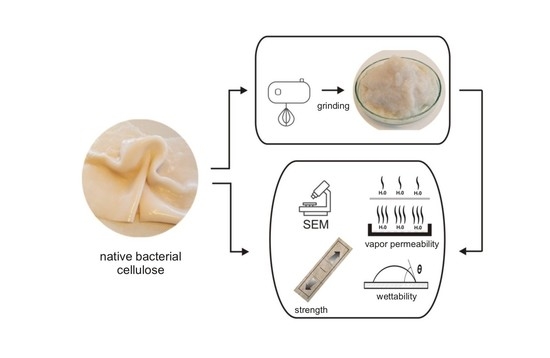
The Impact of the Mechanical Modification of Bacterial Cellulose Films on Selected Quality Parameters
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Tensile Strength Test
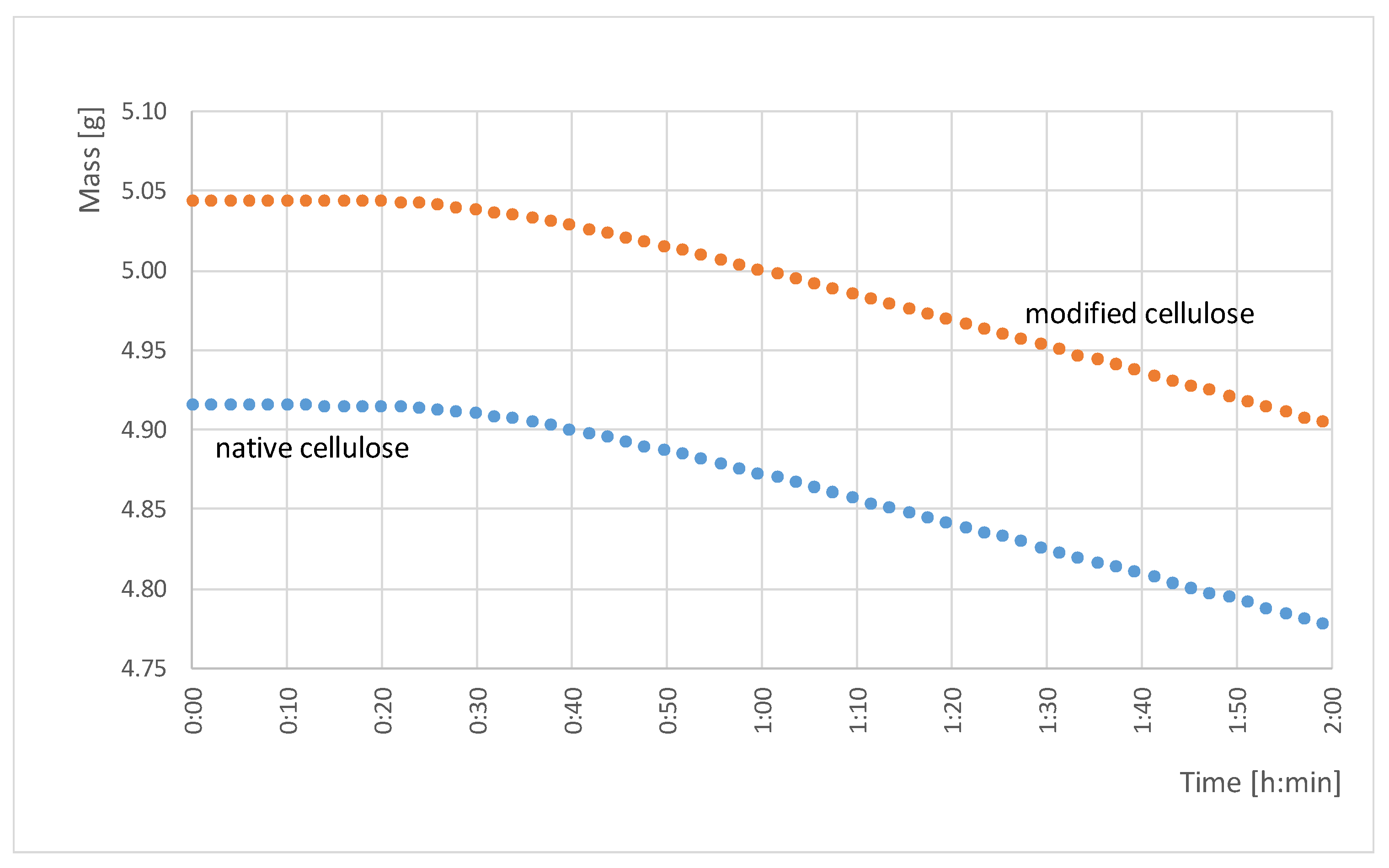
2.3. Water Vapor Transport and Hygroscopicity of Bacterial Cellulose
2.4. Determination of the Contact Angle and Surface Free Energy (SFE)
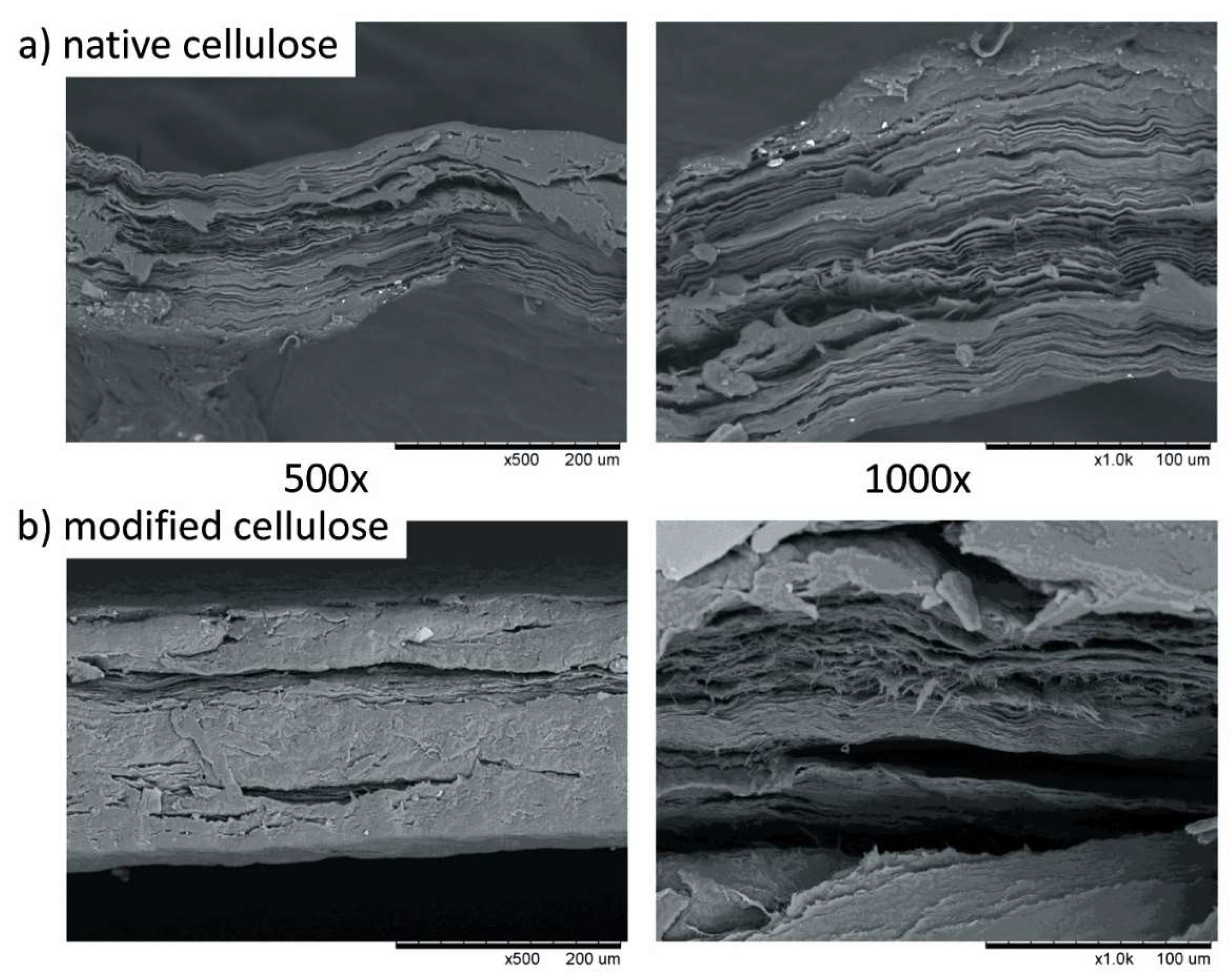
2.5. Scanning Electron Microscopy (SEM)
2.6. Test Standards
3. Results and Discussion
3.1. Assessment of Mechanical Properties
3.2. Water Vapor Permeability
3.3. Contact Angle and Surface Free Energy (SFE)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abba, M.; Abdullahi, M.; Nor, M.H.M.; Chong, C.S.; Ibrahim, Z. Isolation and characterisation of locally isolated Gluconacetobacter xylinus BCZM sp. with nanocellulose producing potentials. IET Nanobiotechnol. 2018, 12, 52–56. [Google Scholar] [CrossRef]
- Premjet, S.; Premjet, D.; Ohtani, Y. The effect of ingredients of sugar cane molasses on bacterial cellulose production by Acetobacter xylinum ATCC 10245. Sen-iGakkaishi 2007, 63, 193–199. [Google Scholar] [CrossRef] [Green Version]
- Skocaj, M. Bacterial nanocellulose in papermaking. Cellulose 2019, 26, 6477–6488. [Google Scholar] [CrossRef]
- Araújo, S.; Moreira da Silva, F.; Gouveia, I.C. The role of technology towards a new bacterial-cellulose-based material for fashion design. J. Ind. Intell. Inf. 2015, 3, 168–172. [Google Scholar] [CrossRef] [Green Version]
- Wacikowski, B.; Michałowski, M. The possibility of using bacterial cellulose in particleboard technology. Ann WULS—SGGW Wood Technol. 2020, 109, 16–23. [Google Scholar] [CrossRef]
- Swingler, S.; Gupta, A.; Gibson, H.; Kowalczuk, M.; Heaselgrave, W.; Radecka, I. Recent Advances and Applications of Bacterial Cellulose in Biomedicine. Polymers 2021, 13, 412. [Google Scholar] [CrossRef] [PubMed]
- Frone, A.N.; Panaitescu, D.M.; Chiulan, I.; Nicolae, C.A.; Casarica, A.; Gabor, A.R.; Trusca, R.; Damian, C.M.; Purcar, V.; Alexandrescu, E.; et al. Surface treatment of bacterial cellulose in mild eco-friendly conditions. Coatings 2018, 8, 221. [Google Scholar] [CrossRef] [Green Version]
