Hybrid Zinc-Based Multilayer Systems with Improved Protective Ability against Localized Corrosion Incorporating Polymer-Modified ZnO or CuO Particles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Preparation of Stable ZnO Suspension
2.2. Materials and Preparation of Stable CuO Suspension
2.3. Characterization of ZnO and CuO Nanoparticles
2.4. Electrodeposition of Hybrid Coatings Containing ZnO
- (1)
- First step is to electrodeposit a layer of PEI coated ZnO nanoparticles on low-carbon steel sample at pH 7.5 (to minimize the ZnO solubility effects) at a cathodic current (direct current—DC) density of 0.02 A/dm2 and application of non-soluble anodes. The duration of this step is 10 min. As already discussed, the role of PEI is to provide large electrostatic repulsive forces between the positively charged ZnO nanoparticles stabilizing in such a way that the ZnO suspension is promoting their further electrodeposition on the cathode.
- (2)
- Second step is to electrodeposit zinc coating (thickness ~14 µm) on the obtained ZnO nanoparticles layer from slightly acidic zinc electrolyte (pH 4.5–5.0) at a cathodic current (direct current—DC) density of 2 A/dm2 by using soluble zinc anodes. The duration of this step is 20 min.
2.5. Electrodeposition of Hybrid Coatings Containing CuO
2.6. Corrosion Characterization
2.7. Corrosive Medium
2.8. Reproducibility
3. Results and Discussion
3.1. Characterization of ZnO Nanoparticles Dispersion
3.2. Characterization of CuO Nanoparticles
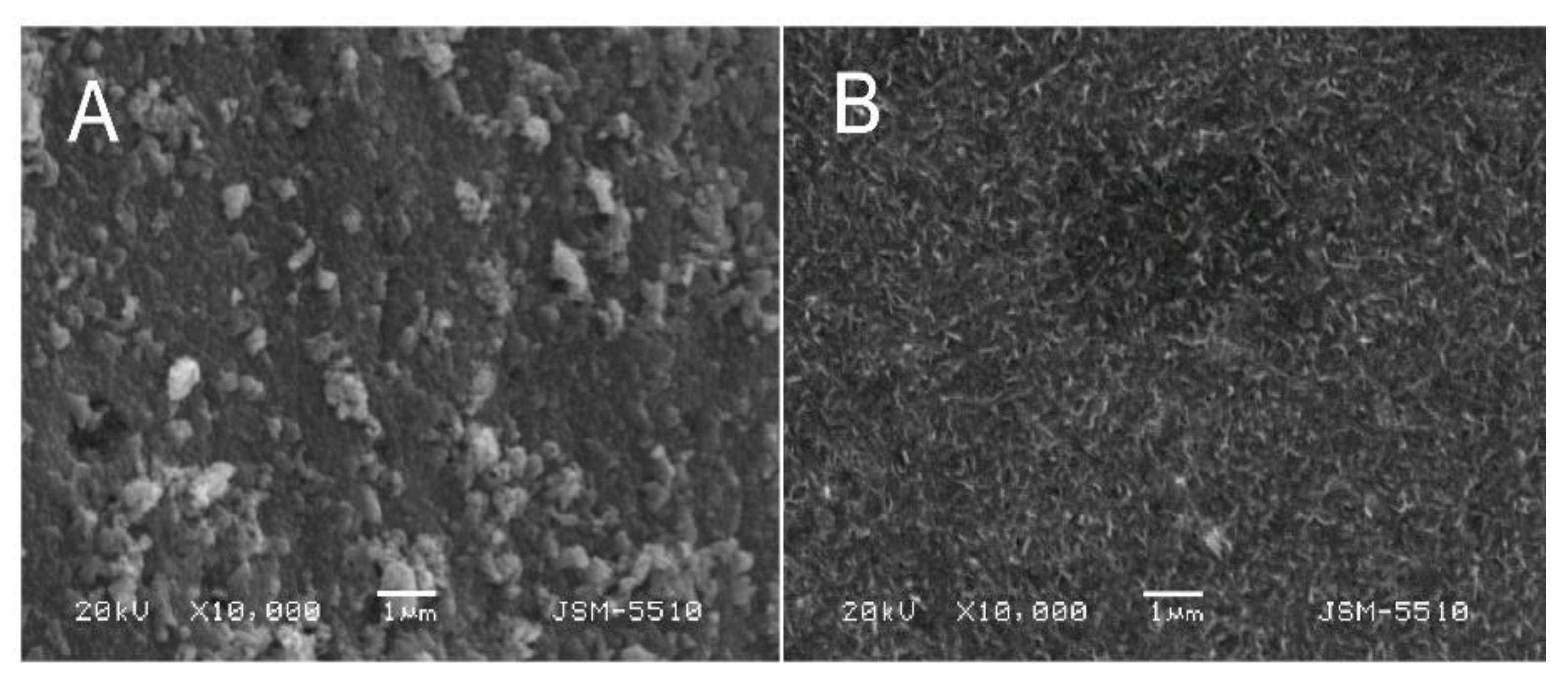
