Preparation of Degradable Superhydrophobic Mg/P/Z/F/H Composite Materials and Their Anticorrosion
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Sample Preparation
2.3. Preparation of Mg/P/Z/F/H Composite Materials
2.4. Surface Characterization and Property Tests
3. Results and Discussion
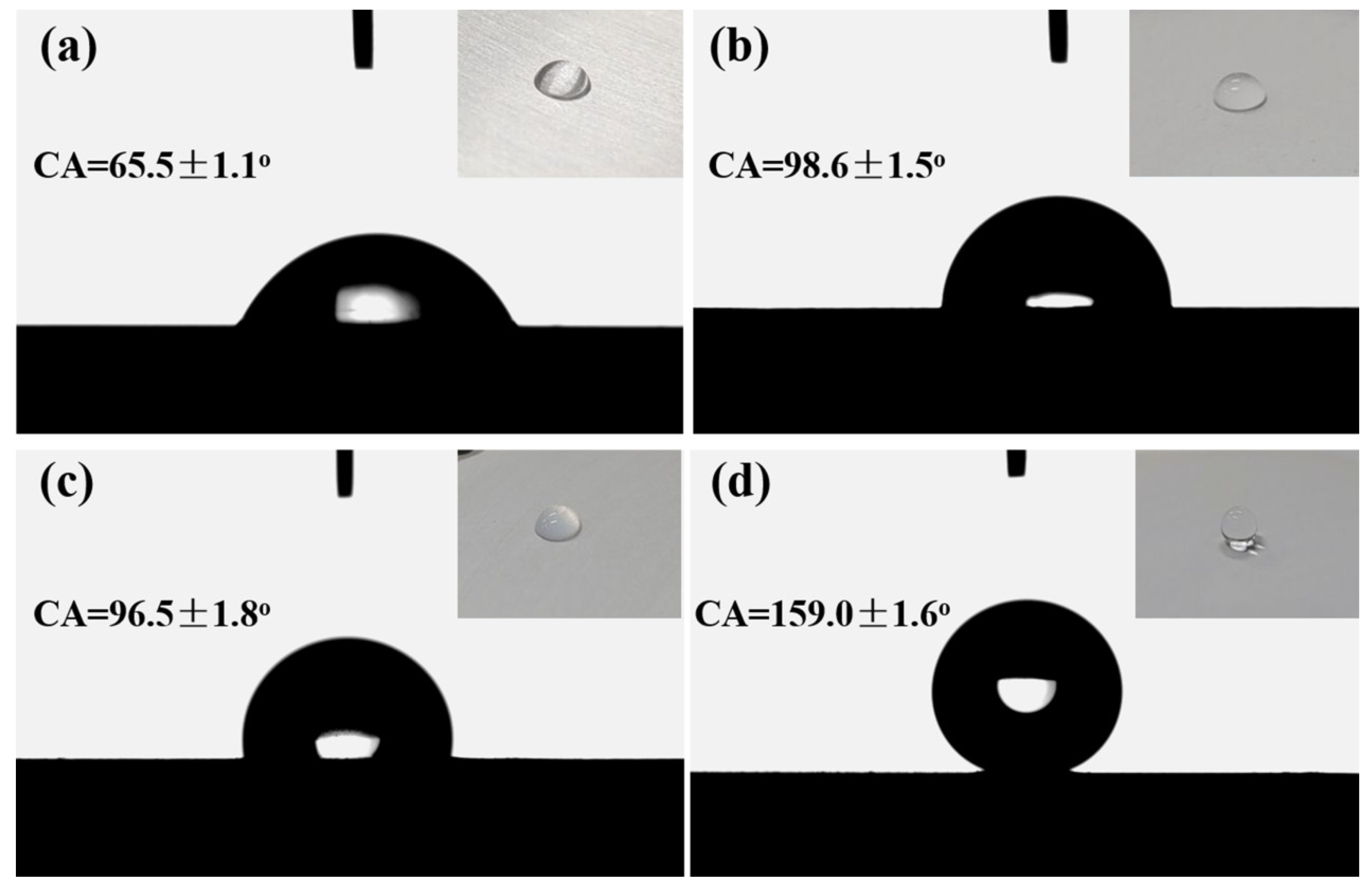
3.1. Wetting Behaviors
3.2. Surface Characteristics
3.3. Electrochemical Corrosion Behavior
3.4. Interface Model and Anticorrosion Mechanism
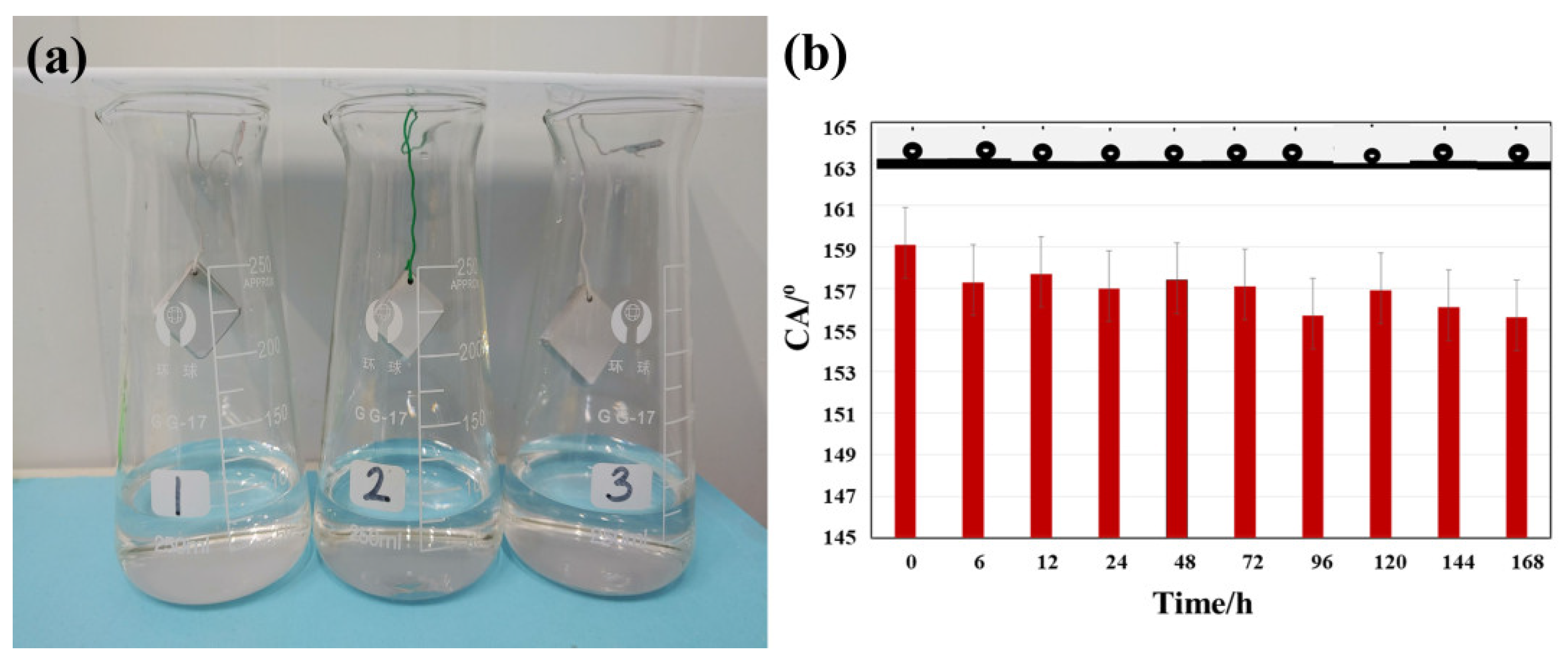
3.5. Stability and Adhesion
3.6. Self-Cleaning Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wei, J.; Zhang, G.; Dong, J.; Wang, H.; Guo, Y.; Zhuo, X.; Li, C.; Liang, H.; Gu, S.; Li, C.; et al. Facile, scalable spray-coating of stable emulsion for transparent self-cleaning surface of cellulose-based materials. ACS Sustain. Chem. Eng. 2018, 6, 11335–11344. [Google Scholar] [CrossRef]
- Xiao, L.; Deng, M.; Zeng, W.; Zhang, B.; Xu, Z.; Yi, C.; Liao, G. Novel robust superhydrophobic coating with self-cleaning properties in air and oil based on rare earth metal oxide. Ind. Eng. Chem. Res. 2017, 56, 12354–12361. [Google Scholar] [CrossRef]
- Tavakoli, M.M.; Tsui, K.H.; Zhang, Q.; He, J.; Yao, Y.; Li, D.; Fan, Z. Highly efficient flexible perovskite solar cells with antireflection and self-cleaning nanostructures. ACS Nano 2015, 9, 10287–10295. [Google Scholar] [CrossRef]
- Chen, B.; Qiu, J.; Sakai, E.; Kanazawa, N.; Liang, R.; Feng, H. Robust and superhydrophobic surface modification by a “paint + adhesive” method: Applications in self-cleaning after oil contamination and oil-water separation. ACS Appl. Mater. Interfaces 2016, 8, 17659–17667. [Google Scholar] [CrossRef]
- Shi, T.; Li, X.; Zhang, C.; Wang, H.; He, Z.; Zhou, X.; Yang, D.; Yang, H.; Zhang, B.; Yang, K. One-step preparation of the superhydrophobic Al alloy surface with enhanced corrosion and wear resistance. Mater. Corros. 2020, 72, 904–911. [Google Scholar] [CrossRef]
- Zhang, J.; Gu, C.; Tu, J. Robust slippery coating with superior corrosion resistance and anti-icing performance for AZ31B Mg alloy protection. ACS Appl. Mater. Interfaces 2017, 9, 11247–11257. [Google Scholar] [CrossRef]
