Adsorption Features of Loess Calcareous Nodules to Heavy-Metal Ions in Aqueous Solution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Scanning Electron Microscopy and Microwave Digestion of Calcareous Tuberculosis
2.2.2. Adsorption Kinetics
2.2.3. Calcium Nodule Adsorption to Heavy-Metal Ions
2.3. Data Processing
3. Results and Analysis
3.1. Adsorption Isotherm Experiment
3.2. Adsorption Kinetics Experiment
3.3. Micromorphology and Chemical Composition of Calcareous Nodules
3.4. Impact of the Particle Size on the Adsorption of Heavy-Metal Ions by Calcium Nodules
3.5. Impact of the Adsorption Time on the Adsorption of Heavy-Metal Ions by Calcium Nodules
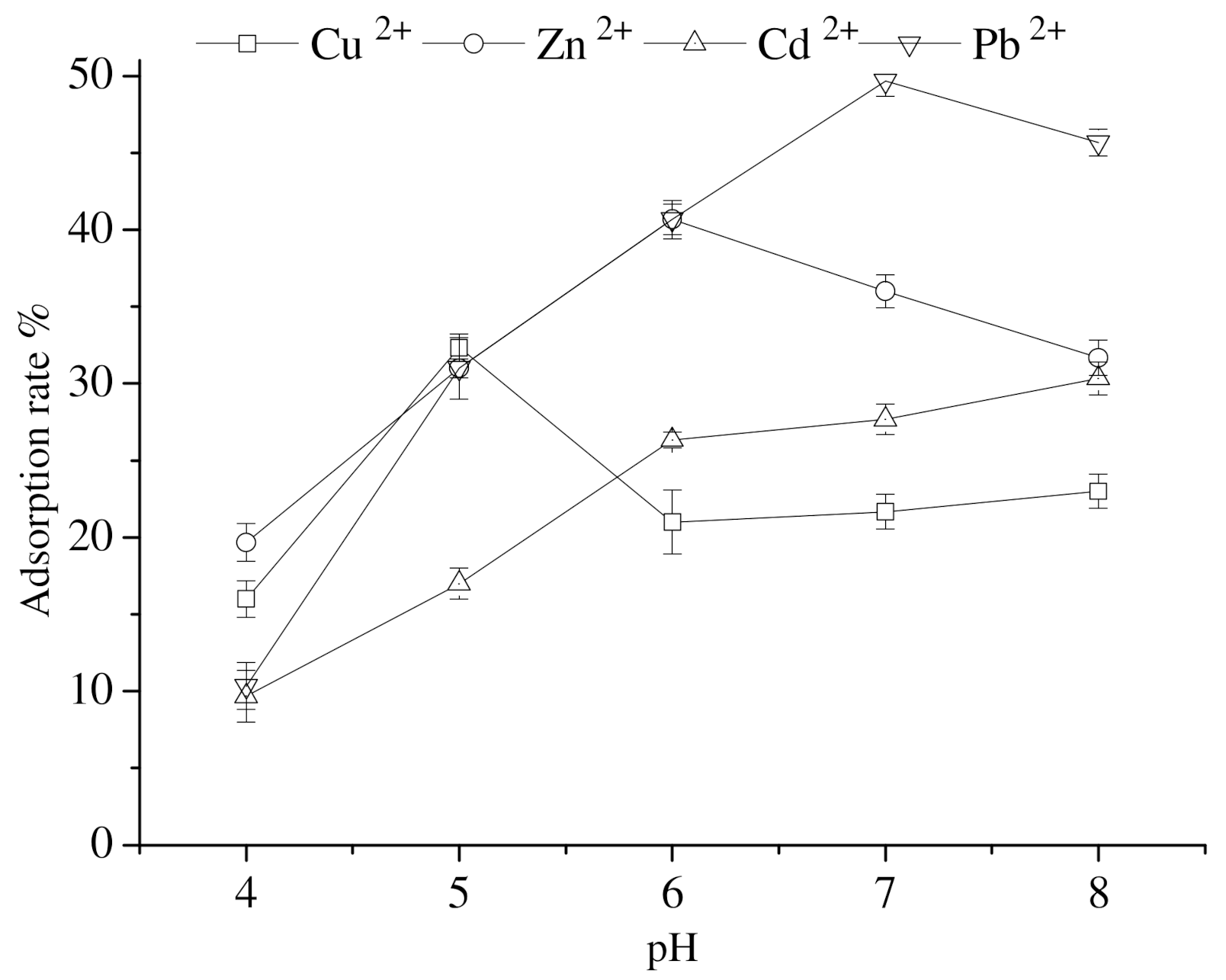
3.6. Impact of pH Value on the Adsorption of Heavy-Metal Ions by Calcium Nodules
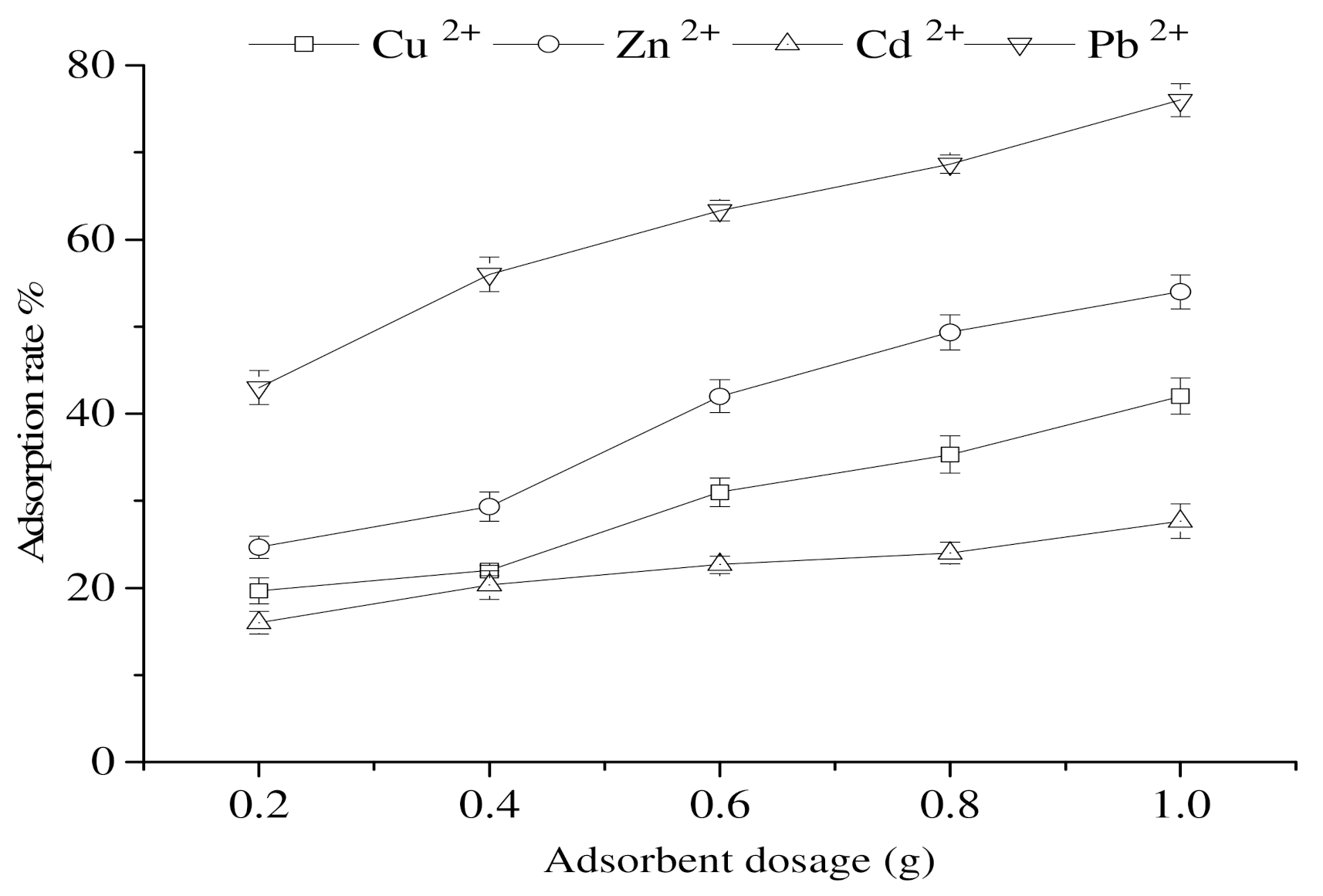
3.7. Impact of the Amount of Adsorbent on the Adsorption of Heavy-Metal Ions by Calcium Nodules
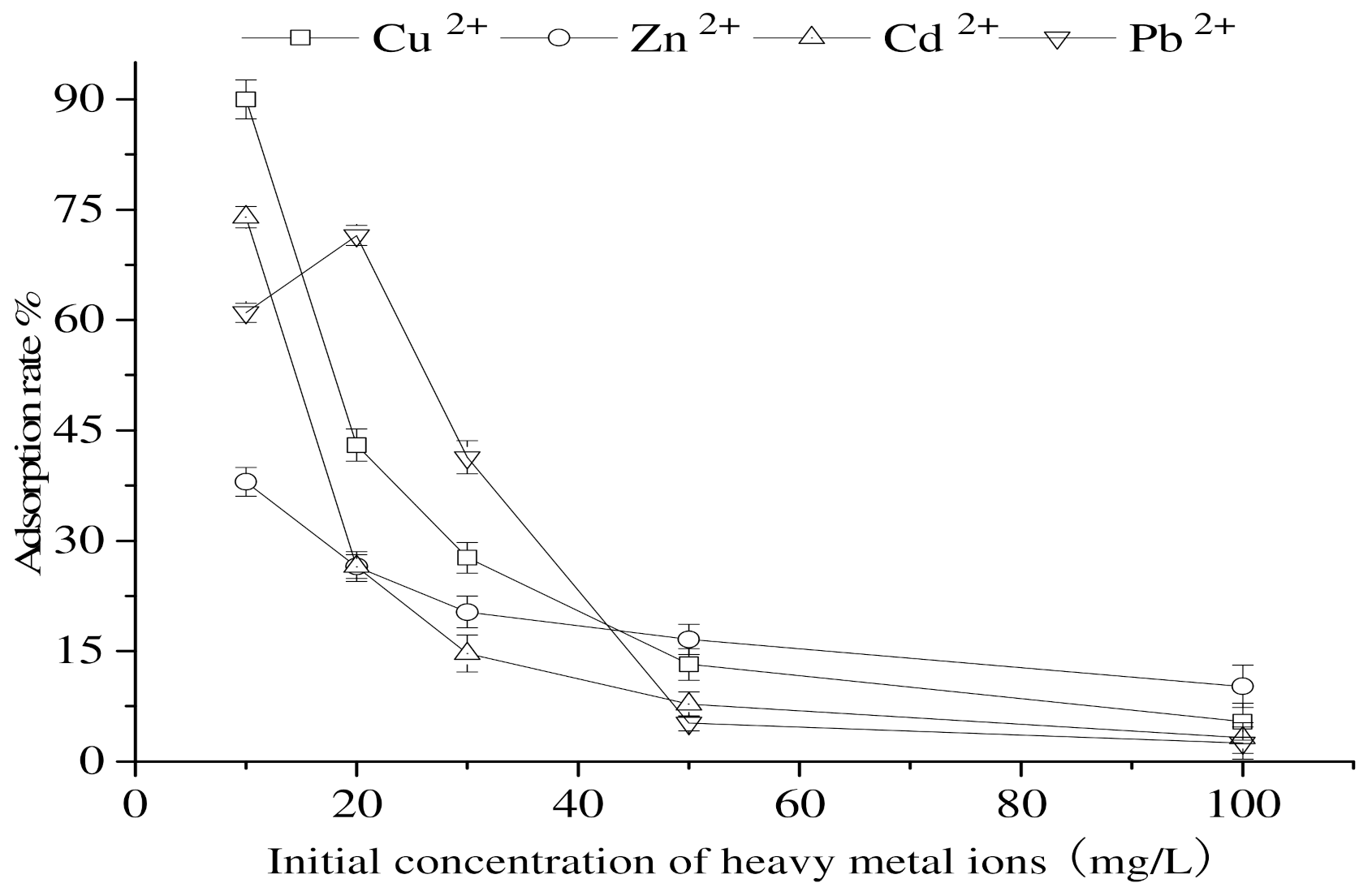
3.8. Impact of the Initial Concentration of Heavy Metals on the Adsorption of Heavy-Metal Ions by Calcium Nodules
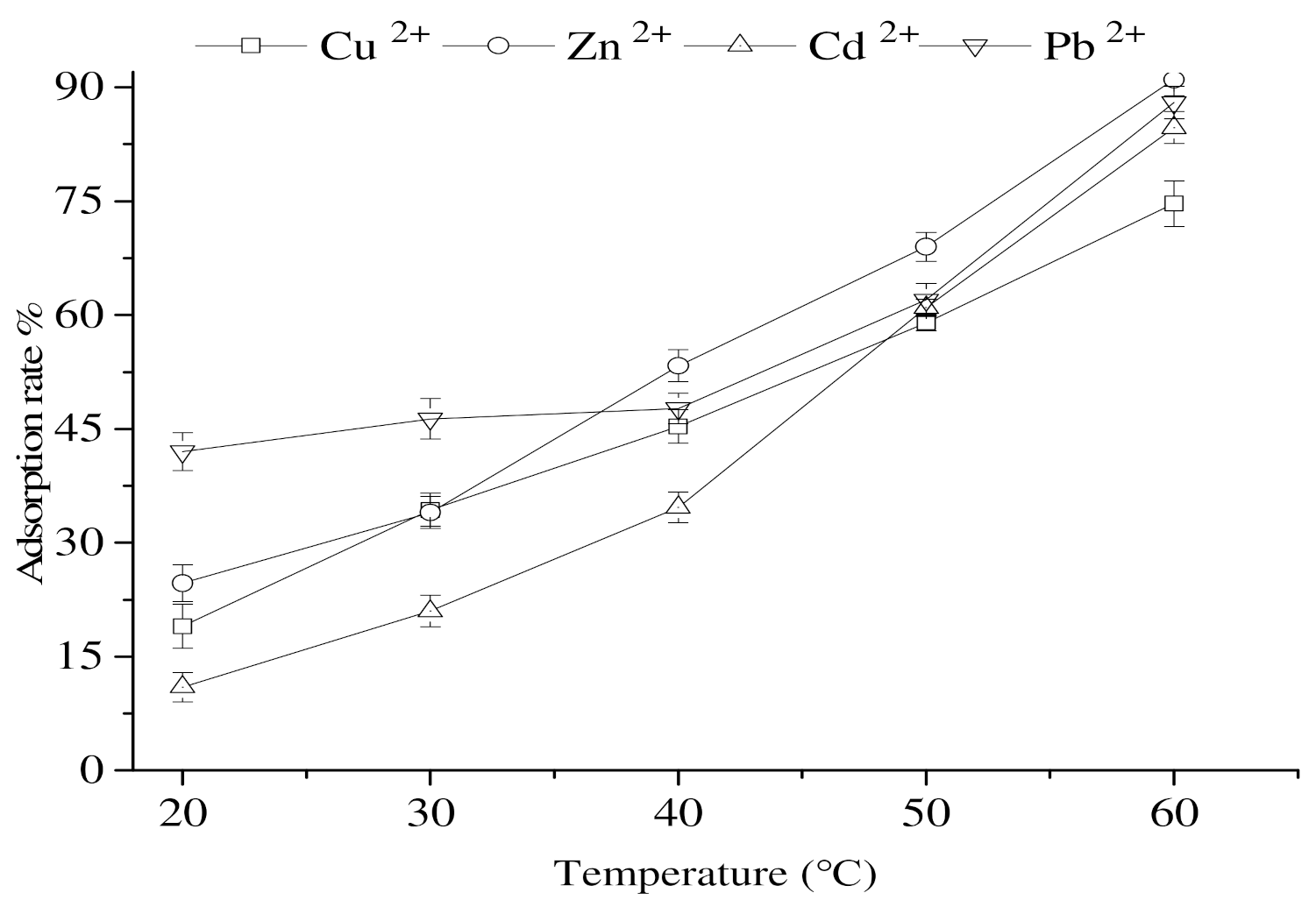
3.9. Impact of the Temperature on the Adsorption of Heavy-Metal Ions by Calcium Nodules
