Properties of Spark Plasma Sintered Compacts and Magnetron Sputtered Coatings Made from Cr, Mo, Re and Zr Alloyed Tungsten Diboride
Abstract
:1. Introduction
2. Material and Methods
2.1. Spark Plasma Sintering
2.2. Radio Frequency Magnetron Sputtering (RF-MS)
2.3. Characterization
3. Results and Discussion
3.1. Targets
3.1.1. Density
3.1.2. Microstructure
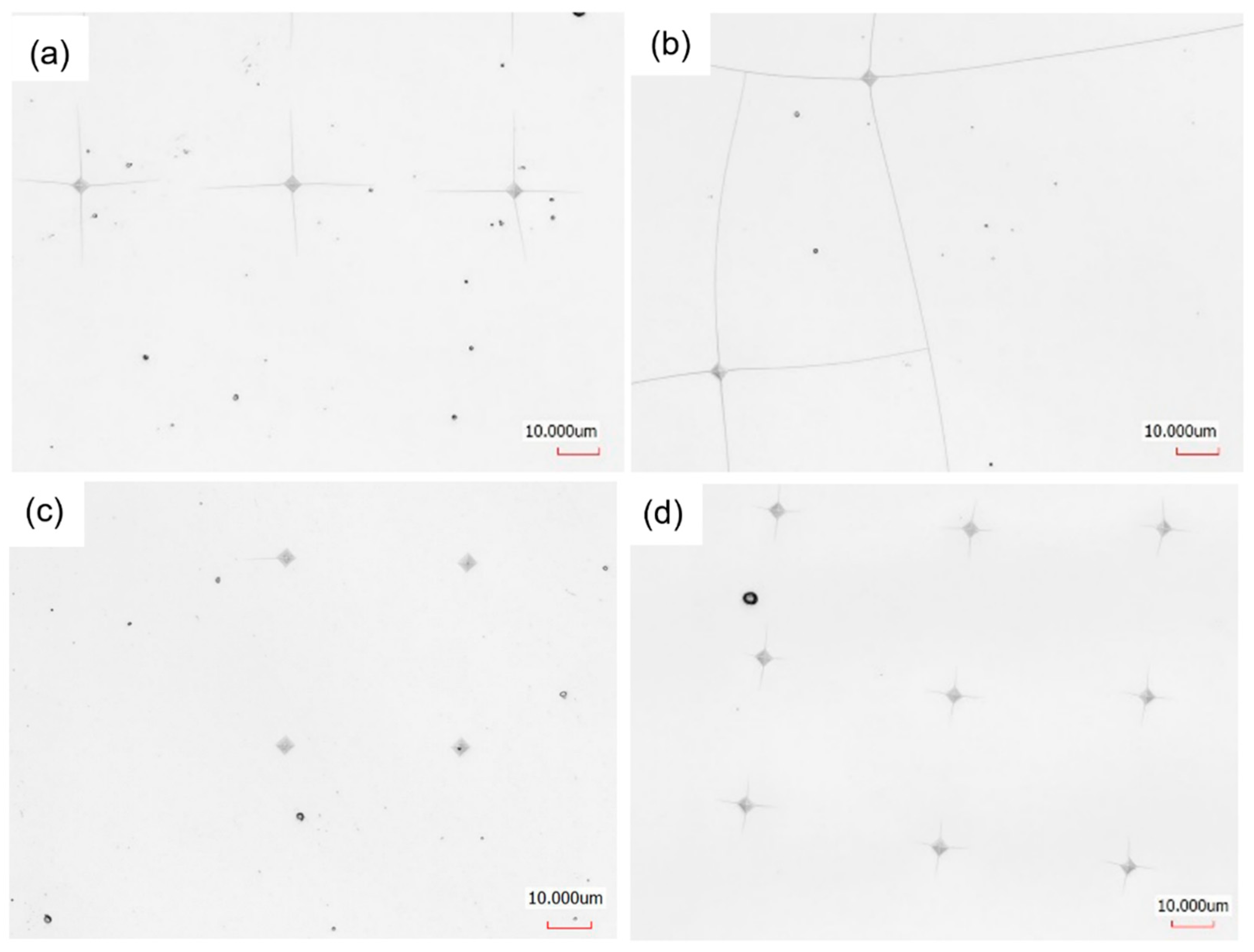
3.1.3. Hardness
3.1.4. Electrical Conductivity
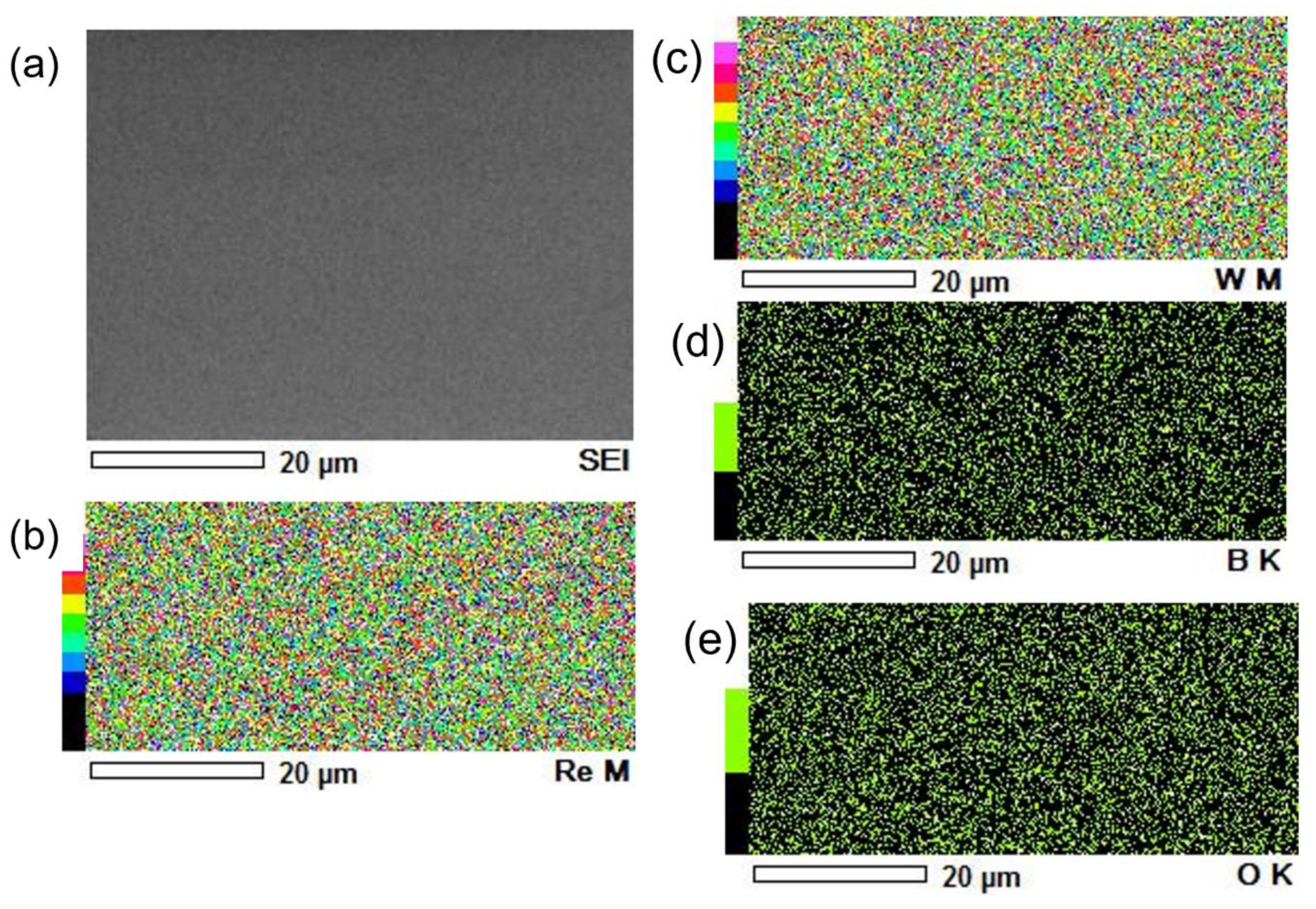
3.2. Coatings
4. Conclusions
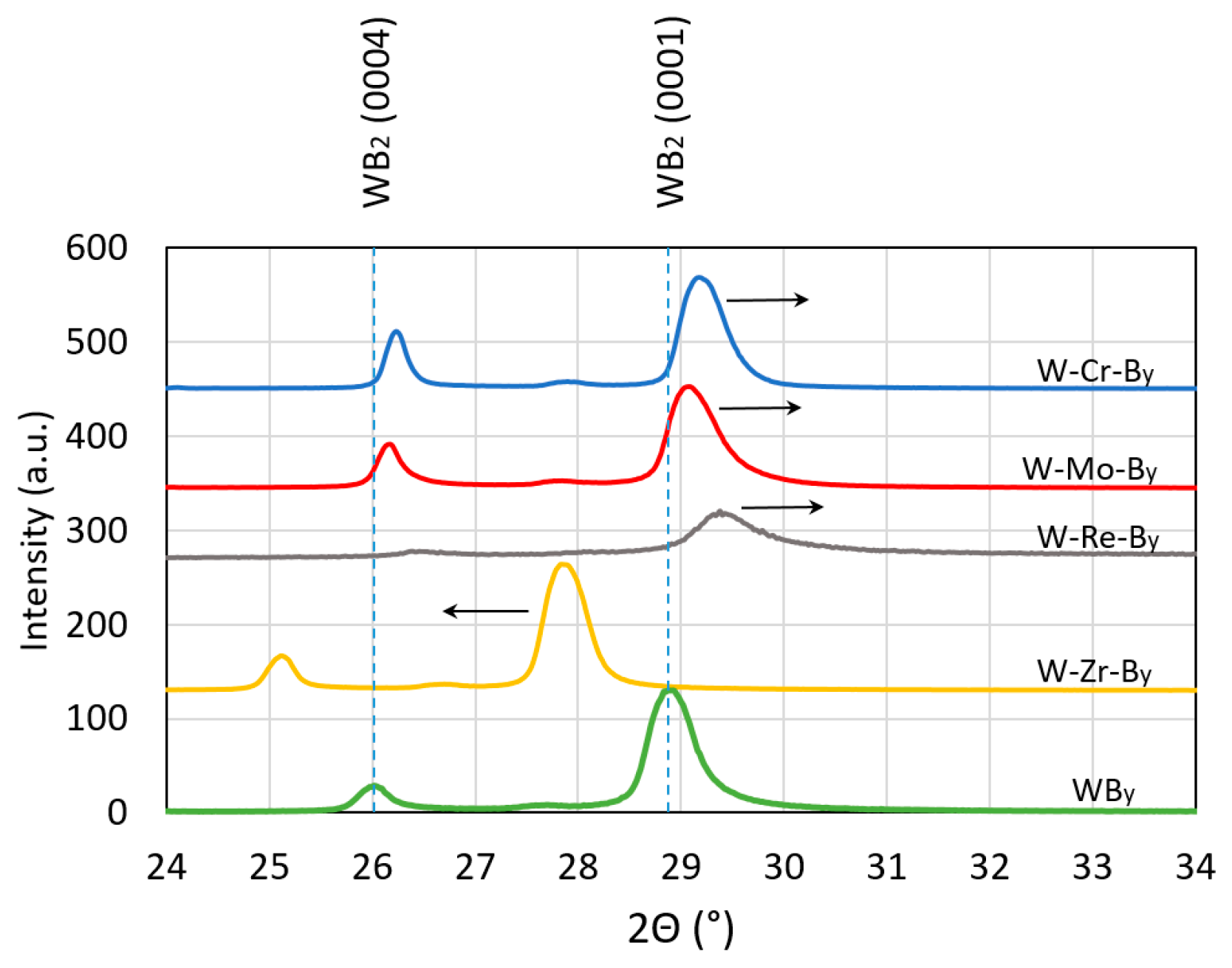
- X-ray diffraction results of SPS-ed compacts indicate that the solubility limit is below 8 at.% for molybdenum, rhenium and zirconium and below 16 at.% for chromium. Above this limit, both diborides (W,TM)B2 are created.
- The addition of transition metal caused decrease of density and increase of hardness and electrical conductivity of sintered compacts.
- Deposited coatings W1−xTMxBy (TM = Cr, Mo, Re, Zr; x = ~0.2; y = 1.7–2.0) are homogenous, smooth and hard. The maximal hardness was measured for W-Cr-B2 coatings and under the load 10 g was 50.4 ± 4.7 GPa. Deposited films possess relatively high fracture toughness and for WB2 coatings alloyed with zirconium it was K1c = 2.11 MPa m1/2. The connection of very high hardness with high fracture toughness places these coatings in a group of novel materials with improved mechanical properties.