Defective Microwave Photonic Crystals for Salinity Detection
Abstract
:1. Introduction
2. Materials and Methods
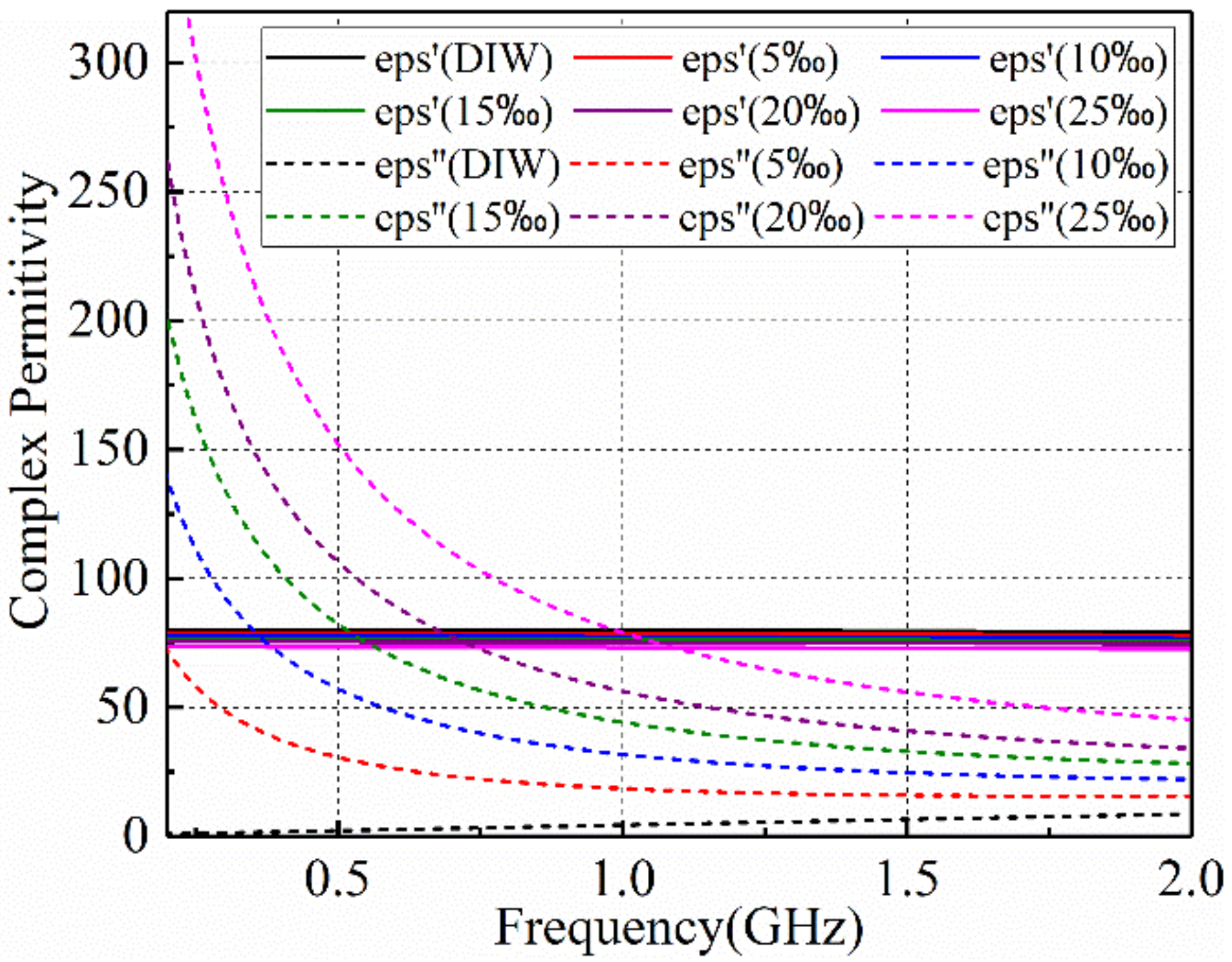
2.1. Microwave Dielectric Properites of Salt Solution
2.2. Defective Microwave Photonic Crystal Design