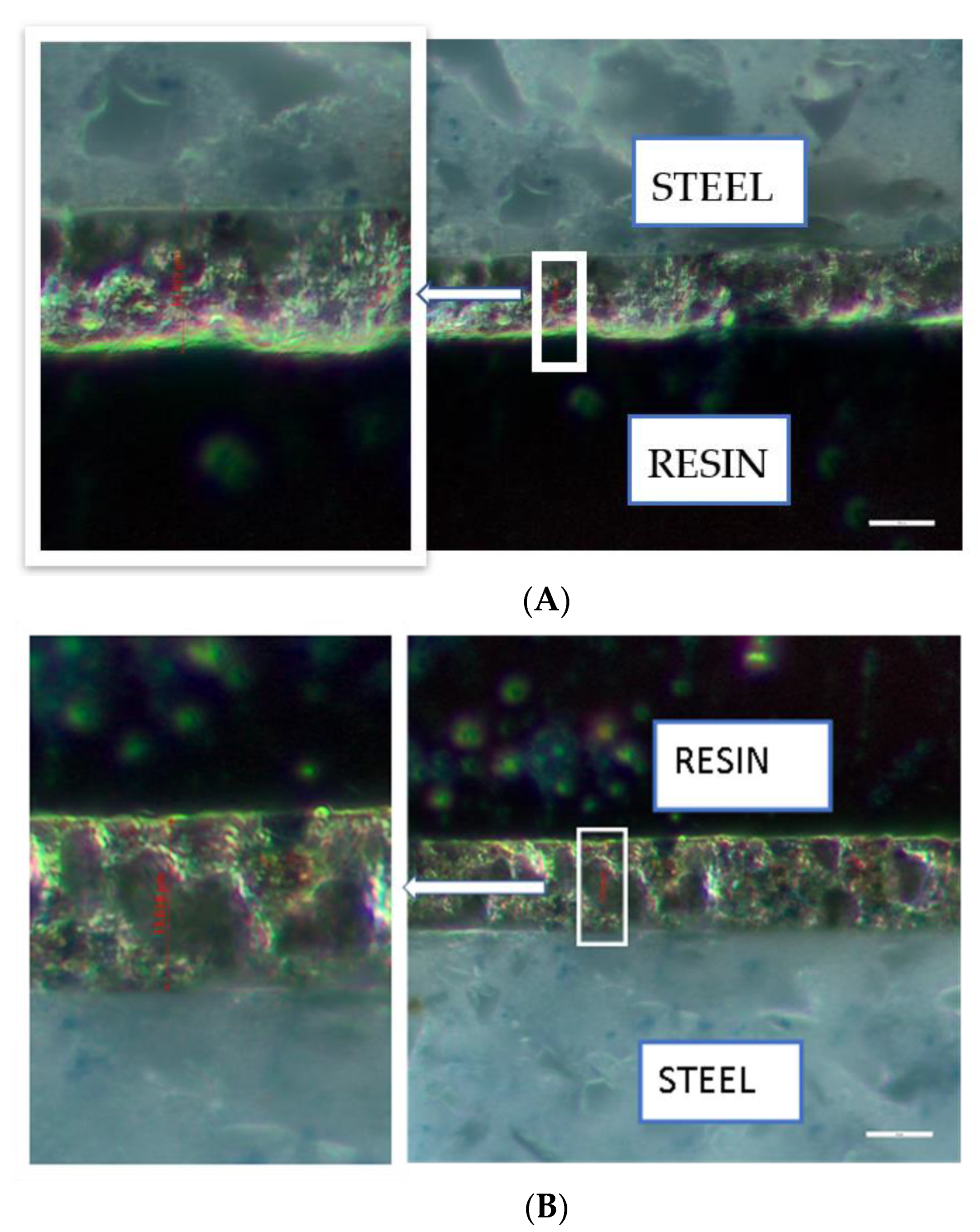
3.3. Surface Morphology and Cross-Sections
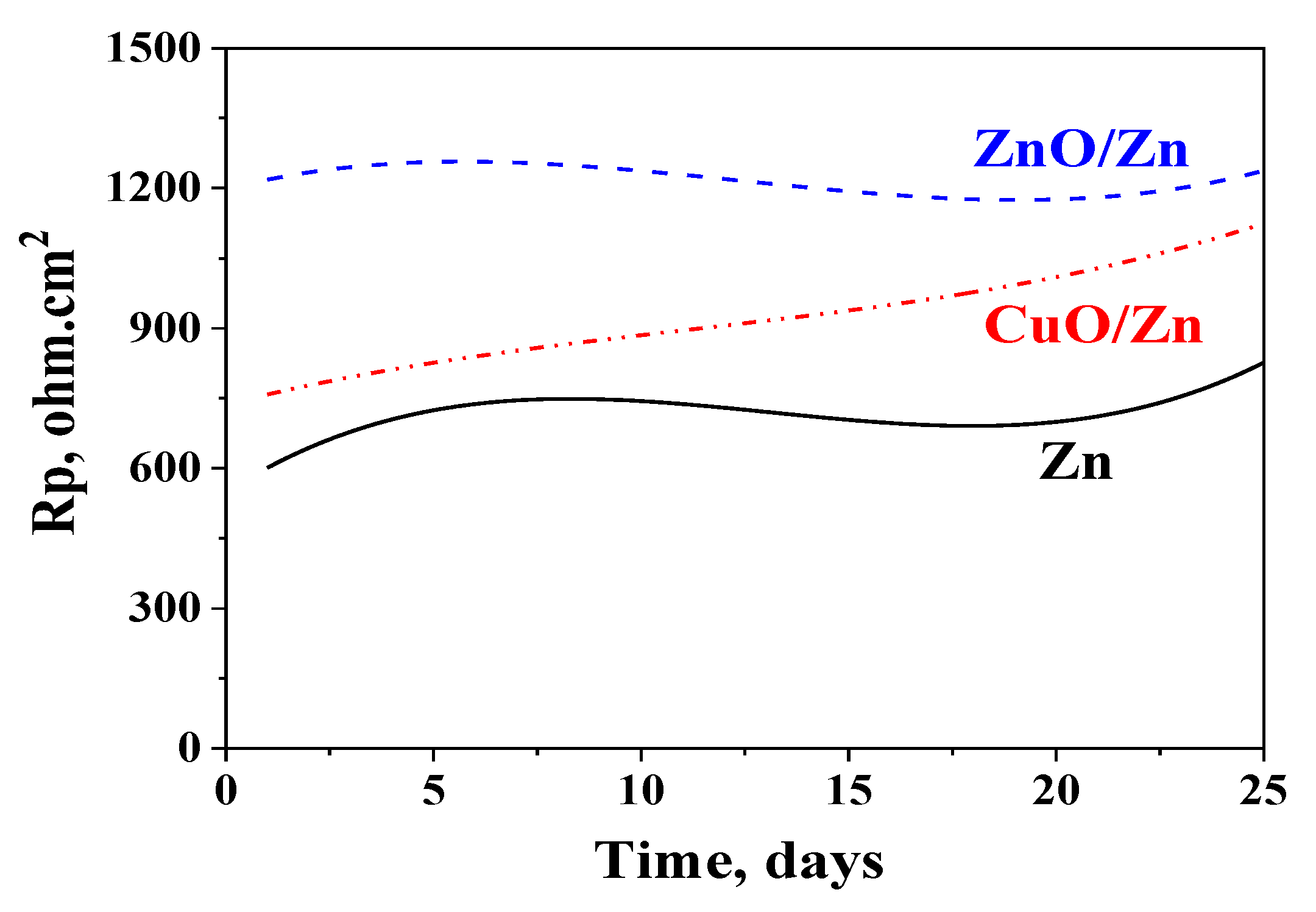
3.4. Polarization Resistance Measurements
3.5. Open Circuit Potential
3.6. Polarization Resistance Measurements
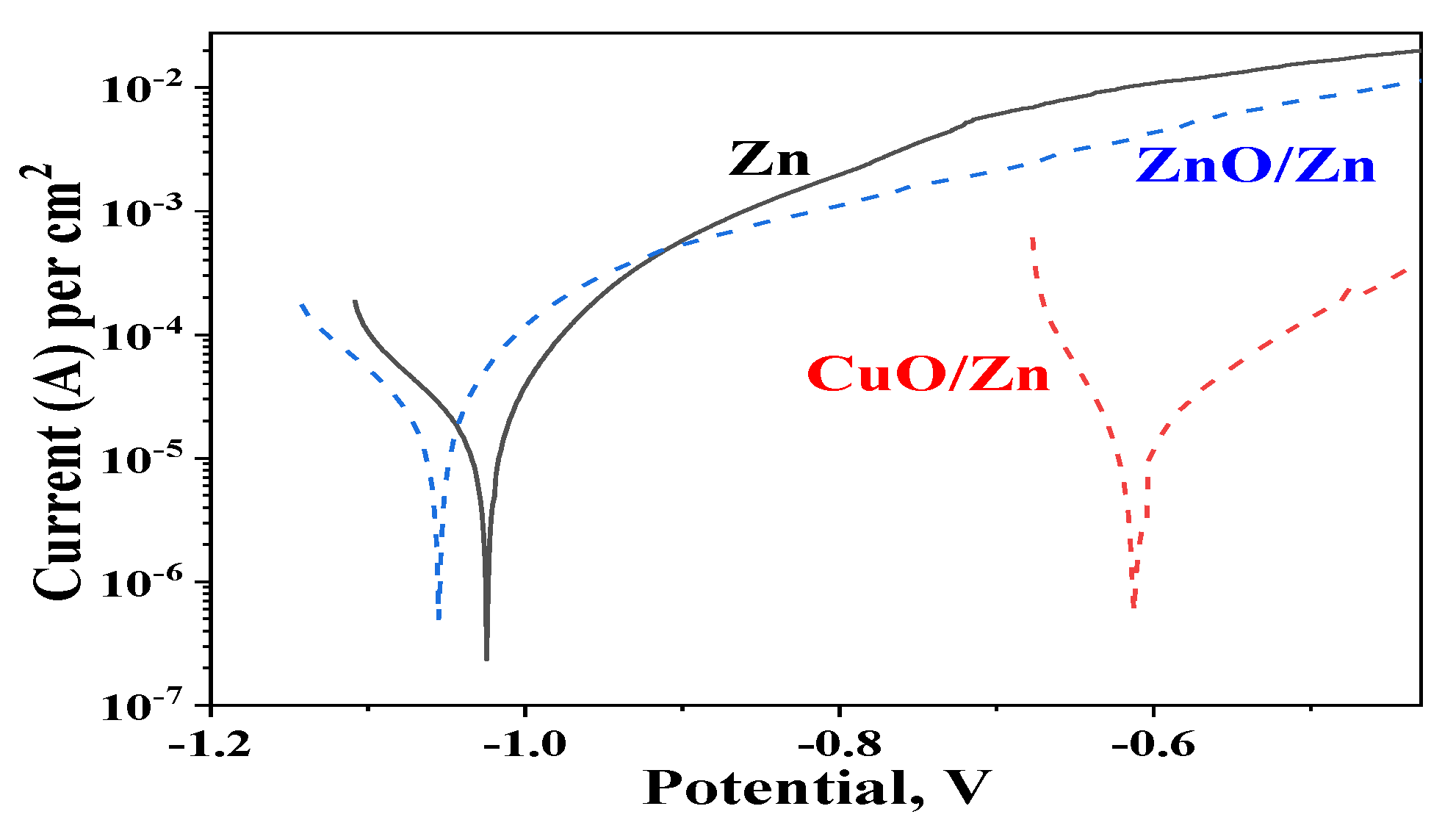
3.7. Cyclic Voltammetry
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koch, G.; Brongers, M.; Thomson, N.; Virmani, Y.; Payer, J. Corrosion Cost and Preventive Strategies in the United States; NACE International: Houston, TX, USA, 2016. [Google Scholar]
- Fragata, F.; Salai, R.; Amorin, C.; Almeida, E. Compatibility and incompatibility in anticorrosive painting: The particular case of maintenance painting. Prog. Org. Coat. 2006, 56, 257–268. [Google Scholar] [CrossRef]
- Zheludkevich, M.L.; Shchukin, D.G.; Yasakau, K.A.; Moehwald, H.; Ferreira, M.G.S. Anticorrosion coatings with self-healing effect based on nanocontainers impregnated with corrosion inhibitor. Chem. Mater. 2007, 19, 402. [Google Scholar] [CrossRef]
- Zheludkevich, M.L.; Tedim, J.; Ferreira, M.G.S. “Smart” coatings for active corrosion protection based on multi-functional micro and nanocontainers. Electrochim. Acta 2012, 82, 314–323. [Google Scholar] [CrossRef]
- Praveen, B.M.; Venkatesha, T.V.; Arthoba Naik, Y. Corrosion studies of carbon nanotubes—Zn composite coating. Surf. Coat. Technol. 2007, 201, 5836–5842. [Google Scholar] [CrossRef]
