Microstructure and Oxidation Behavior of Metal-Modified Mo-Si-B Alloys: A Review
Abstract
1. Introduction
2. Mo-Si-B Ternary Alloys
2.1. Low Silicon Content of the Mo-Si-B Ternary Alloys
2.2. High Silicon Content of the Mo-Si-B Ternary Alloys
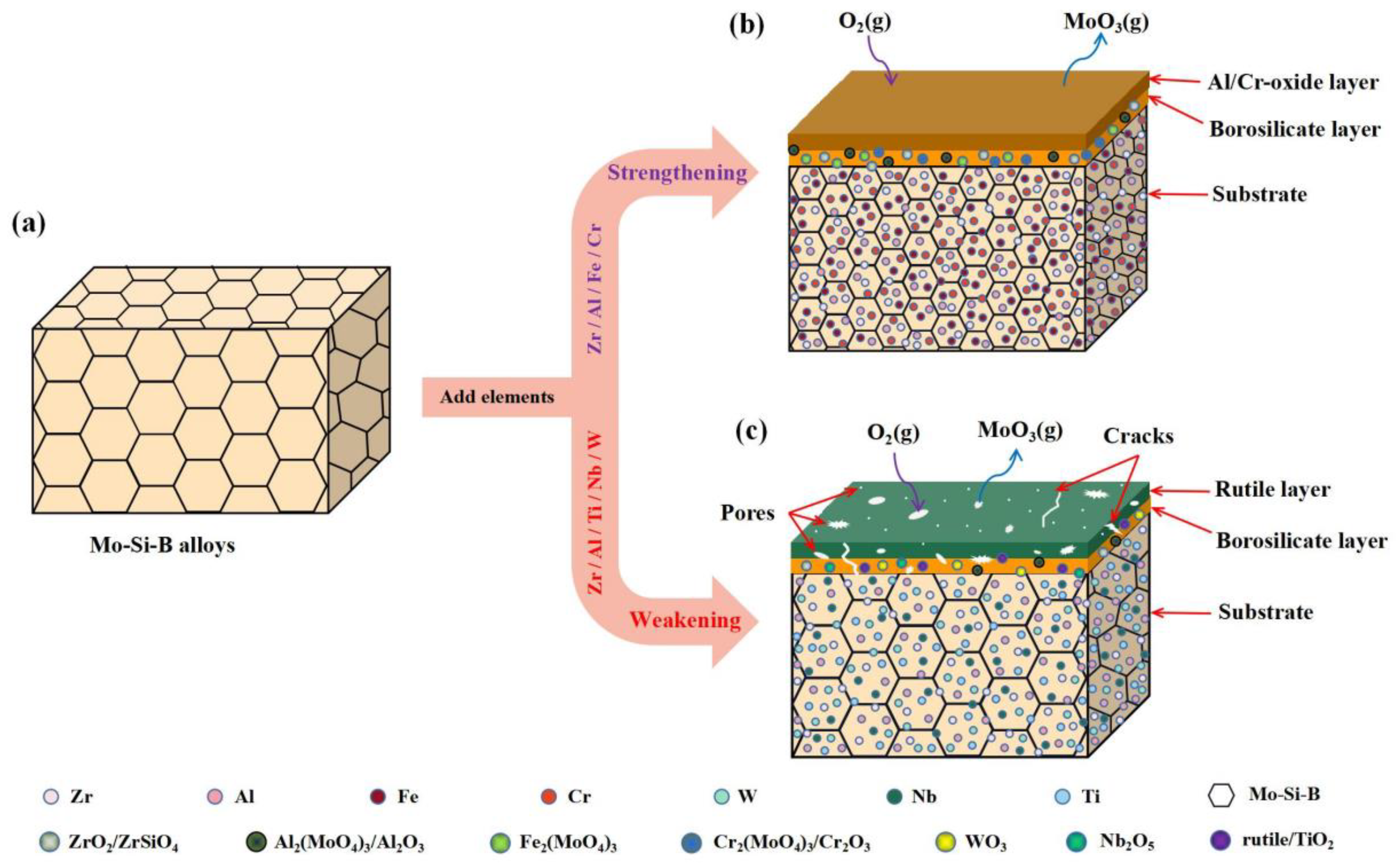
3. Metallic Elements Modified Mo-Si-B Alloys
3.1. Single Metallic Elements Modified Mo-Si-B Alloys
3.1.1. Zr Element Modified Mo-Si-B Alloys
3.1.2. Ti Element Modified Mo-Si-B Alloys
3.1.3. Al Element Modified Mo-Si-B Alloys
3.1.4. W Element Modified Mo-Si-B Alloys
3.1.5. Other Single Metallic Elements Modified Mo-Si-B Alloys
3.2. Multiple Metallic Elements Co-Modified Mo-Si-B Alloys
3.3. Modification Mechanism of Metal Elements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yoshimi, K.; Nakatani, S.; Suda, T.; Hanada, S.; Habazaki, H. Oxidation behavior of Mo5SiB2-based alloy at elevated tempera-tures. Intermetallics 2002, 10, 407–414. [Google Scholar] [CrossRef]
- Liu, L.; Shi, C.; Zhang, C.; Voyles, P.; Fournelle, J.; Perepezko, J. Microstructure, microhardness and oxidation behavior of Mo-Si-B alloys in the Moss+Mo2B+Mo5SiB2 three phase region. Intermetallics 2020, 116, 106618. [Google Scholar] [CrossRef]
- Zhang, Y.; Cui, K.; Gao, Q.; Hussain, S.; Lv, Y. Investigation of morphology and texture properties of WSi2 coatings on W substrate based on contact-mode AFM and EBSD. Surf. Coat. Technol. 2020, 396, 125966. [Google Scholar] [CrossRef]
- Zhang, X.; Fu, T.; Cui, K.; Zhang, Y.; Shen, F.; Wang, J.; Yu, L.; Mao, H. The Protection, Challenge, and Prospect of Anti-Oxidation Coating on the Surface of Niobium Alloy. Coatings 2021, 11, 742. [Google Scholar] [CrossRef]
- Cui, K.; Fu, T.; Zhang, Y.; Wang, J.; Mao, H.; Tan, T. Microstructure and mechanical properties of CaAl12O19 reinforced Al2O3-Cr2O3 composites. J. Eur. Ceram. Soc. 2021, 41, 7935–7945. [Google Scholar] [CrossRef]
- Jung, J.; Zhou, N.X.; Jian, L. Effects of sintering aids on the densification of Mo–Si–B alloys. J. Mater. Sci. 2012, 47, 8308–8319. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Cui, K.; Fu, T.; Gao, J.; Hussain, S.; AlGarni, T.S. Pyrometallurgical recovery of zinc and valuable metals from electric arc furnace dust—A review. J. Clean. Prod. 2021, 298, 126788. [Google Scholar] [CrossRef]
- Kramer, M.; Thom, A.; Degirmen, O.; Behrani, V.; Akinc, M. Oxidation Behavior of Mo-Si-B Alloys in Wet Air. Mater. Sci. Eng. A 2004, 371, 335–342. [Google Scholar] [CrossRef][Green Version]
