Film Characterization And Antifungal Properties. J. Food Sci. Technol. 2019, 56, 4045–4056. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.C.; Zhang, H.; Ren, K.; Ying, Z.; Zhu, F.; Qian, J.; Ji, J.; Wu, Z.L.; Zheng, Q. Ultrathin κ-Carrageenan/Chitosan Hydrogel Films with High Toughness and Antiadhesion Property. ACS Appl. Mater. Interfaces 2018, 10, 9002–9009. [Google Scholar] [CrossRef] [PubMed]
- Dehnad, D.; Mirzaei, H.; Emam-Djomeh, Z.; Jafari, S.-M.; Dadashi, S. Thermal and Antimicrobial Properties Of Chitosan–Nanocellulose Films For Extending Shelf Life Of Ground Meat. Carbohydr. Polym. 2014, 109, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.R.; Sadiq, M.B.; Mehmood, Z. Development of Edible Gelatin Composite Films Enriched With Polyphenol Loaded Nanoemulsions As Chicken Meat Packaging Material. CyTA-J. Food 2020, 18, 137–146. [Google Scholar] [CrossRef] [Green Version]
- Sanadgol, N.; Wackerlig, J. Developments of Smart Drug-Delivery Systems Based on Magnetic Molecularly Imprinted Polymers for Targeted Cancer Therapy: A Short Review. Pharmaceutics 2020, 12, 831. [Google Scholar] [CrossRef]
- Ustunol, Z. Edible Films and Coatings for Meat and Poultry. In Edible Films and Coatings for Food Applications; Huber, K.C., Embuscado, M.E., Eds.; Springer: New York, NY, USA, 2009; pp. 245–268. [Google Scholar] [CrossRef]
- Zhao, Y.; Teixeira, J.S.; Saldaña, M.D.A.; Gänzle, M.G. Antimicrobial Activity Of Bioactive Starch Packaging Films Against Listeria Monocytogenes And Reconstituted Meat Microbiota On Ham. Int. J. Food Microbiol. 2019, 305, 108253. [Google Scholar] [CrossRef]
- Kumar, R.; Ghoshal, G.; Goyal, M. Synthesis and Functional Properties Of Gelatin/CA–Starch Composite Film: Excellent Food Packaging Material. J. Food Sci. Technol. 2019, 56, 1954–1965. [Google Scholar] [CrossRef]
- Panrong, T.; Karbowiak, T.; Harnkarnsujarit, N. Thermoplastic Starch And Green Tea Blends With LLDPE Films For Active Packaging Of Meat And Oil-Based Products. Food Packag. Shelf Life 2019, 21, 100331. [Google Scholar] [CrossRef]
- Marcos, B.; Gou, P.; Arnau, J.; Guàrdia, M.D.; Comaposada, J. Co-Extruded Alginate As An Alternative To Collagen Casings In The Production Of Dry-Fermented Sausages: Impact Of Coating Composition. Meat Sci. 2020, 169, 108184. [Google Scholar] [CrossRef] [PubMed]
- Moosavi, M.H.; Khani, M.R.; Shokri, B.; Hosseini, S.M.; Shojaee-Aliabadi, S.; Mirmoghtadaie, L. Modifications of Protein-Based Films Using Cold Plasma. Int. J. Biol. Macromol. 2020, 142, 769–777. [Google Scholar] [CrossRef] [PubMed]
- Bojorges, H.; Ríos-Corripio, M.A.; Hernández-Cázares, A.S.; Hidalgo-Contreras, J.V.; Contreras-Oliva, A. Effect of the application Of An Edible Film With Turmeric (Curcuma Longa L.) On The Oxidative Stability Of Meat. Food Sci. Nutr. 2020, 8, 4308–4319. [Google Scholar] [CrossRef]
- Abdallah, M.R.; Mohamed, M.A.; Mohamed, H.; Emara, M.T. Application of Alginate And Gelatin-Based Edible Coating Materials As Alternatives To Traditional Coating For Improving The Quality Of Pastirma. Food Sci. Biotechnol. 2018, 27, 1589–1597. [Google Scholar] [CrossRef]
