Preparation of Wear-Resistant Coating on Ti6Al4V Alloy by Cold Spraying and Plasma Electrolytic Oxidation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Coatings Preparation and Process Parameters
2.3. Characterization Methods
3. Results and Discussion
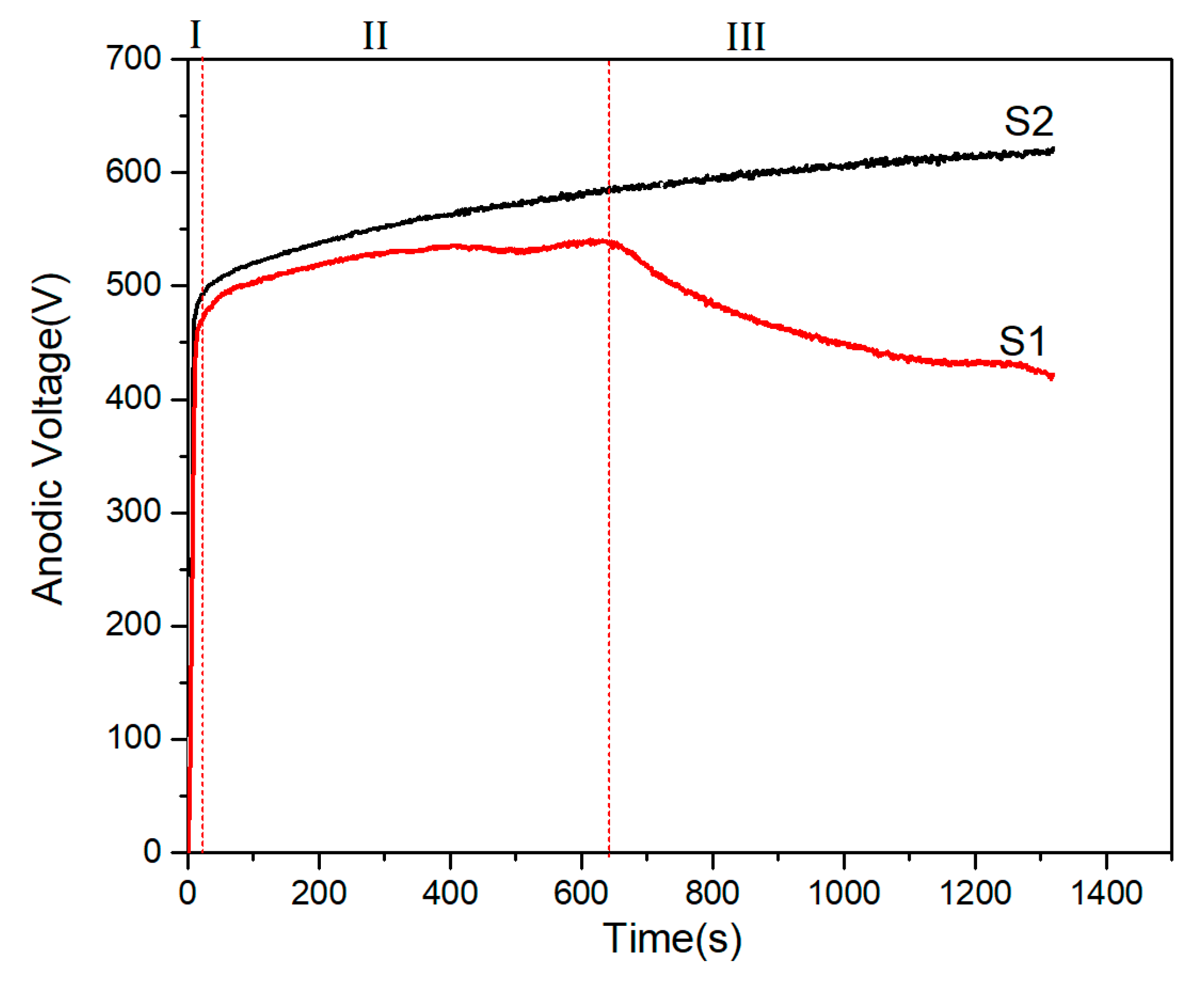
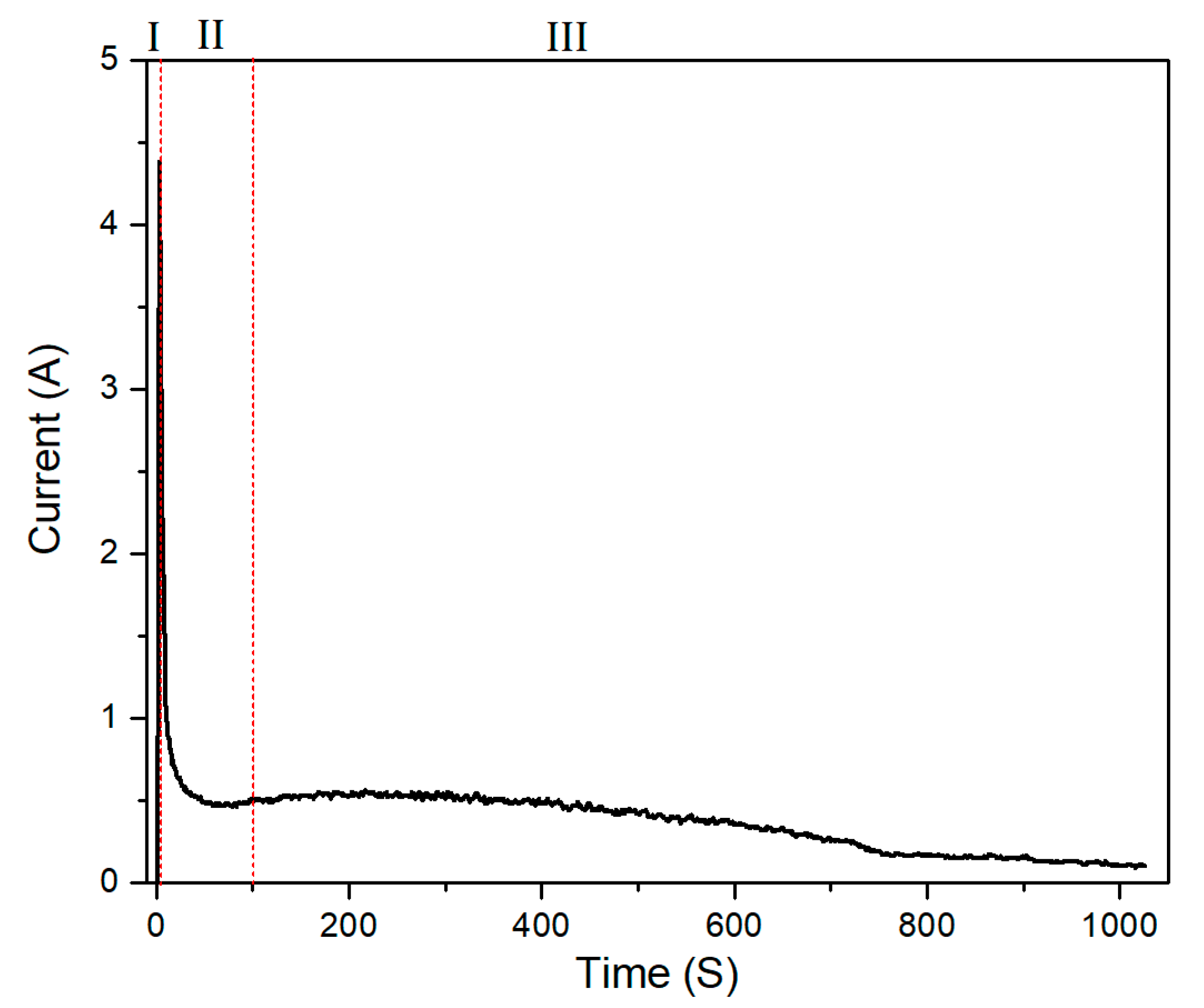
3.1. The Cell Voltage–Time and Current Density–Time Responses during PEO Treatment