2.3. Transfer Matrix Method for Lossy Medium
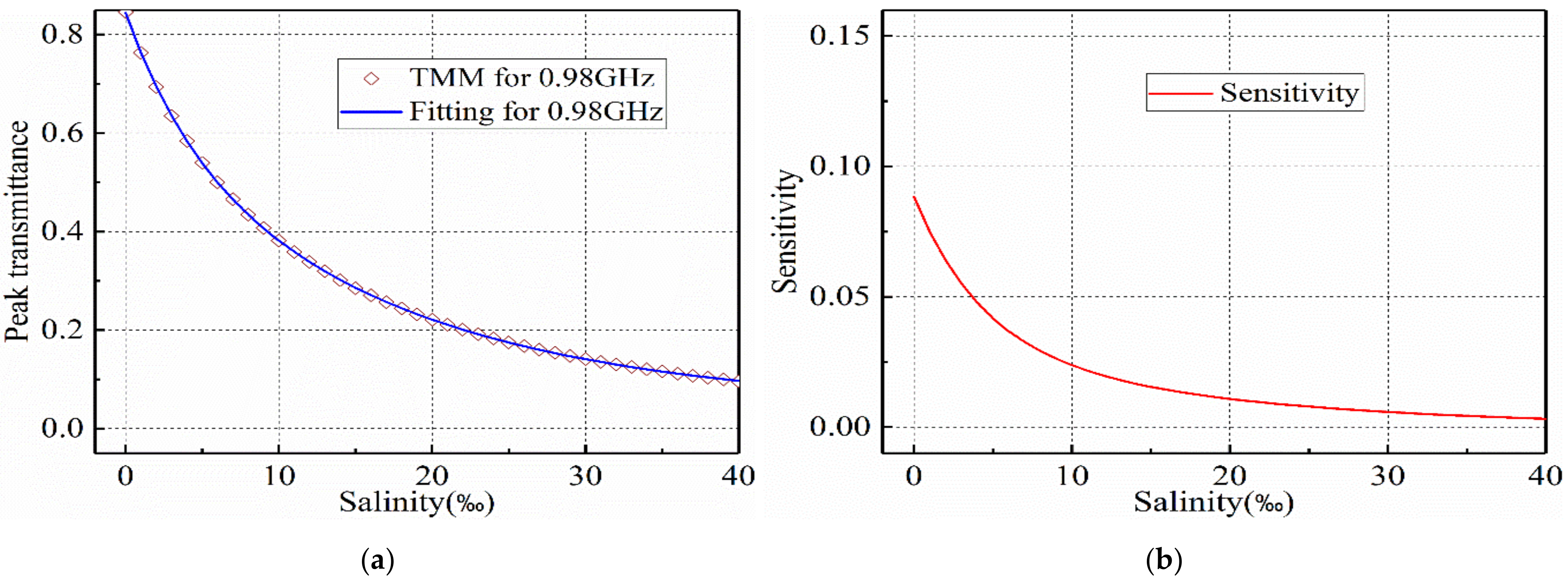
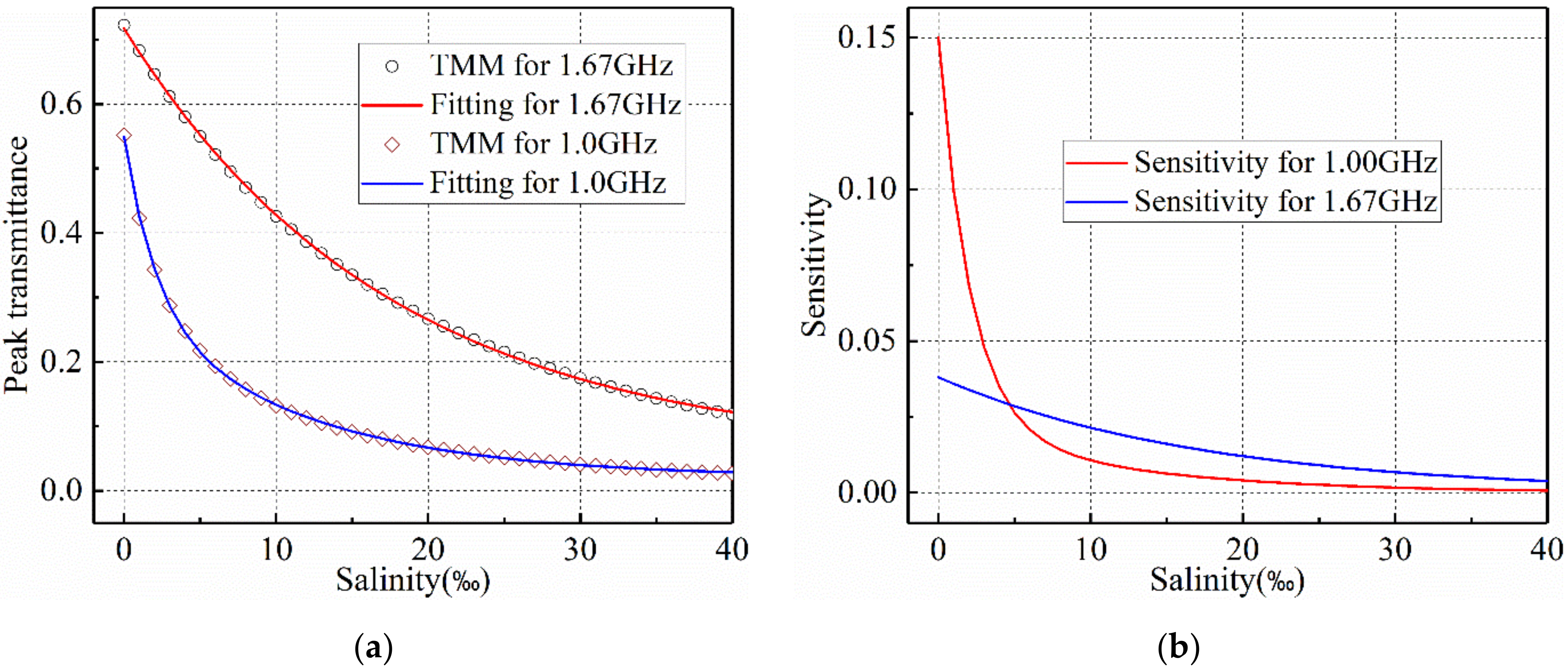
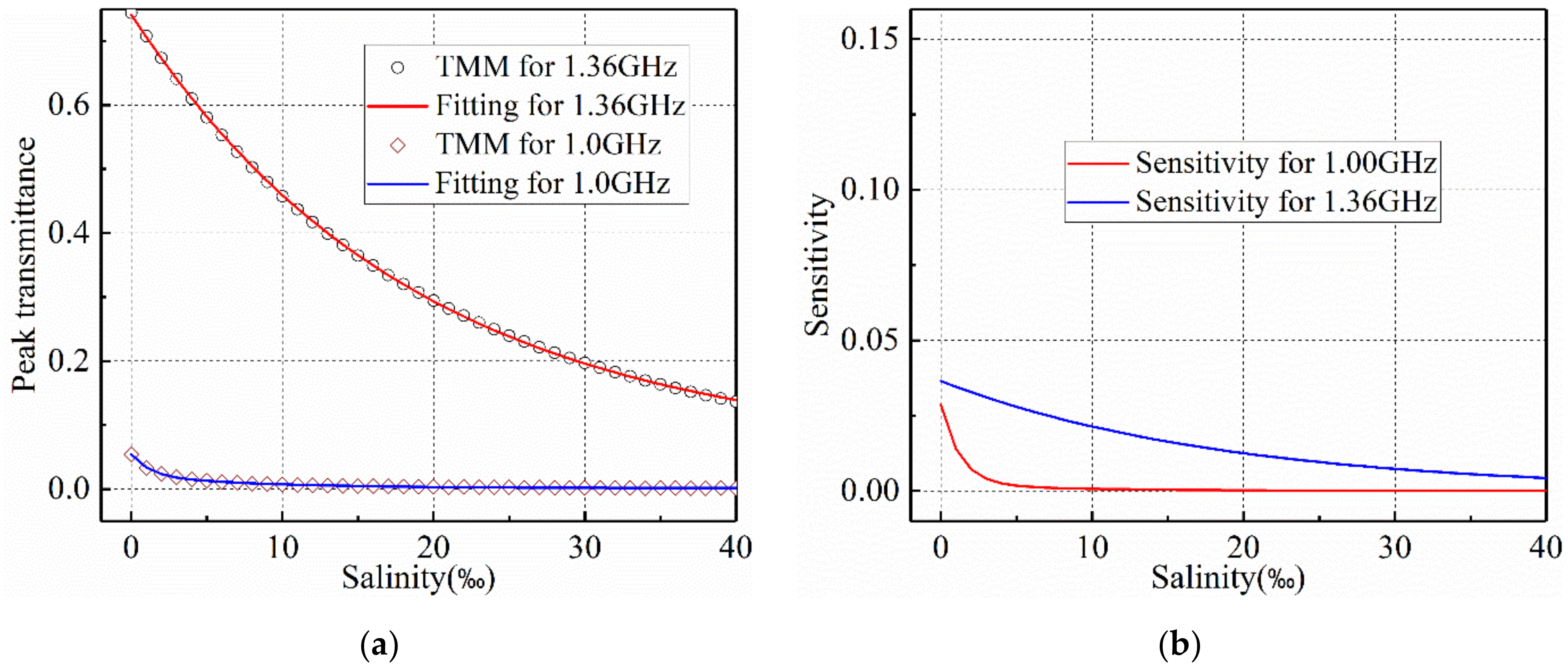
2.4. Detecting Sensitivity
2.5. Quality Factor
3. Results
3.1. Microwave Dielectric Properites of Salt Solution