- Badshah, M.; Ullah, H.; Khan, A.R.; Khan, S.; Park, J.K.; Khan, T. Surface modification and evaluation bacterial cellulose for drug delivery. Int. J. Biol. Macromol. 2018, 113, 526–533. [Google Scholar] [CrossRef]
- Hu, W.; Chen, S.; Yang, Z.; Li, Z.; Wang, H. Functionalized bacterial cellulose derivatives and nanocomposites. Carbohydr. Polym. 2014, 101, 1043–1060. [Google Scholar] [CrossRef]
- Gao, X.; Sözümert, E.; Zhi, Z.; Yang, G.; Silberchmidt, V.V. Mechanical modification of bacterial cellulose hydrogel under biaxial cyclic tension. Mech. Mater. 2020, 142, 103272. [Google Scholar] [CrossRef]
- Betlej, I.; Salerno-Kochan, R.; Krajewski, K.J.; Zawadzki, J.; Boruszewski, P. The influence of culture medium components on the physical and mechanical properties of cellulose synthesized by kombucha microorganisms. BioResources 2020, 15, 3125–3135. [Google Scholar] [CrossRef]
- Betlej, I. Studies on the diversity of substrate composition in the culture medium of Kombucha microorganisms and its influence on the quality of synthesized cellulose. Ann WULS—SGGW Wood Technol. 2019, 108, 21–25. [Google Scholar] [CrossRef]
- Vigentini, I.; Fabrizio, V.; Dellacà, F.; Rossi, S.; Azario, I.; Mondi, C.; Benaglia, M.; Foschino, R. Set-up of bacterial cellulose production from the genus Komagataeibacter and its use in a gluten-free bakery product as a case study. Front Microbiol. 2019, 10, 1953. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Wu, G.; Chen, L.; Wang, W.; Hong, F.F.; Jönsson, L.J. Comparison of productivity and quality of bacterial nanocellulose synthesized using culture media based on seven sugars from biomass. Microb. Biotechnol. 2019, 12, 677–687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stanisławska, A.; Staroszczyk, H.; Szkodo, M. The effect of dehydration/rehydration of bacterial nanocellulose on its tensile strength and physicochemical properties. Carbohydr. Polym. 2020, 236, 116023. [Google Scholar] [CrossRef]
- Domskiene, J.; Sederaviciute, F.; Simonaityte, J. Kombucha bacterial cellulose for sustainable fashion. Int. J. Cloth. Sci. Technol. 2019, 31, 644–652. [Google Scholar] [CrossRef]
