Development of Dithieno[3,2-b:2′,3′-d]thiophene (DTT) Derivatives as Solution-Processable Small Molecular Semiconductors for Organic Thin Film Transistors
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Materials and Methods
2.2. Synthesis
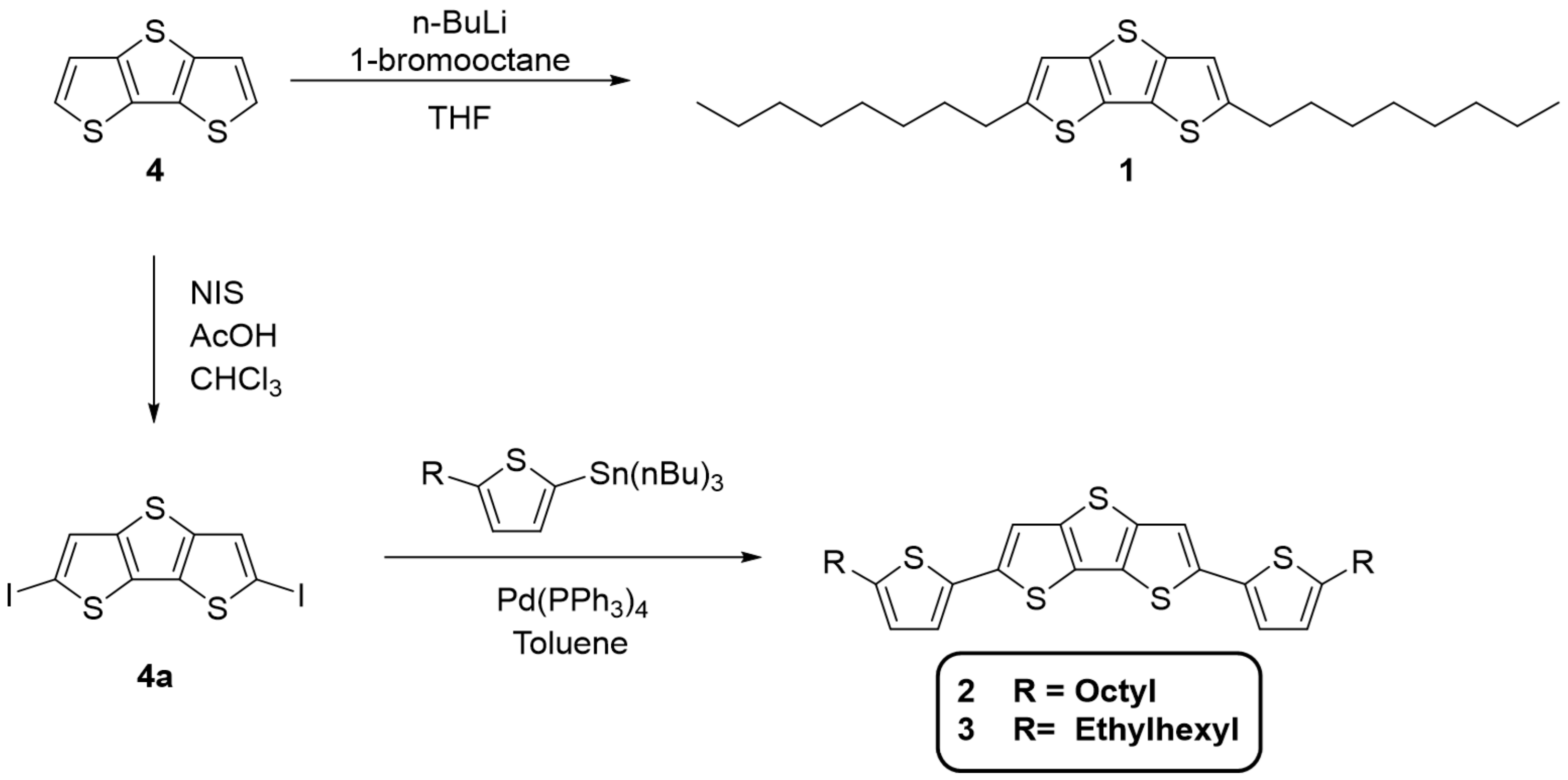
2.2.1. Synthesis of Dithieno[3,2-b:2′,3′-d]thiophene (4)
2.2.2. Synthesis of 2,6-Diiododithieno[3,2-b:2′,3′-d]thiophene (4a)
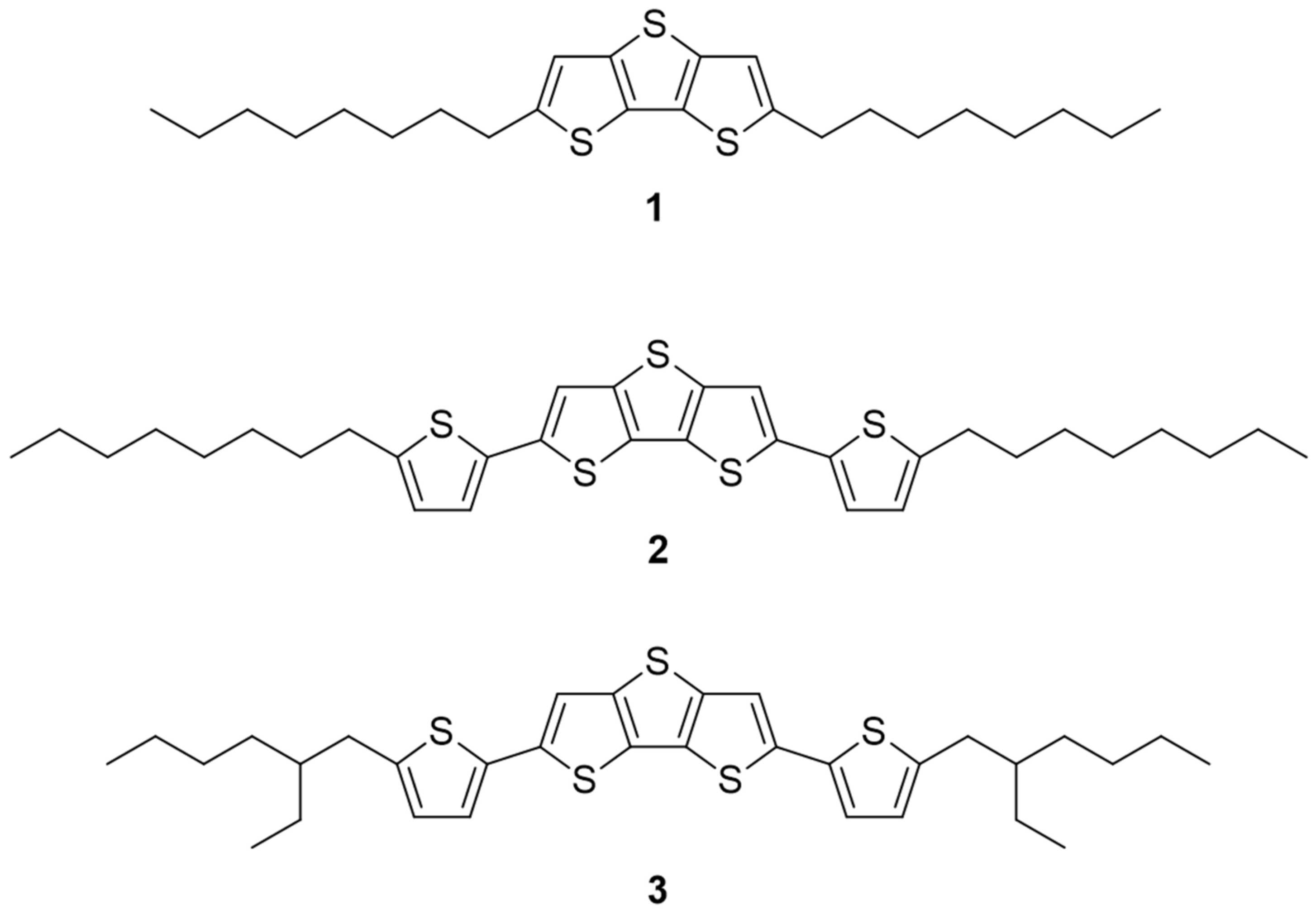
2.2.3. Synthesis of 2,6-Dioctyldithieno[3,2-b:2′,3′-d]thiophene (1)
2.2.4. Synthesis of 2,6-Bis(5-octylthiophen-2-yl)dithieno[3,2-b:2′,3′-d]thiophene (2)
2.2.5. Synthesis of 2,6-Bis(5-(2-ethylhexyl)thiophen-2-yl)dithieno[3,2-b:2′,3′-d]thiophene (3)
2.3. Theoretical Calculation
