Influence of α-Al2O3 Template and Process Parameters on Atomic Layer Deposition and Properties of Thin Films Containing High-Density TiO2 Phases
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
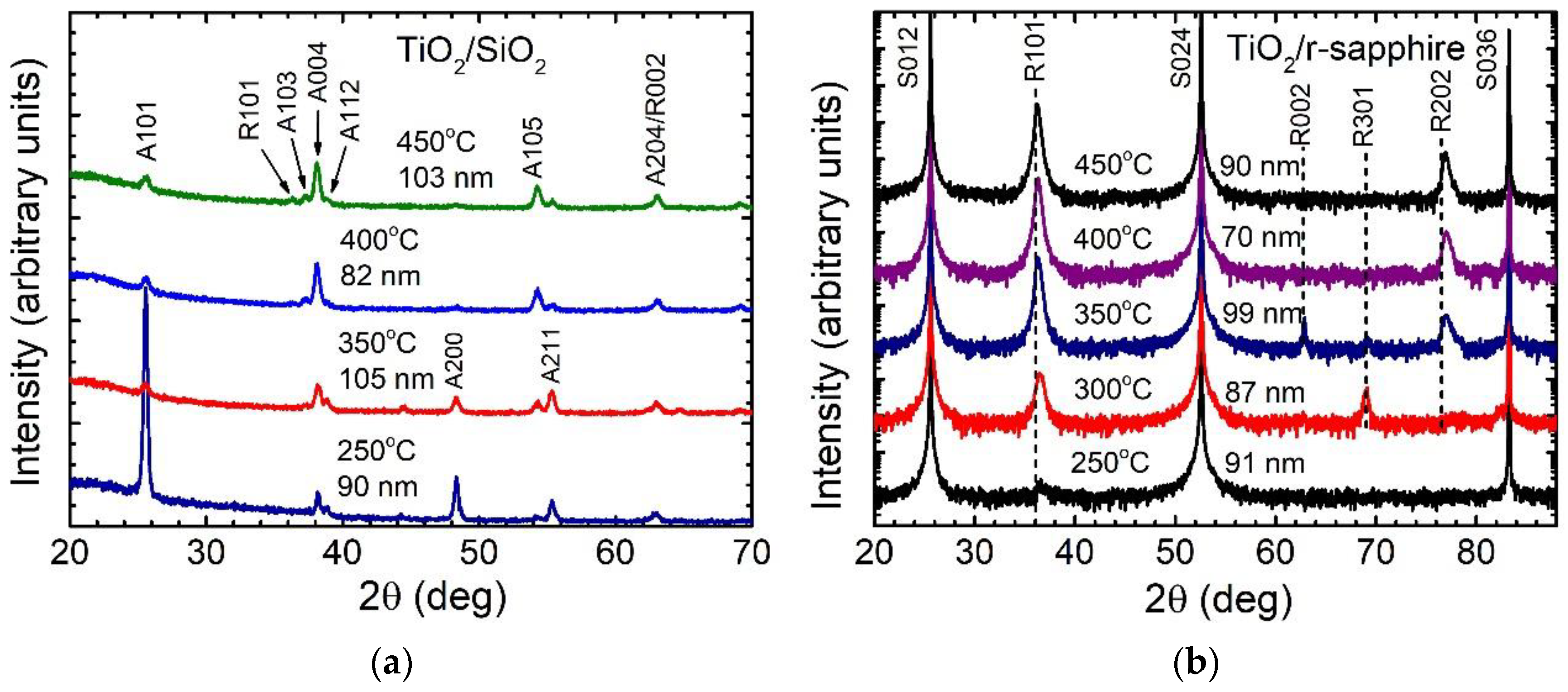
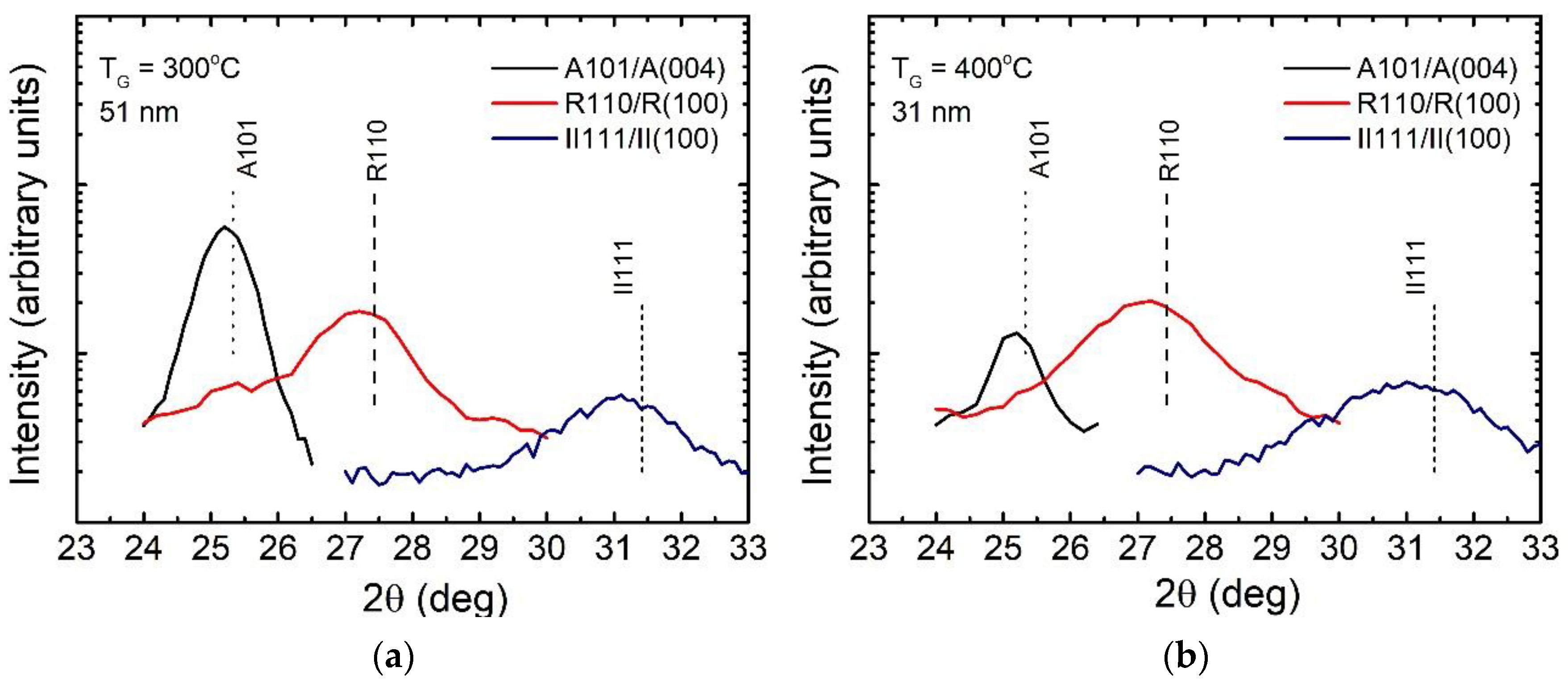
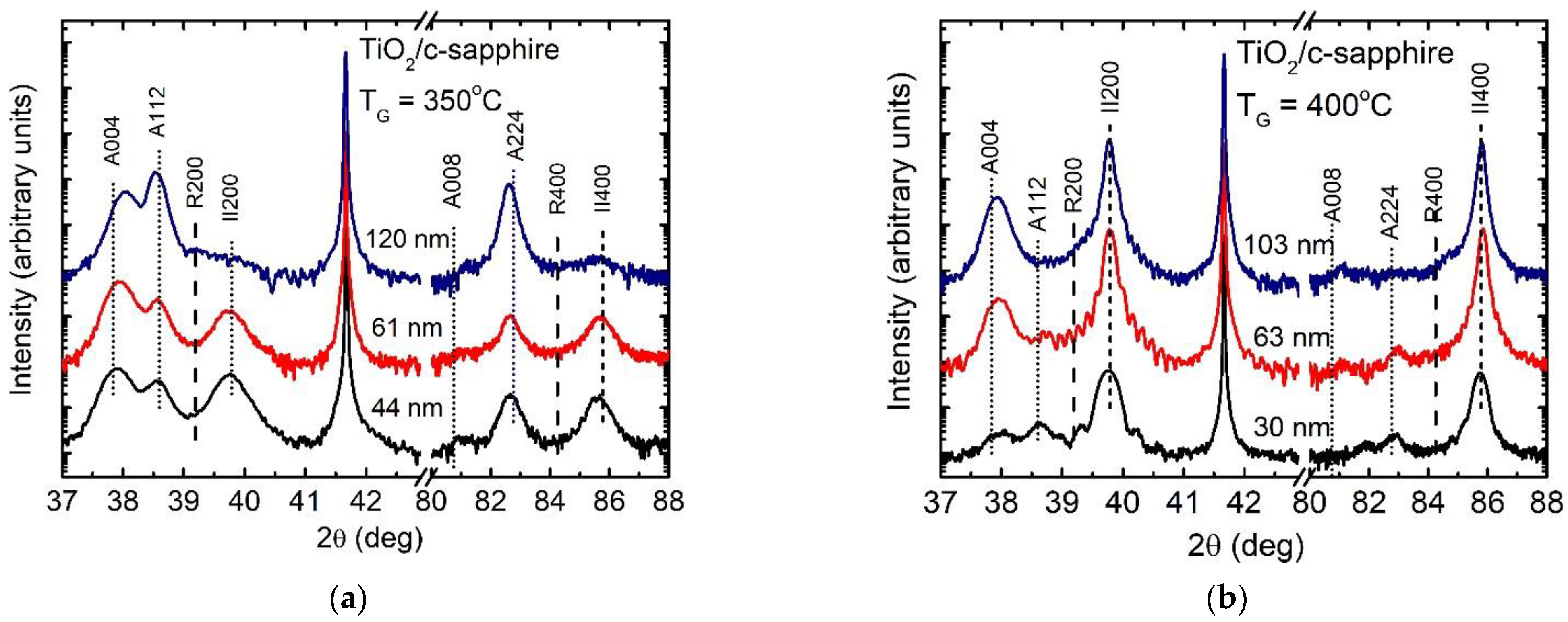
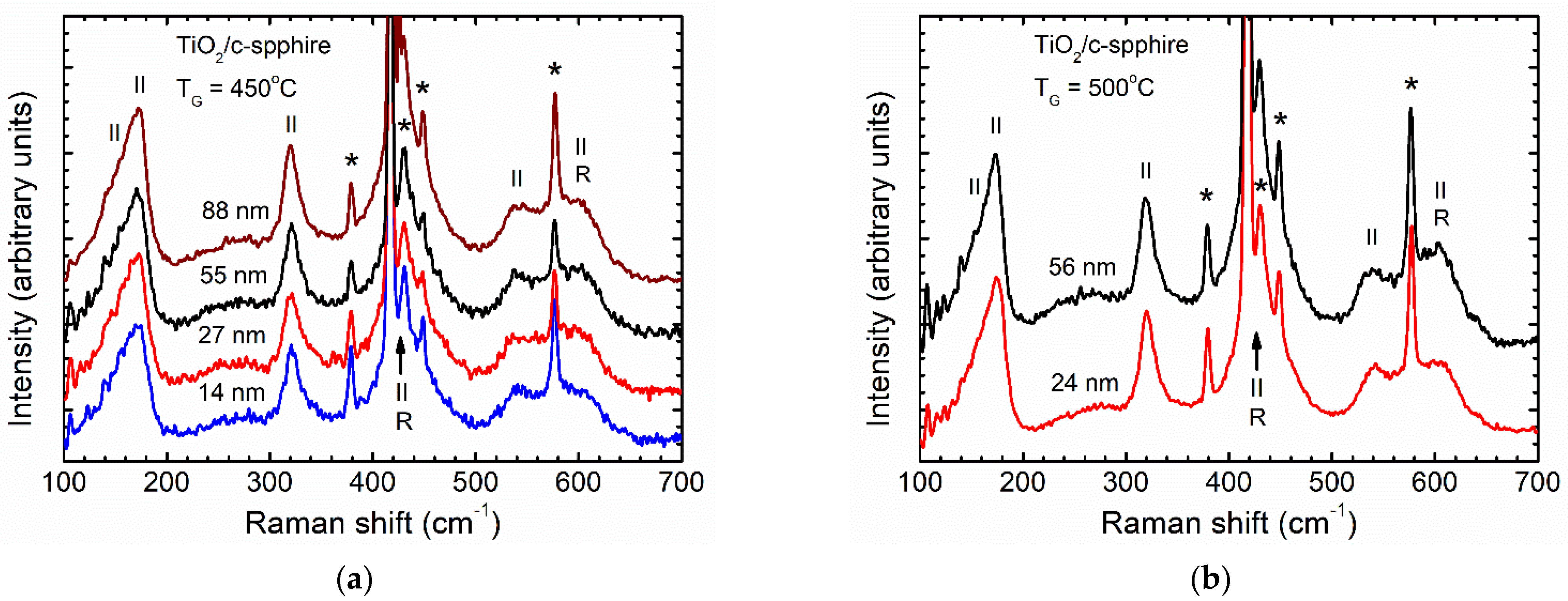
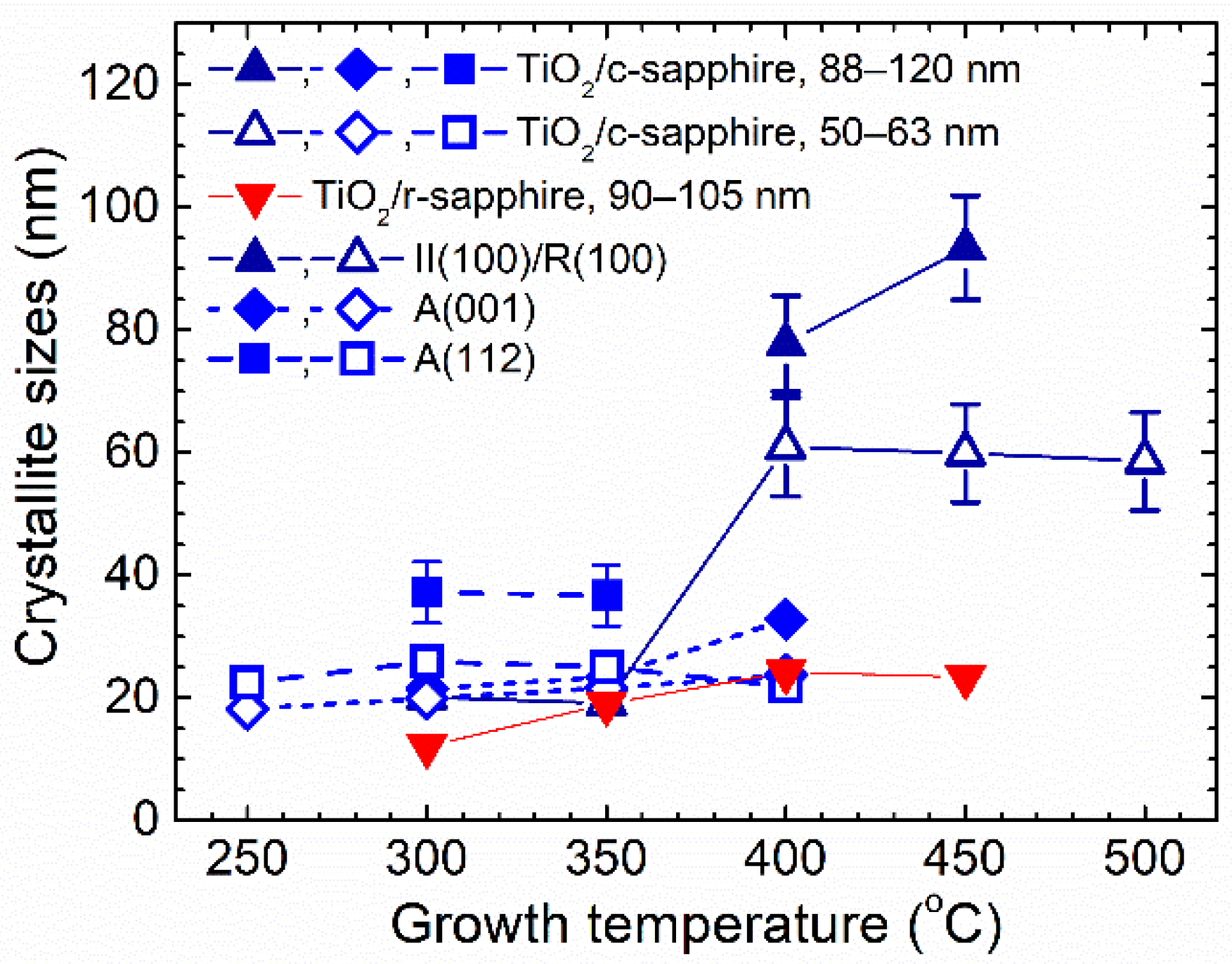
3.1. Effect of Growth Temperature and Film Thickness on Crystal Structure
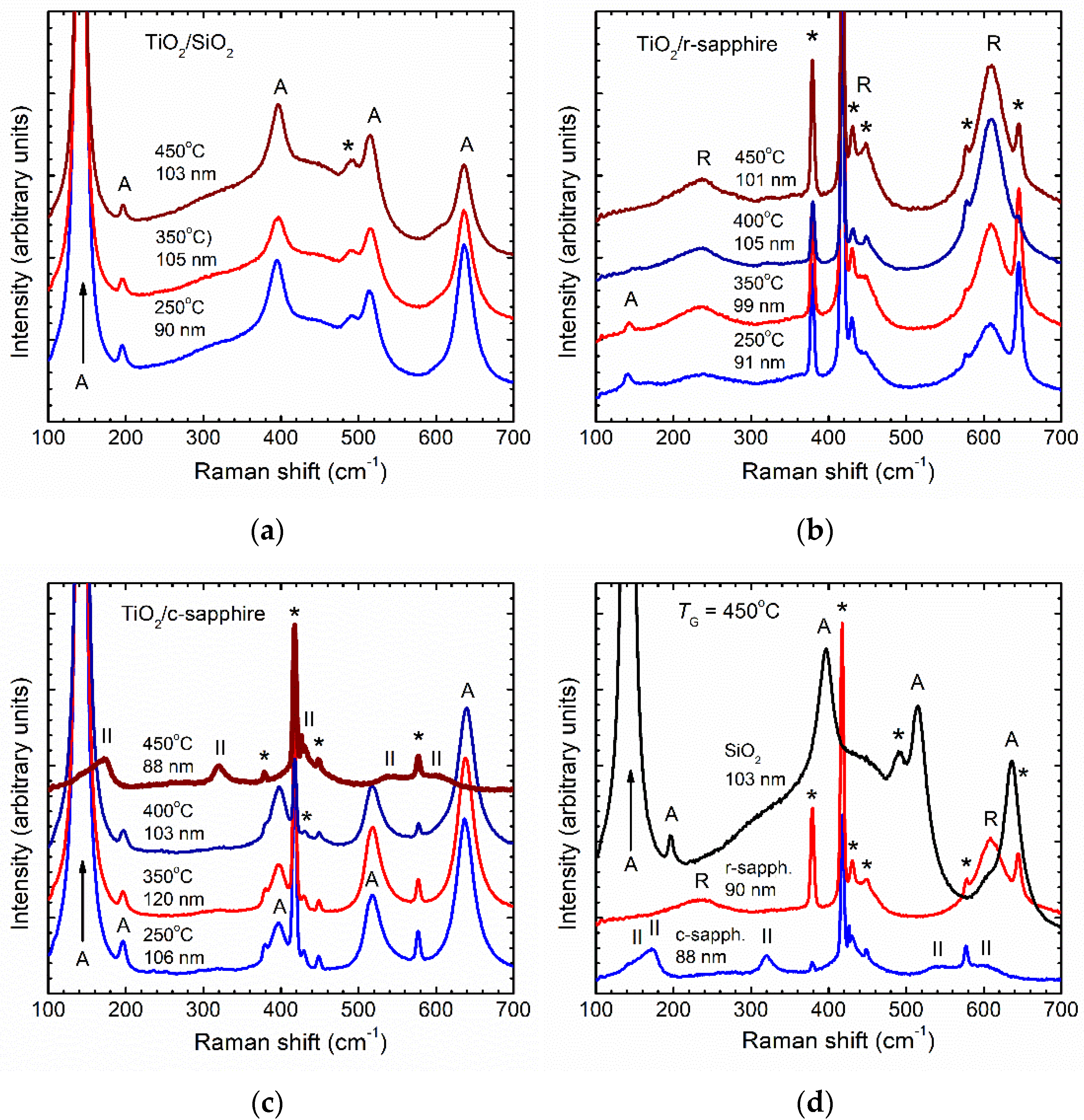
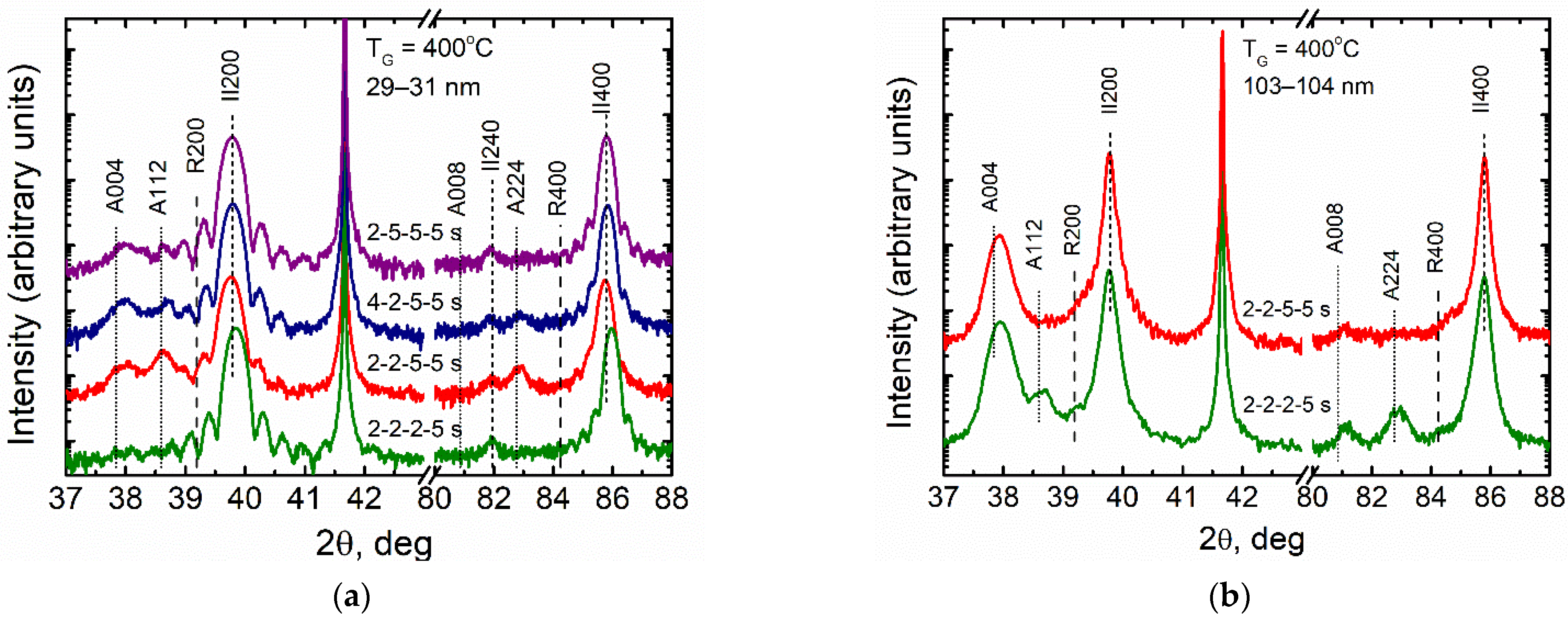
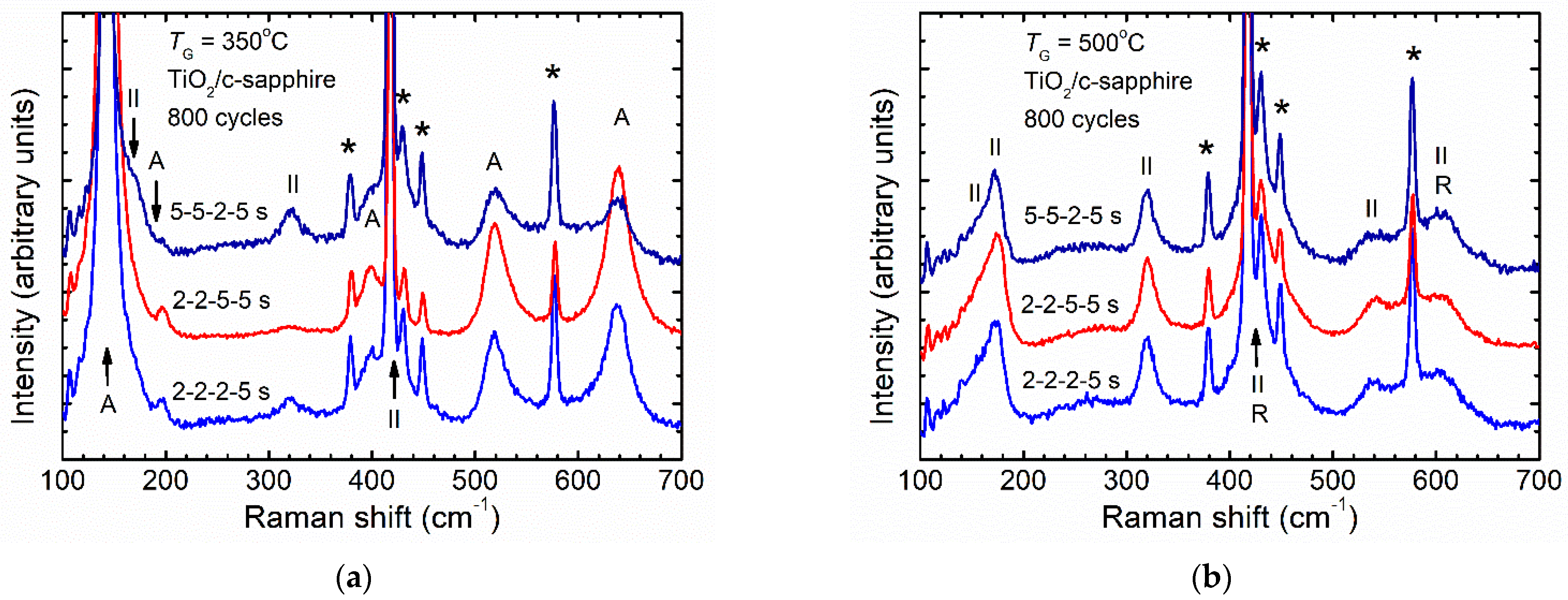
3.2. Effect of ALD Process Time Parameters on Phase Composition
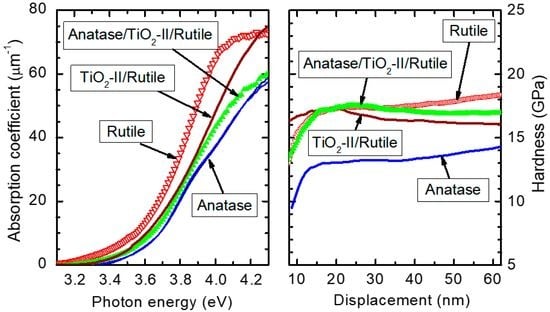
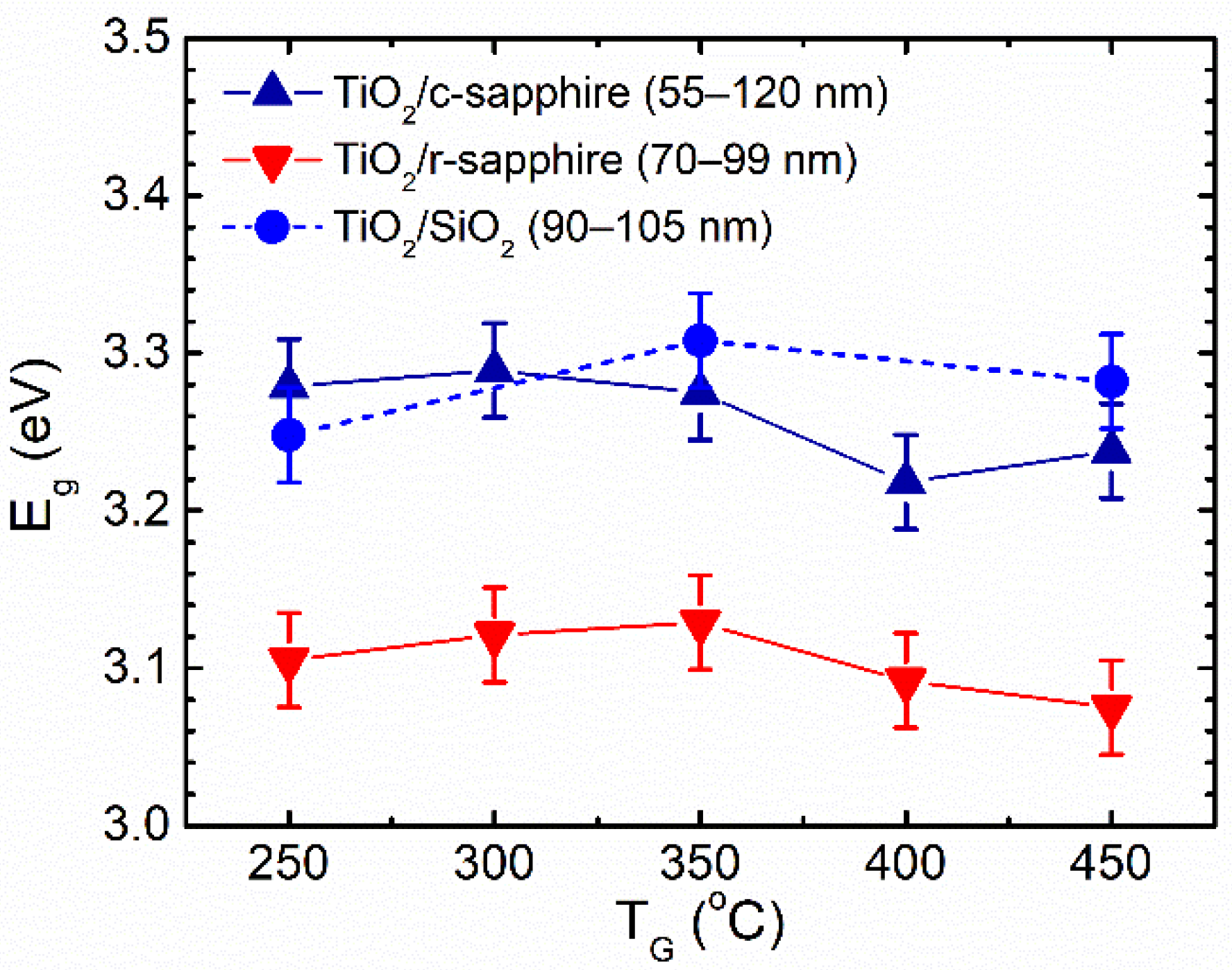
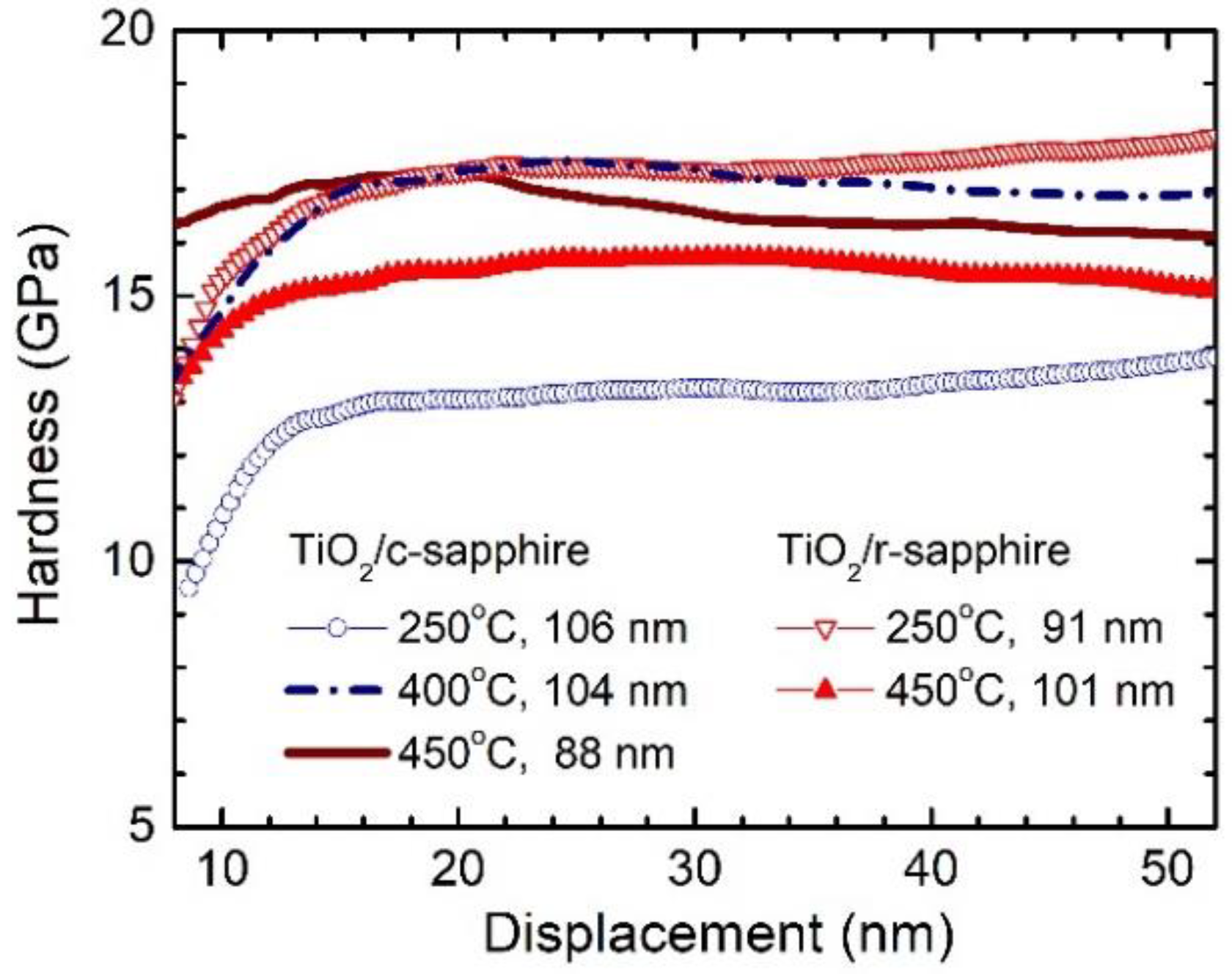
3.3. Influence of Phase Composition on the Growth Rate and Properties of Films
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Šubrt, J.; Criado, J.M.; Szatmáry, L.; Diánez-Millán, M.J.; Murafa, N.; Péres-Maqueda, L.A.; Brezová, V. Mechanochemical synthesis of visible light sensitive titanium dioxide photocatalyst. Int. J. Photoenergy 2011, 2011, 156941. [Google Scholar] [CrossRef] [Green Version]
- Murata, H.; Kataoka, Y.; Kawamoto, T.; Tanaka, I.; Taniguchi, T. Photocatalytic activity of α-PbO2-type TiO2. Phys. Status Solidi RRL 2014, 10, 822–826. [Google Scholar] [CrossRef]
- Razavi-Khosroshahi, H.; Edalati, K.; Hirayama, M.; Emami, H.; Arita, M.; Yamauchi, M.; Hagiwara, H.; Ida, S.; Ishihara, T.; Akiba, E.; et al. Visible-light-driven photocatalytic hydrogen generation on nanosized TiO2-II stabilized by high-pressure torsion. ACS Catal. 2016, 6, 5103–5107. [Google Scholar] [CrossRef]
- Edalati, K.; Wang, Q.; Eguchi, H.; Razavi-Khosroshahi, H.; Emami, H.; Yamauchi, M.; Fuji, M.; Horita, Z. Impact of TiO2-II phase stabilized in anatase matrix by high-pressure torsion on electrocatalytic hydrogen production. Mater. Res. Lett. 2019, 7, 334–339. [Google Scholar] [CrossRef] [Green Version]
- Hidalgo-Jimenes, J.; Wang, Q.; Edalati, K.; Cubero-Sesín, J.M.; Razavi-Khosroshahi, H.; Ikoma, Y.; Gutiérrez-Fallas, D.; Dittel-Meza, F.A.; Rodríguez-Rufino, J.C.; Fuji, M.; et al. Phase transformations, vacancy formation and variations of optical and photocatalytic properties in TiO2-ZnO composites by high-pressure torsion. Int. J. Plast. 2020, 124, 170–185. [Google Scholar] [CrossRef]