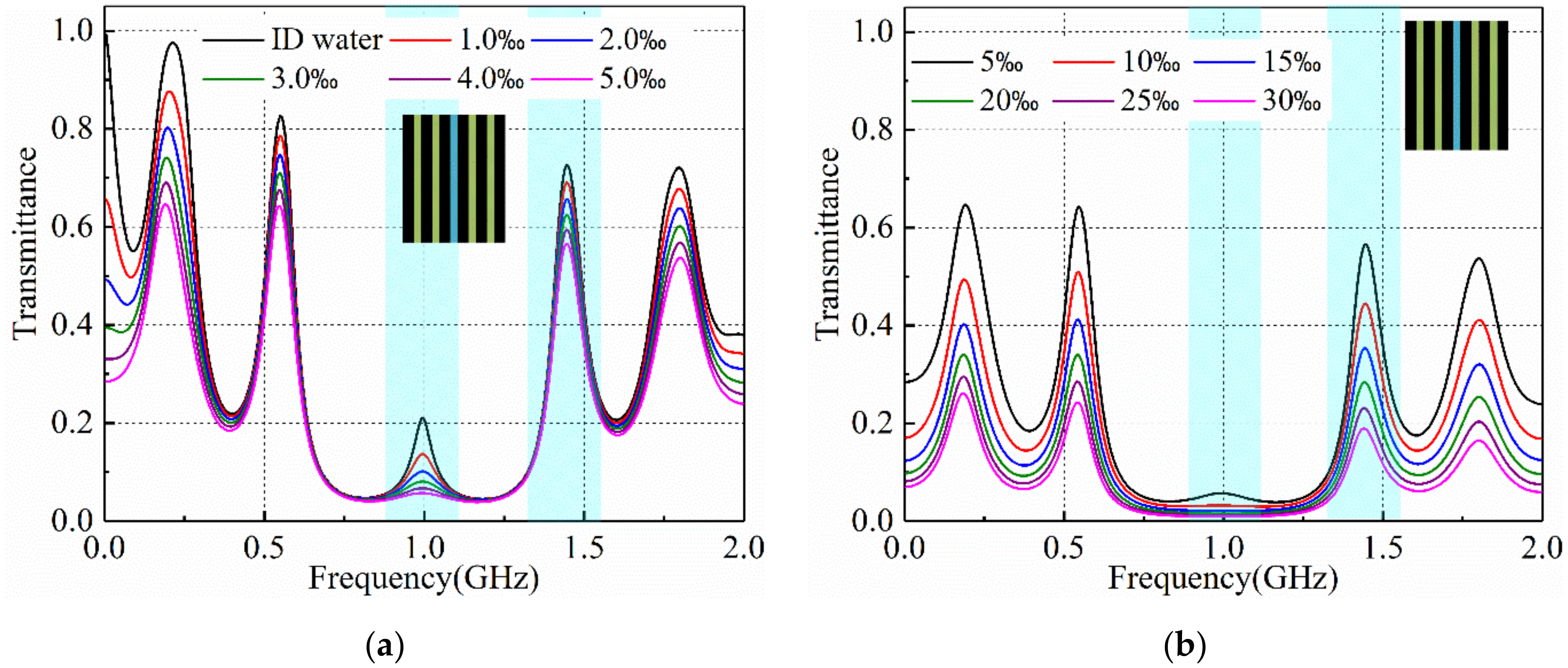
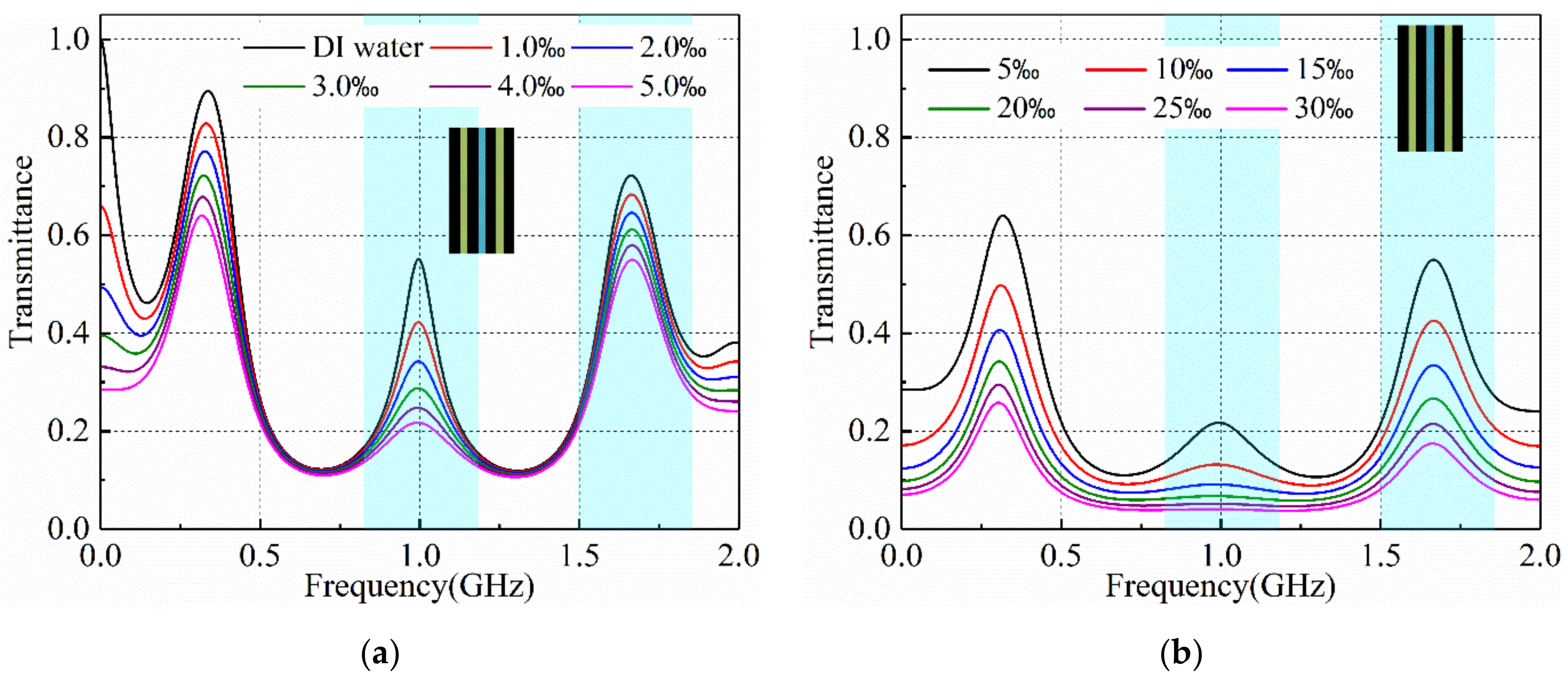
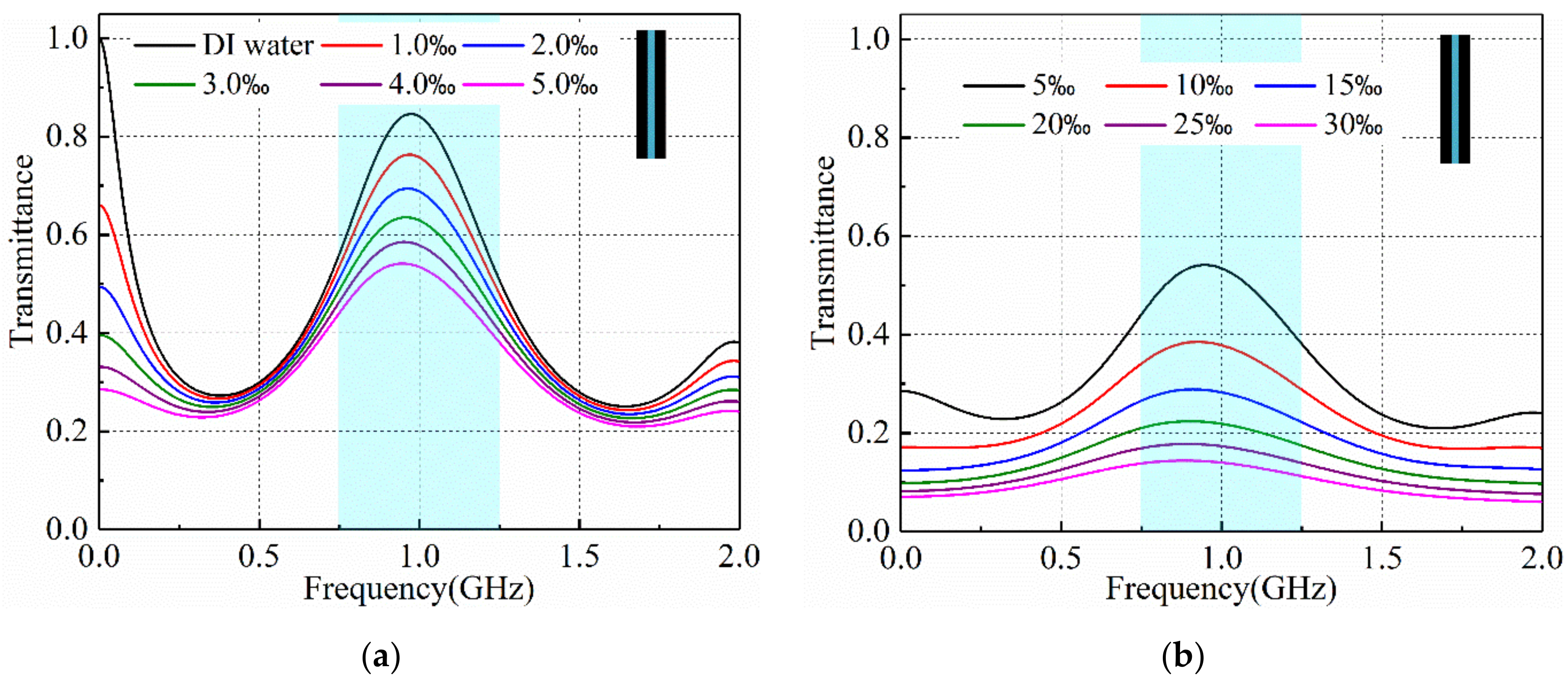
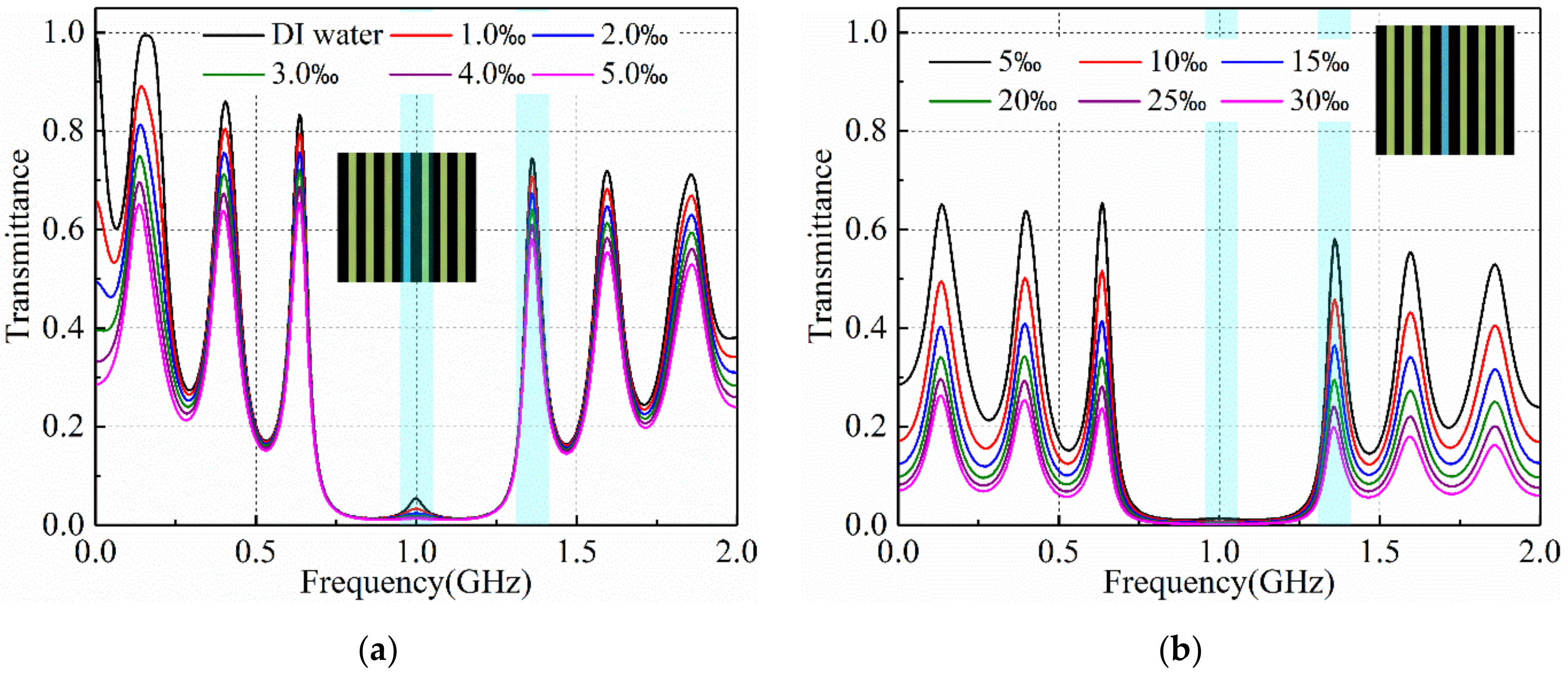
3.2. Salinity Sensoring Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Durack, E.; Alonso-Gomez, M.; Wilkinson, M.G. Salt: A review of its role in food science and public health. Curr. Nutr. Food Sci. 2008, 4, 290–297. [Google Scholar] [CrossRef]
- Harnsoongnoen, S.; Wanthong, A. A non-contact planar microwave sensor for detection of high-salinity water containing NaCl, KCl, CaCl2, MgCl2 and Na2CO3. Sens. Actuators B Chem. 2021, 331, 129355. [Google Scholar] [CrossRef]
- Islam, M.T.; Rahman, M.N.; Singh, M.S.J. Detection of salt and sugar contents in water on the basis of dielectric properties using microstrip antenna-based sensor. IEEE Access 2018, 6, 4118–4126. [Google Scholar] [CrossRef]
- GB 2760–2014 (2014) National Food Safety Standard, Standards for the Use of Food Additives; National Health and Family Planning Commission of the People’s Republic of China & China Food and Drug Administration: Beijing, China, 2014.