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gu, X.; Liu, C.; Guo, H.; Zhang, K.; Chen, C. Sorting transition-metal diborides: New descriptor for mechanical properties. Acta Mater. 2021, 207, 116685. [Google Scholar] [CrossRef]
- Ivanovskii, A.L. The search for novel superhard and incompressible materials on the basis of higher borides of s, p, d metals. J. Superhard Mater. 2011, 33, 73–87. [Google Scholar] [CrossRef]
- Yeung, M.T.; Mohammadi, R.; Kaner, R.B. Ultraincompressible, superhard materials. Annu. Rev. Mater. Res. 2016, 46, 465–485. [Google Scholar] [CrossRef]
- Wuchina, E.; Opila, E.; Opeka, M.; Fahrenholtz, B.; Talmy, I. UHTCs: Ultra-high temperature ceramic materials for extreme environment applications. Electrochem. Soc. Interface 2007, 16, 30–36. [Google Scholar] [CrossRef]
- Moraes, V.; Riedl, H.; Fuger, C.; Polcik, P.; Bolvardi, H.; Holec, D.; Mayrhofer, P.H. Ab initio inspired design of ternary boride thin films. Sci. Rep. 2018, 8, 9288. [Google Scholar] [CrossRef] [PubMed]
- Moscicki, T.; Psiuk, R.; Słomińska, H.; Levintant-Zayonts, N.; Garbiec, D.; Pisarek, M.; Bazarnik, P.; Nosewicz, S.; Chrzanowska-Giżyńska, J. Influence of overstoichiometric boron and titanium addition on the properties of RF magnetron sputtered tungsten borides. Surf. Coat. Technol. 2020, 390, 125689. [Google Scholar] [CrossRef]
- Wicher, B.; Chodun, R.; Trzciński, M.; Lachowski, A.; Kubiś, M.; Nowakowska-Langier, K.; Zdunek, K. Design of pulsed neon injection in the synthesis of W-B-C films using magnetron sputtering from a surface-sintered single powder cathode. Thin Solid Film. 2020, 716, 138426. [Google Scholar] [CrossRef]
- Radziejewska, J.; Psiuk, R.; Mościcki, T. Characterization and wear response of magnetron sputtered W–B and W–Ti–B coatings on WC–Co tools. Coatings 2020, 10, 1231. [Google Scholar] [CrossRef]
- Garbiec, D.; Wiśniewska, M.; Psiuk, R.; Denis, P.; Levintant-Zayonts, N.; Leshchynsky, V.; Rubach, R.; Mościcki, T. Zirconium alloyed tungsten borides synthesized by spark plasma sintering. Arch. Civ. Mech. Eng. 2021, 21, 37. [Google Scholar] [CrossRef]
- Shaw, A.H. Physical Properties of Various Conductive Diborides and Their Binaries. Graduate Theses and Dissertations, Iowa State University, USA, 2015; p. 14496. Available online: https://lib.dr.iastate.edu/etd/14496 (accessed on 4 November 2021).