- Shajudheen, V.P.M.; Rani, K.A.; Kumar, V.S.; Maheswari, A.U.; Sivakumar, M.; Kumar, S.S. Optical and corrosion studies of spray pyrolysis coated titanium dioxide thin films. Adv. Sci. Lett. 2018, 24, 5836–5842. [Google Scholar] [CrossRef]
- Radhakrishnan, S.; Siju, C.R.; Mahanta, D.; Patil, S. Conducting polyaniline-nano-TiO2 composites for smart corrosion resistant coatings. Electrochim. Acta 2009, 54, 1249–1254. [Google Scholar] [CrossRef]
- Shajudheen, V.P.M.; Kumar, S.S.; Kumar, V.S.; Maheswari, A.U.; Sivakumar, M.; Mohan, R.S. Enhancement of anticorrosion properties of stainless steel 304L using nanostructured ZnO thin films. AIMS Mater. Sci. 2018, 5, 932–944. [Google Scholar] [CrossRef]
- Karbowniczek, J.; Cordero-Arias, L.; Virtanen, S.; Misra, S.K.; Valsami-Jones, E.; Rutkowski, B.; Górecki, K.; Bała, P.; Czyrska-Filemonowicz, A.; Boccaccini, A.R. Electrophoretic deposition of organic/inorganic composite coatings containing ZnO nanoparticles exhibitiong antibacterial properties. Mater. Sci. Eng. C 2017, 77, 780–789. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Wang, Y.; Zhang, D.; Li, X. Corrosion resistance research of ZnO/polyelectrolyte composite film. Int. J. Electrochem. Sci. 2016, 11, 8512–8519. [Google Scholar] [CrossRef]
- Cordero-Arias, L.; Cabanas-Polo, S.; Goudouri, O.M.; Misra, S.K.; Gilabert, J.; Valsami-Jones, E.; Sanchez, E.; Virtanen, S.; Boccaccini, A.R. Electrophoretic deposition of ZnO/alginate and ZnO-bioactive glass/alginate composite coatings for antimicrobial applications. Mater. Sci. Eng. C 2015, 55, 137–144. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Wu, K.; Zhitomirsky, I. Electrodeposition of composite zinc oxide–chitosan films. Colloids Surf. A Physicochem. Eng. Asp. 2010, 356, 63–70. [Google Scholar] [CrossRef]