- Li, X.B.; Kang, Y.H. Agricultural utilization and vegetation establishment on saline-sodic soils using a water–salt regulation method for scheduled drip irrigation. Agric. Water Manag. 2020, 231, 105995. [Google Scholar] [CrossRef]
- Chiang, C.T.; Chang, C.W. Design of a calibrated salinity sensor transducer for monitoring salinity of ocean environment and aquaculture. IEEE Sens. J. 2015, 15, 5151–5157. [Google Scholar] [CrossRef]
- You, B.; Yue, Y.D.; Sun, M.X.; Li, J.Y.; Jia, D.L. Design of a real-time salinity detection system for water injection wells based on fuzzy control. Sensors 2021, 21, 3086. [Google Scholar] [CrossRef]
- Stadler, A. Analyzing UV/Vis/NIR spectra—Correct and efficient parameter extraction. IEEE Sens. J. 2010, 10, 1921–1931. [Google Scholar] [CrossRef]
- Stadler, A. Analyzing UV/Vis/NIR spectra—Part II: Correct and efficient parameter extraction. IEEE Sens. J. 2011, 11, 897–904. [Google Scholar] [CrossRef]
- Kapilevich, B.; Litvak, B. Optimized microwave sensor for online concentration measurements of binary liquid mixtures. IEEE Sens. J. 2011, 11, 2611–2616. [Google Scholar] [CrossRef]
- Diamond, J.M. An inductive conductivity meter for monitoring the salinity of dialysis Water. IEEE Trans. Biomed. Eng. 1970, 17, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Hyldgard, A.; Mortensen, D.; Birkelund, K.; Hansen, O.; Thomsen, E.V. Autonomous multi-sensor micro-system for measurement of ocean water salinity. Sens. Actuators A Phys. 2008, 147, 474–484. [Google Scholar] [CrossRef]
- Huang, X.; Pascal, R.W.; Chamberlain, K.; Banks, C.J.; Mowlem, M.; Morgan, H. A Miniature, high precision conductivity and temperature sensor system for ocean monitoring. IEEE Sens. J. 2011, 11, 3246–3252. [Google Scholar] [CrossRef]
- Cong, J.; Zhang, X.M.; Chen, K.S.; Xu, J. Fiber optic Bragg grating sensor based on hydrogels for measuring salinity. Sens. Actuators B Chem. 2002, 87, 487–490. [Google Scholar] [CrossRef]
- Kauffmann, T.H.; Fontana, M.D. Optical sensor of salt concentration: Uncertainty evaluation. Sens. Actuators B Chem. 2012, 161, 21–27. [Google Scholar] [CrossRef]
- Yin, Y.; Li, S.; Ren, J.; Farrell, G.; Lewis, E.; Wang, P. High-sensitivity salinity sensor based on optical microfiber coil resonator. Opt. Express 2018, 26, 34633–34640. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.Z.; Qiao, X.G.; Lim, K.S.; Harun, S.W.; Chong, W.Y.; Islam, M.R.; Ahmad, H. Optical fiber sensing of salinity and liquid level. IEEE Photonics Technol. Lett. 2014, 26, 1742–1745. [Google Scholar] [CrossRef]
- Durickovic, I.; Marchetti, M.; Claverie, R.; Bourson, P.; Chassot, J.M. Experimental study of NaCl aqueous solutions by Raman spectroscopy: Towards a new optical sensor. Appl. Spectrosc. 2010, 64, 853–857. [Google Scholar] [CrossRef]
- Kleis, S.J.; Sanchez, L.A. Dependence of sound velocity on salinity and temperature in saline solutions. Sol. Energy 1991, 46, 371–375. [Google Scholar] [CrossRef]
- Cheng, E.M.; Fareq, M.; Shahriman, A.B.; Mohd Afendi, R.; Lee, Y.S.; Khor, S.F.; Tan, W.H.; Nashrul Fazli, M.N.; Abdullah, A.Z.; Jusoh, M.A. Development of microstrip patch antenna sensing system for salinity and sugar detection in water. Int. J. Mech. Mechatron. Eng. 2014, 15, 31–36. [Google Scholar]
- Rahman, M.N.; Hassan, S.A.; Samsuzzaman, M.; Singh, M.S.J.; Islam, M.T. Determination of salinity and sugar concentration using microwave sensor. Microw. Opt. Technol. Lett. 2019, 61, 361–364. [Google Scholar] [CrossRef]
- Harnsoongnoen, S.; Wanthong, A.; Charoen-In, U.; Siritaratiwat, A. Planar microwave sensor for detection and discrimination of aqueous organic and inorganic solutions. Sens. Actuators B Chem. 2018, 271, 300–305. [Google Scholar] [CrossRef]
- Kilpijärvi, J.; Halonen, N.; Juuti, J.; Hannu, J. Microfluidic microwave sensor for detecting saline in biological range. Sensors 2019, 19, 819. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.Y.; Chen, B.X. Experimental research of optical fiber sensor for salinity measurement. Sens. Actuators A Phys. 2012, 184, 53–56. [Google Scholar] [CrossRef]
- Rahman, H.A.; Harun, S.W.; Yasin, M.; Phang, S.W.; Damanhuri, S.S.A.; Arof, H.; Ahmad, H. Tapered plastic multimode fiber sensor for salinity detection. Sens. Actuators A Phys. 2011, 171, 219–222. [Google Scholar] [CrossRef]
- Wu, C.; Guan, B.O.; Lu, C.; Tam, H.Y. Salinity sensor based on polyimide-coated photonic crystal fiber. Opt. Express 2011, 19, 20003–20008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vigneswarana, D.; Ayyanarb, N.; Sharmac, M.; Sumathib, M.; Rajand, M.S.M.; Porsezian, K. Salinity sensor using photonic crystal fiber. Sens. Actuators A Phys. 2018, 269, 22–28. [Google Scholar] [CrossRef]