- Cazón, P.; Velázquez, G.; Vázques, M. Bacterial cellulose films: Evaluation of the water interaction. Food Packag. Shelf Life 2020, 25, 100529. [Google Scholar] [CrossRef]
- Stanisławska, A. Bacteria nanocellulose as a microbiological derived nanomaterial. Adv. Mater. Sci. 2016, 16, 45–57. [Google Scholar] [CrossRef] [Green Version]
- Indriyati, I.; Indrati, L. Incorporation of citrus essential oils into bacterial cellulose-based edible films and assessment of their physical properties. IOP Conference Series: Earth Environ. Sci. 2017, 60, 0120180. [Google Scholar] [CrossRef] [Green Version]
- Yano, S.; Maeda, H.; Nakajima, M.; Hagiwara, T.; Sawaguchi, T. Preparation and mechanical properties of bacterial cellulose nanocomposites loaded with silica nanoparticles. Cellulose 2008, 15, 111–120. [Google Scholar] [CrossRef]
- Cazón, P.; Vázquez, M.; Velazquez, G. Composite films with UV-Barrier properties of bacterial cellulose with glycerol and poly(vinyl alcohol): Puncture properties, solubility, and swelling degree. Biomacromolecules 2019, 20, 3115–3125. [Google Scholar] [CrossRef] [PubMed]
- Sommer, A.; Staroszczyk, H.; Sinkiewicz, I.; Bruździak, P. Preparation and characterization of films based on disintegrated bacterial cellulose and montmorillonite. J. Polym. Environ. 2020, 29, 1526–1541. [Google Scholar] [CrossRef]
- Luddee, M.; Pivsa-Art, S.; Sirisansaneeyakul, S.; Pechyen, C. Particle size of ground bacterial cellulose affecting mechanical, thermal, and moisture barrier properties of PLA/BC biocomposites. Energy Procedia 2014, 56, 211–218. [Google Scholar] [CrossRef] [Green Version]
- Yousefi, H.; Faezipour, M.; Hedjazi, S.; Mousavi, M.W.; Azusa, Y.; Heidair, A.H. Comparative study of paper and nanopaper properties prepared from bacterial cellulose nanofibers and fibers/ground cellulose nanofibers of canola straw. Ind. Crops Prod. 2014, 43, 732–737. [Google Scholar] [CrossRef]
- Balquinta, M.L.; Andrés, S.C.; Cerrutti, P.; Califano, A.N.; Lorenzo, G. Effect of bacterial nanocellulose post-synthetic processing on powders and rehydrated suspensions characteristics. J Food Eng. 2020, 280, 109994. [Google Scholar] [CrossRef]