- Fu, T.; Cui, K.; Zhang, Y.; Wang, J.; Shen, F.; Yu, L.; Qie, J.; Zhang, X. Oxidation protection of tungsten alloys for nuclear fusion applications: A comprehensive review. J. Alloys Compd. 2021, 884, 161057. [Google Scholar] [CrossRef]
- Obert, S.; Kauffmann, A.; Seils, S.; Boll, T.; Weiss, S.K.; Chen, H.; Anton, R.; Heilmaier, M. Microstructural and chemical consti-tution of the oxide scale formed on a pesting-resistant Mo-Si-Ti alloy. Corros. Sci. 2021, 178, 109081. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Cui, K.K.; Fu, T.; Wang, J.; Shen, F.Q.; Zhang, X.; Yu, L.H. Formation of MoSi2 and Si/MoSi2 coatings on TZM (Mo-0.5Ti-0.1Zr-0.02C) alloy by hot dip silicon-plating method. Ceram. Int. 2021, 47, 23053–23065. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Li, Y.G.; Bai, C.G. Microstructure and oxidation behavior of Si-MoSi2 functionally graded coating on Mo substrate. Ceram. Int. 2017, 43, 6250–6256. [Google Scholar] [CrossRef]
- Wirkus, C.D.; Wilder, D.R. High-Temperature Oxidation of Molybdenum Disilicide. J. Am. Ceram. Soc. 1966, 49, 173–177. [Google Scholar] [CrossRef]
- Fu, T.; Cui, K.; Zhang, Y.; Wang, J.; Zhang, X.; Shen, F.; Yu, L.; Mao, H. Microstructure and Oxidation Behavior of Anti-Oxidation Coatings on Mo-Based Alloys through HAPC Process: A Review. Coatings 2021, 11, 883. [Google Scholar] [CrossRef]
- Thom, A.J.; Summers, E.; Akinc, M. Oxidation behavior of extruded Mo5Si3Bx–MoSi2–MoB intermetallics from 600–1600 °C. Intermetallics 2002, 10, 555–570. [Google Scholar] [CrossRef]
- Majumdar, S.; Schliephake, D.; Gorr, B.; Christ, H.-J.; Heilmaier, M. Effect of Yttrium Alloying on Intermediate to High-Temperature Oxidation Behavior of Mo-Si-B Alloys. Met. Mater. Trans. A 2013, 44, 2243–2257. [Google Scholar] [CrossRef]
- Majumdar, S. A study on microstructure development and oxidation phenomenon of arc consolidated Mo-Nb-Si-(Y) alloys. Int. J. Refract. Met. Hard Mater. 2018, 78, 76–84. [Google Scholar] [CrossRef]
- Halvarsson, M.; Jonsson, T.; Ingemarsson, L.; Sundberg, J.M.; Svensson, E.; Johansson, L.G. Microstructural investigation of the initial oxidation at 1450 °C and 1500 °C of a Mo(Si,Al)2-based composite. Mater. High Temp. 2009, 26, 137–143. [Google Scholar] [CrossRef]
- Zhang, Y.; Hussain, S.; Cui, K.; Fu, T.; Wang, J.; Javed, M.S.; Lv, Y.; Aslam, B. Microstructure and Mechanical Properties of MoSi2 Coating Deposited on Mo Substrate by Hot Dipping Processes. J. Nanoelectron. Optoelectron. 2019, 14, 1680–1685. [Google Scholar] [CrossRef]