- Wang, Q.; Watanabe, M.; Edalati, K. Visible-light photocurrent in nanostructured high-pressure TiO2-II (columbite) phase. J. Phys. Chem. C 2020, 124, 13930–13935. [Google Scholar] [CrossRef]
- Wang, Y.; Saitow, K. Mechanochemical synthesis of red-light-active green TiO2 photocatalysts with disorder: Defect-rich, with polymorphs, and no metal loading. Chem. Mater. 2020, 32, 9190–9200. [Google Scholar] [CrossRef]
- Mukai, K.; Yamada, I. Columbite-type TiO2 as a negative electrode material for lithium-ion batteries. J. Electrochem. Soc. 2017, 164, A3590–A3594. [Google Scholar] [CrossRef]
- Tang, Y.; Li, Y.; Guo, W.; Wang, J.; Li, X.; Chen, S.; Mu, S.; Zhao, Y.; Gao, F. A highly ordered multi-layered hydrogenated TiO2-II phase nanowire array negative electrode for 2.4 eV aqueous asymmetric supercapacitors with high energy density and long cycle life. J. Mater. Chem. A 2018, 6, 623–632. [Google Scholar] [CrossRef]
- Das, A.; Chowdhury, B.N.; Saha, R.; Sikdar, S.; Bhunia, S.; Chattopadyay, S. Ultrathin vapor-liquid-solid grown titanium dioxide-II film on bulk GaAs substrates for advanced metal-oxide-semiconductor device applications. IEEE Trans. Electron Devices 2018, 65, 1466–1472. [Google Scholar] [CrossRef]
- Pushpa, M.D.; Crespo, M.S.; Cristopher, M.M.; Karthick, P.; Sridharam, M.; Sanjeeviraja, C.; Jeyadheepan, K. Influence of pyrolytic temperature on optoelectronic properties and the energy harvesting applications of high pressure TiO2 thin films. Vacuum 2019, 161, 81–91. [Google Scholar] [CrossRef]
- Bendeliany, N.A.; Popova, S.V.; Vereshchagin, L.F. New modification of titanium dioxide obtained at high pressures. Geochemistry 1966, 5, 499–501. [Google Scholar]
- Simons, P.Y.; Dachille, F. The structure of TiO2 II, a high-pressure phase of TiO2. Acta Crystallogr. 1967, 23, 334–336. [Google Scholar] [CrossRef]
- Mcqueen, R.G.; Jamieson, J.C.; Marsh, S.P. Shock-wave compression and x-ray studies of titanium dioxide. Science 1967, 155, 1401–1404. [Google Scholar] [CrossRef] [PubMed]
- Linde, R.K.; DeCarli, P.S. Polymorphic behaviour of titania under dynamic loading. J. Chem. Phys. 1969, 50, 319–325. [Google Scholar] [CrossRef]
- Gerward, L.; Olsen, J.S. Post-rutile phases in TiO2. J. Appl. Cryst. 1997, 30, 259–264. [Google Scholar] [CrossRef]