- Gao, W.H.; Chen, K.F.; Yang, R.D.; Yang, F.; Han, W.J. Properties of bacterial cellulose and its influence on the physical properties of paper. Bioresources 2011, 6, 144–153. [Google Scholar] [CrossRef]
- Yamada, S.; Ishihara, M.; Sugiyama, J. Structural modification of bacterial cellulose. Cellulose 2000, 7, 213–225. [Google Scholar]
- Boruszewski, P.; Betlej, I. Płyta Wiórowa Modyfikowana Celulozą Bakteryjną. Patent Application No. P.433630, 21 April 2020. [Google Scholar]
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Liptáková, E.; and Kúdela, J. Analysis of the wood-wetting process. Holzforschung 1994, 48, 139–144. [Google Scholar] [CrossRef]
- Stefaniuk, E.; Bosacka, K.; Hryniewicz, W. Walidacja i weryfikacja metod i testów diagnostycznych w laboratorium mikrobiologicznym. Post Microbiol. 2015, 54, 415–424. [Google Scholar]
- Ul-Islam, M.; Khattak, W.A.; Kang, M.; Kim, S.M.; Khan, T.; Park, J.K. Effect of post-synthetic processing conditions on structural variations and applications of bacterial cellulose. Cellulose 2013, 20, 253–263. [Google Scholar] [CrossRef]
- Yang, S.; Yao, X.; Li, J.; Wang, Z.; Zhang, C.; Wu, S.; Wang, K.; Wang, W. Preparation and properties of ready-to-use low-density foamed concretederived from industrial solid wastes. Constr. Build. Mater. 2021, 287, 122964. [Google Scholar] [CrossRef]
- Boruszewski, P.; Borysiuk, P.; Mamiński, M.; Czechowska, J. Mat compression measurements during low-density 2 particleboard manufacturing. Bioresources 2016, 11, 6909–6919. [Google Scholar] [CrossRef] [Green Version]
- Tome, L.C.; Brandão, L.; Mendes, A.M.; Silvestre, A.J.D.; Neto, C.P.; Gandini, A.; Freire, C.S.R.; Marrucho, I.M. Preparation and characterization of bacterial cellulose membranes with tailored surface and barrier properties. Cellulose 2010, 17, 1203–1211. [Google Scholar] [CrossRef]
- Mamiński, M.; Mierzejewska, K.; Borysiuk, P.; Parzuchowski, P.; Boruszewski, P. Surface properties of octadecanol—Grafted pine veneers. Int. J. Adhes. Adhes. 2009, 29, 781–784. [Google Scholar] [CrossRef]
- Petrile, R.A.N. Estudo in vitro da interação da linhagem de fibroblastos L929 com membranas de cellulose bacteriana para aplicaçõesemengenharia de tecidos. Ph. D. Dissertation, Universidade Federal de Santa Catarina, Florianópolis, Brazil, 2007. [Google Scholar]