- Pan, Y.; Pu, D.; Yu, E. Structural, electronic, mechanical and thermodynamic properties of Cr–Si binary silicides from first-principles investigations. Vacuum 2021, 185, 110024. [Google Scholar] [CrossRef]
- Seidenschnur, P. The intermediate and high-temperature oxidation behaviour of Mo(Si1-xAlx)2 intermetallic alloys. Intermetallics 1997, 5, 69–81. [Google Scholar]
- Sharif, A. Effects of Re- and Al-alloying on mechanical properties and high-temperature oxidation of MoSi2. J. Alloys Compd. 2012, 518, 22–26. [Google Scholar] [CrossRef]
- Ingemarsson, L.; Halvarsson, M.; Hellström, K.; Jonsson, T.; Sundberg, M.; Johansson, L.G.; Svensson, J.E. Oxidation behavior at 300–1000 °C of a (Mo,W)Si2-based composite containing boride. Intermetallics 2010, 18, 77–86. [Google Scholar] [CrossRef]
- Samadzadeh, M.; Oprea, C.; Sharif, H.K.; Troczynski, T. Comparative studies of the oxidation of MoSi 2 based materials: High-temperature oxidation (1000–1600 °C). Int. J. Refract. Met. Hard Mater. 2017, 69, 31–39. [Google Scholar] [CrossRef]
- Zhang, L.T.; Zhu, O.; Zhang, F.; Shan, A.D.; Wu, J.S. High-temperature oxidation of Mo-rich (Mo1-xNbx)Si2 pseudo-binary compounds. Scr. Mater. 2007, 57, 305–308. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Zhao, J.; Li, J.H.; Lei, J.; Cheng, X. KEffect of hot-dip siliconizing time on phase composition and microstructure of Mo-MoSi2 high temperature structural materials. Ceram. Int. 2019, 45, 5588–5593. [Google Scholar] [CrossRef]
- Sharif, A.A. High-temperature oxidation of MoSi2. J. Mater. Sci. 2010, 45, 865–870. [Google Scholar] [CrossRef]
- Hellström, K.; Persson, P.; Ström, E. Oxidation behaviors and microstructural alterations of a Mo(Si,Al)2-based composite after heating at 1580°C either in a furnace (ex-situ) or via alternating current (in-situ). J. Eur. Ceram. Soc. 2015, 35, 513–523. [Google Scholar] [CrossRef]
- Ingemarsson, L.; Halvarsson, M.; Engkvist, J.; Jonsson, T.; Hellström, K.; Johansson, L.G.; Svensson, J.E. Oxidation behavior of a Mo(Si,Al)2-based composite at 300–1000 °C. Intermetallics 2010, 18, 633–640. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, Y.; Fu, T.; Wang, J.; Cui, K.; Shen, F. Rare Earth Elements Enhanced the Oxidation Resistance of Mo-Si-Based Alloys for High Temperature Application: A Review. Coatings 2021, 11, 1144. [Google Scholar] [CrossRef]