- Qi, C.; Chen, H.; Shen, L.; Li, X.; Fu, Q.; Zhang, Y.; Sun, Y.; Liu, Y. Superhydrophobic surface based on assembly of nanoparticles for application in anti-icing under ultralow temperature. ACS Appl. Nano Mater. 2020, 3, 2047–2057. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, L.; Lin, Z.; Ding, L.; Zhang, X.; Dai, R.; Yan, Q.; Wang, X.-D. Highly sensitive dissolved oxygen sensor with a sustainable antifouling, antiabrasion, and self-cleaning superhydrophobic surface. ACS Omega 2019, 4, 1715–1721. [Google Scholar] [CrossRef]
- Liu, R.; Chi, Z.; Cao, L.; Weng, Z.; Wang, L.; Li, L.; Saeed, S.; Lian, Z.; Wang, Z. Fabrication of biomimetic superhydrophobic and anti-icing Ti6Al4V alloy surfaces by direct laser interference lithography and hydrothermal treatment. Appl. Surf. Sci. 2020, 534, 147576. [Google Scholar] [CrossRef]
- Lin, J.; Zheng, C.; Ye, W.; Wang, H.; Feng, D.; Li, Q.; Huan, B. A facile dip-coating approach to prepare SiO2/fluoropolymer coating for superhydrophobic and superoleophobic fabrics with self-cleaning property. J. Appl. Polym. Sci. 2015, 132, 41458. [Google Scholar] [CrossRef]
- Darmanin, T.; de Givenchy, E.T.; Amigoni, S.; Guittard, F. Superhydrophobic Surfaces by electrochemical processes. Adv. Mater. 2013, 25, 1378–1394. [Google Scholar] [CrossRef]
- Zang, D.M.; Zhu, R.W.; Zhang, W.; Yu, X.Q.; Lin, L.; Guo, X.L.; Liu, M.J.; Jiang, L. Corrosion resistance: Corrosion-resistant super hydrophobic coatings on Mg alloy surfaces inspired by lotus seedpod. Adv. Funct. Mater. 2017, 27, 1605446. [Google Scholar]
- Liu, Q.; Chen, D.; Kang, Z. One-step electrodeposition process to fabricate corrosion-resistant superhydrophobic surface on magnesium alloy. ACS Appl. Mater. Interfaces 2015, 7, 1859–1867. [Google Scholar] [CrossRef]
- Suryaprabha, T.; Sethuraman, M.G. Fabrication of copper-based superhydrophobic self-cleaning antibacterial coating over cotton fabric. Cellulose 2016, 24, 395–407. [Google Scholar] [CrossRef]
- Han, C.H.; Min, B.G. Superhydrophobic and antibacterial properties of cotton fabrics coated with copper nanoparticles through sonochemical process. Fibers Polym. 2020, 21, 785–791. [Google Scholar]
- Ghasemi, N.; Seyfi, J.; Asadollahzadeh, M.J. Imparting superhydrophobic and antibacterial properties onto the cotton fabrics: Synergistic effect of zinc oxide nanoparticles and octadecanethiol. Cellulose 2018, 25, 4211–4222. [Google Scholar] [CrossRef]