- Šulčiūtė, A.; Ostachavičiūtė, S.; Valatka, E. Synthesis, structure and photoelectrochemical behaviour of ZnO coatings on AISI 304 type steel. Chemija 2017, 28, 85–92. [Google Scholar]
- Verde, M.; Peiteado, M.; Caballero, A.C.; Villegas, M.; Ferrar, B. Electrophoretic deposition of transparent ZnO thin films from highly stabilized colloidal suspensions. J. Colloid Interface Sci. 2012, 373, 27–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarkar, P.; Huang, X.; Nicholson, P.S. Zirconia/alumina functionally gradiented composites by electrophoretic deposition tech-niques. J. Am. Ceram. Soc. 1993, 76, 1055–1056. [Google Scholar] [CrossRef]
- Dinamini, M.; Kamath, P.V.; Seshadri, R. Electrochemical deposition of BaSO4 coatings on stainless steel substrates. Chem. Mater. 2001, 13, 3981–3985. [Google Scholar] [CrossRef]
- Dange, C.; Phan, T.N.T.; André, V.; Rieger, J.; Persello, J.; Foissy, A. Adsorption mechanism and dispersion efficiency of three anionic additives [poly(acrylic acid), poly(styrene sulfonate) and HEDP] on zinc oxide. J. Colloid Interface Sci. 2007, 315, 107–115. [Google Scholar] [CrossRef]
- Fatehah, M.O.; Aziz, H.A.; Stoll, S. Stability of ZnO nanoparticles in solution. Influence of pH, dissolution, aggregation and disaggregation effects. J. Colloid Sci. Biotechnol. 2014, 3, 75–84. [Google Scholar] [CrossRef]