- Ingemarsson, L.; Hellström, K.; Canovic, S.; Jonsson, T.; Halvarsson, M.; Johansson, L.G.; Svensson, J.E. Oxidation behavior of a Mo(Si,Al)2 composite at 900–1600 °C in dry air. J. Mater. Sci. 2013, 48, 1511–1523. [Google Scholar] [CrossRef]
- Roy, B.; Das, J.; Mitra, R. Transient stage oxidation behavior of Mo76Si14B10 alloy at 1150 °C. Corros. Sci. 2012, 68, 231–237. [Google Scholar] [CrossRef]
- Supatarawanich, V.; Johnson, D.R.; Liu, C.T. Oxidation behavior of multiphase Mo–Si–B alloys. Intermetallics 2004, 12, 721–725. [Google Scholar] [CrossRef]
- Supatarawanich, V.; Johnson, D.R.; Liu, C.T. Effects of microstructure on the oxidation behavior of multiphase Mo–Si–B alloys. Mater. Sci. Eng. A 2003, 344, 328–339. [Google Scholar] [CrossRef]
- Becker, J.; Fichtner, D.; Schmigalla, S.; Schultze, S.; Heinze, C.; Küsters, Y.; Hasemann, G.; Schmelzer, J.; Krüger, M. Oxidation response of additively manufactured eutectic Mo-Si-B alloys. IOP Conf. Ser. Mater. Sci. Eng. 2020, 882, 12002. [Google Scholar] [CrossRef]
- Mendiratta, M.G.; Parthasarathy, T.A.; Dimiduk, D.M. Oxidation behavior of αMo–Mo3Si–Mo5SiB2 (T2) three phase system. Intermetallics 2002, 10, 225–232. [Google Scholar] [CrossRef]
- Parthasarathy, T.; Mendiratta, M.; Dimiduk, D. Oxidation mechanisms in Mo-reinforced Mo5SiB2(T2)–Mo3Si alloys. Acta Mater. 2002, 50, 1857–1868. [Google Scholar] [CrossRef]
- Wang, F.; Shan, A.D.; Dong, X.P.; Wu, J.S. Oxidation behavior of Mo–12.5Si–25B alloy at high temperature. J. Alloys Compd. 2008, 459, 362–368. [Google Scholar] [CrossRef]
- Wang, F.; Shan, A.-D.; Dong, X.-P.; Wu, J.-S. Oxidation behavior of multiphase Mo5SiB2 (T2)-based alloys at high temperatures. Trans. Nonferrous Met. Soc. 2007, 17, 1242–1247. [Google Scholar] [CrossRef]
- Wang, F.; Shan, A.D.; Dong, X.P.; Wu, J.S. Microstructure and oxidation behavior of directionally solidifified Mo–Mo5SiB2 (T2)–Mo3Si alloys. J. Alloys Compd. 2008, 462, 436–441. [Google Scholar] [CrossRef]
- Wang, F.; Shan, A.D.; Dong, X.P.; Wu, J.S. Microstructure and oxidation resistance of laser-remelted Mo-Si-B alloy. Scr. Mater. 2007, 56, 737–740. [Google Scholar] [CrossRef]
- Rioult, F.; Imhoff, S.; Sakidja, R.; Perepezko, J. Transient oxidation of Mo–Si–B alloys: Effect of the microstructure size scale. Acta Mater. 2009, 57, 4600–4613. [Google Scholar] [CrossRef]
- Choi, W.J.; Park, C.W.; Park, J.H.; Kim, Y.D.; Byun, J.M. Volume and size effects of intermetallic compounds on the high-temperature oxidation behavior of Mo-Si-B alloys. Int. J. Refract. Met. Hard Mater. 2019, 81, 94–99. [Google Scholar] [CrossRef]