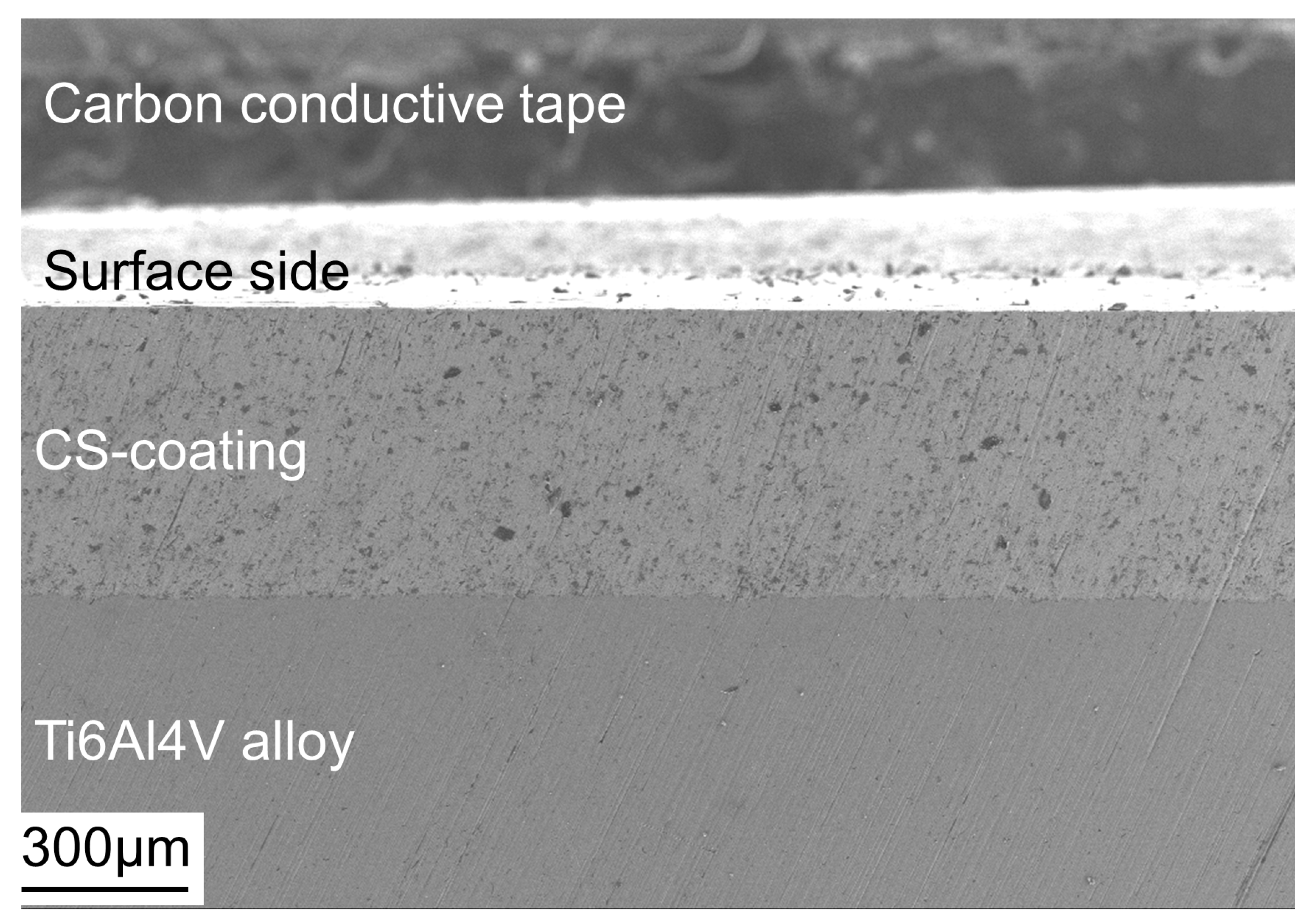
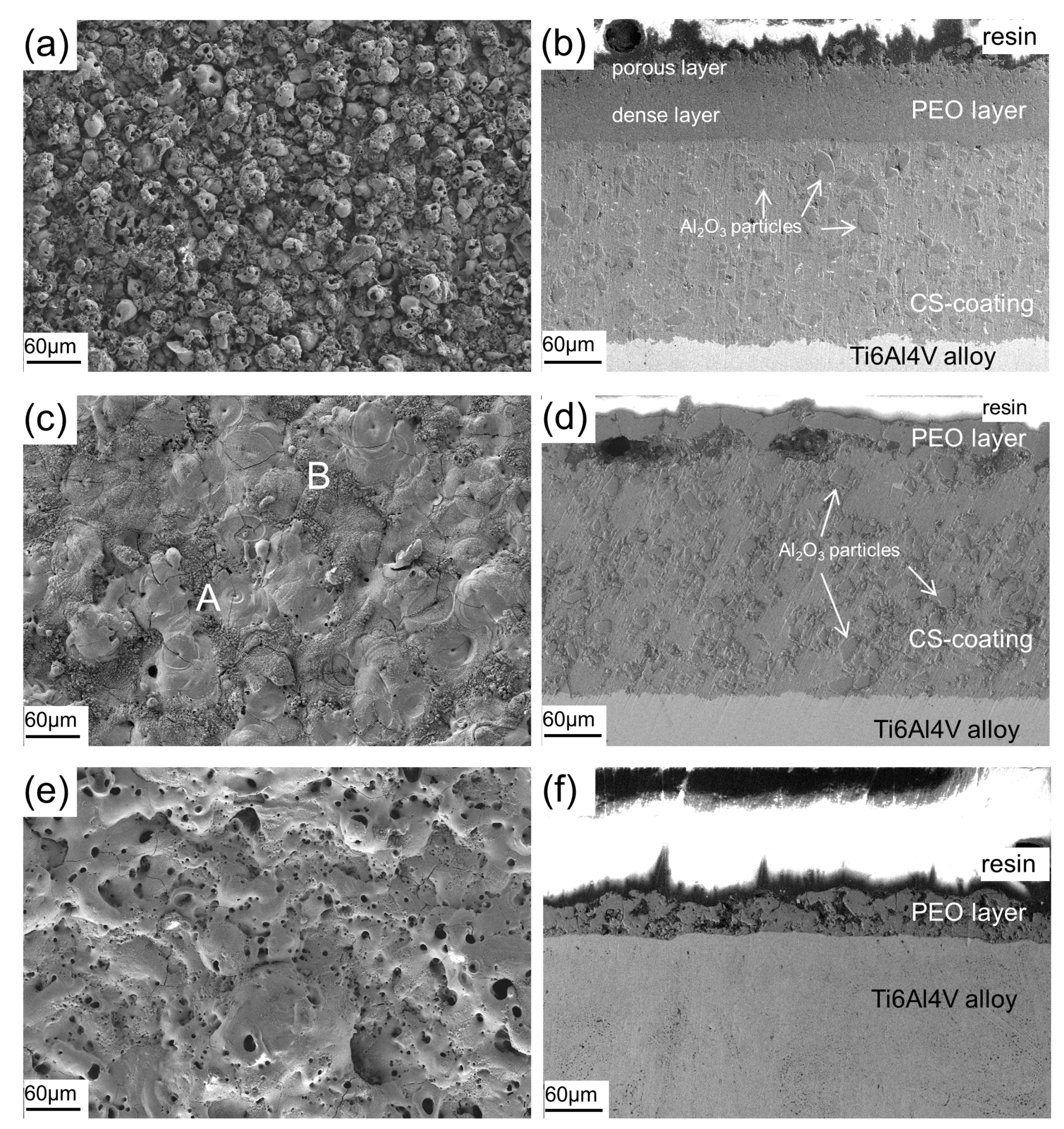
3.2. Morphology of Coatings
3.3. Phase Composition of Coatings
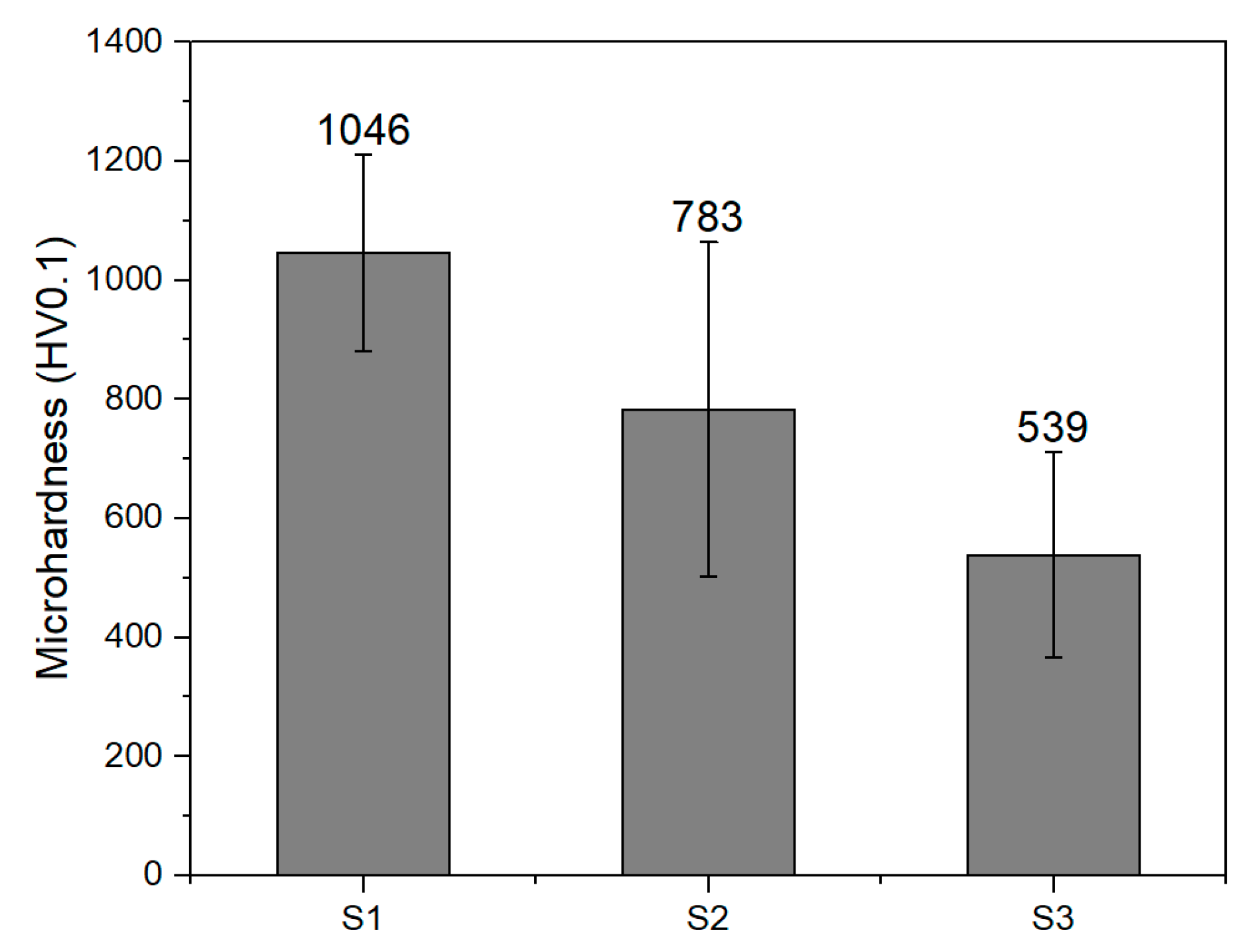
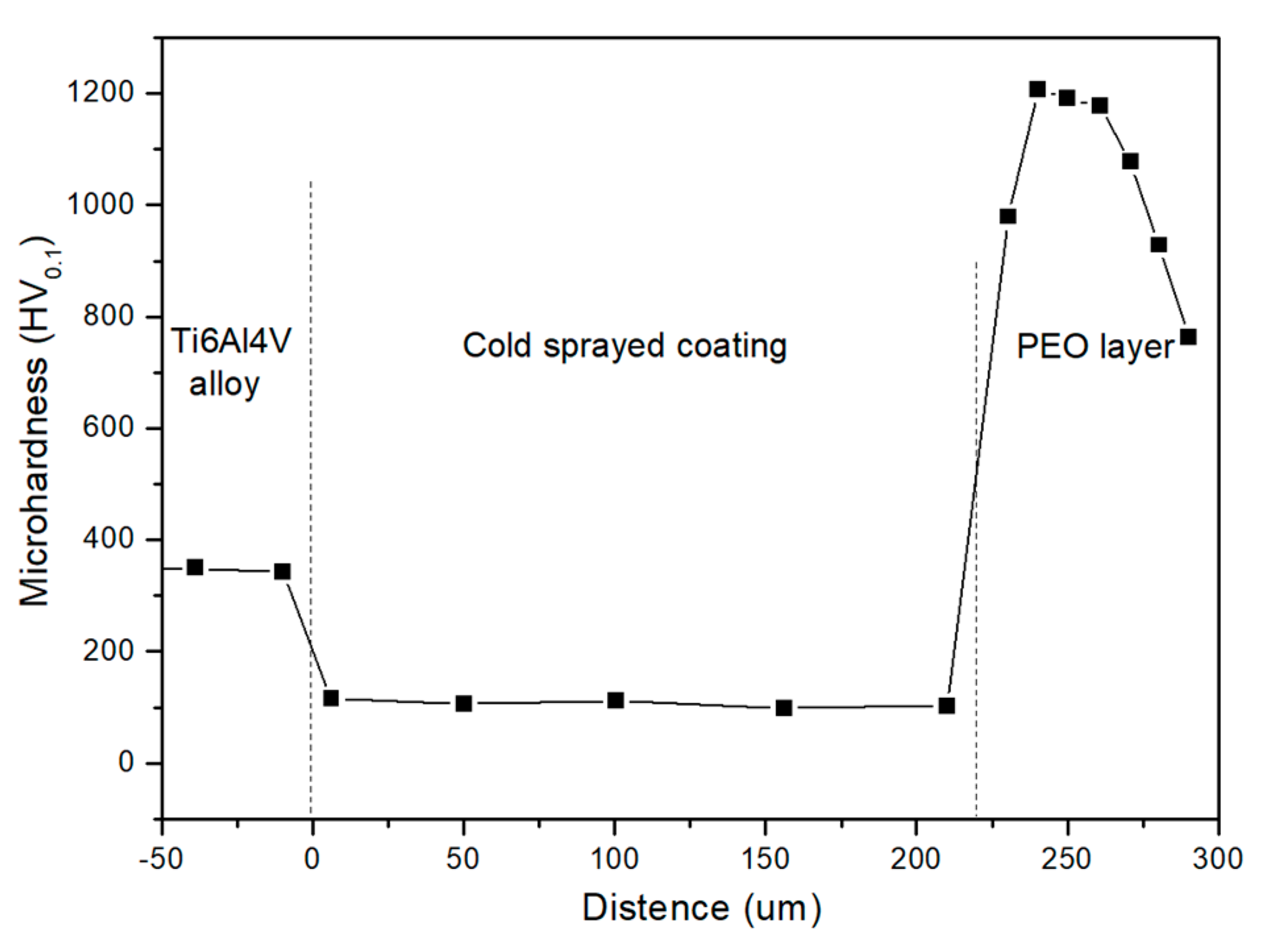