- Tang, F.; Uchikoshi, T.; Sakka, Y. Electrophoretic deposition behavior of aqueous nanosized zinc oxide suspensions. J. Am. Ceram. Soc. 2002, 85, 2161–2165. [Google Scholar] [CrossRef]
- Olsen, S.M.; Pedersen, L.T.; Laursen, M.H.; Kiil, S.; Dam-Johansen, K. Enzyme-based antifouling coatings: A review. Biofouling 2007, 23, 369–383. [Google Scholar] [CrossRef]
- Almeida, E.; Diamantino, T.C.; de Sousa, O. Marine paints: The particular case of antifouling paints. Prog. Org. Coat. 2007, 59, 2–20. [Google Scholar] [CrossRef]
- Jia, Z.; Liu, Y.; Wang, Y.; Gong, Y.; Jin, P.; Suo, X.; Li, H. Flame spray fabrication of polyethylene-Cu composite coatings with enwrapped structures: A new route for constructing antifouling layers. Surf. Coat. Technol. 2017, 309, 872–879. [Google Scholar] [CrossRef]
- He, X.; Abdoli, L.; Li, H. Participation of copper ions in formation of alginate conditioning layer: Evolved structure and regulated microbial adhesion. Colloids Surf. B Biointerfaces 2018, 162, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Piola, R.F.; Dafforn, K.; Johnston, E. The influence of antifouling practices on marine invasions. Biofouling 2009, 25, 633–644. [Google Scholar] [CrossRef]
- Vucko, M.J.; King, P.C.; Poole, A.J.; Hu, Y.; Jahedi, M.Z.; de Nys, R. Assessing the antifouling properties of cold-spray metal em-bedment using loading density gradients of metal particles. Biofouling 2014, 30, 651–666. [Google Scholar] [CrossRef]