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Z.; Bao, L.I.; Liang, R. Comparison of sediment heavy metal fractions at estuary and center of Nanyang Zone from Nansi Lake, China. Huanjing Kexue Xuebao/Acta Sci. Circumstantiae 2015, 35, 1408–1416. [Google Scholar]
- Wang, Y.Y.; Liu, Y.X.; Lu, H.H.; Yang, R.Q.; Yang, S.M. Competitive adsorption of Pb (Ⅱ), Cu (Ⅱ), and Zn (Ⅱ) ions onto hydroxyapatite-biochar nano composite in aqueous solutions. J. Solid State Chem. 2018, 261, 53–61. [Google Scholar] [CrossRef]
- Zou, J.; Sun, D.; Zhao, J.; Wang, S.; Li, S.; Li, W.; Lv, Y.; Dou, H. Adsorption of heavy metal Cd, Pb by maize straw Biochar. J. Beihua Univ. 2018, 281, 96–99. [Google Scholar]
- Li, L.; Lu, Y.; Liu, Y.; Sun, H.W.; Liang, Z.Y. Adsorption mechanisms of cadmium (Ⅱ) on biochars derived from corn straw. J. Agro-Environ. Sci. 2012, 31, 2277–2283. [Google Scholar]
- Liu, J.; Liu, L.; Xue, J. Research progress on treatment of heavy metal wastewater by adsorption. Environ. Chem. 2018, 37, 2016–2024. [Google Scholar]
- Hu, H.; Liu, G. The trend of heavy metal wastewater treatment by adsorption method. In Proceedings of the 2012 2nd International Conference on Remote Sensing, Environment and Transportation Engineering, Nanjing, China, 1–3 June 2012. [Google Scholar]
- Ji, Z.; Wu, X.F.; Li, Y.; Feng, C. Kinetic adsorption and change in chemical potential of heavy metal ions in aqueous solutions. Environ. Chem. 2015, 11, 53–61. [Google Scholar]