- Alexandre, S.; Vital, A.C.P.; Mottin, C.; do Prado, R.M.; Ornaghi, M.G.; Ramos, T.R.; Guerrero, A.; Pilau, E.J.; do Prado, I.N. Use of Alginate Edible Coating And Basil (Ocimum Spp) Extracts On Beef Characteristics During Storage. J. Food Sci. Technol. 2021, 58, 3835–3843. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, G.P.; Dutra, M.P.; Fontes, P.R.; Ramos, A.d.L.S.; Gomide, L.A.d.M.; Ramos, E.M. Selection of a Chitosan Gelatin-Based Edible Coating for Color Preservation of Beef in Retail Display. Meat Sci. 2016, 114, 85–94. [Google Scholar] [CrossRef]
- Ruan, C.; Zhang, Y.; Sun, Y.; Gao, X.; Xiong, G.; Liang, J. Effect of Sodium Alginate And Carboxymethyl Cellulose Edible Coating With Epigallocatechin Gallate On Quality And Shelf Life Of Fresh Pork. Int. J. Biol. Macromol. 2019, 141, 178–184. [Google Scholar] [CrossRef]
- Noori, S.; Zeynali, F.; Almasi, H. Antimicrobial and antioxidant Efficiency of Nanoemulsion-Based Edible Coating Containing Ginger (Zingiber officinale) Essential Oil and Its Effect on Safety and Quality Attributes of Chicken Breast Fillets. Food Control 2018, 84, 312–320. [Google Scholar] [CrossRef]
- Meindrawan, B.; Putri, S.; Susanto, C.S.; Ofe, O.; Mangindaan, D.; Ayman, A.; Kasih, T.P. Bionanocomposite of Gelatin–ZnO Nanoparticles as Potential Edible Coating for Broiler Chicken Fillet. Macromol. Symp. 2020, 391, 1900165. [Google Scholar] [CrossRef]
- Alizadeh Behbahani, B.; Falah, F.; Vasiee, A.; Tabatabaee Yazdi, F. Control of Microbial Growth And Lipid Oxidation In Beef Using A Lepidium Perfoliatum Seed Mucilage Edible Coating Incorporated With Chicory Essential Oil. Food Sci. Nutr. 2021, 9, 2458–2467. [Google Scholar] [CrossRef]
- Noshad, M.; Alizadeh Behbahani, B.; Jooyandeh, H.; Rahmati-Joneidabad, M.; Hemmati Kaykha, M.E.; Ghodsi Sheikhjan, M. Utilization of Plantago Major Seed Mucilage Containing Citrus Limon Essential Oil as an Edible Coating to Improve Shelf-Life of Buffalo Meat under Refrigeration Conditions. Food Sci. Nutr. 2021, 9, 1625–1639. [Google Scholar] [CrossRef] [PubMed]
- Matiacevich, S.; Acevedo, N.; López, D. Characterization of Edible Active Coating Based on Alginate–Thyme Oil–Propionic Acid for the Preservation of Fresh Chicken Breast Fillets. J. Food Process. Preserv. 2015, 39, 2792–2801. [Google Scholar] [CrossRef]
- Guerrero, A.; Ferrero, S.; Barahona, M.; Boito, B.; Lisbinski, E.; Maggi, F.; Sañudo, C. Effects of Active Edible Coating Based On Thyme And Garlic Essential Oils On Lamb Meat Shelf Life After Long-Term Frozen Storage. J. Sci. Food Agric. 2020, 100, 656–664. [Google Scholar] [CrossRef] [PubMed]
- Soni, A.; Gurunathan, K.; Mendiratta, S.K.; Talukder, S.; Jaiswal, R.K.; Sharma, H. Effect of Essential Oils Incorporated Edible Film On Quality And Storage Stability Of Chicken Patties At Refrigeration Temperature (4 ± 1 °C). J. Food Sci. Technol. 2018, 55, 3538–3546. [Google Scholar] [CrossRef] [PubMed]