- Amiri, I.S.; Paul, B.K.; Ahmed, K.; Aly, A.H.; Zakzria, R.; Yupapin, P.; Vigneswaran, D. Tri-core photonic crystal fiber based refractive index dual sensor for salinity and temperature detection. Microw. Opt. Technol. Lett. 2019, 61, 847–852. [Google Scholar] [CrossRef]
- Ramya, K.C.; Monfared, Y.E.; Maheswar, R.; Dhasarathan, V. Dual-core twisted photonic crystal fiber salinity sensor: A numerical investigation. IEEE Photonics Technol. Lett. 2020, 32, 616–619. [Google Scholar] [CrossRef]
- Zhang, Y.; Xie, X.; Yang, Z.K.; Hao, J.J.; Xu, Z.G.; Yang, H.W. Study on the spectrum of photonic crystal cavity and its application in measuring the concentration of NaCl Solution. Z. Nat. A J. Phys. Sci. 2017, 72, 345–349. [Google Scholar] [CrossRef]
- Ben Ali, N.; Alsaif, H.; Trabelsi, Y.; Chughtai, M.T.; Dhasarathan, V.; Kanzari, M. High sensitivity to salinity-temperature using one-dimensional deformed photonic crystal. Coatings 2021, 11, 713. [Google Scholar]
- Qutb, S.R.; Aly, A.H.; Sabra, W. Salinity and temperature detection for seawater based on a 1d-defective photonic crystal material. Int. J. Mod. Phys. B 2021, 35, 2150012. [Google Scholar] [CrossRef]
- Sayed, H.; Aly, A.H. Salinity optical sensor by using two-dimensional photonic crystals: Computational study. Mater. Sci. Eng. B 2021, 269, 115169. [Google Scholar] [CrossRef]
- Zaky, Z.A.; Aly, A.H. Highly sensitive salinity and temperature sensor using tamm resonance. Plasmonics 2021. early access. [Google Scholar] [CrossRef]
- Lee, K.; Hassan, A.; Lee, C.H.; Bae, J. Microstrip patch sensor for salinity determination. Sensors 2017, 17, 2941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Velez, P.; Grenier, K.; Mata-Contreras, J.; Dubuc, D.; Martín, F. Highly-sensitive microwave sensors based on open complementary split ring resonators (OCSRRs) for dielectric characterization and solute concentration measurement in liquids. IEEE Access 2018, 6, 48324–48338. [Google Scholar] [CrossRef]
- Velez, P.; Munoz-Enano, J.; Grenier, K.; Mata-Contreras, J.; Dubuc, D.; Martín, F. Split ring resonator-based microwave fluidic sensors for electrolyte concentration measurements. IEEE Sens. J. 2019, 19, 2562–2569. [Google Scholar] [CrossRef]
- Chudpooti, N.; Duangrit, N.; Sangpet, P.; Akkaraekthalin, P.; Imberg, B.U.; Robertson, I.D.; Somjit, N. In-situ self-aligned NaCl-solution fluidic-integrated microwave sensors for industrial and biomedical applications. IEEE Access 2020, 8, 188897–188907. [Google Scholar] [CrossRef]
- Stogryn, A. Equations for calculating the dielectric constant of saline water. IEEE Trans. Microw. Theory Tech. 1971, 19, 733–736. [Google Scholar] [CrossRef]
- Klein, L.; Swift, C. An improved model for the dielectric constant of sea water at microwave frequencies. IEEE Trans. Antennas Propag. 1977, 25, 104–111. [Google Scholar] [CrossRef]
- Gadani, D.H.; Rana, V.A.; Bhatnagar, S.P.; Prajapati, A.N.; Vyas, A.D. Effect of salinity on the dielectric properties of water. Indian J. Pure Appl. Phys. 2012, 50, 405–410. [Google Scholar]
- John, S. Strong localization of photons in certain disordered dielectric superlattices. Phys. Rev. Lett. 1987, 58, 2486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.Y.; Liu, S.B. Tunable filter using plasma defect in one-dimensional microwave photonic crystal. Acta Phys. Sin. 2009, 58, 7062–7066. [Google Scholar]
- Delfino, F.; Procopio, R.; Rossi, M.; Rachidi, F. Prony series representation for the lightning channel base current. IEEE Trans. Electromagn. Compat. 2012, 54, 308–315. [Google Scholar] [CrossRef]
- Nocedal, J.; Wright, S.J. Numerical Optimization; Springer: Berlin/Heidelberg, Germany, 1999. [Google Scholar]


| Sensor Structure | Defective Resonance | 1st Transmitting Mode |
|---|---|---|
| ACA | 1.364 | - |
| ABACABA | 5.332 | 5.612 |
| ABABACABABA | 10.345 | 12.146 |
| ABABABACABABABA | 11.089 | 21.143 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Y.; Yang, H. Defective Microwave Photonic Crystals for Salinity Detection. Coatings 2021, 11, 1243. https://doi.org/10.3390/coatings11101243
Zhu Y, Yang H. Defective Microwave Photonic Crystals for Salinity Detection. Coatings. 2021; 11(10):1243. https://doi.org/10.3390/coatings11101243
Chicago/Turabian StyleZhu, Yuxia, and Hongwei Yang. 2021. "Defective Microwave Photonic Crystals for Salinity Detection" Coatings 11, no. 10: 1243. https://doi.org/10.3390/coatings11101243
APA StyleZhu, Y., & Yang, H. (2021). Defective Microwave Photonic Crystals for Salinity Detection. Coatings, 11(10), 1243. https://doi.org/10.3390/coatings11101243