- Sheikhhosseini, A.; Shirvani, M.; Shariatmadari, H. Competitive sorption of nickel, cadmium, zinc and copper on palygorskite and sepiolite silicate clay minerals. Geoderma 2013, 192, 249–253. [Google Scholar] [CrossRef]
- He, H.P.; Guo, J.G.; Zhu, J.X.; Yang, D. An experimental study of adsorption capacity of montmorillonite, kaolinite and illite for heavy metals. Acta Mineral. Petrol. 2001, 20, 573–578. [Google Scholar]
- Gong, T.; Zhu, Y. Spatial pattern of caliche nodule in surface soil of the hillslopes in Liudaogou catchment in the wind-water erosion crisscross zone of the Loess Plateau. Sci. Soil Water Conserv. 2016, 14, 42–49. [Google Scholar]
- Teng, Z.H.; Liu, R.M. Research on calcareous nodules in the loess strata of China. Chin. Sci. Bull. 1990, 192, 1008–1011. [Google Scholar]
- Wang, S.Y. Study on the Petrological Characteristics of N2 Red Clay in Northern Shanxi. Master’s Thesis, China University of Mining and Technology, Xuzhou, China, 2017. [Google Scholar]
- Gong, T.X. Study on the Distribution of Calcareous Nodules and Their Water Characteristics on Slopes in Loess Area. Master’s Thesis, University of Chinese Academy of Sciences, Beijing, China, 2017. [Google Scholar]