- Lee, K.Y.; Blaker, J.J.; Bismarck, A. Surface functionalisation of bacterial cellulose as the route to produce green polylactide nanocomposites with improved properties. Compos. Sci. Technol. 2009, 69, 2724–2733. [Google Scholar] [CrossRef] [Green Version]
- da Silva, C.M.; Bottene, M.K.; de Oliveria Barud, H.G.; da Silva Barud, H.; Ligabue, R.A.; Jahno, V.D. Wettability and morphological characterization of a polymeric bacterial cellulose/corn starch membrane. Mater. Res. 2015, 18, 10–113. [Google Scholar] [CrossRef] [Green Version]
- Cunha, A.G.; Gandini, A. Turning polysaccharides into hydrophobic materials: A critical review. Part 1.Cellulose. Cellulose 2010, 17, 875–889. [Google Scholar] [CrossRef]
- Wenzel, R.N. Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Kim, J.; Choi, S.O. Superhydrophobicity. In Waterproof and Water Repellent Textiles and Clothing; Williams, J., Ed.; Woodhead Publishing: Cambridge, UK, 2018; pp. 267–297. [Google Scholar]
- Barnat-Hunek, D. Swobodna Energia Powierzchniowa Jako Czynnik Kształtujący Skuteczność Hydrofobizacji w Ochronie Konstrukcji Budowlanych, 1st ed.; Politechnika Lubelska: Lublin, Poland, 2016; pp. 23–38. [Google Scholar]
- Celia, E.; Darmanin, T.; de Givenchy, E.T.; Amigoni, S.; Guittard, F. Recent Advances in Designing Superhydrophobic Surfaces. J. Colloid. Interf. Sci. 2013, 402, 1–18. [Google Scholar] [CrossRef]
- Kowalski, M. Trwałość Właściwości Biofizycznych Wielowarstwowych Materiałów Odzieżowych w Procesach Konserwacji. Ph.D. Thesis, Cracow University of Economics, Cracow, Poland, 2021. [Google Scholar]
- Cassie, A.B.D.; Baxter, S. Wettability of Porous Surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]

| Parameter | Native Cellulose * | Modified Cellulose * | Sum of Squares | Fisher’s F-Test | Significance Level |
|---|---|---|---|---|---|
| SS | F | p | |||
| Breaking load (N) | 269.90 (56.51) | 461.38 (78.88) | 125868 | 28.6218 | 0.000132 ** |
| Elongation (mm) | 2.413 (1.110) | 4.234 (1.430) | 10.5571 | 6.5903 | 0.023420 ** |
| Young’s modulus (MPa) | 70.12 (5.16) | 89.51 (8.17) | 952.97 | 21.959 | 0.001569 ** |
| Tensile strength (MPa) | 90.14 (11.69) | 103.70 (21.29) | 626.3 | 2.2067 | 0.163207 NS |