2.4. Device Fabrication
2.5. Device Characterization
3. Results
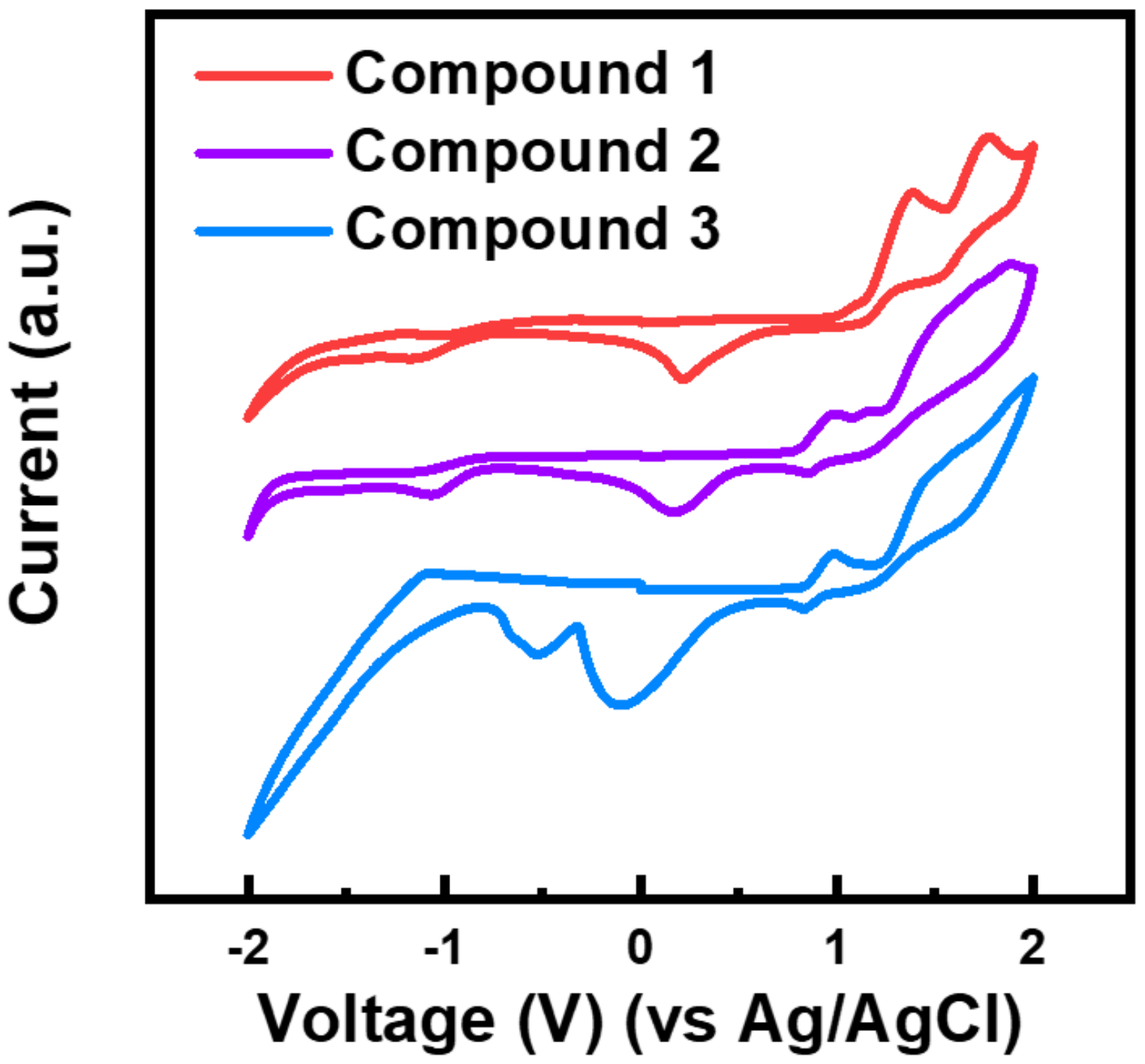
3.1. Thermal, Optical, and Electrochemical Properties
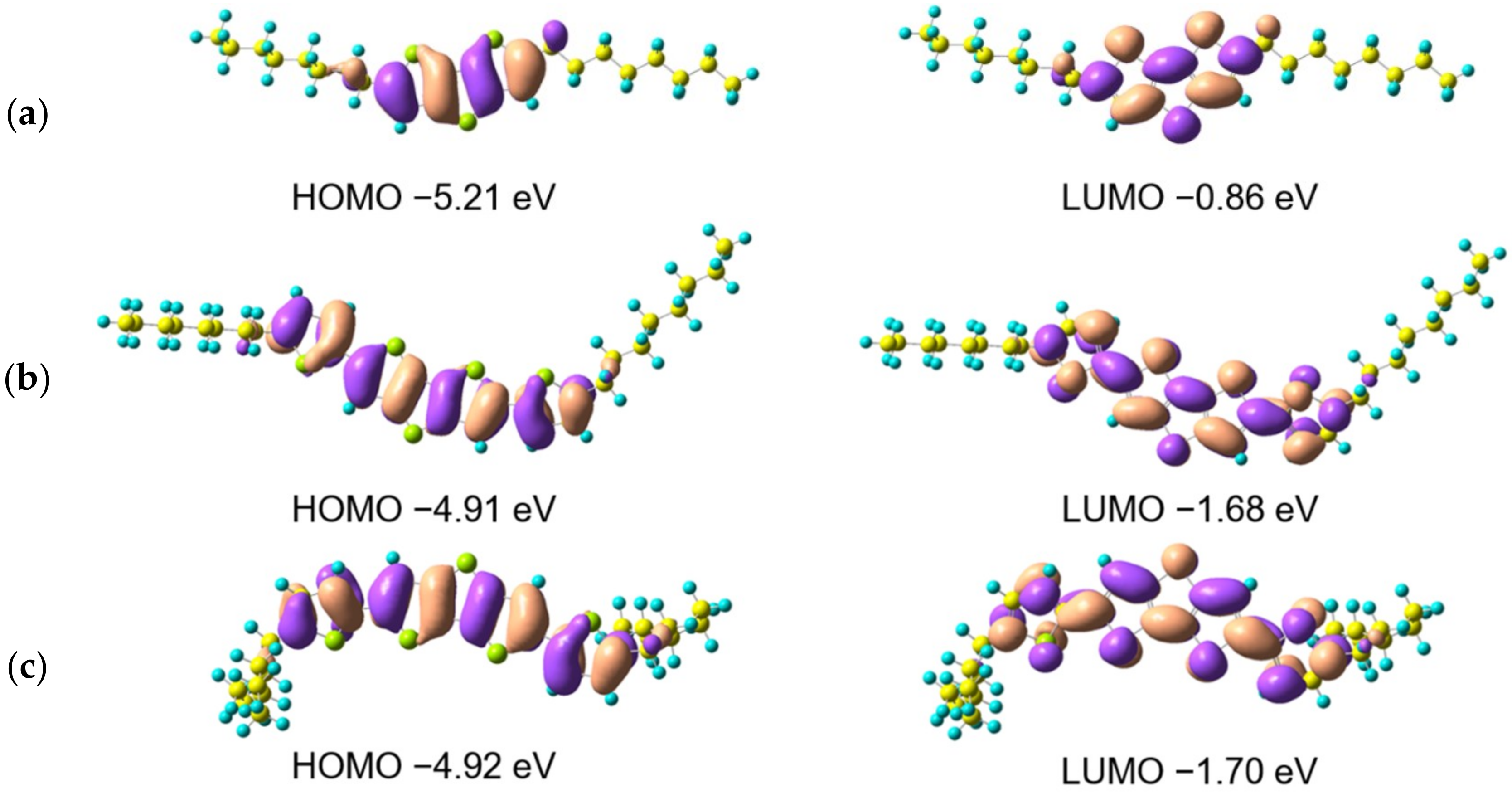
3.2. Theoretical Calculation
3.3. Thin-Film Transistor Characterization
3.4. Thin-Film Microstructure and Morphology
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chan, C.-Y.; Tanaka, M.; Lee, Y.-T.; Wong, Y.-W.; Nakanotani, H.; Hatakeyama, T.; Adachi, C. Stable pure-blue hyperfluorescence organic light-emitting diodes with high-efficiency and narrow emission. Nat. Photonics 2021, 15, 203–207. [Google Scholar] [CrossRef]
- Chen, L.-H.; Lin, P.; Chen, M.-C.; Huang, P.-Y.; Kim, C.; Ho, J.-C.; Lee, C.