- Li, C.A.; Wu, J.P.; Cao, J.X. The morphology of loess calcareous nodules in northwest Hebei and their genesis dynamic characteristics and stratigraphic environmental significance. Earth Sci. 1995, 38, 511–514. [Google Scholar]
- Yang, L.G.; Qiao, D.Y.; Hu, P.Y. Determination of thirteen elements in soil by inductively coupled plasma mass spectrometry with automatic digestion instrument. Soil Fertil. Sci. China 2019, 38, 89–93. [Google Scholar]
- Wu, H.Z.; Meng, L.F. Liquid chromatography-UV determination of heavy metal ions in environmental samples using dispersive liquid-liquid microextraction coupled with magnetic nanoparticles. Appl. Ecol. Environ. Res. 2019, 17, 1571–1584. [Google Scholar] [CrossRef]
- Lee, J.Y.; Chen, C.H.; Cheng, S.; Li, H.Y. Adsorption of Pb(II) and Cu(II) metal ions on functionalized large-pore mesoporous silica. Int. J. Environ. Sci. Technol. 2016, 13, 65–76. [Google Scholar] [CrossRef] [Green Version]
- Melnyk, I.V.; Gdula, K.; Dbrowski, A.; Zub, Y.L. Magneto-sensitive adsorbents modified by functional nitrogen-containing groups. Nanoscale Res. Lett. 2016, 11, 61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, B.Y.; Qiu, T.; Li, R.H.; Qin, R.; Zhang, G.; Li, X.; Zhang, Z. Investigation of heavy metal ions adsorption ability by thiol-modified corn stalk powder. J. Northwest A F Univ. -Nat. Sci. Ed. 2012, 40, 185–190. [Google Scholar]