- Arlt, T.; Bermejo, M.; Blanco, M.A.; Gerward, L.; Jiang, J.Z.; Olsen, J.S.; Recio, J.M. High-pressure polymorphs of anatase TiO2. Phys. Rev. B 2000, 61, 14414–14419. [Google Scholar] [CrossRef] [Green Version]
- Sekiya, T.; Ohta, S.; Kamei, S.; Hanakawa, M.; Kurita, S. Raman spectroscopy and phase transition of anatase TiO2 under high pressure. J. Phys. Chem. Solids 2001, 62, 717–721. [Google Scholar] [CrossRef]
- Ohta, S.; Sekiya, T.; Kurita, S. Pressure dependence of optical properties of anatase TiO2 single crystal. Phys. Stat. Sol. B 2001, 223, 265–269. [Google Scholar] [CrossRef]
- Liu, W.; Chen, J.; Zhang, X.; Yan, J.; Hou, M.; Kunz, M.; Zhang, D.; Zhang, H. Pressure-induced phase transitions of natural brookite. ACS Earth Space Chem. 2019, 3, 844–853. [Google Scholar] [CrossRef] [Green Version]
- Begin-Colin, S.; Le Caer, G.; Mocellin, A.; Zadona, M. Polymorphic transformations of titania induced by ball milling. Philos. Mag. Lett. 1994, 69, 1–7. [Google Scholar] [CrossRef]
- Rinaudo, M.G.; Beltrán, A.M.; Fernández, M.A.; Cadús, L.E.; Morales, M.R. Tailoring materials by high-energy ball milling: TiO2 mixtures for catalyst support applications. Mater. Today Chem. 2020, 17, 100340. [Google Scholar] [CrossRef]
- Dubrovinskaia, N.A.; Dubrovinsky, L.S.; Ahuja, R.; Prokopenko, V.B.; Dmitriev, V.; Weber, H.-P.; Osorio-Guillen, J.M.; Johansson, B. Experimental and theoretical identification of a new high-pressure TiO2 polymorph. Phys. Rev. Lett. 2001, 87, 275501. [Google Scholar] [CrossRef]
- Dyuzheva, T.I.; Lityagina, L.M.; Bendeliani, N.A. hydrothermal crystal growth of the high-pressure phases of α-PbO2 and TiO2-II. J. Alloys Compunds 2004, 377, 17–20. [Google Scholar] [CrossRef]
- Filatov, S.K.; Bendeliani, N.A.; Albert, B.; Kopf, J.; Dyuzheva, T.I.; Lityagina, L.M. Crystalline Structure of the TiO2II High-Pressure Phase at 293, 223, and 133 K According to Single-Crystal X-ray Diffraction Data. Dokl. Phys. 2007, 52, 195–199. [Google Scholar] [CrossRef]
- Spektor, K.; Tran, K.S.; Leinenweber, K.; Häussermann, U. Transformation of rutile to TiO2-II in a high pressure hydrothermal environment. J. Solid State Chem. 2013, 206, 209–216. [Google Scholar] [CrossRef]
- Manuputty, M.Y.; Dreyer, J.A.H.; Sheng, Y.; Bringley, E.J.; Botero, M.L.; Akroyd, J.; Kraft, M. Polymorphism of nanocrystalline TiO2 prepared in a stagnation flame: Formation of the TiO2-II phase. Chem. Sci. 2019, 10, 1342. [Google Scholar] [CrossRef] [Green Version]