- Fuger, C.; Moraes, V.; Hahn, R.; Bolvardi, H.; Polcik, P.; Riedl, H.; Mayrhofer, P.H. Influence of Tantalum on phase stability and mechanical properties of WB2. MRS Commun. 2019, 9, 375–380. [Google Scholar] [CrossRef]
- Maździarz, M.; Mościcki, T. Structural, mechanical, optical, thermodynamical and phonon properties of stable ReB2 polymorphs from density functional calculations. J. Alloys Compd. 2016, 657, 878–888. [Google Scholar] [CrossRef]
- Mohammadi, R.; Lech, A.T.; Xie, M.; Weaver, B.E.; Yeung, M.; Tolbert, S.H.; Kaner, R.B. Tungsten tetraboride, an inexpensive superhard material. Proc. Natl. Acad. Sci. USA 2011, 108, 10958–10962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, S.; Li, X.; Su, L.; Li, H.; Yang, H.; Cheng, X. Ab initio study on structural, electronic properties, and hardness of re-doped W2B5. Solid State Commun. 2016, 245, 60–64. [Google Scholar] [CrossRef]
- Lech, A.T.; Turner, C.L.; Lei, J.; Mohammadi, R.; Tolbert, S.H.; Kaner, R.B. Superhard rhenium/tungsten diboride solid solutions. J. Am. Chem. Soc. 2016, 138, 14398–14408. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.; Wang, Y. First-principles study of the elastic properties of OsxW1−xB2 and RexW1−xB2 alloys. Solid State Commun. 2011, 151, 238–241. [Google Scholar] [CrossRef]
- Smolik, J.; Kacprzyńska-Gołacka, J.; Sowa, S.; Piasek, A. The analysis of resistance to brittle cracking of tungsten doped tib2 coatings obtained by magnetron sputtering. Coatings 2020, 10, 807. [Google Scholar] [CrossRef]