- Li, R.; Li, B.; Chen, X.; Wang, J.; Wang, T.; Gong, Y.; Ren, S.; Zhang, G. Variation of phase composition of Mo-Si-B alloys induced by boron and their mechanical properties and oxidation resistance. Mater. Sci. Eng. A 2019, 749, 196–209. [Google Scholar] [CrossRef]
- Zhang, G.J.; Kou, H.; Dang, Q.; Liu, G.; Sun, J. Microstructure and oxidation resistance behavior of lanthanum oxide-doped Mo-12Si-8.5B alloys. Int. J. Refract. Met. Hard Mater. 2021, 30, 6–11. [Google Scholar]
- Majumdar, S.; Gorr, B.; Christ, H.J.; Schliephake, D.; Heilmaier, M. Oxidation mechanisms of lanthanum-alloyed Mo–Si–B. Corros. Sci. 2014, 88, 360–371. [Google Scholar] [CrossRef]
- Majumdar, S.; Burk, S.; Schliephake, D.; Krüger, M.; Christ, H.-J.; Heilmaier, M. A Study on Effect of Reactive and Rare Earth Element Additions on the Oxidation Behavior of Mo–Si–B System. Oxid. Met. 2013, 80, 219–230. [Google Scholar] [CrossRef]
- Burk, S.; Gorr, B.; Trindade, V.B.; Christ, H.J. High temperature oxidation of mechanically alloyed Mo-Si-B alloys. Br. Corros. J. 2009, 44, 168–175. [Google Scholar] [CrossRef]
- Burk, S.; Christ, H.J. High-Temperature Oxidation Performance of Mo-Si-B Alloys: Current Results, Developments and Opportunities. Adv. Mater. Res. 2011, 278, 587–592. [Google Scholar] [CrossRef]
- Wen, S.H.; Zhou, C.G.; Sha, J.B. Improvement of oxidation resistance of a Mo-62Si-5B (at.%) alloy at 1250 °C and 1350 °C via an in situ pre-formed SiO2 fabricated by spark plasma sintering. Corros. Sci. 2017, 127, 175–185. [Google Scholar] [CrossRef]
- Pan, K.; Yang, Y.; Wei, S.; Wu, H.; Dong, Z.; Wu, Y.; Wang, S.; Zhang, L.; Lin, J.; Mao, X. Oxidation behavior of Mo-Si-B alloys at medium-to-high temperatures. J. Mater. Sci. Technol. 2021, 60, 113–127. [Google Scholar] [CrossRef]
- Wang, J.; Li, B.; Li, R.; Chen, X.; Zhang, G. Bimodal α-Mo grain structure inducing excellent oxidation resistance in Mo-12Si-8.5B alloy at 1100 °C. Int. J. Refract. Met. Hard Mater. 2021, 98, 105533. [Google Scholar] [CrossRef]
- Wang, J.; Li, B.; Li, R.; Chen, X.; Wang, T.; Zhang, G.J. Unprecedented oxidation resistance at 900 °C of Mo–Si–B composite with addition of ZrB2. Ceram. Int. 2020, 46, 14632–14639. [Google Scholar] [CrossRef]
- Wang, J.; Li, B.; Shuai, R.; Wang, T.; Zhang, G. Enhanced oxidation resistance of Mo-12Si-8.5B alloys with ZrB2 addition at 1300 °C. J. Mater. Sci. Technol. 2018, 34, 635–642. [Google Scholar] [CrossRef]