- Šuput, D.; Lazić, V.; Pezo, L.; Gubić, J.; Šojić, B.; Plavšić, D.; Lončar, B.; Nićetin, M.; Filipović, V.; Knežević, V. Shelf Life And Quality Of Dehydrated Meat Packed In Edible Coating Under Modified Atmosphere. Rom. Biotechnol. Lett. 2019, 24, 545–553. [Google Scholar] [CrossRef]
- Marchiore, N.G.; Manso, I.J.; Kaufmann, K.C.; Lemes, G.F.; Pizolli, A.P.d.O.; Droval, A.A.; Bracht, L.; Gonçalves, O.H.; Leimann, F.V. Migration Evaluation Of Silver Nanoparticles From Antimicrobial Edible Coating To Sausages. LWT-Food Sci. Technol. 2017, 76, 203–208. [Google Scholar] [CrossRef]
- Chawla, R.; Sivakumar, S.; Kaur, H. Antimicrobial Edible Films In Food Packaging: Current Scenario And Recent Nanotechnological Advancements—A Review. Carbohydr. Polym. Technol. Appl. 2021, 2, 100024. [Google Scholar] [CrossRef]
- Pauer, E.; Tacker, M.; Gabriel, V.; Krauter, V. Sustainability of Flexible Multilayer Packaging: Environmental Impacts And Recyclability Of Packaging For Bacon In Block. Clean. Environ. Syst. 2020, 1, 100001. [Google Scholar] [CrossRef]
- Kanatt, S.R. Development of Active/Intelligent Food Packaging Film Containing Amaranthus Leaf Extract For Shelf Life Extension Of Chicken/Fish During Chilled Storage. Food Packag. Shelf Life 2020, 24, 100506. [Google Scholar] [CrossRef]
- Alizadeh-Sani, M.; Tavassoli, M.; Mohammadian, E.; Ehsani, A.; Khaniki, G.J.; Priyadarshi, R.; Rhim, J.-W. pH-Responsive Color Indicator Films Based On Methylcellulose/Chitosan Nanofiber And Barberry Anthocyanins For Real-Time Monitoring Of Meat Freshness. Int. J. Biol. Macromol. 2021, 166, 741–750. [Google Scholar] [CrossRef]
- Heydari, S.; Jooyandeh, H.; Alizadeh Behbahani, B.; Noshad, M. The Impact Of Qodume Shirazi Seed Mucilage-Based Edible Coating Containing Lavender Essential Oil On The Quality Enhancement And Shelf Life Improvement Of Fresh Ostrich Meat: An Experimental And Modeling Study. Food Sci. Nutr. 2020, 8, 6497–6512. [Google Scholar] [CrossRef] [PubMed]
- Comaposada, J.; Marcos, B.; Bou, R.; Gou, P. Influence of Surfactants And Proteins On The Properties Of Wet Edible Calcium Alginate Meat Coatings. Food Res. Int. 2018, 108, 539–550. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Song, Z.; Zhi, X.; Du, B. Photoinduced Antimicrobial Activity of Curcumin-Containing Coatings: Molecular Interaction, Stability and Potential Application in Food Decontamination. ACS Omega 2020, 5, 31044–31054. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, T.; Nakano, K.; Katiyar, V. Curcumin doped Functionalized Cellulose Nanofibers Based Edible Chitosan Coating On Kiwifruits. Int. J. Biol. Macromol. 2021, 184, 936–945. [Google Scholar] [CrossRef]
- Nieto-Suaza, L.; Acevedo-Guevara, L.; Sánchez, L.T.; Pinzón, M.I.; Villa, C.C. Characterization of Aloe Vera-Banana Starch Composite Films Reinforced With Curcumin-Loaded Starch Nanoparticles. Food Struct. 2019, 22, 100131. [Google Scholar] [CrossRef]