- Pennington, A.M.; Halim, H.; Shi, J.; Kear, B.H.; Celik, F.E.; Tse, S.D. Low-pressure flame synthesis of carbon-stabilized TiO2-II (srilankite) nanoparticles. J. Aerosol Sci. 2021, 156, 105775. [Google Scholar] [CrossRef]
- Chang, H.L.M.; You, H.; Guo, J.; Lam, D.J. Epitaxial TiO2 and VO2 films prepared by MOCVD. Appl. Surf. Sci. 1991, 48–49, 12–18. [Google Scholar] [CrossRef]
- Mitchell, D.R.G.; Triani, G.; Attard, D.J.; Finnie, K.S.; Evans, P.J.; Barbé, C.J.; Bartlett, J.R. Atomic layer deposition of TiO2 and Al2O3 thin films and nanolaminates. Smart Mater. Struct. 2006, 15, S57–S64. [Google Scholar] [CrossRef]
- Kasikov, A.; Aarik, J.; Mändar, H.; Moppel, M.; Pärs, M.; Uustare, T. Refractive index gradients in TiO2 thin films grown by atomic layer deposition. J. Phys. D Appl. Phys. 2006, 39, 54–60. [Google Scholar] [CrossRef]
- Tarre, A.; Möldre, K.; Niilisk, A.; Mändar, H.; Aarik, J.; Rosental, A. Atomic layer deposition of epitaxial TiO2 II on c-sapphire. J. Vac. Sci. Technol. A 2013, 31, 01A118. [Google Scholar] [CrossRef]
- Möldre, K.; Aarik, L.; Mändar, H.; Niilisk, A.; Rammula, R.; Tarre, A.; Aarik, J. Atomic layer deposition of rutile and TiO2-II from TiCl4 and O3 on sapphire: Influence of substrate orientation on thin film structure. J. Crystallogr. Growth 2015, 428, 86–92. [Google Scholar] [CrossRef]
- Möls, K.; Aarik, L.; Mändar, H.; Kasikov, A.; Niilisk, A.; Rammula, R.; Aarik, J. Influence of phase composition on optical properties of TiO2: Dependence of refractive index and band gap on formation of TiO2-II phase in thin films. Opt. Mater. 2019, 96, 109335. [Google Scholar] [CrossRef]
- Zhao, W.N.; Zhu, S.-C.; Li, Y.-F.; Liu, Z.-P. Three-phase junction for modulating electron-hole migration in anatase–rutile photocatalysts. Chem. Sci. 2015, 6, 3483–3494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shang, C.; Zhao, W.N.; Liu, Z.-P. Searching for new TiO2 crystal phases with better photoactivity. J. Phys. Condens. Matter 2015, 27, 134203-1-8. [Google Scholar] [CrossRef] [PubMed]
- Dewhurst, J.K.; Lowther, J.E. High-pressure structural phases of titanium dioxide. Phys. Rev. B 1996, 54, R3673–R3675. [Google Scholar] [CrossRef]