- Burk, S.; Gorr, B.; Trindade, V.B.; Christ, H.-J. Effect of Zr Addition on the High-Temperature Oxidation Behaviour of Mo–Si–B Alloys. Oxid. Met. 2009, 73, 163–181. [Google Scholar] [CrossRef]
- Burk, S.; Gorr, B.; Christ, H.-J. High temperature oxidation of Mo–Si–B alloys: Effect of low and very low oxygen partial pressures. Acta Mater. 2010, 58, 6154–6165. [Google Scholar] [CrossRef]
- Kumar, N.K.; Das, J.; Mitra, R. Effect of Zr Addition on Microstructure, Hardness and Oxidation Behavior of Arc-Melted and Spark Plasma Sintered Multiphase Mo-Si-B Alloys. Met. Mater. Trans. A 2019, 50, 2041–2060. [Google Scholar] [CrossRef]
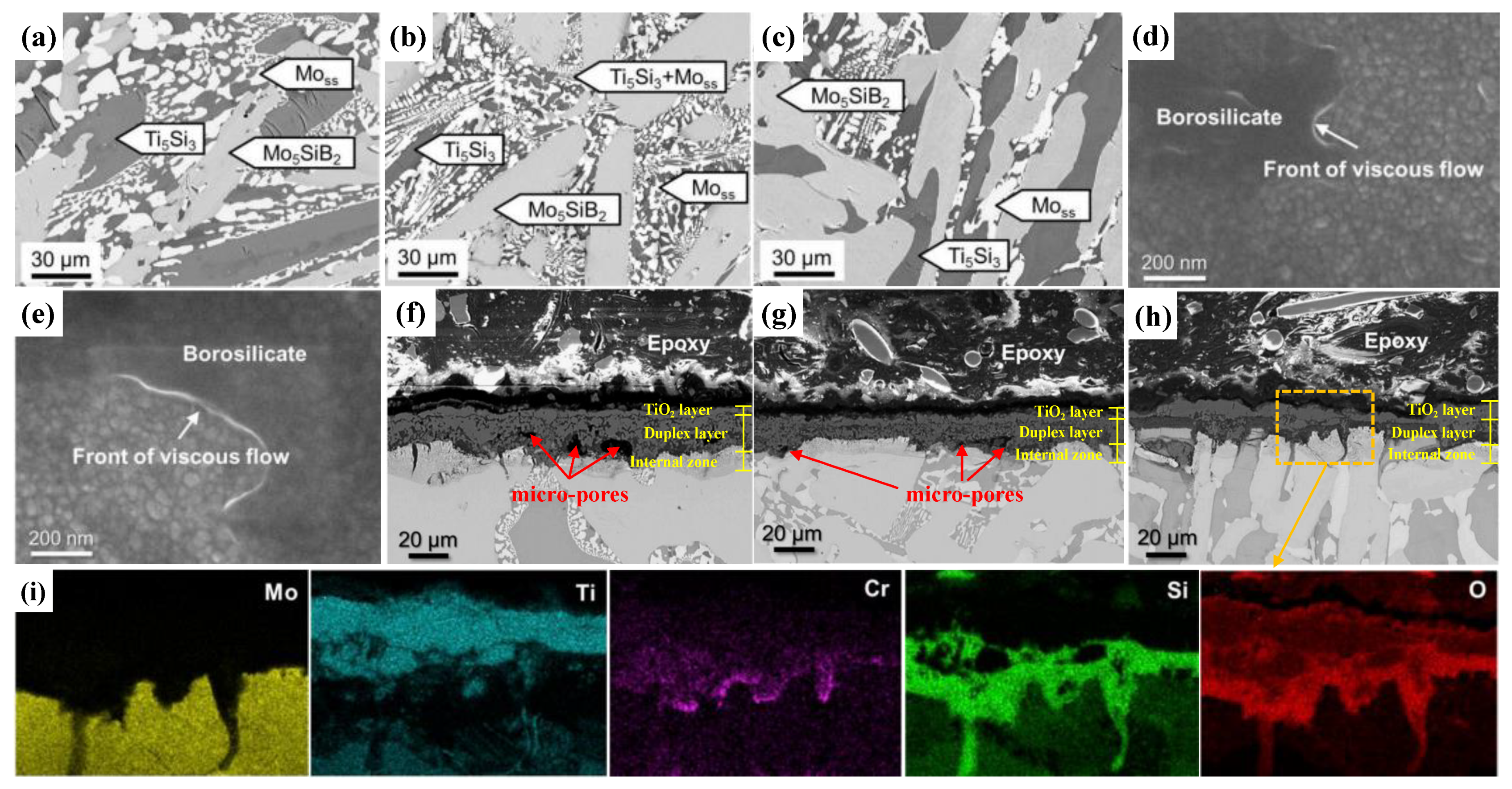
- Schliephake, D.; Azim, M.; Von Klinski-Wetzel, K.; Gorr, B.; Christ, H.-J.; Bei, H.; George, E.; Heilmaier, M. High-Temperature Creep and Oxidation Behavior of Mo-Si-B Alloys with High Ti Contents. Met. Mater. Trans. A 2013, 45, 1102–1111. [Google Scholar] [CrossRef]
- Azim, M.; Burk, S.; Gorr, B.; Christ, H.J.; Schliephake, D.; Heilmaier, M.; Bornemann, R.; Bolívar, P.H. Effect of Ti (macro-) alloying on the high-temperature oxidation behavior of ternary Mo-Si-B alloys at 820–1300 °C. Oxid. Met. 2013, 80, 231–242. [Google Scholar] [CrossRef]
- Schliephake, D.; Gombola, C.; Kauffmann, A.; Heilmaier, M.; Perepezko, J.H. Enhanced oxidation resistance of Mo–Si–B–Ti alloys by pack cementation. Oxid. Met. 2017, 88, 267–277. [Google Scholar] [CrossRef]
- Das, J.; Mitra, R.; Roy, S.K. Oxidation behaviour of Mo-Si-B-(Al, Ce) ultrafine-eutectic dendrite composites in the temperature range of 500–700 °C. Intermetallics 2011, 19, 1–8. [Google Scholar] [CrossRef]
- Das, J.; Roy, B.; Kumar, N.K.; Mitra, R. High temperature oxidation response of Al/Ce doped Mo-Si-B composites. Intermetallics 2017, 83, 101–109. [Google Scholar] [CrossRef]
- Cui, K.K.; Zhang, Y.Y.; Fu, T.; Hussain, S.; Al Garni, T.S.; Wang, J.; Zhang, X.; Ali, S. Effects of Cr2O3 content on microstructure and mechanical properties of Al2O3 matrix composites. Coatings 2021, 11, 234. [Google Scholar] [CrossRef]
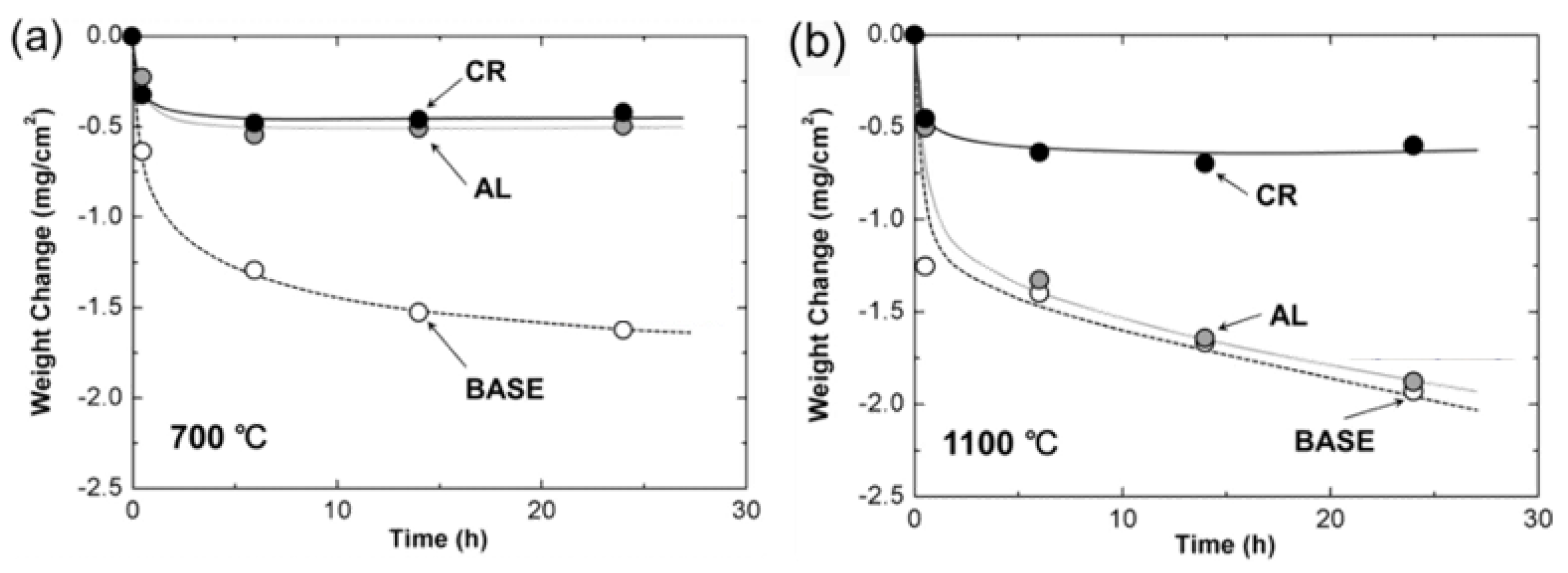
- Paswan, S.; Mitra, R.; Roy, S.K. Isothermal oxidation behaviour of Mo-Si-B and Mo-Si-B-Al alloys in the temperature range of 400–800 °C. Mater. Sci. Eng. A 2006, 424, 251–265. [Google Scholar] [CrossRef]
- Paswan, S.; Mitra, R.; Roy, K.S. Oxidation behaviour of the Mo-Si-B and Mo-Si-B-Al alloys in the temperature range of 700–1300 °C. Intermetallics 2007, 15, 1217–1227. [Google Scholar] [CrossRef]