- Qin, H.F.; Zhang, W.M. Adsorption of lead and cadmium by hydroxyapatite coated quartz sands. Sci. Technol. Eng. 2018, 20, 183–189. [Google Scholar]
- Kang, J.; Kim, T.; Park, J.; Lee, K.Y.; Park, D.H.; Park, S.; Kim, S.; Jung, Y. A mesoporous chelating polymer-carbon composite for the hyper-efficient separation of heavy metal ions. J. Nanosci. Nanotechnol. 2020, 20, 3042–3046. [Google Scholar] [CrossRef]
- Tsao, T.M.; Chen, Y.M.; Sheu, H.S.; Zhuang, S.Y.; Shao, P.H.; Chen, H.W.; Shea, K.S.; Wang, M.K.; Shau, Y.H.; Chiang, K.Y. Red soil chemistry and mineralogy reflect uniform weathering environments in fluvial sediments, Taiwan. J. Soils Sediments 2012, 12, 1054–1065. [Google Scholar] [CrossRef]
- Zhu, X.; Chen, H.; Li, W.; He, Y.; Brookes, P.C.; Xu, J. Aggregation kinetics of natural soil nanoparticles in different electrolytes. Eur. J. Soil Sci. 2014, 65, 206–217. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Huang, L.; Liu, F.; Kuang Wang, M.; Ling Fu, Q.; Zhu, J. The properties of clay minerals in soil particles from two Ultisols, China. Clays Clay Miner. 2017, 65, 273–285. [Google Scholar] [CrossRef]
- Chou, Y.M.; Song, S.R.; Tsao, M.T.; Lin, C.S.; Wang, M.K.; Lee, J.J.; Chen, F.J. Identification and tectonic implications of nano-particle quartz (<50 nm) by synchrotron X-ray diffraction in the Chelungpu fault gouge, Taiwan. Tectonophysics 2014, 619, 36–43. [Google Scholar]