- Leon-Bejarano, M.; Durmus, Y.; Ovando-Martínez, M.; Simsek, S. Physical, Barrier, Mechanical, and Biodegradability Properties of Modified Starch Films with Nut By-Products Extracts. Foods 2020, 9, 226. [Google Scholar] [CrossRef] [Green Version]
- Gagaoua, M.; Terlouw, E.M.C.; Picard, B. The Study Of Protein Biomarkers To Understand The Biochemical Processes Underlying Beef Color Development In Young Bulls. Meat Sci. 2017, 134, 18–27. [Google Scholar] [CrossRef]
- Gagaoua, M.; Picard, B.; Monteils, V. Associations Among Animal, Carcass, Muscle Characteristics, And Fresh Meat Color Traits In Charolais Cattle. Meat Sci. 2018, 140, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Gagaoua, M.; Hughes, J.; Terlouw, E.M.C.; Warner, R.D.; Purslow, P.P.; Lorenzo, J.M.; Picard, B. Proteomic Biomarkers Of Beef Colour. Trends Food Sci. Technol. 2020, 101, 234–252. [Google Scholar] [CrossRef]
- Hastaoğlu, E.; Vural, H. New Approaches to Production of Turkish-type Dry-cured Meat Product. Korean J. Food Sci. Anim. Resour. 2018, 38, 224–239. [Google Scholar]
- Domínguez, R.; Pateiro, M.; Gagaoua, M.; Barba, F.J.; Zhang, W.; Lorenzo, J.M. A Comprehensive Review on Lipid Oxidation in Meat and Meat Products. Antioxidants 2019, 8, 429. [Google Scholar] [CrossRef] [Green Version]
- Jridi, M.; Mora, L.; Souissi, N.; Aristoy, M.-C.; Nasri, M.; Toldrá, F. Effects of Active Gelatin Coated With Henna (L. Inermis) Extract On Beef Meat Quality During Chilled Storage. Food Control 2018, 84, 238–245. [Google Scholar] [CrossRef]
- Wrona, M.; Nerín, C.; Alfonso, M.J.; Caballero, M.Á. Antioxidant Packaging With Encapsulated Green Tea For Fresh Minced Meat. Innov. Food Sci. Emerg. Technol. 2017, 41, 307–313. [Google Scholar] [CrossRef] [Green Version]
- Pateiro, M.; Domínguez, R.; Bermúdez, R.; Munekata, P.E.S.; Zhang, W.; Gagaoua, M.; Lorenzo, J.M. Antioxidant Active Packaging Systems To Extend The Shelf Life Of Sliced Cooked Ham. Curr. Res. Food Sci. 2019, 1, 24–30. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Batlle, R.; Gómez, M. Extension of the Shelf-Life Of Foal Meat With Two Antioxidant Active Packaging Systems. LWT-Food Sci. Technol. 2014, 59, 181–188. [Google Scholar] [CrossRef]
- Domínguez, R.; Barba, F.J.; Gómez, B.; Putnik, P.; Bursać Kovačević, D.; Pateiro, M.; Santos, E.M.; Lorenzo, J.M. Active packaging Films With Natural Antioxidants To Be Used In Meat Industry: A Review. Food Res. Int. 2018, 113, 93–101. [Google Scholar] [CrossRef]
- Gallego, M.; Arnal, M.; Talens, P.; Toldrá, F.; Mora, L. Effect of Gelatin Coating Enriched With Antioxidant Tomato By-Products On The Quality Of Pork Meat. Polymers 2020, 12, 1032. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Hernández, E.; Lira-Moreno, C.Y.; Guerrero-Legarreta, I.; Wild-Padua, G.; Di Pierro, P.; García-Almendárez, B.E.; Regalado-González, C. Effect of Nanoemulsified and Microencapsulated Mexican Oregano (Lippia graveolens Kunth) Essential Oil Coatings on Quality of Fresh Pork Meat. J. Food Sci. 2017, 82, 1423–1432. [Google Scholar] [CrossRef]