- Psiuk, R.; Milczarek, M.; Jenczyk, P.; Denis, P.; Jarząbek, D.M.; Bazarnik, P.; Pisarek, M.; Mościcki, T. Improved mechanical properties of W-Zr-B coatings deposited by hybrid RF magnetron—PLD method. Appl. Surf. Sci. 2021, 570, 151239. [Google Scholar] [CrossRef]
- Chrzanowska, J.; Kurpaska, Ł.; Giżyński, M.; Hoffman, J.; Szymański, Z.; Mościcki, T. Fabrication and characterization of superhard tungsten boride layers deposited by radio frequency magnetron sputtering. Ceram. Int. 2016, 42, 12221–12230. [Google Scholar] [CrossRef]
- Wang, H.L.; Chiang, M.J.; Hon, M.H. Determination of thin film hardness for a film/substrate system. Ceram. Int. 2001, 27, 385–389. [Google Scholar] [CrossRef]
- Palmqvist, S. Occurrence of crack formation during Vickers indentation as a measure of the toughness of hard materials. Arch. Eisenhuettenwes 1962, 33, 629–633. [Google Scholar]
- Akopov, G.; Yeung, M.; Kaner, R.B. Rediscovering the crystal chemistry of borides. Adv. Mater. 2017, 29, 1604506. [Google Scholar] [CrossRef] [PubMed]
- Kittel, C. Introduction to Solid State Physics, 8th ed.; Johnson, S., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2005. [Google Scholar]
- Maździarz, M.; Mościcki, T. New zirconium diboride polymorphs—First-principles calculations. Materials 2020, 13, 3022. [Google Scholar] [CrossRef] [PubMed]
- Ordan’Yan, S.S.; Boldin, A.A.; Suvorov, S.S.; Smirnov, V.V. Phase diagram of the W2B5-ZrB2 system. Inorg. Mater. 2005, 41, 232–234. [Google Scholar] [CrossRef]
- Ken, H.; Endo, T.; Masaki, K.; Nakane, S.; Nishimura, T.; Morisada, Y.; Mizuuchi, K. Simultaneous synthesis and consolidation of W-added ZrB2 by pulsed electric current pressure sintering and their mechanical properties. Mater. Sci. Forum 2007, 561–565, 527–530. [Google Scholar] [CrossRef]