- Ye, T.; Huang, L.; Zhang, K.Q.; Zhang, B.; Chang, H.; Liu, Z.J.; Du, L.Z. Evaluation of the combined removal of heavy metals by saponin and citric acid from municipal sewage sludges and metal stability features. Huanjing Kexue 2017, 38, 4850–4859. [Google Scholar]
- Wang, W.; Han, N.; Yang, C.; Zhang, W.; Miao, J.; Li, W.; Zhang, X. Fabrication of P(AN-MA)/rGO-g-PAO superhydrophilic nanofiber membrane for removal of heavy metal ions. J. Nanosci. Nanotechnol. 2020, 20, 1685–1696. [Google Scholar] [CrossRef]
- Tofighy, M.A.; Mohammadi, T. Adsorption of divalent heavy metal ions from water using carbon nanotube sheets. J. Hazard. Mater. 2011, 185, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Li, H.; Liu, Y.; Song, S. A review on heavy metal ions adsorption from water by graphene oxide and its composites. J. Mol. Liq. 2017, 230, 496–504. [Google Scholar] [CrossRef]
- Gu, D.G.; Fein, J.B. Adsorption of metals onto graphene oxide: Surface complexation modeling and linear free energy relationships. Colloids Surf. A Physicochem. Eng. Asp. 2015, 481, 319–327. [Google Scholar] [CrossRef]