- Naseri, H.R.; Beigmohammadi, F.; Mohammadi, R.; Sadeghi, E. Production and Characterization Of Edible Film Based On Gelatin–Chitosan Containing Ferulago Angulate Essential Oil And Its Application In The Prolongation Of The Shelf Life Of Turkey Meat. J. Food Process. Preserv. 2020, 44, e14558. [Google Scholar] [CrossRef]
- Amjadi, S.; Nazari, M.; Alizadeh, S.A.; Hamishehkar, H. Multifunctional Betanin Nanoliposomes-Incorporated Gelatin/Chitosan Nanofiber/Zno Nanoparticles Nanocomposite Film For Fresh Beef Preservation. Meat Sci. 2020, 167, 108161. [Google Scholar] [CrossRef]
- Rakshit, M.; Ramalingam, C. Gum Acacia Coating with Garlic and Cinnamon as an Alternate, Natural Preservative for Meat and Fish. Afr. J. Biotechnol. 2013, 12, 406–413. [Google Scholar]
- Raeisi, M.; Tabaraei, A.; Hashemi, M.; Behnampour, N. Effect of Sodium Alginate Coating Incorporated with Nisin, Cinnamomum Zeylanicum, and Rosemary Essential Oils on Microbial Quality of Chicken Meat and Fate of Listeria Monocytogenes during Refrigeration. Int. J. Food Microbiol. 2016, 238, 139–145. [Google Scholar] [CrossRef]
- Pattanayaiying, R.; H-Kittikun, A.; Cutter, C.N. Incorporation of Nisin Z and Lauric Arginate into Pullulan Films to Inhibit Foodborne Pathogens Associated with Fresh and Ready-to-Eat Muscle Foods. Int. J. Food Microbiol. 2015, 207, 77–82. [Google Scholar] [CrossRef]
- Da Costa, R.J.; Voloski, F.L.S.; Mondadori, R.G.; Duval, E.H.; Fiorentini, Â.M. Preservation of Meat Products with Bacteriocins Produced by Lactic Acid Bacteria Isolated from Meat. J. Food Qual. 2019, 2019, 4726510. [Google Scholar] [CrossRef] [Green Version]
- Shukla, V.; Mendiratta, S.K.; Zende, R.J.; Agrawal, R.K.; Kumar Jaiswal, R. Effects of Chitosan Coating Enriched with Syzygium Aromaticum Essential Oil on Quality and Shelf-Life of Chicken Patties. J. Food Process. Preserv. 2020, 44, e14870. [Google Scholar] [CrossRef]
- Alessandroni, L.; Caprioli, G.; Faiella, F.; Fiorini, D.; Galli, R.; Huang, X.; Marinelli, G.; Nzekoue, F.; Ricciutelli, M.; Scortichini, S.; et al. A Shelf-Life Study for the Evaluation of a New Biopackaging to Preserve the Quality of Organic Chicken Meat. Food Chem. 2021, 371, 131134. [Google Scholar] [CrossRef] [PubMed]
- Bharti, S.K.; Pathak, V.; Alam, T.; Arya, A.; Singh, V.K.; Verma, A.K.; Rajkumar, V. Materialization of Novel Composite Bio-based Active Edible Film Functionalized with Essential Oils on Antimicrobial and Antioxidative Aspect of Chicken Nuggets during Extended Storage. J. Food Sci. 2020, 85, 2857–2865. [Google Scholar] [CrossRef]
- Ben Braïek, O.; Smaoui, S. Chemistry, Safety, and Challenges of the Use of Organic Acids and Their Derivative Salts in Meat Preservation. J. Food Qual. 2021, 2021, 6653190. [Google Scholar] [CrossRef]