- Wang, C.; Song, L.; Xie, Y. Mechanical and electrical characteristics of WB2 synthesized at high pressure and high temperature. Materials 2020, 13, 1212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bauer, A.; Regnat, A.; Blum, C.G.F.; Gottlieb-Schönmeyer, S.; Pedersen, B.; Meven, M.; Wurmehl, S.; Kunes, J.; Pfleiderer, C. Low-temperature properties of single-crystalCrB2. Phys. Rev. B 2014, 90, 064414. [Google Scholar] [CrossRef] [Green Version]
- Levine, J.B. Synthesis and Characterization of Ultra-Incompressible Superhard Borides; Proquest, Umi Dissertation Publishing: Ann Arbor, MI, USA, 2008. [Google Scholar]
- Rahman, M.; Wang, C.C.; Chen, W.; Akbar, S.A.; Mroz, C. Electrical resistivity of titanium diboride and zirconium diboride. J. Am. Ceram. Soc. 1995, 78, 1380–1382. [Google Scholar] [CrossRef]
- Guimarães, B.; Fernandes, C.; Figueiredo, D.; Cerqueira, M.F.; Carvalho, O.; Silva, F.; Miranda, G. A novel approach to reduce in-service temperature in WC-Co cutting tools. Ceram. Int. 2020, 46, 3002–3008. [Google Scholar] [CrossRef]
- Chrzanowska-Giżyńska, J.; Denis, P.; Giżyński, M.; Kurpaska, Ł.; Mihailescu, I.; Ristoscu, C.; Szymański, Z.; Mościcki, T. Thin WBx and WyTi1−yBx films deposited by combined magnetron sputtering and pulsed laser deposition technique. Appl. Surf. Sci. 2019, 478, 505–513. [Google Scholar] [CrossRef]
- Pauling, L. Atomic Radii and Interatomic Distances in Metals. J. Am. Chem. Soc. 1947, 69, 542–553. [Google Scholar] [CrossRef]