3.4. Micro Hardness of Coatings
3.5. Wear Test
3.5.1. Coefficients of Friction (COF)
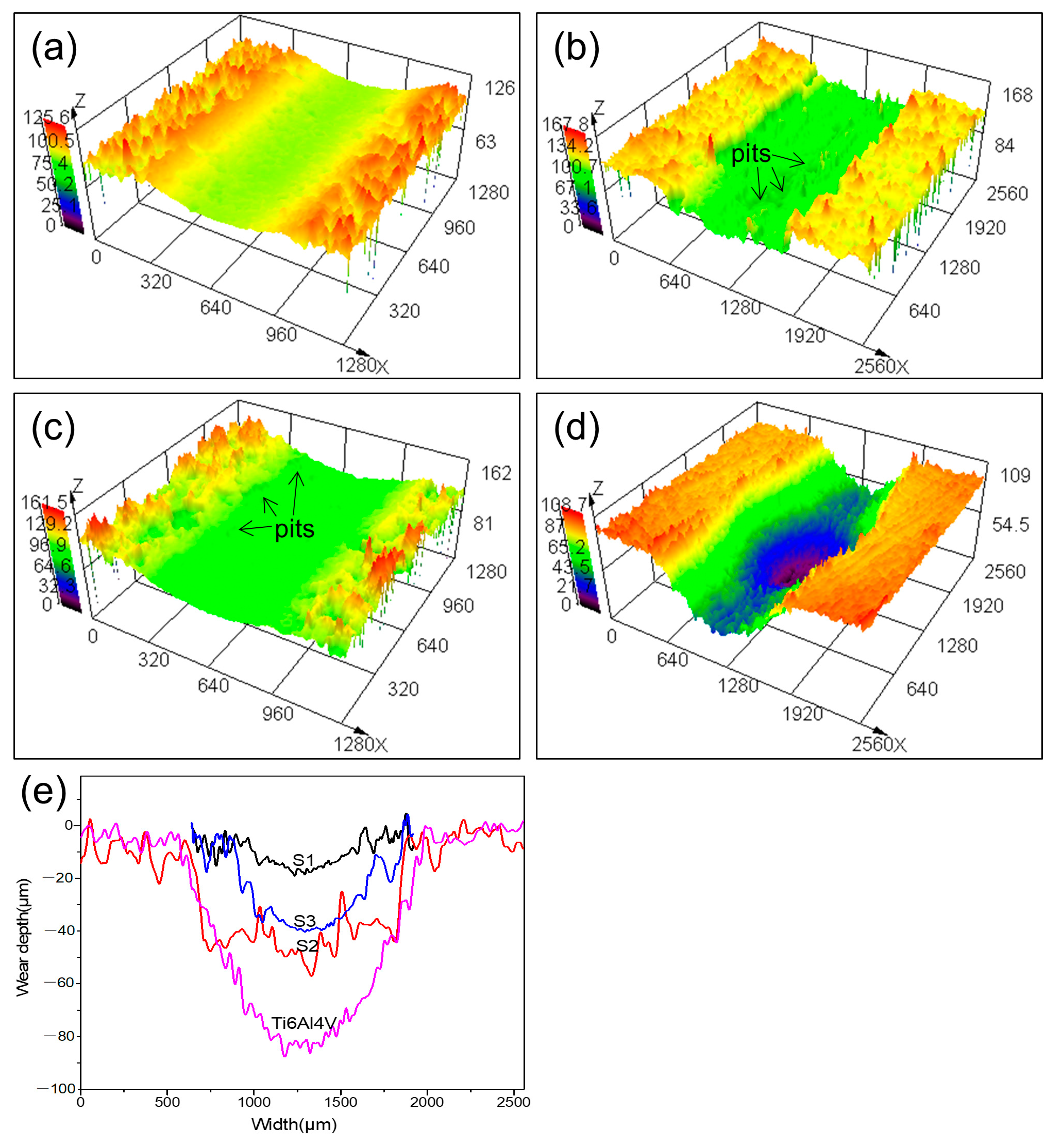
3.5.2. Friction Morphology
3.5.3. Wear Rate
4. Conclusions
- (1)
- The PEO coating that formed on the Ti6Al4V alloy with CS-coating under soft sparking mode had a more compact structure, relatively higher α-Al2O3 content, and higher micro hardness than that formed under unipolar mode.