- Hsu, C.P.; Chang, L.Y.; Chiu, C.W.; Lee, P.T.C.; Lin, J.J. Facile fabrication of robust superhydrophobic epoxy film with polyamine dispersed carbon nanotubes. ACS Appl. Mater. Interfaces 2013, 5, 538–545. [Google Scholar]
- Bonneaud, C.; Howell, J.; Bongiovanni, R.; Joly-Duhamel, C.; Friesen, C.M. Diversity of synthetic approaches to functionalized perfluoropolyalkylether polymers. Macromolecules 2021, 54, 521–550. [Google Scholar] [CrossRef]
- Tang, Y.; Huang, H.; Guo, X.; Zhong, C. Superhydrophobic ether-based porous organic polymer-coated polyurethane sponge for highly efficient oil-water separation. Ind. Eng. Chem. Res. 2020, 59, 13228–13238. [Google Scholar] [CrossRef]
- Tam, J.; Jiao, Z.; Lau, J.; Erb, U. Wear stability of superhydrophobic nano Ni-PTFE electrodeposits. Wear 2017, 374, 1–4. [Google Scholar] [CrossRef]
- Liu, J.; Ouyang, Y.; Qiu, R.; Ma, B.; Zhang, Y.; Hu, S. Compositing fluid infused in superhydrophobic Cu(OH)2 nanoneedle matrix to inhibit abiotic and microbiologically induced corrosion of Cu in seawater environment. Prog. Org. Coat. 2020, 142, 105542. [Google Scholar] [CrossRef]
- Abad, S.N.K.; Ilkhechi, N.N.; Adel, M.; Mozammel, M. Hierarchical architecture of a superhydrophobic Cd-Si co-doped TiO2 thin film. Appl. Surf. Sci. 2020, 533, 147495. [Google Scholar] [CrossRef]
- Pollock, T.M. Weight loss with magnesium alloys. Science 2010, 328, 986–987. [Google Scholar] [CrossRef]
- Tie, D.; Liu, H.; Guan, R.; Holt-Torres, P.; Liu, Y.; Wang, Y.; Hort, N. In vivo assessment of biodegradable magnesium alloy ureteral stents in a pig model. Acta Biomater. 2020, 116, 415–425. [Google Scholar] [CrossRef]
- Xi, Z.X.; Tan, C.; Xu, L.; Yang, N.; Li, Q. Preparation of novel functional Mg/O/PCL/ZnO composite biomaterials and their cor-rosion resistance. Appl. Surf. Sci. 2015, 351, 410–415. [Google Scholar]
- Sterl, F.; Strohfeldt, N.; Walter, R.; Griessen, R.; Tittl, A.; Giessen, H. Magnesium as novel material for active plasmonics in the visible wavelength range. Nano Lett. 2015, 15, 7949–7955. [Google Scholar] [CrossRef] [Green Version]
- Su, H.; Liu, Y.; Gao, X.; Qian, Y.; Li, W.; Ren, T.; Wang, L.; Zhang, J. Corrosion inhibition of magnesium alloy in NaCl solution by ionic liquid: Synthesis, electrochemical and theoretical studies. J. Alloy. Compd. 2019, 791, 681–689. [Google Scholar] [CrossRef]