- Kruk, T.; Szczepanowicz, K.; Stefańska, J.; Socha, R.; Warszyński, P. Synthesis and antimicrobial activity of monodisperse copper nanoparticles. Colloids Surf. B Biointerfaces 2015, 128, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Rui, D.; Xiangbo, L.; Jia, W.; Likun, X. Electrochemical corrosion and mathematical model of cold spray Cu-Cu2O coating in NaCl solution—Part I: Tafel polarization region model. Int. J. Electrochem. Sci. 2013, 8, 5902–5924. [Google Scholar]
- Aruoja, V.; Dubourguier, H.-C.; Kasemets, K.; Kahru, A. Toxicity of nanoparticles of CuO, ZnO and TiO2 to microalgae Pseudokirchneriella subcapitata. Sci. Total. Environ. 2009, 407, 1461–1468. [Google Scholar] [CrossRef] [PubMed]
- Son, J.; Vavra, J.; Forbes, V.E. Effects of water quality parameters on agglomeration and dissolution of copper oxide nanoparticles (CuO-NPs) using a central composite circumscribed design. Sci. Total Environ. 2015, 521, 183–190. [Google Scholar] [CrossRef]
- Khan, R.; Inam, M.A.; Zam, S.Z.; Akram, M.; Shin, S.; Yeom, I.T. Coagulation and dissolution of CuO nanoparticles in the presence of dissolved organic matter under different pH values. Sustainability 2019, 11, 2825. [Google Scholar] [CrossRef] [Green Version]
- Sousa, V.S.; Teixeira, M.R. Aggregation kinetics and surface charge of CuO nanoparticles: The influence of pH, ionic strength and humic acids. Environ. Chem. 2013, 10, 313–322. [Google Scholar] [CrossRef] [Green Version]
- Shehayeb, S.; Deschanels, X.; Lautru, J.; Ghannam, L.; Odorico, M.; Karamé, I.; Toquer, G. Thin polymeric CuO film from EPD designed for low temperature photothermal absorbers. Electrochim. Acta 2019, 305, 295–303. [Google Scholar] [CrossRef]