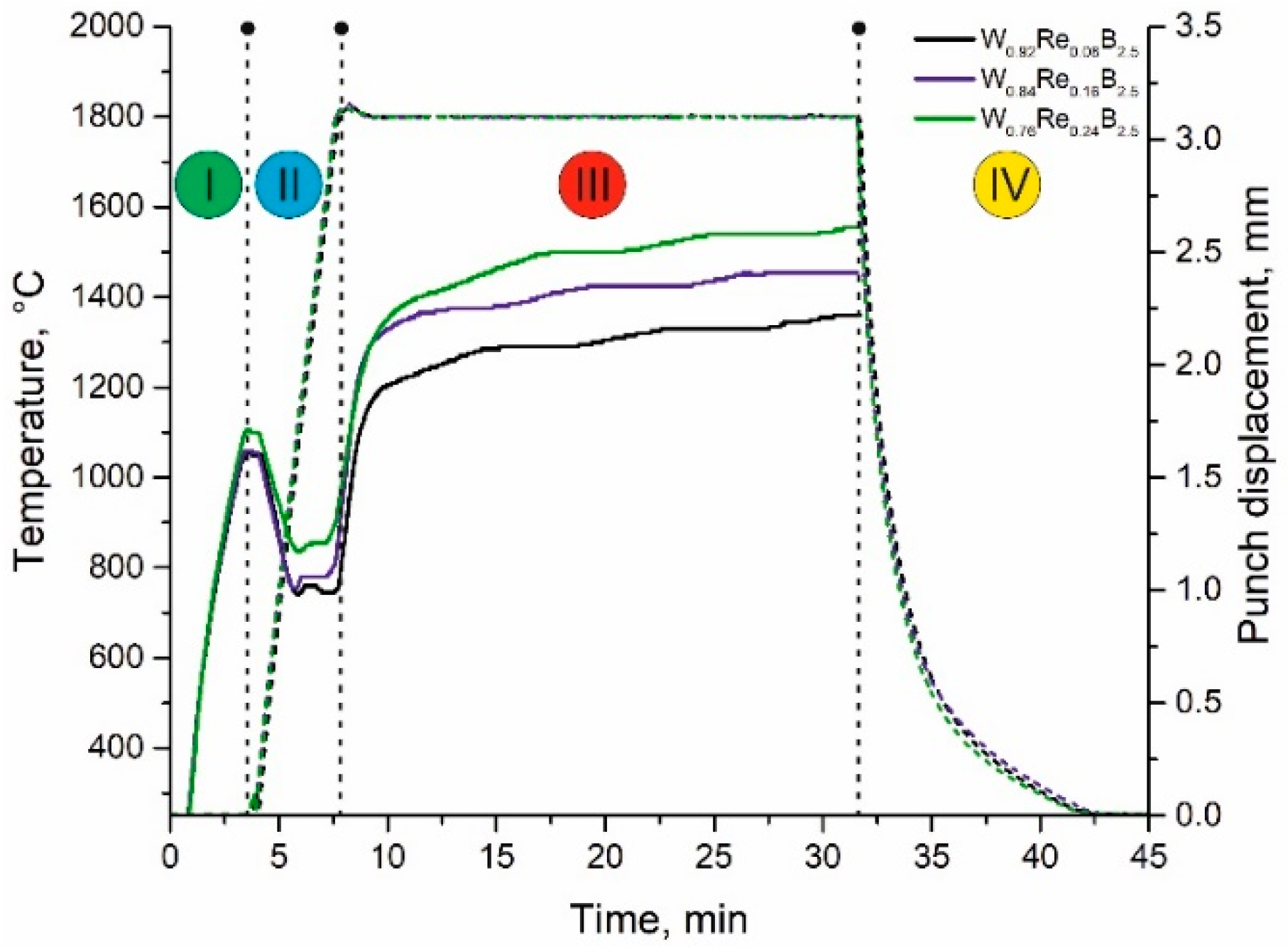
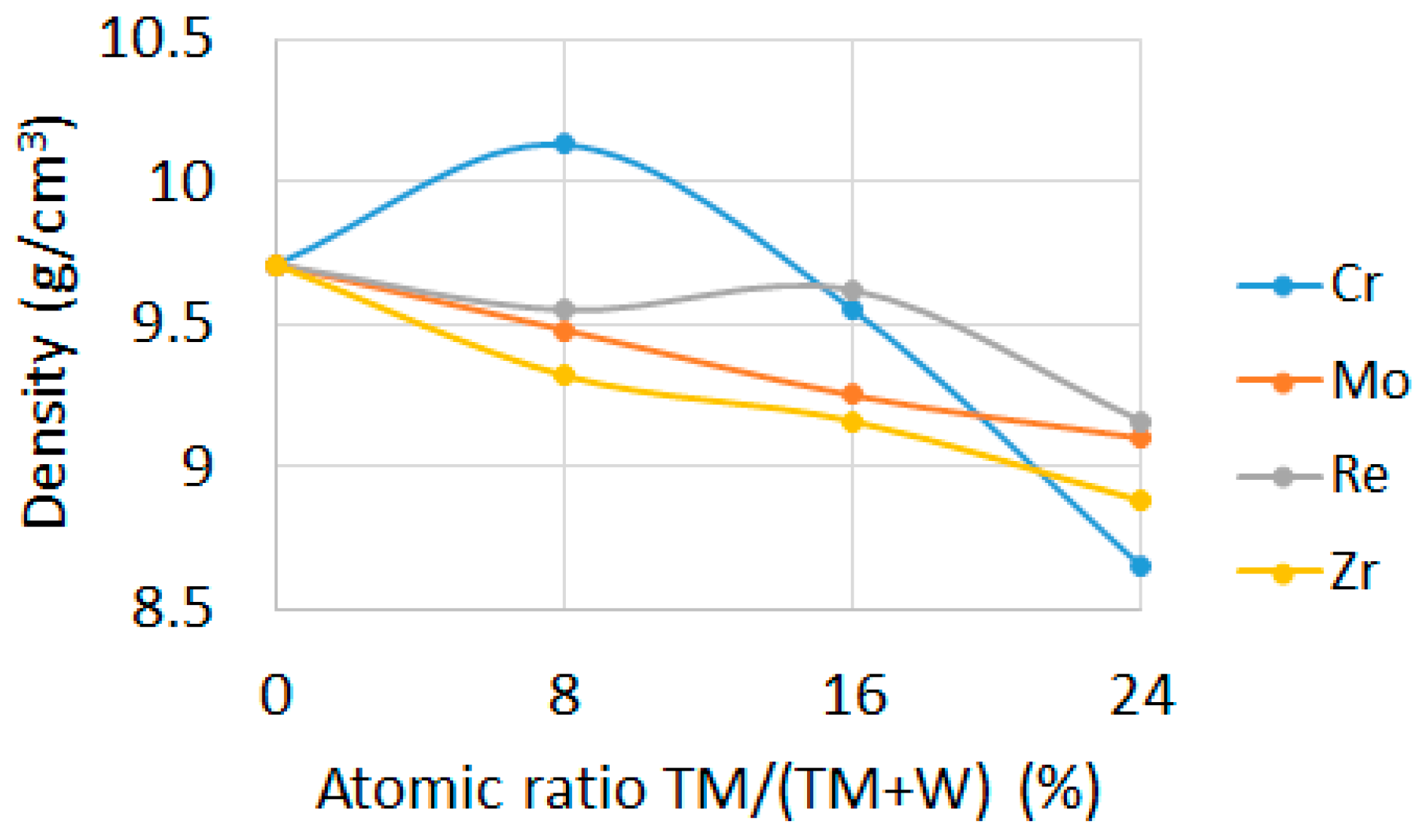
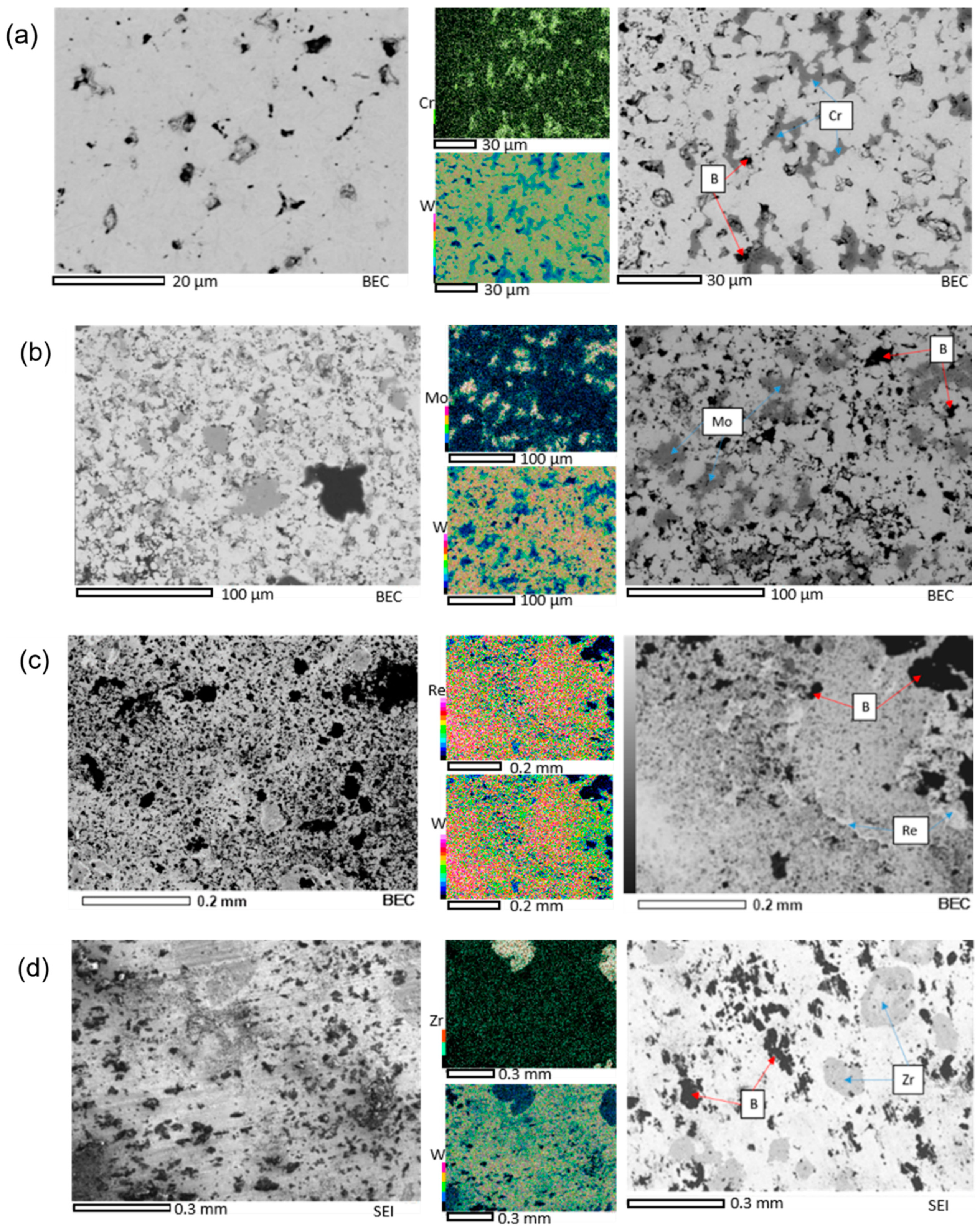


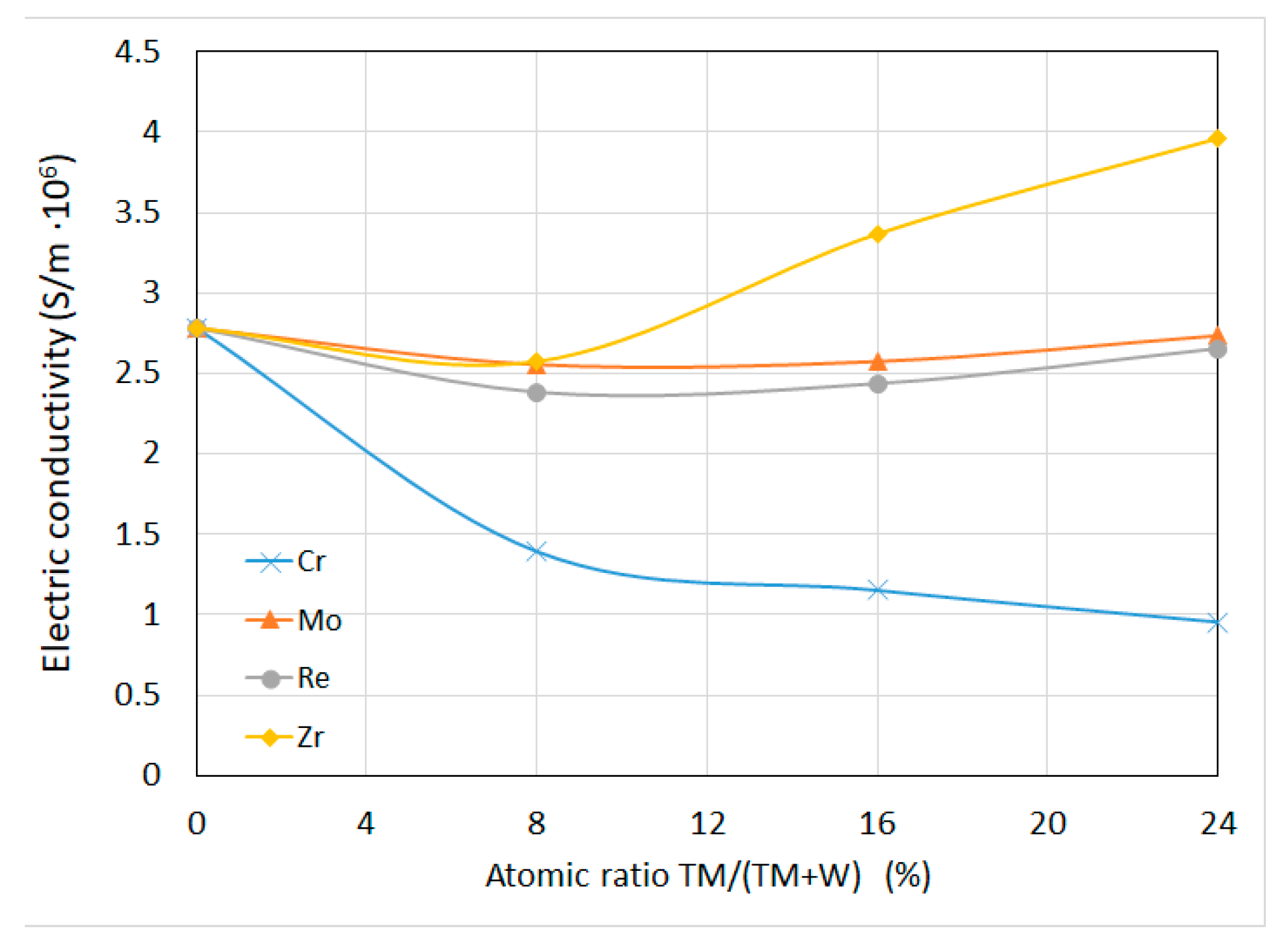
| Material Composition | Tungsten, (g) | TM, (g) | Boron, (g) |
|---|---|---|---|
| WB2.5 | 12.202 | - | 1.795 |
| W0.92Cr0.08B2.5 | 11.817 | 0.291 | 1.890 |
| W0.84Cr0.16B2.5 | 11.389 | 0.614 | 1.995 |
| W0.76Cr0.24B2.5 | 10.911 | 0.975 | 2.112 |
| W0.92Mo0.08B2.5 | 11.613 | 0.527 | 1.857 |
| W0.84Mo0.16B2.5 | 10.982 | 1.092 | 1.924 |
| W0.76Mo0.24B2.5 | 10.305 | 1.698 | 1.995 |
| W0.92Re0.08B2.5 | 11.216 | 0.988 | 1.794 |
| W0.84Re0.16B2.5 | 10.232 | 1.974 | 1.792 |
| W0.76Re0.24B2.5 | 9.249 | 2.958 | 1.791 |
| W0.92Zr0.08B2.5 | 11.635 | 0.502 | 1.861 |
| W0.84Zr0.16B2.5 | 11.025 | 1.042 | 1.931 |
| W0.76Zr0.24B2.5 | 10.366 | 1.624 | 2.007 |
| Material Composition | Sintering Temperature, °C | Heating Rate, °C/min | Holding Time, min | Compacting Pressure, MPa |
|---|---|---|---|---|
| W1−xTMxB2.5 | 1800 | 400 | 24 | 50 |
| Boride | WB2 | CrB2 | MoB2 | ReB2 | ZrB2 | WC-Co |
|---|---|---|---|---|---|---|
| Electrical conductivity (S/m) | 1.1–4.5 × 106 [27] | 0.95–1.42 × 106 [28] | 7.8 × 106 [10] | 2.45 × 106 [29] | 10.9 × 106 [30] | 4.76 × 106 [31] |
| TM | X = TM/(TM + W) | y = B/(TM + W) | at.% O (%) | Thickness (μm) |
|---|---|---|---|---|
| Cr | 0.213 | 1.698 | 2.16 | 2.31 |
| Mo | 0.217 | 2.057 | 3.70 | 2.34 |
| Re | 0.185 | 1.540 | 5.24 | 3.18 |
| Zr | 0.202 | 1.919 | 3.42 | 2.25 |
| TM | HV0.01 | HV0.01 (Recalculated) | HV0.025 | HV0.025 (Recalculated) | HV0.05 | HV0.05 (Recalculated) |