- Salmieri, S.; Islam, F.; Khan, R.A.; Hossain, F.M.; Ibrahim, H.M.M.; Miao, C.; Hamad, W.Y.; Lacroix, M. Antimicrobial Nanocomposite Films made of Poly(Lactic Acid)–Cellulose Nanocrystals (PLA–CNC) in Food Applications—Part B: Effect of Oregano Essential Oil Release on the Inactivation of Listeria Monocytogenes in Mixed Vegetables. Cellulose 2014, 21, 4271–4285. [Google Scholar] [CrossRef]
- Wu, Y.-M.; Wang, Z.-W.; Hu, C.-Y.; Nerín, C. Influence of Factors on Release of Antimicrobials from Antimicrobial Packaging Materials. Crit. Rev. Food Sci. Nutr. 2018, 58, 1108–1121. [Google Scholar] [CrossRef]
- Ahmed, I.; Lin, H.; Zou, L.; Brody, A.L.; Li, Z.; Qazi, I.M.; Pavase, T.R.; Lv, L. A Comprehensive Review on the Application of Active Packaging Technologies to Muscle Foods. Food Control 2017, 82, 163–178. [Google Scholar] [CrossRef]
- Loucanová, E.; Nosál’ová, M.; Olšiaková, M. The Development of the Innovation Status and Impact of Smart Packaging on Slovak Consumers. Acta Logist. 2019, 6, 115–122. [Google Scholar] [CrossRef]
- Ahmed, I.; Lin, H.; Zou, L.; Li, Z.; Brody, A.L.; Qazi, I.M.; Lv, L.; Pavase, T.R.; Khan, M.U.; Khan, S.; et al. An overview of Smart Packaging Technologies for Monitoring Safety and Quality of Meat and Meat Products. Packag. Technol. Sci. 2018, 31, 449–471. [Google Scholar] [CrossRef]
- Nguyen, L.H.; Naficy, S.; McConchie, R.; Dehghani, F.; Chandrawati, R. Polydiacetylene-Based Sensors to Detect Food Spoilage at Low Temperatures. J. Mater. Chem. C 2019, 7, 1919–1926. [Google Scholar] [CrossRef]
- Park, S.; Jeon, Y.; Han, T.; Kim, S.; Gwon, Y.; Kim, J. Nanoscale Manufacturing as an Enabling Strategy for the Design of Smart Food Packaging Systems. Food Packag. Shelf Life 2020, 26, 100570. [Google Scholar] [CrossRef]
- Eom, K.-H.; Hyun, K.-H.; Lin, S.; Kim, J.-W. The Meat Freshness Monitoring System Using the Smart RFID Tag. Int. J. Distrib. Sens. Netw. 2014, 10, 591812. [Google Scholar] [CrossRef] [Green Version]
- Kolbeck, S.; Hilgarth, M.; Vogel, R.F. Proof of concept: Predicting the Onset of Meat Spoilage by an Integrated Oxygen Sensor Spot in MAP Packages. Lett. Appl. Microbiol. 2021, 73, 39–45. [Google Scholar] [CrossRef]
- Lee, K.; Baek, S.; Kim, D.; Seo, J. A Freshness Indicator for Monitoring Chicken-Breast Spoilage using a Tyvek® Sheet and RGB Color Analysis. Food Packag. Shelf Life 2019, 19, 40–46. [Google Scholar] [CrossRef]
- Chayavanich, K.; Thiraphibundet, P.; Imyim, A. Biocompatible Film Sensors Containing Red Radish Extract for Meat Spoilage Observation. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 226, 117601. [Google Scholar] [CrossRef]
- Senturk Parreidt, T.; Müller, K.; Schmid, M. Alginate-Based Edible Films and Coatings for Food Packaging Applications. Foods 2018, 7, 170. [Google Scholar] [CrossRef] [Green Version]
- Mustafa, F.; Andreescu, S. Nanotechnology-Based Approaches for Food Sensing and Packaging Applications. RSC Adv. 2020, 10, 19309–19336. [Google Scholar] [CrossRef]