- Song, G. recent progress in corrosion and protection of magnesium alloys. Adv. Eng. Mater. 2005, 7, 563–586. [Google Scholar] [CrossRef]
- Qiu, R.X.; Li, C.; Tong, W.; Xiong, D.S.; Li, Z.X.; Wu, Z.L. High-speed wire electrical discharge machining to create superhy-drophobic surfaces for magnesium alloys with high corrosion and wear resistance. Mater. Corros. 2020, 71, 1711–1720. [Google Scholar]
- Yang, Y.; Zhou, J.; Chen, Q.; Detsch, R.; Cui, X.; Jin, G.; Virtanen, S.; Boccaccini, A.R. In vitro osteocompatibility and en-hanced biocorrosion resistance of diammonium hydrogen phosphate-pretreated/poly(ether imide) coatings on magnesium for orthopedic application. ACS Appl. Mater. Interfaces 2019, 11, 29667–29680. [Google Scholar]
- Yu, D.; Qiu, H.; Mou, X.; Dou, Z.; Zhou, N.; Guo, Q.; Lyu, N.; Lu, L.; Yang, Y.; Huang, N. One-pot but two-step vapor-based amine- and fluorine-bearing dual-layer coating for improving anticorrosion and biocompatibility of magnesium alloy. ACS Biomater. Sci. Eng. 2019, 5, 4331–4340. [Google Scholar] [CrossRef]
- Wu, F.; Liu, C.S.; O’Neilla, B.; Wei, J.; Ngothai, Y. Fabrication and properties of porous scaffold of magnesium phos-phate/polycaprolactone biocomposite for bone tissue engineering. Appl. Surf. Sci. 2012, 258, 7589–7595. [Google Scholar]
- Hou, L.; Zhang, X.; Mikael, P.E.; Lin, L.; Dong, W.; Zheng, Y.; Simmons, T.J.; Zhang, F.; Linhardt, R.J. Biodegradable and bioactive PCL-PGS core–shell fibers for tissue engineering. ACS Omega 2017, 2, 6321–6328. [Google Scholar] [CrossRef]
- Li, Y.-C.E.; Wang, J.-H.; Wang, Y.-H.; Shao, H.-J.; Young, L.-C.; Young, T.-H. PCL-blended chitosan substrates for patterning the heterotypic cell distribution in an epithelial and mesenchymal coculture system. ACS Biomater. Sci. Eng. 2020, 6, 4225–4235. [Google Scholar] [CrossRef]
- Yuan, W.; Li, C.Y.; Zhao, C.; Sui, C.G.; Yang, W.T.; Xu, F.J.; Ma, J. Facilitation of gene transfection and cell adhesion by gela-tin-functionalized PCL film surfaces. Adv. Funct. Mater. 2012, 22, 1835–1842. [Google Scholar]
- Behl, A.; Parmar, V.S.; Malhotra, S.; Chhillar, A.K. Biodegradable diblock copolymeric PEG-PCL nanoparticles: Synthesis, characterization and applications as anticancer drug delivery agents. Polymer 2020, 207, 122901. [Google Scholar] [CrossRef]