- Wang, Y.; Deen, I.; Zhitomirsky, I. Electrophoretic deposition of polyacrylic acid and composite films containing nanotubes and oxide particles. J. Colloid Interface Sci. 2011, 362, 367–374. [Google Scholar] [CrossRef]
- Kamburova, K.; Boshkova, N.; Boshkov, N.; Radeva, T. Composite coatings with polymeric modified ZnO nanoparticles and nanocontainers with inhibitor for corrosion protection of low carbon steel. Colloids Surf. A Physicochem. Eng. Asp. 2021, 609, 125741. [Google Scholar] [CrossRef]
- Kamburova, K.; Boshkova, N.; Boshkov, N.; Atanassova, G.; Radeva, T. Hybrid zinc coatings for corrosion protection of steel using polyelectrolyte nanocontainers loaded with benzotriazole. Colloids Surf. A Physicochem. Eng. Asp. 2018, 559, 243–250. [Google Scholar] [CrossRef]
- Boshkov, N.; Tsvetkova, N.; Petrov, P.; Koleva, D.; Petrov, K.; Avdeev, G.; Tsvetanov, C.; Raichevski, G.; Raicheff, R. Corrosion behavior and protective ability of Zn and Zn-Co electrodeposits with embedded polymeric nanoparticles. Appl. Surf. Sci. 2008, 254, 5618–5625. [Google Scholar] [CrossRef]
- Finšgar, M.; Fassbender, S.; Nicolini, F.; Milošev, I. Polyethyleneimine as a corrosion inhibitor for ASTM 420 stainless steel in near-neutral saline media. Corros. Sci. 2009, 51, 525–533. [Google Scholar] [CrossRef]



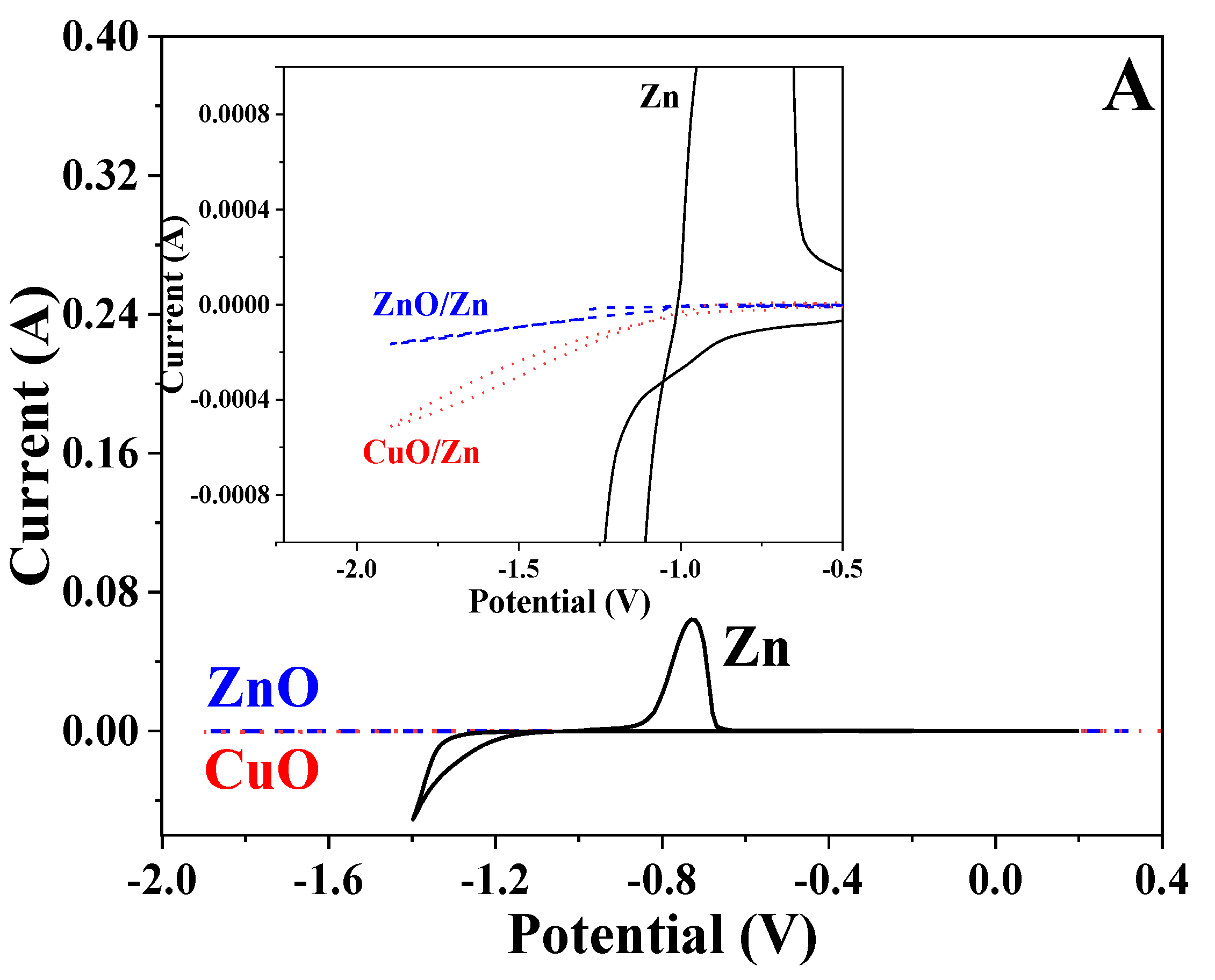
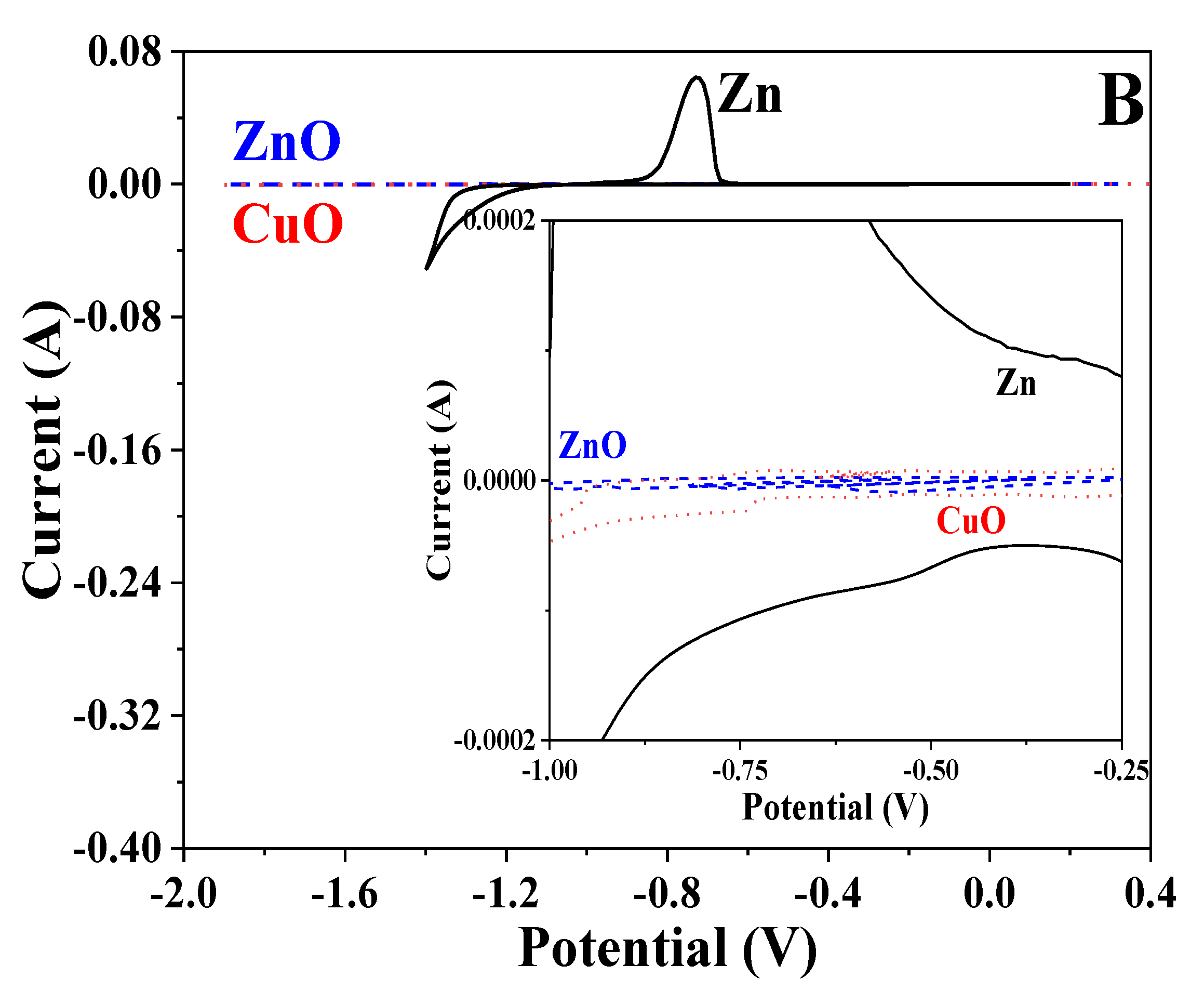
| Sample/Parameter | Icorr, A·cm−2 | Ecorr, V | Ipass, A·cm−2 |