- Schuisky, M.; Hårsta, A.; Aidla, A.; Kukli, K.; Kiisler, A.-A.; Aarik, J. Atomic layer chemical vapor deposition of TiO2: Low temperature epitaxy of rutile and anatase. J. Electrochem. Soc. 2000, 147, 3319–3325. [Google Scholar] [CrossRef]
- Arroval, T.; Aarik, L.; Rammula, R.; Mändar, H.; Aarik, J.; Hudec, B.; Hušekova, K.; Fröhlich, K. Influence of growth temperature on structure and electrical properties of high-permittivity TiO2 films in TiCl4-H2O and TiCl4-O3 atomic layer deposition processes. Phys. Status Solidi A Appl. Mater. Sci. 2014, 211, 425–432. [Google Scholar] [CrossRef]
- Hwang, S.-L.; Shen, P.; Chu, H.-T.; Yui, T.-F. Nanometer-size α-PbO2-type TiO2 in garnet: A thermobarometer for ultrahigh-pressure metamorphism. Science 2000, 288, 321–324. [Google Scholar] [CrossRef]
- Meng, D.W.; Wu, X.L.; Sun, F.; Huang, L.W.; Liu, F.; Han, Y.J.; Zheng, J.P.; Meng, X.; Mason, R. High-pressure polymorphic transformation of rutile to α-PbO2-type TiO2 at {0 1 1}R twin boundaries. Micron 2008, 39, 280–286. [Google Scholar] [CrossRef]
- Smith, F.C.; Glass, B.P.; Simonson, B.M.; Smith, J.P.; Krull-Davatzes, A.E.; Booksh, K.S. Shock-metamorphoused rutile grains containing the high-pressure polymorph TiO2-II in four Neoarchean spherule layers. Geology 2016, 44, 775–778. [Google Scholar] [CrossRef]
- Kusky, T.; Wang, L.; Robinson, P.T.; Huang, Y.; Wirth, R.; Ning, W.; Zhong, Y.; Polat, A. Ultra-high pressure inclusion in Archean ophiolitic podiform chromitite in mélange block suggests deep subduction on early Earth. Precambr. Res. 2021, 362, 106318. [Google Scholar] [CrossRef]
- Miikkulainen, V.; Leskelä, M.; Ritala, M.; Puurunen, R.L. Crystallinity of inorganic films grown by atomic layer deposition: Overview and general trends. J. Appl. Phys. 2013, 113, 021301. [Google Scholar] [CrossRef]
- Niemelä, J.-P.; Marin, G.; Karppinen, M. Titanium dioxide thin films by atomic layer deposition: A review. Semicond. Sci. Technol. 2017, 32, 093005. [Google Scholar] [CrossRef]
- Leskelä, M.; Ritala, M. Atomic layer deposition (ALD): From precursors to thin film structures. Thin Solid Films 2002, 409, 138–146. [Google Scholar] [CrossRef]
- George, S.M. Atomic layer deposition: An overview. Chem. Rev. 2010, 110, 111–131. [Google Scholar] [CrossRef] [PubMed]