- (2)
- The PEO coating that formed on Ti6Al4V base alloy was mainly composed of TiO2 and contained cracks and pores.
- (3)
- Among all the samples, the PEO coating that formed on CS-coating under soft sparking mode exhibited the best wear resistance with a wear rate of 1.18 × 10−5 mm3/(Nm). The excellent wear resistance of the coating depended on the higher α-Al2O3 content and compact structure of the coating.
- (4)
- The investigations indicated that the combination of cold spraying and PEO under soft sparking mode is a promising technique for improving the wear resistance of titanium alloys. Further research is needed to reduce the COF of the PEO coating formed under soft sparking mode.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Leyens, C.; Peters, M. Titanium and Titanium Alloys: Fundamentals and Applications; Wiley-VCH: Weinheim, Germany, 2003; pp. 19–22. [Google Scholar]
- Boyer, R.R. An overview on the use of titanium in the aerospace industry. Mater. Sci. Eng. A-Struct. 1996, 213, 103–114. [Google Scholar] [CrossRef]
- Zhang, T.G.; Zhuang, H.F.; Zhang, Q.; Yao, B.; Yang, F. Influence of Y2O3 on the microstructure and tribological properties of Ti-based wear-resistant laser-clad layers on TC4 alloy. Ceram. Int. 2020, 46, 13711–13723. [Google Scholar] [CrossRef]
- Li, X.; Hu, G.; Tian, J.; Tian, W.; Xie, W.; Li, X. Wear resistance enhancement of Ti-6Al-4 V Alloy by applying Zr-modified silicide coatings. J. Mater. Eng. Perform. 2018, 27, 1073–1082. [Google Scholar] [CrossRef]
- Jin, J.; Duan, H.; Li, X. The influence of plasma nitriding on microstructure and properties of CrN and CrNiN coatings on Ti6Al4V by magnetron sputtering. Vacuum 2017, 136, 112–120. [Google Scholar] [CrossRef]
- Liu, W.; Blawert, C.; Zheludkevich, M.L.; Lin, Y.; Talha, M.; Shi, Y.; Chen, L. Effects of graphene nanosheets on the ceramic coatings formed on Ti6Al4V alloy drill pipe by plasma electrolytic oxidation. J. Alloy Compd. 2019, 789, 996–1007. [Google Scholar] [CrossRef]
- Mu, M.; Liang, J.; Zhou, X.; Xiao, Q. One-step preparation of TiO2/MoS2 composite coating on Ti6Al4V alloy by plasma electrolytic oxidation and its tribological properties. Surf. Coat. Tech. 2013, 214, 124–130. [Google Scholar] [CrossRef]
- Kaseem, M.; Fatimah, S.; Nashrah, N.; Ko, Y.G. Recent progress in surface modification of metals coated by plasma electrolytic oxidation: Principle, structure, and performance. Prog. Mater. Sci. 2021, 117, 100735. [Google Scholar] [CrossRef]
- Bailing, J.; Yaming, W. Plasma electrolytic oxidation treatment of aluminium and titanium alloys. In Surface Engineering of Light Alloys; Dong, H., Ed.; Woodhead Publishing: Cambridge, UK, 2010; Volume 5, pp. 110–154. [Google Scholar]
- Fazel, M.; Salimijazi, H.R.; Golozar, M.A.; Garsivaz Jazi, M.R. A comparison of corrosion, tribocorrosion and electrochemical impedance properties of pure Ti and Ti6Al4V alloy treated by micro-arc oxidation process. Appl. Surf. Sci. 2015, 324, 751–756. [Google Scholar] [CrossRef]
- Qin, Y.K.; Xiong, D.S.; Li, J.L.; Tyagi, R. Compositions and tribological properties of PEO coatings on Ti6Al4V alloy. Surf. Eng. 2017, 33, 895–902. [Google Scholar] [CrossRef]
- Bertuccioli, C.; Garzoni, A.; Martini, C.; Morri, A.; Rondelli, G. Plasma electrolytic oxidation (PEO) layers from silicate/phosphate baths on Ti-6Al-4V for biomedical components: Influence of deposition conditions and surface finishing on dry sliding behaviour. Coatings 2019, 9, 614. [Google Scholar] [CrossRef] [Green Version]
- Simchen, F.; Sieber, M.; Kopp, A.; Lampke, T. Introduction to plasma electrolytic oxidation-an overview of the process and applications. Coatings 2020, 10, 628. [Google Scholar] [CrossRef]
- Tsai, D.; Chen, G.; Chou, C. Probe the micro arc softening phenomenon with pulse transient analysis in plasma electrolytic oxidation. Surf. Coat. Technol. 2019, 357, 235–243. [Google Scholar] [CrossRef]