- Ahmed, M.A.; Al-Kahtani, H.A.; Jaswir, I.; AbuTarboush, H.; Ismail, E.A. Extraction and Characterization of Gelatin from Camel Skin (Potential Halal Gelatin) and Production of Gelatin Nanoparticles. Saudi J. Biol. Sci. 2020, 27, 1596–1601. [Google Scholar] [CrossRef] [PubMed]
- Benassi, L.; Alessandri, I.; Vassalini, I. Assessing Green Methods for Pectin Extraction from Waste Orange Peels. Molecules 2021, 26, 1766. [Google Scholar] [CrossRef] [PubMed]
- Musatti, A.; Cavicchioli, D.; Mapelli, C.; Bertoni, D.; Hogenboom, J.A.; Pellegrino, L.; Rollini, M. From Cheese Whey Permeate to Sakacin A: A Circular Economy Approach for the Food-Grade Biotechnological Production of an Anti-Listeria Bacteriocin. Biomolecules 2020, 10, 597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
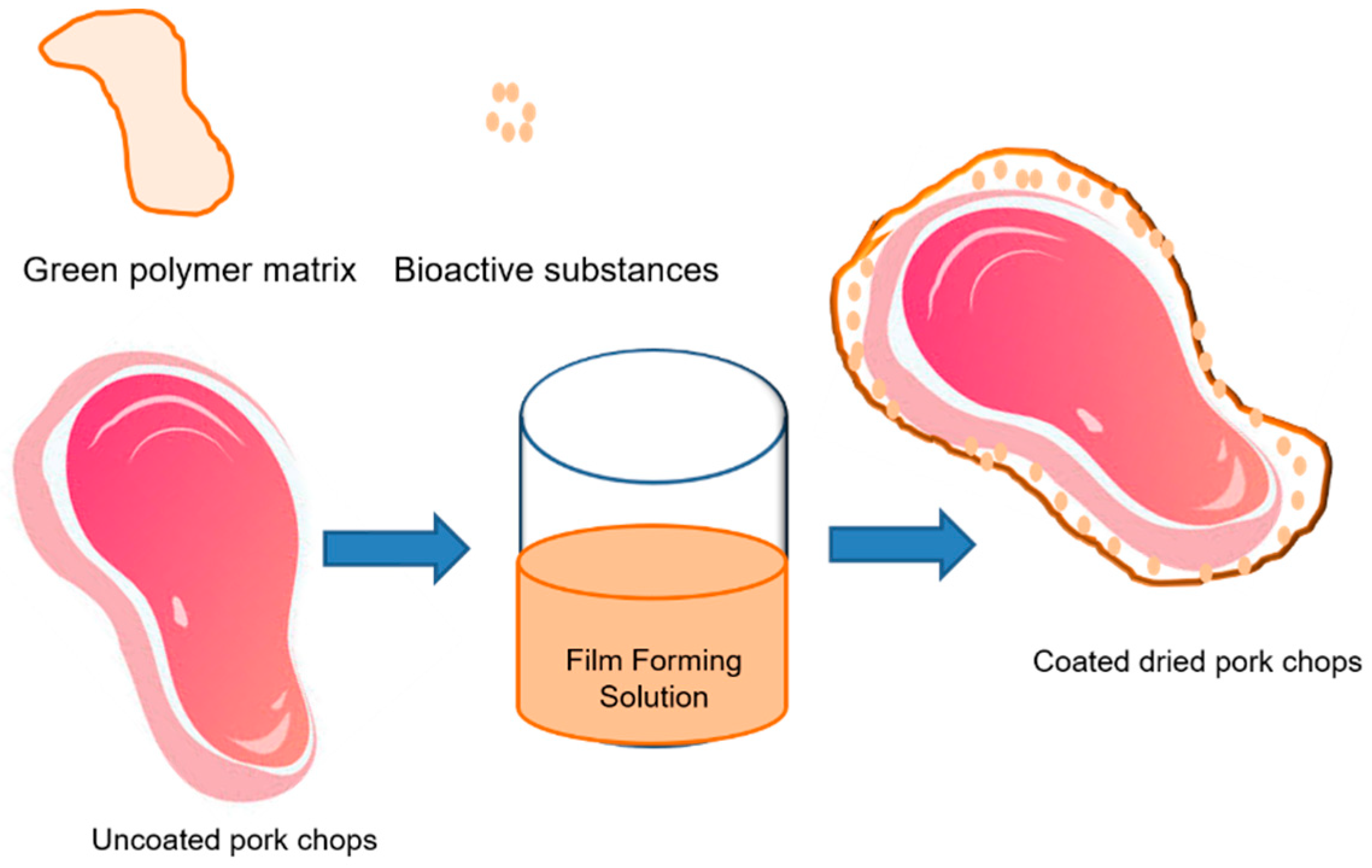
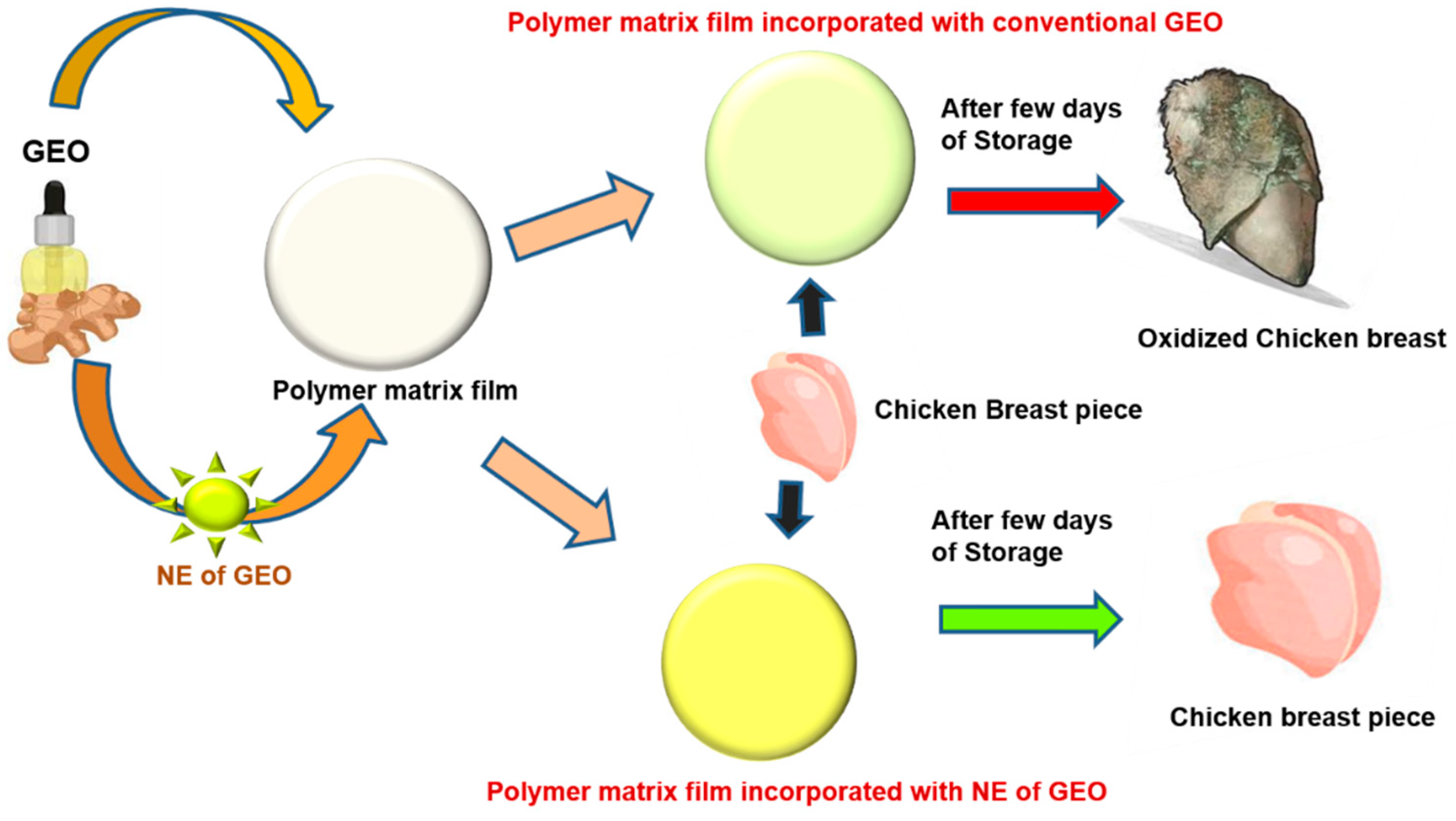
| Types of Films | Additive | Technique | Meat Types | References |
|---|---|---|---|---|
| Chitosan | Green leaf extract | Homogenization followed by dipping | Pork chops | [77] |
| Chitosan | Liquid smoke | Magnetic stirring followed by dipping | Beef | [113] |
| Corn starch | Zataria multiflora root extract essential oil | Nanoemulsion followed by dipping | Chicken breast | [114] |
| Chitosan | N/A | Solution casting | Chilled meat | [115] |
| LLDPE-coated chitosan gel matrix | Composite of allyl isothiocyante β cyclodextrin | Deposition coating layer on plastic films | Beef | [116] |
| LLDPE and cassava composite | Sappan and cinnamon extract | Blow extrusion | Beef | [117] |
| Green Polymers | Additive | Overall Effect/Role | Meat Types | References |
|---|---|---|---|---|
| Alginate | Curcumin | Improvement in mechanical, barrier, antioxidant properties | Pork | [133] |
| Thermoplastic starch (TPS) | Sappan and cinnamon | Controlled oxygen permeability, inhibition of the formation of metmyoglobin due to lipid oxidation, antimicrobial effect | Beef | [117] |
| TPS, acetylated starch (AS), native starch (NS) | Green tea extract | Promotion of the formation of oxymyoglobin, retardation of lipid oxidation and free radical formation, reduction of the total viable count | Bacon | [130] |
| Gelatin, alginate | N/A | Color preservation | Pastirma | [134] |
| Alginate | Basil extract | Antioxidant | Beef sausage | [135] |
| Gelatin | Chitosan | Antioxidant, color preservation | Beef | [136] |
| Carboxymethyl cellulose | Epigallocatechin | Antioxidant | Pork | [137] |
| Sodium caseinate | Ginger extract essential oil nanocapsules | Antioxidant, color preservation | Chicken breast fillets | [138] |
| Gelatin | Zinc nanoparticles | Antimicrobial | Chicken fillets | [139] |
| Lepidium perfoliatum seed mucilage | Chicory extract oil | Antimicrobial | Beef slices | [140] |
| Plantago major seed mucilage | Citrus lemon seed oil | Antimicrobial | Buffalo meat | [141] |
| Alginate | Propionic acid, thyme oil | Antimicrobial | Chicken | [142] |
| Alginate | Thyme and garlic oil | Antimicrobial | Lamb | [143] |
| Carageenan | Oregano oil | Antimicrobial | Chicken patty | [144] |