- Kukli, K.; Aidla, A.; Aarik, J.; Schuisky, M.; Hårsta, A.; Ritala, M.; Leskelä, M. Real-time monitoring in atomic layer deposition of TiO2 from TiI4 and H2O–H2O2. Langmuir 2000, 16, 8122–8128. [Google Scholar] [CrossRef]
- Fröhlich, K.; Ťapajna, M.; Rosová, A.; Dobročka, E.; Hušeková, K.; Aarik, J.; Aidla, A. Growth of high-dielectric-constant TiO2 films in capacitors with RuO2 electrodes. Electrochem. Solid State Lett. 2008, 11, G19–G21. [Google Scholar] [CrossRef]
- Jõgi, I.; Pärs, M.; Aarik, J.; Aidla, A.; Laan, M.; Sundqvist, J.; Oberbeck, L.; Heitmann, J.; Kukli, K. Conformity and structure of titanium oxide films grown by atomic layer deposition on silicon substrates. Thin Solid Films 2008, 516, 4855–4862. [Google Scholar] [CrossRef]
- Raaijmakers, I. Current and future applications of ALD in micro-electronics. ECS Trans. 2011, 41, 3–17. [Google Scholar] [CrossRef]
- Graniel, O.; Weber, M.; Balme, S.; Miele, P.; Bechelany, M. Atomic layer deposition for biosensing applications. Biosens. Bioelectron. 2018, 122, 147–159. [Google Scholar] [CrossRef]
- Weber, M.; Julbe, A.; Kim, S.S.; Bechelany, M. Atomic layer deposition (ALD) on inorganic or polymeric membranes. J. Appl. Phys. 2019, 126, 041101. [Google Scholar] [CrossRef]
- Aarik, L.; Arroval, T.; Rammula, R.; Mändar, H.; Sammelselg, V.; Aarik, J. Atomic layer deposition of TiO2 from TiCl4 and O3. Thin Solid Films 2013, 542, 100–107. [Google Scholar] [CrossRef]
- Arroval, T.; Aarik, L.; Rammula, R.; Kruusla, V.; Aarik, J. Effect of substrate-enhanced and inhibited growth on atomic layer deposition and properties of aluminum-titanium oxide films. Thin Solid Films 2016, 600, 119–125. [Google Scholar] [CrossRef]
- Aarik, L.; Kozlova, J.; Mändar, H.; Aarik, J.; Sammelselg, V. Chemical resistance and protective properties of atomic layer deposited TiO2 and Al2O3 single-layer and multilayer coatings on silicon and stainless steel. Mater. Chem. Phys. 2019, 228, 285–292. [Google Scholar] [CrossRef]
- Aarik, J.; Aidla, A.; Uustare, T.; Kukli, K.; Sammelselg, V.; Ritala, M.; Leskelä, M. Atomic layer deposition of TiO2 thin films from TiI4 and H2O. Appl. Surf. Sci. 2002, 193, 277–286. [Google Scholar] [CrossRef]
- Schuisky, M.; Kukli, K.; Aarik, J.; Lu, J.; Hårsta, A. Epitaxial growth of TiO2 films in a hydroxyl-free ALD process. J. Crystallogr. Growth 2002, 235, 293–299. [Google Scholar] [CrossRef]