- Rogov, A.B.; Yerokhin, A.; Matthews, A. The role of cathodic current in plasma electrolytic oxidation of aluminum: Phenomenological concepts of the “soft sparking” mode. Langmuir 2017, 33, 11059–11069. [Google Scholar] [CrossRef]
- Hakimizad, A.; Raeissi, K.; Santamaria, M.; Asghari, M. Effects of pulse current mode on plasma electrolytic oxidation of 7075 Al in Na2WO4 containing solution: From unipolar to soft-sparking regime. Electrochim. Acta 2018, 284, 618–629. [Google Scholar] [CrossRef]
- Koshuro, V.A.; Fomina, M.A.; Rodionov, I.V.; Fomin, A.A. Nanoporous structure of coatings formed by thermal spraying of aluminum oxide with further microarc oxidation on titanium alloy VT6 implants. Biomed. Eng. 2016, 50, 54–57. [Google Scholar] [CrossRef]
- Koshuro, V.; Fomin, A.; Rodionov, I. Composition, structure and mechanical properties of metal oxide coatings produced on titanium using plasma spraying and modified by micro-arc oxidation. Ceram. Int. 2018, 44, 12593–12599. [Google Scholar] [CrossRef]
- Kang, S.; Tu, W.; Han, J.; Li, Z.; Cheng, Y. A significant improvement of the wear resistance of Ti6Al4V alloy by a combined method of magnetron sputtering and plasma electrolytic oxidation (PEO). Surf. Coat. Technol. 2019, 358, 879–890. [Google Scholar] [CrossRef]
- Hu, C.; Chiu, P. Wear and corrosion resistance of pure titanium subjected to aluminization and coated with a microarc oxidation ceramic coating. Int. J. Electrochem. Sci. 2015, 10, 4290–4302. [Google Scholar]
- Moridi, A.; Hassani-Gangaraj, S.M.; Guagliano, M.; Dao, M. Cold spray coating: Review of material systems and future perspectives. Surf. Eng. 2014, 30, 369–395. [Google Scholar] [CrossRef]
- Huang, G.; Wang, H.; Li, X.; Xing, L.; Zhou, J. Deposition efficiency of low pressure cold sprayed aluminum coating. Mater. Manuf. Process. 2018, 33, 1100–1106. [Google Scholar] [CrossRef]
- Choi, W.B.; Li, L.; Luzin, V.; Neiser, R.; Gnäupel-Herold, T.; Prask, H.J.; Sampath, S.; Gouldstone, A. Integrated characterization of cold sprayed aluminum coatings. Acta Mater. 2007, 55, 857–866. [Google Scholar] [CrossRef]
- Mi, P.; Zhao, H.; Wang, T.; Ye, F. Sliding wear behavior of HVOF sprayed WC-(nano-WC-Co) coating at elevated temperatures. Mater. Chem. Phys. 2018, 206, 1–6. [Google Scholar] [CrossRef]
- Tsai, D.; Chou, C. Review of the soft sparking issues in plasma electrolytic oxidation. Metals 2018, 8, 105. [Google Scholar] [CrossRef] [Green Version]
- Martin, J.; Nominé, A.; Ntomprougkidis, V.; Migot, S.; Bruyère, S.; Soldera, F.; Belmonte, T.; Henrion, G. Formation of a metastable nanostructured mullite during plasma electrolytic oxidation of aluminium in “soft” regime condition. Mater. Des. 2019, 180, 107977. [Google Scholar] [CrossRef]
- Rogov, A.B.; Matthews, A.; Yerokhin, A. Role of cathodic current in plasma electrolytic oxidation of Al: A quantitative approach to in-situ evaluation of cathodically induced effects. Electrochim. Acta 2019, 317, 221–231. [Google Scholar] [CrossRef]
- Patnaik, P. Handbook of Inorganic Chemicals; McGraw-Hill Professional: New York, NY, USA, 2002; pp. 11–12. [Google Scholar]
- Hussein, R.O.; Nie, X.; Northwood, D.O. Effect of current mode on the plasma discharge, microstructure and corrosion resistance of oxide coatings produced on 1100 aluminum alloy by plasma electrolytic oxidation. WIT Trans. Eng. Sci. 2019, 124, 3–16. [Google Scholar]
- Hussein, R.O.; Nie, X.; Northwood, D.O.; Yerokhin, A.; Matthews, A. Spectroscopic study of electrolytic plasma and discharging behaviour during the plasma electrolytic oxidation (PEO) process. J. Phys. D 2010, 43, 105203. [Google Scholar] [CrossRef]
- Kumari, R.; Blawert, C.; Majumdar, J.D. Microstructures and properties of plasma electrolytic oxidized Ti alloy (Ti–6Al–4V) for bio-implant application. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2016, 47, 788–800. [Google Scholar] [CrossRef]