- Paswan, S.; Mitra, R.; Roy, S. Nonisothermal and Cyclic Oxidation Behavior of Mo-Si-B and Mo-Si-B-Al Alloys. Met. Mater. Trans. A 2009, 40, 2644–2658. [Google Scholar] [CrossRef]
- Cui, K.; Zhang, Y.; Fu, T.; Wang, J.; Zhang, X. Toughening Mechanism of Mullite Matrix Composites: A Review. Coatings 2020, 10, 672. [Google Scholar] [CrossRef]
- Yamauchi, A.; Yoshimi, K.; Murakami, Y.; Kurokawa, K.; Hanada, S. Oxidation Behavior of Mo-Si-B In Situ Composites. Solid State Phenom. 2007, 127, 215–220. [Google Scholar] [CrossRef]
- Ray, P.; Ye, Y.; Akinc, M.; Kramer, M. Effect of Nb and W substitutions on the stability of the A15 Mo3Si phase. J. Alloys Compd. 2012, 537, 65–70. [Google Scholar] [CrossRef]
- Karahan, T.; Ouyang, G.; Ray, P.; Kramer, M.; Akinc, M. Oxidation mechanism of W substituted Mo-Si-B alloys. Intermetallics 2017, 87, 38–44. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Qie, J.M.; Cui, K.K.; Fu, T.; Fan, X.L.; Wang, J.; Zhang, X. Effect of hot dip silicon-plating temperature on micro-structure characteristics of silicide coating on tungsten substrate. Ceram. Int. 2020, 46, 5223–5228. [Google Scholar] [CrossRef]
- Zhang, Y.; Cui, K.; Fu, T.; Wang, J.; Qie, J.; Zhang, X. Synthesis WSi2 coating on W substrate by HDS method with various deposition times. Appl. Surf. Sci. 2020, 511, 145551. [Google Scholar] [CrossRef]
- Zhang, Y.; Fu, T.; Cui, K.; Shen, F.; Wang, J.; Yu, L.; Mao, H. Evolution of surface morphology, roughness and texture of tungsten disilicide coatings on tungsten substrate. Vacuum 2021, 191, 110297. [Google Scholar] [CrossRef]
- Ray, P.K.; Akinc, M.; Kramer, M.J. Applications of an extended Miedema’s model for ternary alloys. J. Alloys Compd. 2010, 489, 357–361. [Google Scholar] [CrossRef]
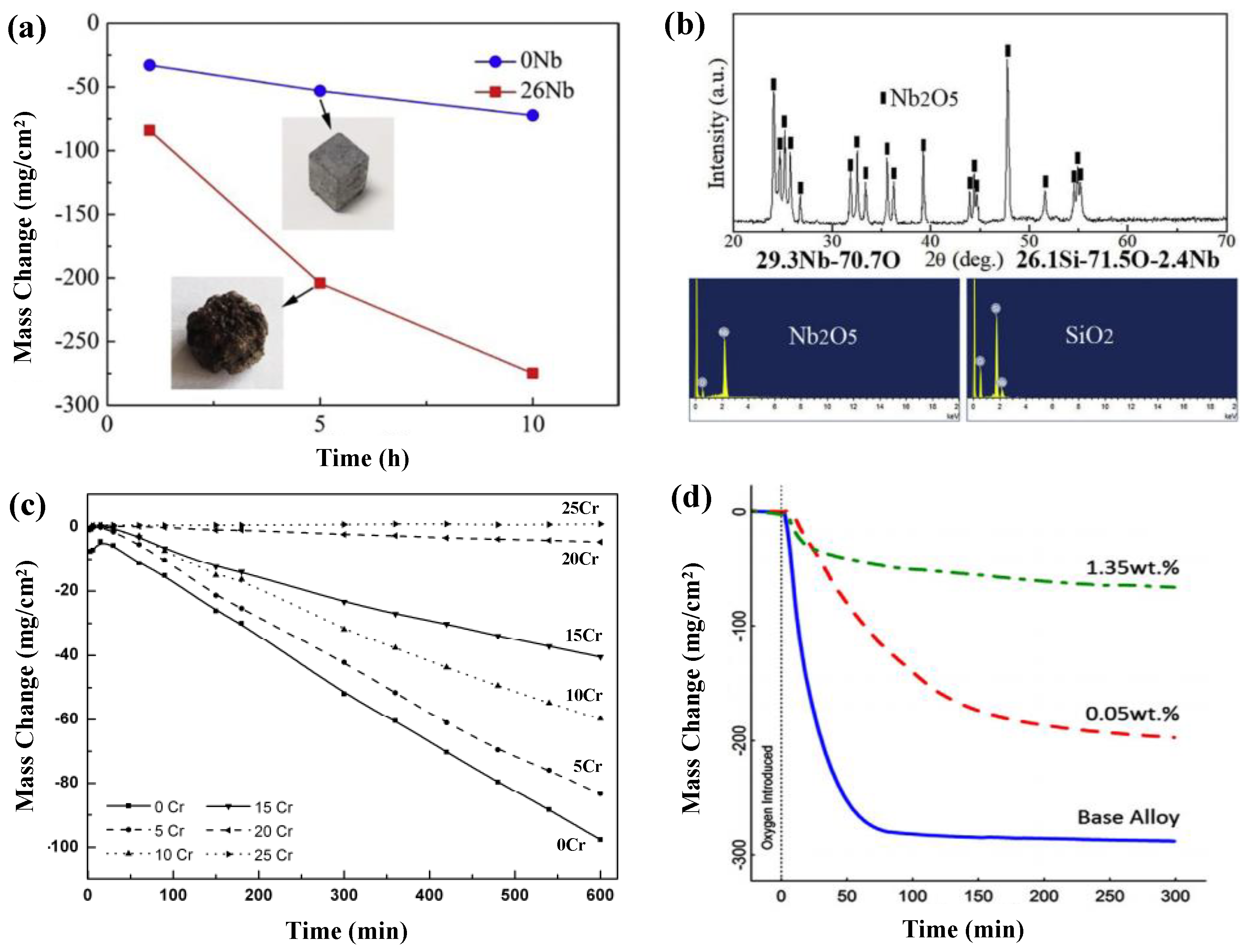
- Yang, T.; Guo, X.P. Comparative studies on densification behavior, microstructure, mechanical properties and oxidation re-sistance of Mo-12Si-10B and Mo3Si-free Mo-26Nb-12Si-10B alloys. Int. J. Refract. Met. Hard Mater. 2019, 84, 104993. [Google Scholar] [CrossRef]
- Behrani, V.; Thom, A.J.; Kramer, M.J.; Akinc, M. Microstructure and oxidation behavior of Nb-Mo-Si-B alloys. Intermetallics 2006, 14, 24–32. [Google Scholar] [CrossRef]
- Burk, S.; Gorr, B.; Krüger, M.; Heilmaier, M.; Christ, H.-J. Oxidation behavior of Mo-Si-B-(X) alloys: Macro- and microalloying (X = Cr, Zr, La2O3). JOM J. Miner. Met. Mater. Soc. 2011, 63, 32–36. [Google Scholar] [CrossRef]