- Aarik, J.; Aidla, A.; Mändar, H.; Uustare, T.; Schuisky, M.; Hårsta, A. Atomic layer growth of epitaxial TiO2 thin films from TiCl4 and H2O on α-Al2O3 substrates. J. Crystal Growth 2002, 242, 189–198. [Google Scholar] [CrossRef]
- Chen, S.; Mason, M.G.; Gysling, H.J.; Paz-Pujalt, G.R.; Blanton, T.N.; Castro, T.; Chen, K.M.; Fictorie, C.P.; Gladfelter, W.L.; Franciosi, A.; et al. Ultrahigh vacuum metalorganic chemical vapor deposition growth and in situ characterization of epitaxial TiO2 films. J. Vac. Sci. Technol. A 1993, 11, 2419–2429. [Google Scholar] [CrossRef]
- Roch, T.; Dobročka, E.; Mikula, M.; Pidik, A.; Durina, P.; Haidry, A.A.; Plecenik, T.; Truchlý, M.; Grancic, B.; Plecenik, A.; et al. Strong biaxial texture and polymorph nature in TiO2 thin film formed by ex-situ annealing on c-plane Al2O3 surface. J. Crystallogr. Growth 2012, 338, 118–124. [Google Scholar] [CrossRef]
- Scherrer, P. Bestimmung der Größe und der inneren Struktur von Kolloidteilchen mittels Röntgenstrahlen. Nach. Ges. Wiss. Göttingen. 1918, 1918, 98–100. [Google Scholar]
- Grey, I.E.; Li, C.; Madsen, I.C.; Braunshausen, G. TiO2-II. Ambient pressure preparation and structure refinement. Mater. Res. Bull. 1988, 23, 743–753. [Google Scholar] [CrossRef]
- McCartney, M.R.; Smith, D.J. Studies of electron irradiation and annealing effects on TiO2 surfaces in ultrahigh vacuum using high-resolution electron microscopy. Surf. Sci. 1991, 250, 169–178. [Google Scholar] [CrossRef]
- Aarik, J.; Aidla, A.; Mändar, H.; Sammelselg, V. Anomalous effect of temperature on atomic layer deposition of titanium dioxide. J. Crystallogr. Growth 2000, 220, 531–537. [Google Scholar] [CrossRef]
- Aarik, J.; Karlis, J.; Mändar, H.; Uustare, T.; Sammelselg, V. Influence of structure development on atomic layer deposition of TiO2 thin films. Appl. Surf. Sci. 2001, 181, 339–348. [Google Scholar] [CrossRef]
- Rams, J.; Tejeda, A.; Cabrera, J.M. Refractive indices of rutile as a function of temperature and wavelength. J. Appl. Phys. 1997, 82, 994–997. [Google Scholar] [CrossRef]
- Jellison, G.E.; Boatner, L.A.; Budai, J.D.; Jeong, B.-S.; Norton, D.P. Spectroscopic ellipsometry of thin film and bulk anatase (TiO2). J. Appl. Phys. 2003, 93, 9537–9541. [Google Scholar] [CrossRef]
- Hong, W.Q. Extraction of extinction coefficient of weak absorbing thin films from special absorption. J. Phys. D Appl. Phys. 1989, 22, 1384–1385. [Google Scholar] [CrossRef]