- Rahnama, E.; Bazrafshan, O.; Asadollahfardi, G. Application of data-driven methods to predict the sodium adsorption rate (SAR) in different climates in Iran. Arab. J. Geosci. 2020, 13, 1160. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, J.; Liu, J.; Nasir, M.S.; Zhu, J.; Li, S.; Liang, J.; Yan, W. Smart formaldehyde detection enabled by metal organic framework-derived doped electrospun hollow nanofibers. Sens. Actuators B Chem. 2021, 326, 128819. [Google Scholar] [CrossRef]
- Liu, J.W.; Wang, J.N.; Zhu, L.; Chen, X.; Ma, Q.; Wang, L.; Wang, X.; Yan, W. A high-safety and multifunctional MOFs modified aramid nanofiber separator for lithium-sulfur batteries. Chem. Eng. J. 2021, 411, 128540. [Google Scholar] [CrossRef]
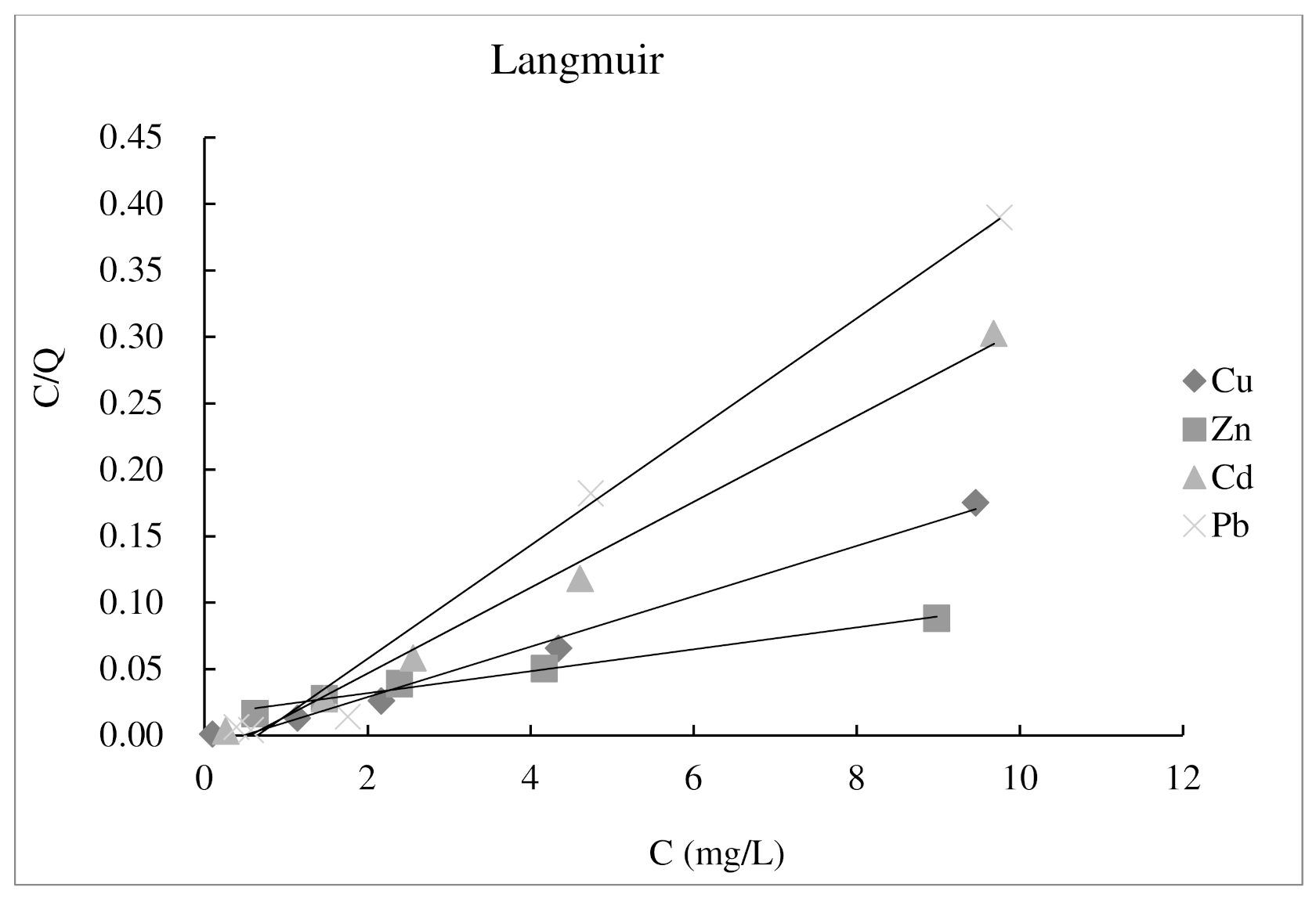
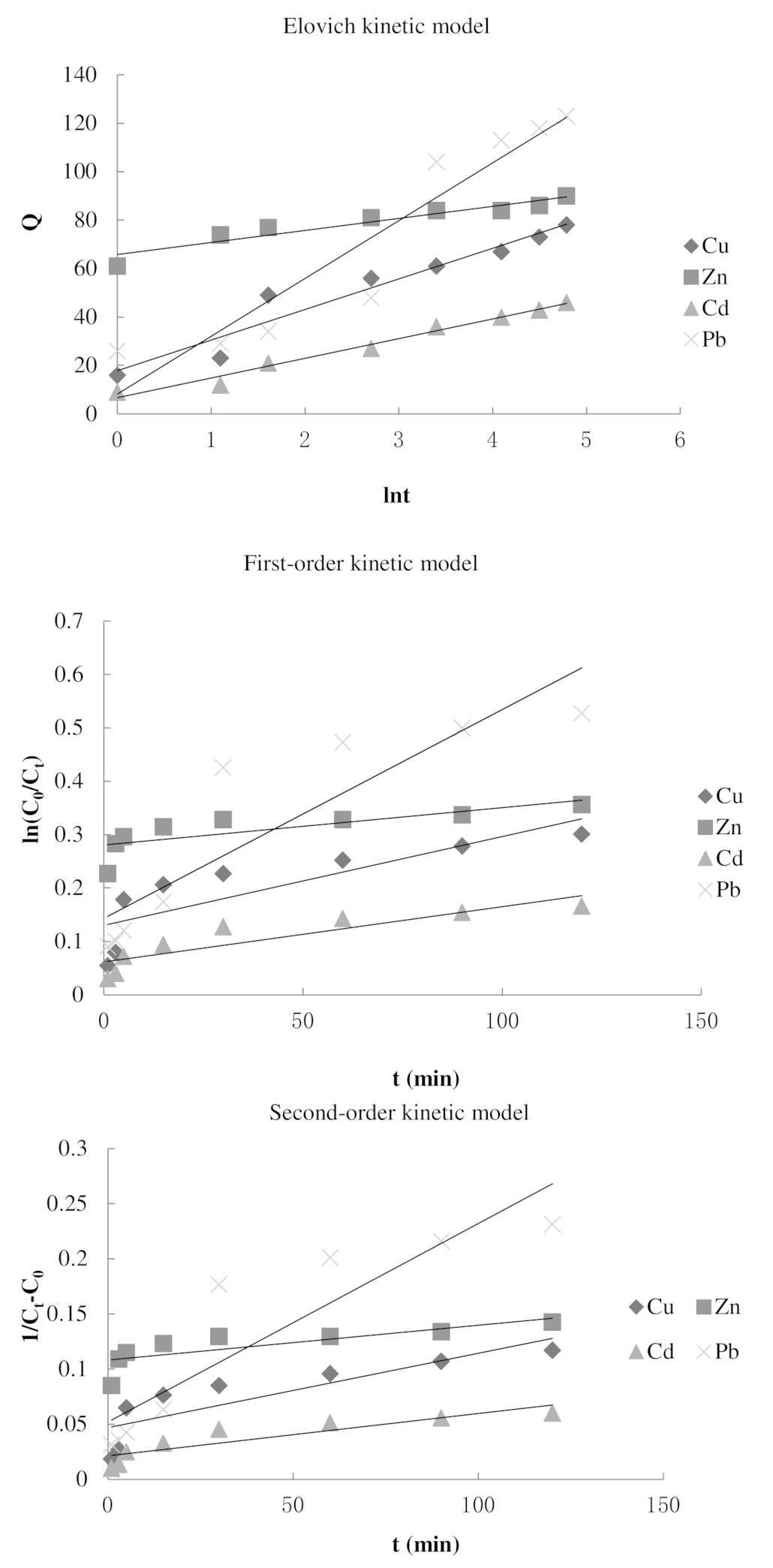
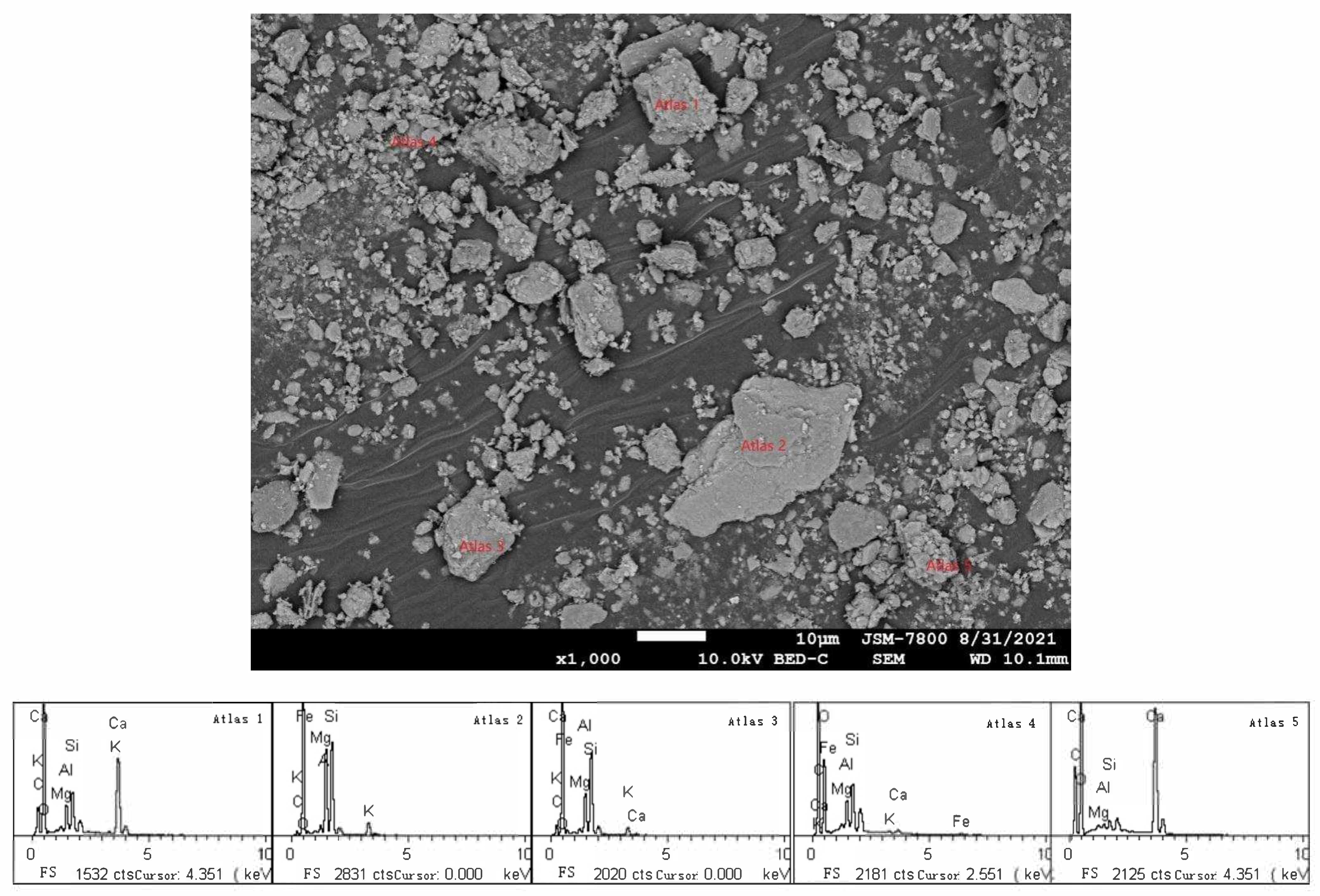

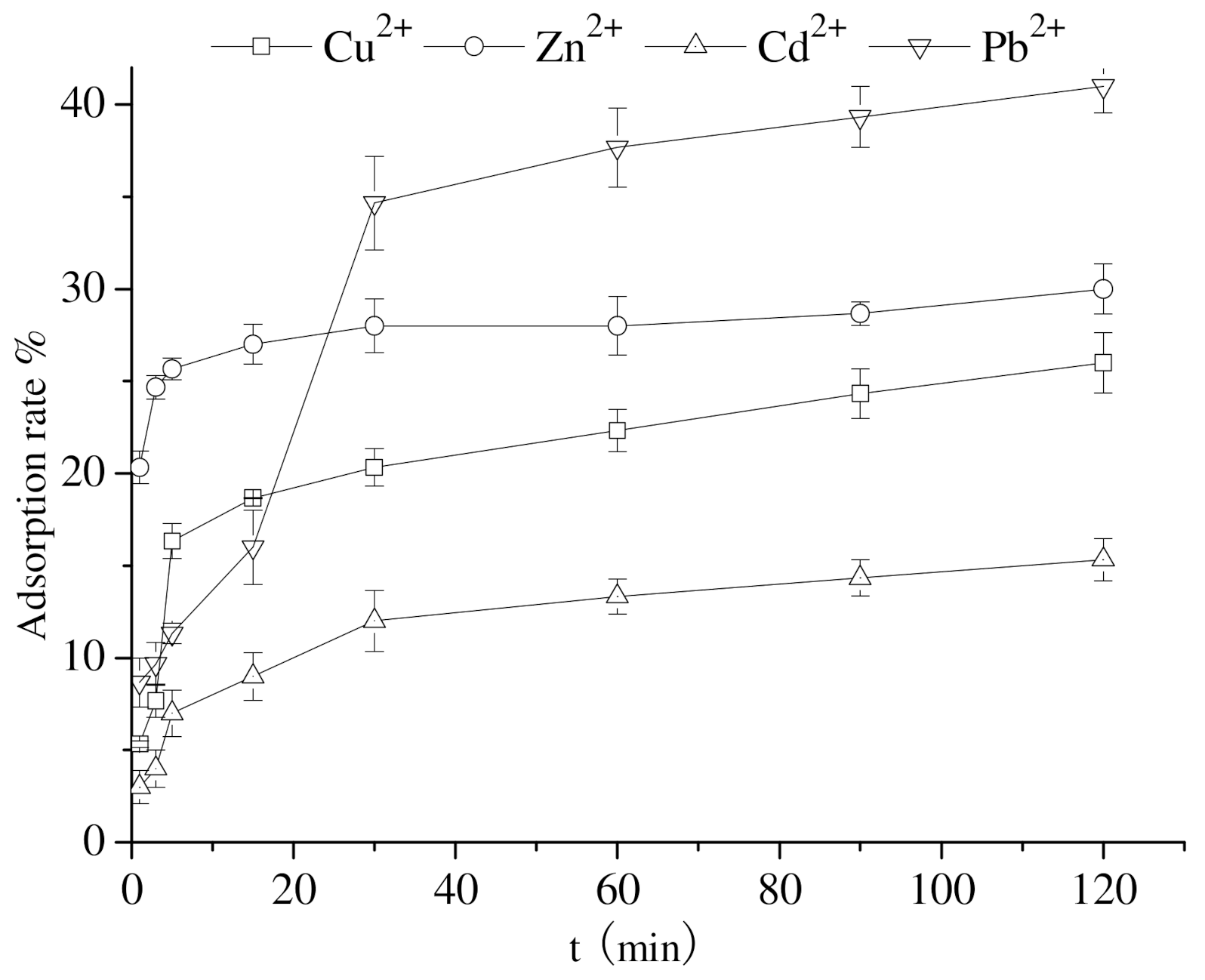
| Heavy Metal | Langmuir Model | Freundlich Model | ||||
|---|---|---|---|---|---|---|
| K (L/mg) | Qm (mg·g−1) | R2 | KF (mg/g)/(mg/L)1/n | n | R2 | |
| Cu2+ | −2.1111111 | 52.6315789 | 0.9908 | 78.072844 | −0.1023 | 0.677 |
| Zn2+ | 0.53947368 | 121.95122 | 0.988 | 45.593192 | 0.378 | 0.9906 |
| Cd2+ | −1.7845304 | 30.9597523 | 0.9924 | 55.309534 | −0.2322 | 0.9921 |
| Pb2+ | −1.5471014 | 23.4192037 | 0.9867 | 76.155277 | −0.4468 | 0.5419 |
| Heavy Metal | Elovich | First-Order Dynamics | Second-Order Kinetics | ||||
|---|---|---|---|---|---|---|---|