- Katea, S.N.; Hajduk, Z.C.; Orel, Z.C.; Westin, G. Low cost, fast solution synthesis of 3D framework ZnO nanosponges. Inorg. Chem. 2017, 56, 15150–15158. [Google Scholar] [CrossRef]
- Peter, R.; Salamon, K.; Omerzu, A.; Grenzer, J.; Badovinac, I.J.; Saric, I.; Petravic, M. Role of hydrogen-related defects in pho-tocatalytic activity of ZnO films grown by atomic layer deposition. J. Phys. Chem. C 2020, 124, 8861–8868. [Google Scholar]
- Farhadi, S.; Aliofkhazraei, M.; Darband, G.B.; Abolhasani, A.; Aghdam, A.S.R. Corrosion and wettability of PEO coatings on magnesium by addition of potassium stearate. J. Magnes. Alloy. 2017, 5, 210–216. [Google Scholar] [CrossRef]
- Wang, Z.-H.; Zhang, J.-M.; Li, Y.; Bai, L.-J.; Zhang, G.-J. Enhanced corrosion resistance of micro-arc oxidation coated magnesium alloy by superhydrophobic Mg-Al layered double hydroxide coating. Trans. Nonferr. Met. Soc. China 2019, 29, 2066–2077. [Google Scholar] [CrossRef]
- Wang, L.; Xiao, X.; Liu, E.; Yu, S.; Yin, X.; Wang, J.; Zhu, G.; Li, Q.; Li, J. Fabrication of superhydrophobic needle-like Ca-P coating with anti-fouling and anti-corrosion properties on AZ31 magnesium alloy. Colloids Surf. A Physicochem. Eng. Asp. 2021, 620, 126568. [Google Scholar] [CrossRef]
- Zhang, S.J.; Cao, D.L.; Xu, L.K.; Tang, J.K.; Meng, R.Q.; Li, H.D. Corrosion resistance of a superhydrophobic dodecyltrimethox-ysilane coating on magnesium hydroxide-pretreated magnesium alloy AZ31 by electrodeposition. Colloids Surf. A Physicochem. Eng. Asp. 2021, 625, 126914. [Google Scholar]
- Zhang, Z.-Q.; Zeng, R.-C.; Yan, W.; Lin, C.-G.; Wang, L.; Wang, Z.-L.; Chen, D.-C. Corrosion resistance of one-step superhydrophobic polypropylene coating on magnesium hydroxide-pretreated magnesium alloy AZ31. J. Alloy. Compd. 2019, 821, 153515. [Google Scholar] [CrossRef]
- Yang, N.; Li, J.C.; Bai, N.N.; Xu, L.; Li, Q. One step phase separation process to fabricate superhydrophobic PVC films and its corrosion prevention for AZ91D magnesium alloy. Mater. Sci. Eng. B 2016, 209, 1–9. [Google Scholar]
- Zhou, H.M.; Chen, R.R.; Liu, Q.; Liu, J.Y.; Yu, J.; Wang, C.; Zhang, M.L.; Liu, P.L.; Wang, J. Fabrication of ZnO/epoxy resin su-perhydrophobic coating on AZ31 magnesium alloy. Chem. Eng. J. 2019, 368, 261–272. [Google Scholar]