- Rodríguez, J.; Gómez, M.; Ederth, J.; Niklasson, G.A.; Granqvist, C.G. Thickness dependence of the optical properties of sputter deposited Ti oxide films. Thin Solid Films 2000, 365, 119–125. [Google Scholar] [CrossRef]
- Makula, P.; Pacia, M.; Macyk, W. How to correctly determine the band gap energy of modified semiconductor photocatalysts based on UV–Vis spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef] [Green Version]
- Kulikovsky, V.; Ctvrtlik, R.; Vorlicek, V.; Filip, J.; Bohac, P.; Jastrabik, L. Mechanical properties and structure of TiO2 films deposited on quartz and silicon substrates. Thin Solid Films 2013, 542, 91–99. [Google Scholar] [CrossRef]
- Ylivaara, O.M.E.; Langner, A.; Liu, X.; Schneider, D.; Julin, J.; Arstila, K.; Sintonen, S.; Ali, S.; Lipsanen, H.; Sajavaara, T.; et al. Mechanical and optical properties of as-grown and thermally annealed titanium dioxide from titanium tetrachloride and water by atomic layer deposition. Thin Solid Films 2021, 732, 138758. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Möls, K.; Aarik, L.; Mändar, H.; Kasikov, A.; Jõgiaas, T.; Tarre, A.; Aarik, J. Influence of α-Al2O3 Template and Process Parameters on Atomic Layer Deposition and Properties of Thin Films Containing High-Density TiO2 Phases. Coatings 2021, 11, 1280. https://doi.org/10.3390/coatings11111280
Möls K, Aarik L, Mändar H, Kasikov A, Jõgiaas T, Tarre A, Aarik J. Influence of α-Al2O3 Template and Process Parameters on Atomic Layer Deposition and Properties of Thin Films Containing High-Density TiO2 Phases. Coatings. 2021; 11(11):1280. https://doi.org/10.3390/coatings11111280
Chicago/Turabian StyleMöls, Kristel, Lauri Aarik, Hugo Mändar, Aarne Kasikov, Taivo Jõgiaas, Aivar Tarre, and Jaan Aarik. 2021. "Influence of α-Al2O3 Template and Process Parameters on Atomic Layer Deposition and Properties of Thin Films Containing High-Density TiO2 Phases" Coatings 11, no. 11: 1280. https://doi.org/10.3390/coatings11111280
APA StyleMöls, K., Aarik, L., Mändar, H., Kasikov, A., Jõgiaas, T., Tarre, A., & Aarik, J. (2021). Influence of α-Al2O3 Template and Process Parameters on Atomic Layer Deposition and Properties of Thin Films Containing High-Density TiO2 Phases. Coatings, 11(11), 1280. https://doi.org/10.3390/coatings11111280