|---|---|---|---|
| CuO/Zn | 1.02 × 10−5 | −1.094 | 6.3 × 10−4 |
| ZnO/Zn | 9.9 × 10−6 | −1.104 | 1.3 × 10−4 |
| Zn | 1.8 × 10−5 | −1.065 | – |
| Sample/Parameter | Icorr, A·cm−2 | Ecorr, V |
|---|---|---|
| CuO/Zn | 1.2 × 10−5 | −0.612 |
| ZnO/Zn | 1.3 × 10−5 | −1.053 |
| Zn | 1.4 × 10−5 | −1.024 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boshkova, N.; Kamburova, K.; Radeva, T.; Boshkov, N. Hybrid Zinc-Based Multilayer Systems with Improved Protective Ability against Localized Corrosion Incorporating Polymer-Modified ZnO or CuO Particles. Coatings 2021, 11, 1223. https://doi.org/10.3390/coatings11101223
Boshkova N, Kamburova K, Radeva T, Boshkov N. Hybrid Zinc-Based Multilayer Systems with Improved Protective Ability against Localized Corrosion Incorporating Polymer-Modified ZnO or CuO Particles. Coatings. 2021; 11(10):1223. https://doi.org/10.3390/coatings11101223
Chicago/Turabian StyleBoshkova, Nelly, Kamelia Kamburova, Tsetska Radeva, and Nikolai Boshkov. 2021. "Hybrid Zinc-Based Multilayer Systems with Improved Protective Ability against Localized Corrosion Incorporating Polymer-Modified ZnO or CuO Particles" Coatings 11, no. 10: 1223. https://doi.org/10.3390/coatings11101223
APA StyleBoshkova, N., Kamburova, K., Radeva, T., & Boshkov, N. (2021). Hybrid Zinc-Based Multilayer Systems with Improved Protective Ability against Localized Corrosion Incorporating Polymer-Modified ZnO or CuO Particles. Coatings, 11(10), 1223. https://doi.org/10.3390/coatings11101223