- Sossaman, T.; Sakidja, R.; Perepezko, J. Influence of minor Fe addition on the oxidation performance of Mo–Si–B alloys. Scr. Mater. 2012, 67, 891–894. [Google Scholar] [CrossRef]
- Woodard, S.R.; Raban, R.; Myers, J.F.; Berczik, D.M. Oxidation Resistant Molybdenum. U.S. Patent 6652674-B1, 25 November 2003. [Google Scholar]
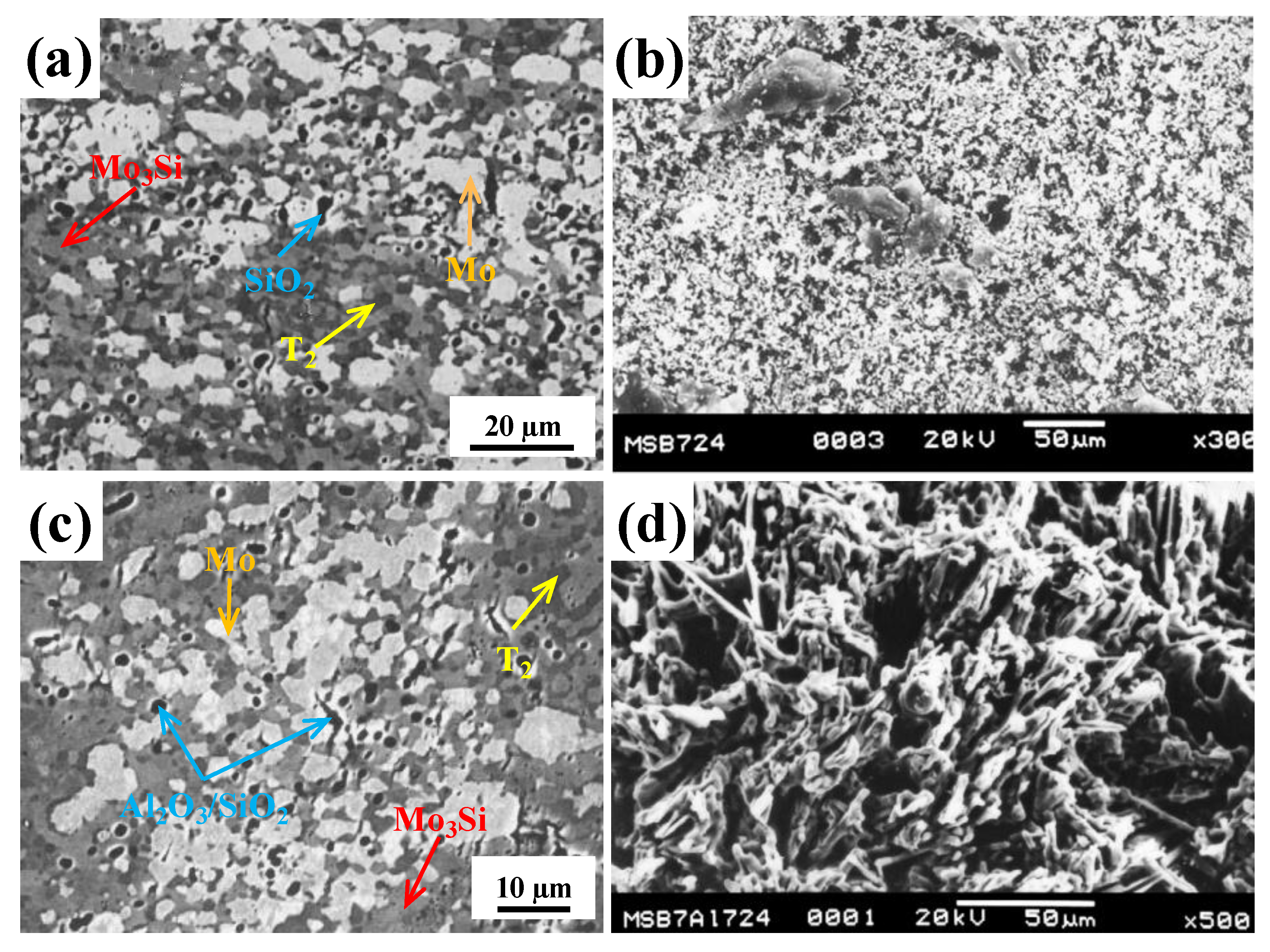
- Zhao, M.; Xu, B.; Shao, Y.; Liang, J.; Wu, S.; Yan, Y. Oxidation behavior of Moss–Ti5Si3–T2 composites at intermediate and high temperatures. Intermetallics 2020, 118, 106702. [Google Scholar] [CrossRef]

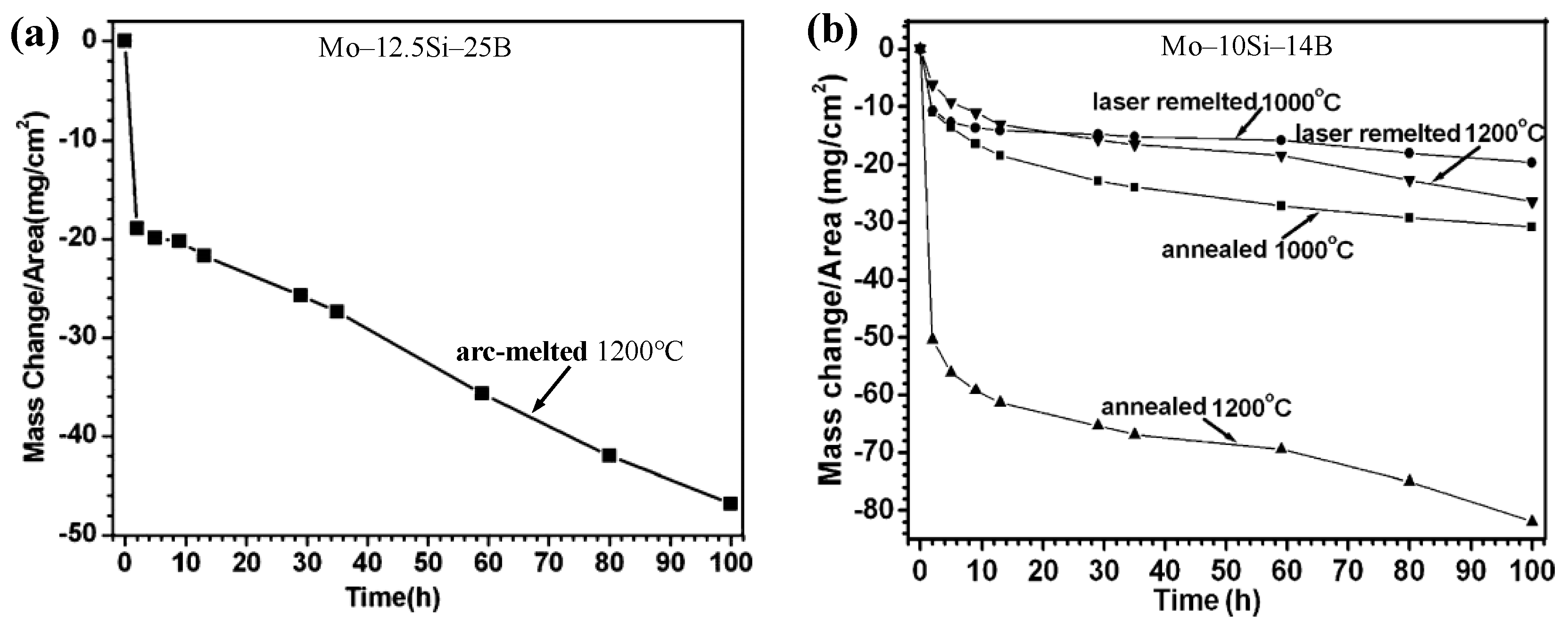
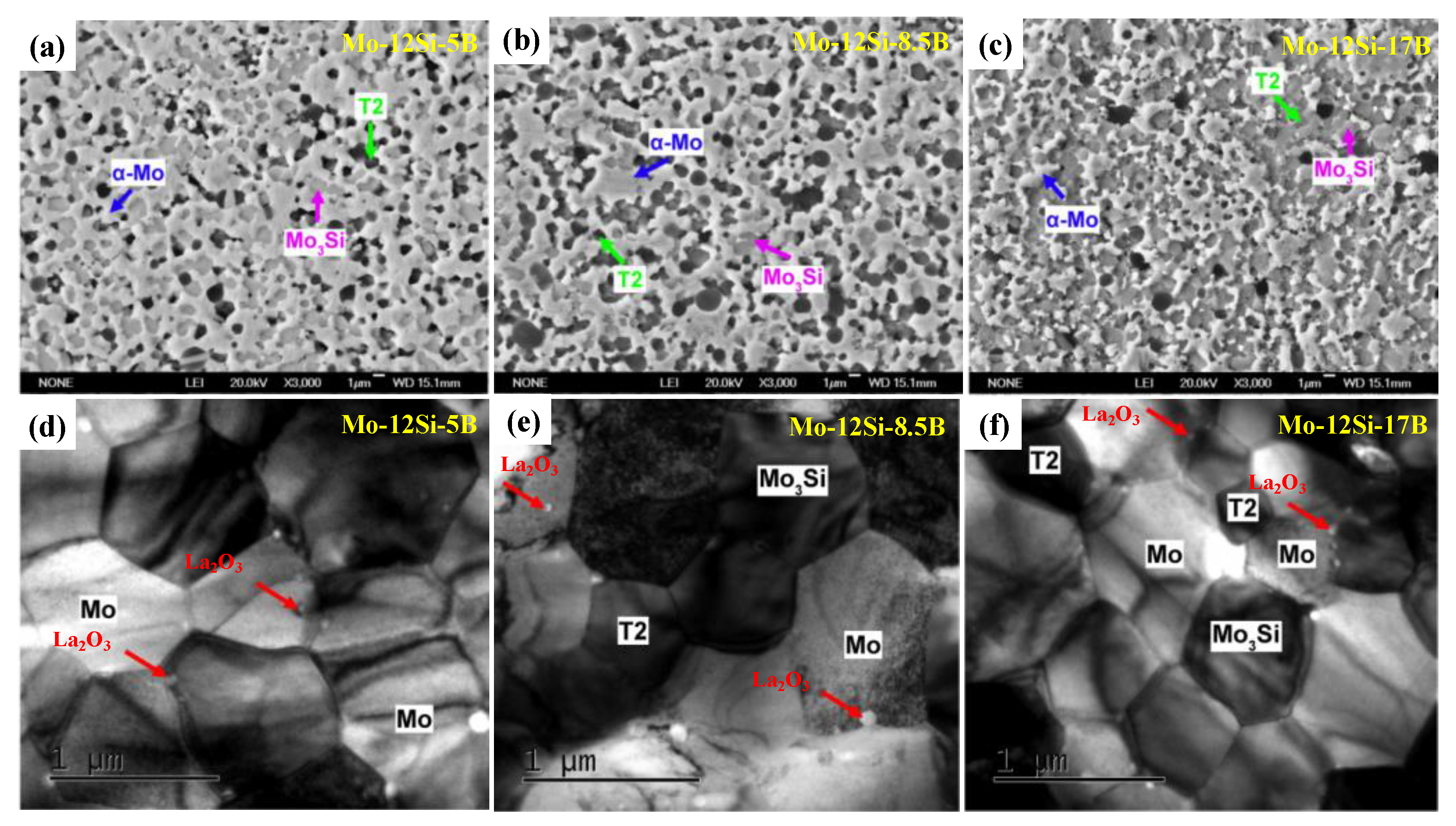

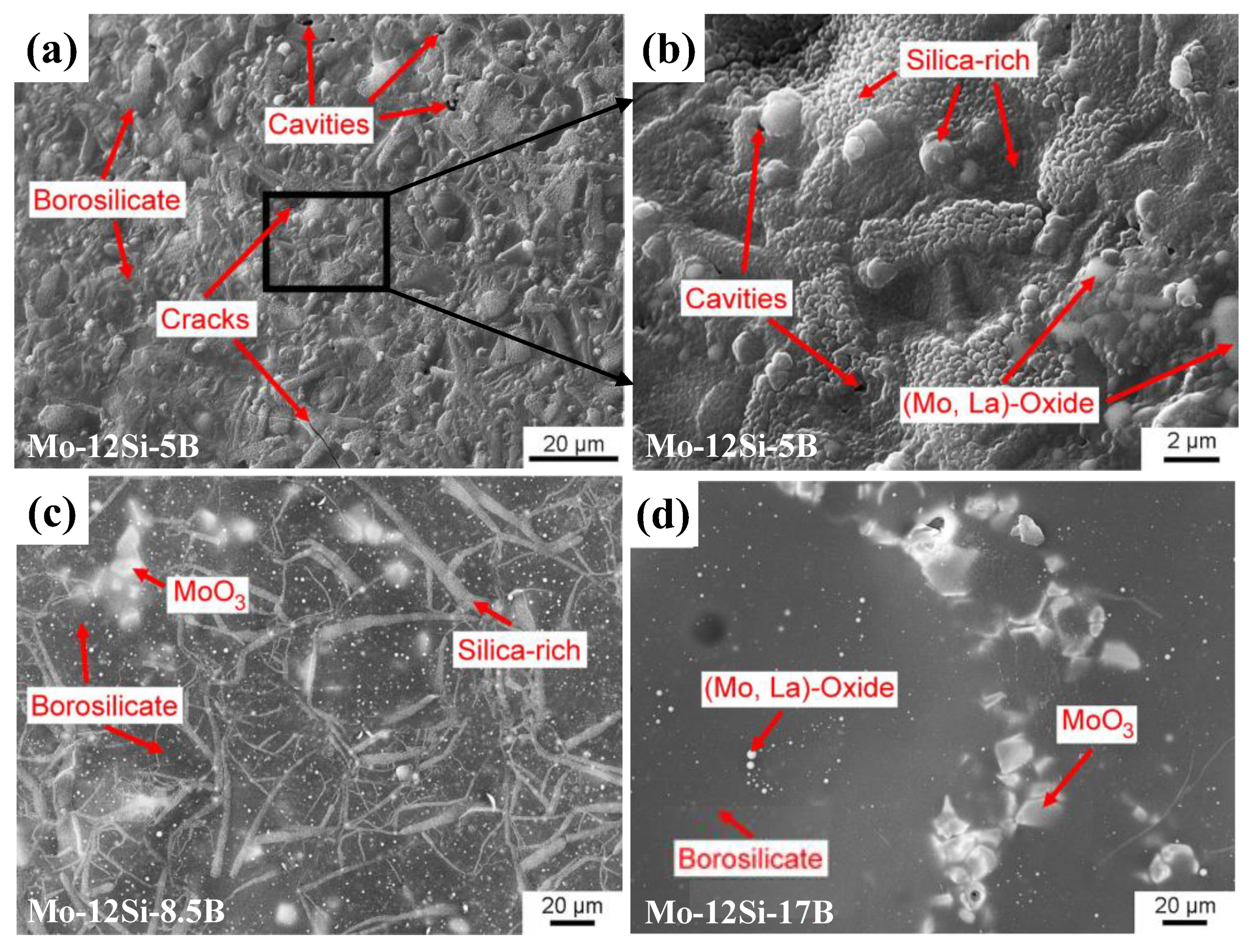
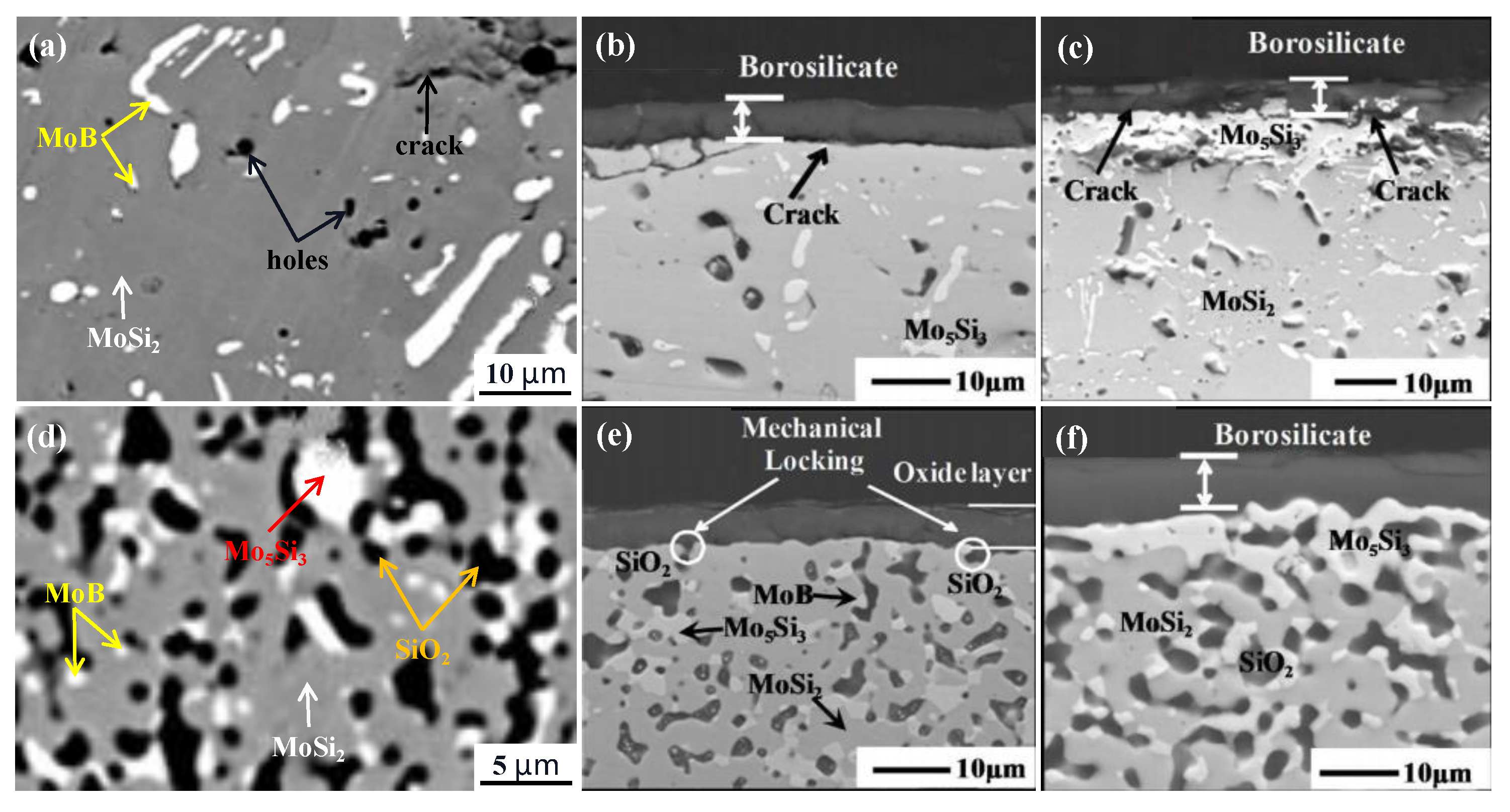
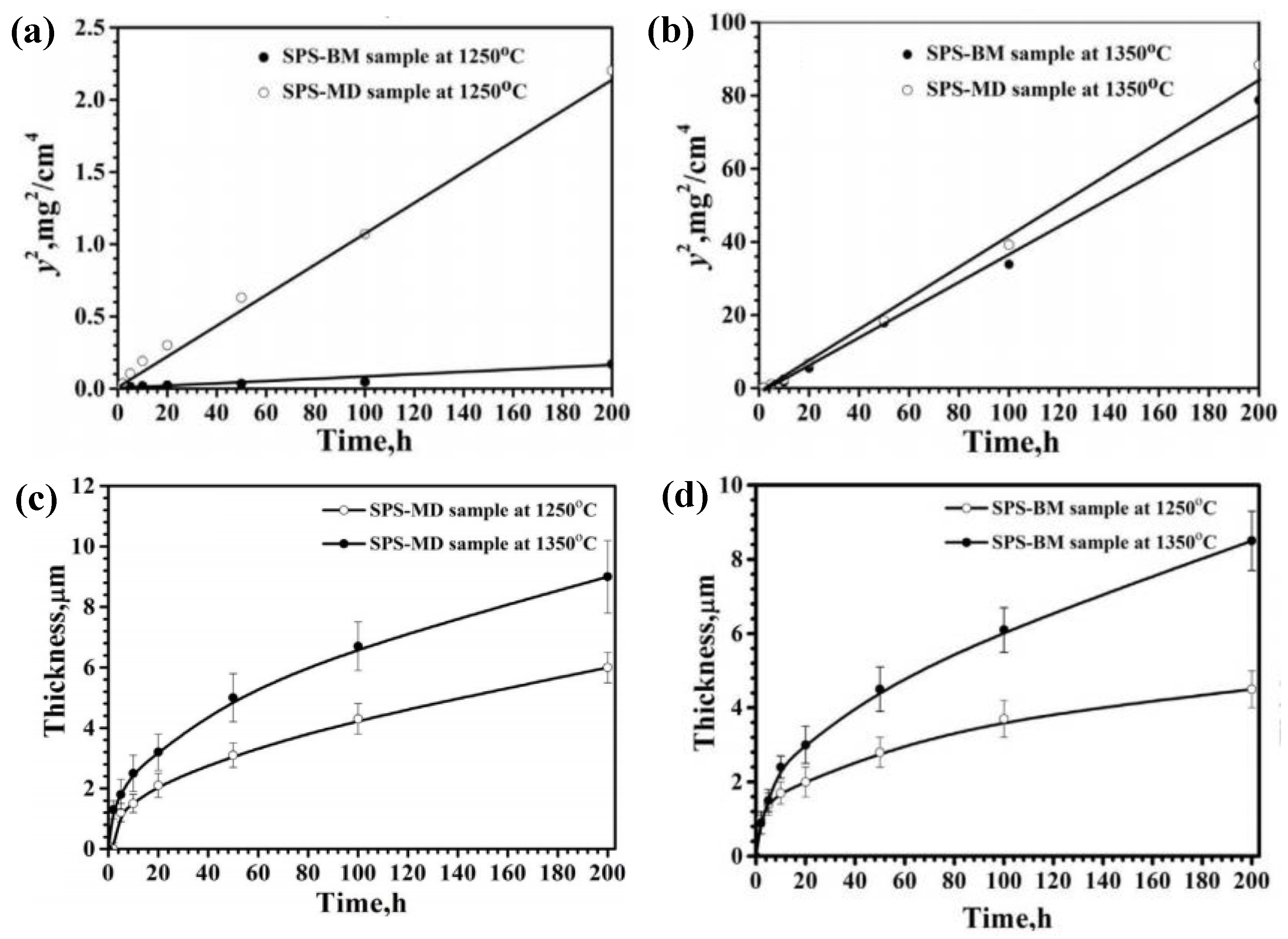
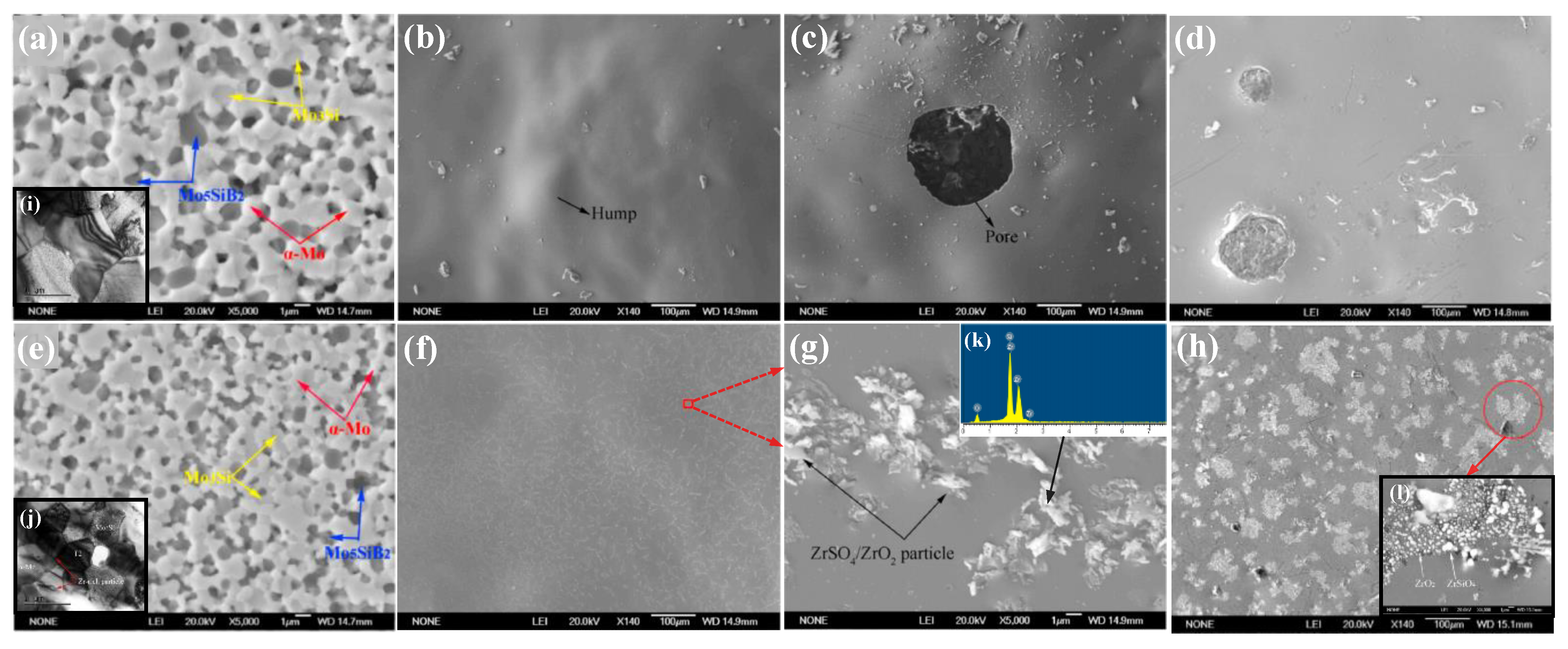

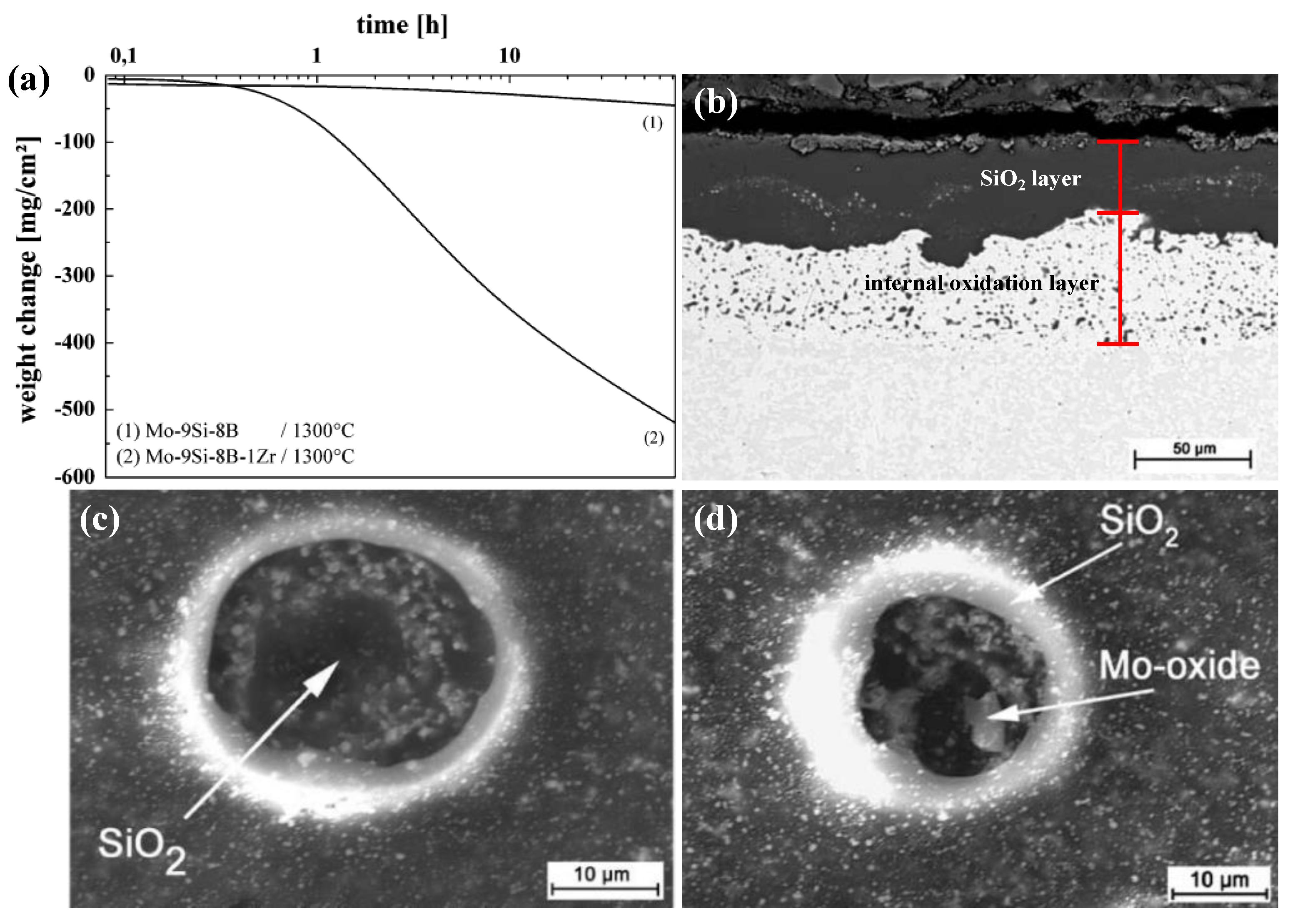

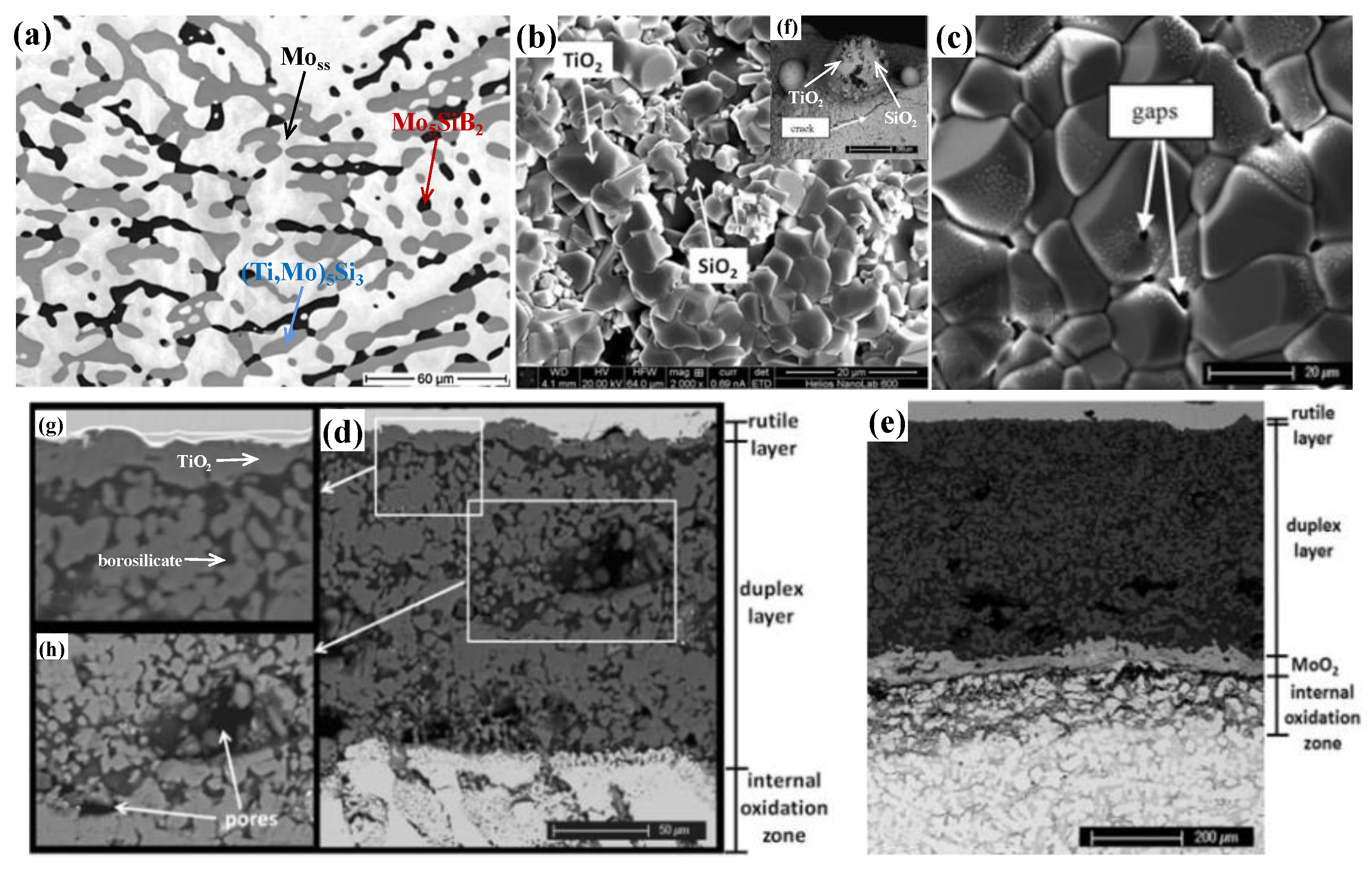



| Temperature (°C) | Oxidation Rate Constants (kg2/m4s) | |
|---|---|---|
| Mo-9Si-8B | Mo-9Si-8B-1Zr | |
| 1000 | 1.0 × 10−9 | 2.78 × 10−12 |
| 1100 | 5.85 × 10−11 | 9.0 × 10−12 |
| 1150 | - | 1.22 × 10−10 |
| 1200 | 1.2 × 10−8 | 3.0 × 10−8 |
| S. No. | T (°C) | Mass Loss (mg/cm2) | |
|---|---|---|---|
| Mo-14Si-10B | Mo-11.2Si-8.1B-7.3Al | ||
| 1 | 500 | +0.6 | −0.7 |
| 2 | 600 | +1.2 | −4 |
| 3 | 700 | −92.43 | −369.45 |
| 4 | 800 | −36.97 | −677.43 |
| 5 | 900 | −18.83 | −621.54 |
| 6 | 1150 | −1.11 | −131.44 |
| 7 | 1300 | −112.81 | −192.37 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, L.; Shen, F.; Fu, T.; Zhang, Y.; Cui, K.; Wang, J.; Zhang, X. Microstructure and Oxidation Behavior of Metal-Modified Mo-Si-B Alloys: A Review. Coatings 2021, 11, 1256. https://doi.org/10.3390/coatings11101256
Yu L, Shen F, Fu T, Zhang Y, Cui K, Wang J, Zhang X. Microstructure and Oxidation Behavior of Metal-Modified Mo-Si-B Alloys: A Review. Coatings. 2021; 11(10):1256. https://doi.org/10.3390/coatings11101256
Chicago/Turabian StyleYu, Laihao, Fuqiang Shen, Tao Fu, Yingyi Zhang, Kunkun Cui, Jie Wang, and Xu Zhang. 2021. "Microstructure and Oxidation Behavior of Metal-Modified Mo-Si-B Alloys: A Review" Coatings 11, no. 10: 1256. https://doi.org/10.3390/coatings11101256
APA StyleYu, L., Shen, F., Fu, T., Zhang, Y., Cui, K., Wang, J., & Zhang, X. (2021). Microstructure and Oxidation Behavior of Metal-Modified Mo-Si-B Alloys: A Review. Coatings, 11(10), 1256. https://doi.org/10.3390/coatings11101256