- Riau, A.K.; Mondal, D.; Setiawan, M.; Palaniappan, A.; Yam, G.H.F.; Liedberg, B.; Venkatraman, S.S.; Mehta, J.S. Functionalization of the polymeric surface with bioceramic nanoparticles via a novel, nonthermal dip coating method. ACS Appl. Mater. Interfaces 2016, 8, 35565–35577. [Google Scholar]
- Fu, J.; Li, X.B.; Wang, L.X.; Lv, X.H.; Lu, Z.; Wang, F.; Xia, Q.; Yu, L.; Li, C.M. One-step dip-coating-fabricated core–shell silk fibroin rice paper fibrous scaffolds for 3D tumor spheroid formation. ACS Appl. Bio Mater. 2020, 3, 7462–7471. [Google Scholar] [CrossRef]
- Bindini, E.; Naudin, G.; Faustini, M.; Grosso, D.; Boissière, C. Critical role of the atmosphere in dip-coating process. J. Phys. Chem. C 2017, 121, 14572–14580. [Google Scholar] [CrossRef] [Green Version]
- Mirri, F.; Ma, A.; Hsu, T.T.; Behabtu, N.; Eichmann, S.L.; Young, C.C.; Tsentalovich, D.E.; Pasquali, M. High-performance carbon nanotube transparent conductive films by scalable dip coating. ACS Nano 2012, 6, 9737–9744. [Google Scholar] [CrossRef]
- Xie, Y.; Zhou, X.; Mi, H.; Ma, J.; Yang, J.; Cheng, J. High efficiency ZnO-based dye-sensitized solar cells with a 1H,1H,2H,2H-perfluorodecyltriethoxysilane chain barrier for cutting on interfacial recombination. Appl. Surf. Sci. 2018, 434, 1144–1152. [Google Scholar] [CrossRef]
- Martins, P.; Lopes, A.C.; Lanceros-Mendez, S. Electroactive phases of poly (vinylidene fluoride): Determination, processing and applications. Prog. Polym. Sci. 2014, 39, 683–706. [Google Scholar]
- Loh, L.; Briscoe, J.; Dunn, S. Chemical protection of ZnO nanorods at ultralow pH to form a hierarchical BiFeO3/ZnO core–shell structure. ACS Appl. Mater. Interfaces 2014, 7, 152–157. [Google Scholar] [CrossRef]
- Felice, B.; Sánchez, M.A.; Socci, M.C.; Sappia, L.D.; Gómez, M.I.; Cruz, M.K.; Felice, C.J.; Martí, M.; Pividori, M.I.; Simonelli, G.; et al. Controlled degradability of PCL-ZnO nanofibrous scaffolds for bone tissue engineering and their antibacterial activity. Mater. Sci. Eng. C 2018, 93, 724–738. [Google Scholar]
- Mallakpour, S.; Lormahdiabadi, M. Polycaprolactone/ZnO-folic acid nanocomposite films: Fabrication, characterization, in-vitro bioactivity, and antibacterial assessment. Mater. Chem. Phys. 2021, 263, 124378. [Google Scholar]