|---|---|---|---|---|---|---|
| Cr | 3610 ± 390 | 5140 ± 480 | 2760 ± 190 | 5570 ± 390 | 2140 ± 120 | 5490 ± 380 |
| Mo | 3250 ± 500 | 4630 ± 680 | 2330 ± 120 | 4610 ± 270 | 2170 ± 210 | 5540 ± 660 |
| Zr | 3050 ± 240 | 4480 ± 330 | 2690 ± 130 | 5540 ± 260 | 2010 ± 110 | 5150 ± 370 |
| Re | 2710 ± 540 | 3330 ± 650 | 1940 ± 210 | 3070 ± 400 | 1750 ± 60 | 3360 ± 180 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mościcki, T.; Psiuk, R.; Radziejewska, J.; Wiśniewska, M.; Garbiec, D. Properties of Spark Plasma Sintered Compacts and Magnetron Sputtered Coatings Made from Cr, Mo, Re and Zr Alloyed Tungsten Diboride. Coatings 2021, 11, 1378. https://doi.org/10.3390/coatings11111378
Mościcki T, Psiuk R, Radziejewska J, Wiśniewska M, Garbiec D. Properties of Spark Plasma Sintered Compacts and Magnetron Sputtered Coatings Made from Cr, Mo, Re and Zr Alloyed Tungsten Diboride. Coatings. 2021; 11(11):1378. https://doi.org/10.3390/coatings11111378
Chicago/Turabian StyleMościcki, Tomasz, Rafał Psiuk, Joanna Radziejewska, Maria Wiśniewska, and Dariusz Garbiec. 2021. "Properties of Spark Plasma Sintered Compacts and Magnetron Sputtered Coatings Made from Cr, Mo, Re and Zr Alloyed Tungsten Diboride" Coatings 11, no. 11: 1378. https://doi.org/10.3390/coatings11111378
APA StyleMościcki, T., Psiuk, R., Radziejewska, J., Wiśniewska, M., & Garbiec, D. (2021). Properties of Spark Plasma Sintered Compacts and Magnetron Sputtered Coatings Made from Cr, Mo, Re and Zr Alloyed Tungsten Diboride. Coatings, 11(11), 1378. https://doi.org/10.3390/coatings11111378