| Thickness (mm) | 0.117 (0.015) | 0.175 (0.011) | 0.009779 | 56.229 | 0.000007 ** |
| Density (g/mm3) | 0.0040 (0.0005) | 0.0026 (0.0002) | 0.000005 | 29.4344 | 0.000154 ** |
| Parameter | Native Cellulose * | Modified Cellulose * | Sum of Squares | Fisher’s F-Test | Significance Level |
|---|---|---|---|---|---|
| SS | F | p | |||
| WVP (g/m2·24 h) | 1084.3 (36.0) | 1174.0 (59.3) | 21933 | 9.612 | 0.012717 ** |
| Hg (%) | 18.38 (0.4) | 29.89 (2.23) | 302.124 | 1077.42 | 0.000000 ** |
| Parameter | Time [s] | Native Cellulose * | Modified Cellulose * | Mean Sum of Squares | Fisher’s F-Test | Significance Level |
|---|---|---|---|---|---|---|
| MS | F | p | ||||
| Contact angle (°) for water | 5 | 63.3 (7.9) | 64.4 (5.9) | 9.8 | 0.205 | 0.653411 NS |
| 20 | 60.4 (8.3) | 61.3 (6.4) | 5.9 | 0.110 | 0.742232 NS | |
| 40 | 58.2 (8.5) | 59.6 (6.8) | 17.1 | 0.291 | 0.593234 NS | |
| 60 | 57.3 (9.3) | 58.5 (7.0) | 12.5 | 0.188 | 0.667767 NS | |
| Contact angle (°) for diiodomethane | 5 | 30.2 (6.6) | 44.9 (6.4) | 2124.9 | 50.645 | 0.000000 ** |
| Free surface energy (SFE) *** (mJ × m−2) | γtot = 53.391 | γtot = 48.073 | ||||
| γD = 44.139 | γD = 37.064 | |||||
| γP = 9.252 | γP = 11.009 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Betlej, I.; Salerno-Kochan, R.; Jankowska, A.; Krajewski, K.; Wilkowski, J.; Rybak, K.; Nowacka, M.; Boruszewski, P. The Impact of the Mechanical Modification of Bacterial Cellulose Films on Selected Quality Parameters. Coatings 2021, 11, 1275. https://doi.org/10.3390/coatings11111275
Betlej I, Salerno-Kochan R, Jankowska A, Krajewski K, Wilkowski J, Rybak K, Nowacka M, Boruszewski P. The Impact of the Mechanical Modification of Bacterial Cellulose Films on Selected Quality Parameters. Coatings. 2021; 11(11):1275. https://doi.org/10.3390/coatings11111275
Chicago/Turabian StyleBetlej, Izabela, Renata Salerno-Kochan, Agnieszka Jankowska, Krzysztof Krajewski, Jacek Wilkowski, Katarzyna Rybak, Malgorzata Nowacka, and Piotr Boruszewski. 2021. "The Impact of the Mechanical Modification of Bacterial Cellulose Films on Selected Quality Parameters" Coatings 11, no. 11: 1275. https://doi.org/10.3390/coatings11111275