- Kim, J.; Mousa, H.M.; Park, C.H.; Kim, C.S. Enhanced corrosion resistance and biocompatibility of AZ31 Mg alloy using PCL/ZnO NPs via electrospinning. Appl. Surf. Sci. 2017, 396, 249–258. [Google Scholar] [CrossRef]
- Zhao, M.; Dai, Y.K.; Li, X.B.; Li, Y.; Zhang, Y.; Wu, H.R.; Wen, Z.H.; Dai, C.S. Evaluation of long-term biocompatibility and oste-ogenic differentiation of graphene nanosheet doped calcium phosphate-chitosan AZ91D composites. Mater. Sci. Eng. C 2018, 90, 365–378. [Google Scholar]
- She, Z.; Li, Q.; Wang, Z.; Li, L.; Chen, F.; Zhou, J. Novel method for controllable fabrication of a superhydrophobic CuO surface on AZ91D magnesium alloy. ACS Appl. Mater. Interfaces 2012, 4, 4348–4356. [Google Scholar] [CrossRef]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]









| Substrates | Dip-Coating Concentrations | Treatment Condition |
|---|---|---|
| Mg/P | 5 wt.% PCL | None |
| Mg/P/Z | 5 wt.% PCL; 5 wt.% ZnO | None |
| Mg/P/Z/F | 5 wt.% PCL; 5 wt.% ZnO; 1.5 mL PFDTES | None |
| Mg/P/Z/F/H | 5 wt.% PCL; 5 wt.% ZnO; 1.5 mL PFDTES | 50 °C, 30 min |
| Substrates | Icorr (A/cm2) | Ecorr (V) | Corrosion Rate (mm/y) |
|---|---|---|---|
| Mg | 1.0237 × 10−3 | −1.7694 | 22.0580 |
| Mg/P | 1.7464 × 10−5 | −1.6203 | 0.3763 |
| Mg/P/Z | 1.6043 × 10−5 | −1.7200 | 0.3457 |
| Mg/P/Z/F | 7.1288 × 10−6 | −1.3073 | 0.1536 |
| Mg/P/Z/F/H | 8.7914 × 10−8 | −1.1752 | 1.9152 × 10−3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xi, Z.; Yuan, C.; Bai, X.; Wang, C.; Neville, A. Preparation of Degradable Superhydrophobic Mg/P/Z/F/H Composite Materials and Their Anticorrosion. Coatings 2021, 11, 1239. https://doi.org/10.3390/coatings11101239
Xi Z, Yuan C, Bai X, Wang C, Neville A. Preparation of Degradable Superhydrophobic Mg/P/Z/F/H Composite Materials and Their Anticorrosion. Coatings. 2021; 11(10):1239. https://doi.org/10.3390/coatings11101239
Chicago/Turabian StyleXi, Zhongxian, Chengqing Yuan, Xiuqin Bai, Chun Wang, and Anne Neville. 2021. "Preparation of Degradable Superhydrophobic Mg/P/Z/F/H Composite Materials and Their Anticorrosion" Coatings 11, no. 10: 1239. https://doi.org/10.3390/coatings11101239
APA StyleXi, Z., Yuan, C., Bai, X., Wang, C., & Neville, A. (2021). Preparation of Degradable Superhydrophobic Mg/P/Z/F/H Composite Materials and Their Anticorrosion. Coatings, 11(10), 1239. https://doi.org/10.3390/coatings11101239