| A | b | R2 | K1 (min−1) | R2 | K2 (10−5g·mg−1·min−1) | R2 | |
| Cu2+ | 17.817 | 12.634 | 0.938 | 0.0017 | 0.6995 | 0.0007 | 0.7299 |
| Zn2+ | 65.842 | 4.9672 | 0.9042 | 0.0007 | 0.6062 | 0.0003 | 0.6242 |
| Cd2+ | 6.7267 | 8.1169 | 0.9817 | 0.001 | 0.7941 | 0.0004 | 0.8068 |
| Pb2+ | 8.1764 | 23.856 | 0.8958 | 0.0039 | 0.8061 | 0.0018 | 0.8268 |
| Chemical Components | K2O | CaO | NaO | MgO | Al2O3 | Fe2O3 | SiO2 | SiO2/Al2O3 |
|---|---|---|---|---|---|---|---|---|
| Content (%) | 2.16 | 34.57 | 1.23 | 1.78 | 14.36 | 4.34 | 41.56 | 2.89 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Li, Y. Adsorption Features of Loess Calcareous Nodules to Heavy-Metal Ions in Aqueous Solution. Coatings 2021, 11, 1314. https://doi.org/10.3390/coatings11111314
Li Q, Li Y. Adsorption Features of Loess Calcareous Nodules to Heavy-Metal Ions in Aqueous Solution. Coatings. 2021; 11(11):1314. https://doi.org/10.3390/coatings11111314
Chicago/Turabian StyleLi, Qi, and Yanan Li. 2021. "Adsorption Features of Loess Calcareous Nodules to Heavy-Metal Ions in Aqueous Solution" Coatings 11, no. 11: 1314. https://doi.org/10.3390/coatings11111314
APA StyleLi, Q., & Li, Y. (2021). Adsorption Features of Loess Calcareous Nodules to Heavy-Metal Ions in Aqueous Solution. Coatings, 11(11), 1314. https://doi.org/10.3390/coatings11111314