- Ríos, J.M.; Quintero, D.; Castaño, J.G.; Echeverría, F.; Gómez, M.A. Comparison among the lubricated and unlubricated tribological behavior of coatings obtained by PEO on the Ti6Al4V alloy in alkaline solutions. Tribol. Int. 2018, 128, 1–8. [Google Scholar] [CrossRef]
- Tekin, K.C.; Malayoglu, U.; Shrestha, S. Tribological behaviour of plasma electrolytic oxide coatings on Ti6Al4V and cp-Ti alloys. Surf. Eng. 2016, 32, 435–442. [Google Scholar] [CrossRef]
- Wheeler, J.M.; Collier, C.A.; Paillard, J.M.; Curran, J.A. Evaluation of micromechanical behaviour of plasma electrolytic oxidation (PEO) coatings on Ti-6Al-4V. Surf. Coat. Technol. 2010, 204, 3399–3409. [Google Scholar] [CrossRef]
- Dehnavi, V.; Liu, X.Y.; Luan, B.L.; Shoesmith, D.W.; Rohani, S. Phase transformation in plasma electrolytic oxidation coatings on 6061 aluminum alloy. Surf. Coat. Technol. 2014, 251, 106–114. [Google Scholar] [CrossRef]
- Cheng, Y.; Cao, J.; Mao, M.; Peng, Z.; Skeldon, P.; Thompson, G.E. High growth rate, wear resistant coatings on an Al-Cu-Li alloy by plasma electrolytic oxidation in concentrated aluminate electrolytes. Surf. Coat. Technol. 2015, 269, 74–82. [Google Scholar] [CrossRef]
- Sieber, M.; Simchen, F.; Morgenstern, R.; Scharf, I.; Lampke, T. Plasma electrolytic oxidation of high-strength aluminium alloys—substrate effect on wear and corrosion performance. Metals 2018, 8, 356. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.-k.; Yang, Z.; Wang, R.-q.; Wu, G.-r.; Chen, D.; Wang, D.-d.; Liu, X.-t.; Li, D.-l.; Guo, C.-h.; Yu, S.-x.; et al. An investigation of microstructure evolution for plasma electrolytic oxidation (PEO) coated Al in an alkaline silicate electrolyte. Surf. Coat. Technol. 2018, 351, 136–152. [Google Scholar] [CrossRef]
- Krishna, L.R.; Gupta, P.S.V.N.; Sundararajan, G. The influence of phase gradient within the micro arc oxidation (MAO) coatings on mechanical and tribological behaviors. Surf. Coat. Technol. 2015, 269, 54–63. [Google Scholar] [CrossRef]
- Benea, L.; Mardare-Danaila, E.; Celis, J. Increasing the tribological performances of Ti–6Al–4V alloy by forming a thin nanoporous TiO2 layer and hydroxyapatite electrodeposition under lubricated conditions. Tribol. Int. 2014, 78, 168–175. [Google Scholar] [CrossRef]
- Yetim, A.F.; Yildiz, F.; Vangolu, Y.; Alsaran, A.; Celik, A. Several plasma diffusion processes for improving wear properties of Ti6Al4V alloy. Wear 2009, 267, 2179–2185. [Google Scholar] [CrossRef]
- Archard, J.F. Contact and rubbing of flat surfaces. J. Appl. Phys. 1953, 24, 981–988. [Google Scholar] [CrossRef]



| Al | V | O | Fe | Ti |
|---|---|---|---|---|
| 5.5~6.9 | 3.5~4.5 | <0.2 | <0.4 | balance |
| Sample Code | Mode | Time (s) | I+ (A) | I− (A) | (μs) | (μs) | (μs) | (μs) | Qp/Qn |
|---|---|---|---|---|---|---|---|---|---|
| S1 | Soft sparking | 1320 | 1.2 | 1.33 | 900 | 100 | 900 | 100 | 0.9 |
| S2 | unipolar | 1320 | 1.2 | N/A | 900 | 1100 | N/A | N/A | ∞ |
| Sample Code | Time (s) | U+ (V) | U− (V) | (μs) | (μs) | (μs) | |
|---|---|---|---|---|---|---|---|
| S3 | 1020 | 350 | 60 | 900 | 100 | 900 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shao, M.; Wang, W.; Yang, H.; Zhang, X.; He, X. Preparation of Wear-Resistant Coating on Ti6Al4V Alloy by Cold Spraying and Plasma Electrolytic Oxidation. Coatings 2021, 11, 1288. https://doi.org/10.3390/coatings11111288
Shao M, Wang W, Yang H, Zhang X, He X. Preparation of Wear-Resistant Coating on Ti6Al4V Alloy by Cold Spraying and Plasma Electrolytic Oxidation. Coatings. 2021; 11(11):1288. https://doi.org/10.3390/coatings11111288
Chicago/Turabian StyleShao, Mingzeng, Wei Wang, Hongbo Yang, Xueer Zhang, and Xiaomei He. 2021. "Preparation of Wear-Resistant Coating on Ti6Al4V Alloy by Cold Spraying and Plasma Electrolytic Oxidation" Coatings 11, no. 11: 1288. https://doi.org/10.3390/coatings11111288