| Maize starch guar gum and xanthan composite | Oregano oil | Enhanced vitamin and mineral content, antimicrobial | Osmotically treated pork slices | [145] |
| Starch and D-glucose | AgNP | Antimicrobial | Chicken sausage | [146] |
| Chitosan, polyethelene oxide | Pomegranate peel extract | Antimicrobial | Meat | [147] |
| Chitosan | Nanocellulose | Antimicrobial | Ground meat | [124] |
| Turmeric starch | Gelatin | Antimicrobial | Frankfurter sausages | [38] |
| Polylactic acid | Nisin | Antimicrobial | Fresh beef | [52] |
| Bacterial cellulose | Zinc nanocomposite | Antimicrobial | Chicken breast | [58] |
| Bacterio nanocellulose | Sakacin | Antimicrobial | Beef patties | [59] |
| Pectin fish gelatin blend | Olive oil | Significant reduction of lipid oxidation and increased meat preservation | Stored beef | [11] |
| Pectin | Oregano oil and resveratrol | Antioxidant and antimicrobial | Pork loin | [62] |
| Alginate | Pineapple peel bioactive hydralcoholic acid | Antimicrobial | Beef steaks | [67] |
| Chitosan | Bamboo vinegar | Antioxidant | Pork chops | [78] |
| Chitosan | Thyme oil | Antimicrobial | Ready-to-eat pork | [79] |
| Pullulan | Nisin, thymol, lauric arginate | Antimicrobial | Raw beef, turkey, chicken | [86] |
| Yuba | Oregano essential oil, grapefruit seed extract, and their mixture | Antimicrobial | Beef patties | [91] |
| Whey protein | Phytochemicals from Laurus nobilis L. and Salvia officinalis | Antioxidant | Cooked meatballs | [93] |
| Acetylated starch | Green tea extract | Color protective, antioxidant | Bacon | [148] |
| Soya protein isolate/guar gum blend | Nisin | Antimicrobial | Chicken breast fillets | [118] |
| Gelatin/polyvinyl alcohol composites | Amaranthus leaf extract | Color protective, antioxidant | Chicken | [149] |
| Methylcellulose/chitosan nanofibers | Barberry anthocyanin | Freshness indicator | Meat | [150] |
| Qodume Shirazi seed mucilage | Lavender essential oil | Antimicrobial | Ostrich meat | [151] |
| Gelatin | Curcumin nanoemulsions | Antimicrobial | Chicken | [125] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gagaoua, M.; Bhattacharya, T.; Lamri, M.; Oz, F.; Dib, A.L.; Oz, E.; Uysal-Unalan, I.; Tomasevic, I. Green Coating Polymers in Meat Preservation. Coatings 2021, 11, 1379. https://doi.org/10.3390/coatings11111379
Gagaoua M, Bhattacharya T, Lamri M, Oz F, Dib AL, Oz E, Uysal-Unalan I, Tomasevic I. Green Coating Polymers in Meat Preservation. Coatings. 2021; 11(11):1379. https://doi.org/10.3390/coatings11111379
Chicago/Turabian StyleGagaoua, Mohammed, Tanima Bhattacharya, Melisa Lamri, Fatih Oz, Amira Leila Dib, Emel Oz, Ilke Uysal-Unalan, and Igor Tomasevic. 2021. "Green Coating Polymers in Meat Preservation" Coatings 11, no. 11: 1379. https://doi.org/10.3390/coatings11111379
APA StyleGagaoua, M., Bhattacharya, T., Lamri, M., Oz, F., Dib, A. L., Oz, E., Uysal-Unalan, I., & Tomasevic, I. (2021). Green Coating Polymers in Meat Preservation. Coatings, 11(11), 1379. https://doi.org/10.3390/coatings11111379