-C. Silver nanowire-polymer composite electrode for high performance solution-processed thin-film transistors. Org. Electron. 2012, 13, 1881–1886. [Google Scholar] [CrossRef]
- Gélinas, S.; Rao, A.; Kumar, A.; Smith, S.L.; Chin, A.W.; Clark, J.; Van Der Poll, T.S.; Bazan, G.C.; Friend, R.H. Ultrafast long-range charge separation in organic semiconductor photovoltaic diodes. Science 2014, 343, 512–516. [Google Scholar] [CrossRef] [Green Version]
- Ho, D.; Ozdemir, R.; Kim, H.; Earmme, T.; Usta, H.; Kim, C. BODIPY-based semiconducting materials for organic bulk heterojunction photovoltaics and thin-film transistors. Chempluschem 2019, 84, 18–37. [Google Scholar] [CrossRef]
- Kondo, Y.; Yoshiura, K.; Kitera, S.; Nishi, H.; Oda, S.; Gotoh, H.; Sasada, Y.; Yanai, M.; Hatakeyama, T. Narrowband deep-blue organic light-emitting diode featuring an organoboron-based emitter. Nat. Photonics 2019, 13, 678–682. [Google Scholar] [CrossRef]
- Kwon, G.; Kim, K.; Choi, B.D.; Roh, J.; Lee, C.; Noh, Y.Y.; Seo, S.; Kim, M.G.; Kim, C. Multifunctional organic-semiconductor interfacial layers for solution-processed oxide-semiconductor thin-film transistor. Adv. Mater. 2017, 29, 1607055. [Google Scholar] [CrossRef]
- Li, H.; Shi, W.; Song, J.; Jang, H.J.; Dailey, J.; Yu, J.; Katz, H.E. Chemical and biomolecule sensing with organic field-effect transistors. Chem. Rev. 2019, 119, 3–35. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sonar, P.; Murphy, L.; Hong, W. High mobility diketopyrrolopyrrole (DPP)-based organic semiconductor materials for organic thin film transistors and photovoltaics. Energy Environ. Sci. 2013, 6, 1684–1710. [Google Scholar] [CrossRef]
- Nikolka, M.; Nasrallah, I.; Rose, B.; Ravva, M.K.; Broch, K.; Sadhanala, A.; Harkin, D.; Charmet, J.; Hurhangee, M.; Brown, A.; et al. High operational and environmental stability of high-mobility conjugated polymer field-effect transistors through the use of molecular additives. Nat. Mater. 2017, 16, 356–362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, Y.; Baeg, K.J.; Kim, C. Solution-processed nonvolatile organic transistor memory based on semiconductor blends. ACS Appl. Mater. Interfaces 2019, 11, 8327–8336. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Yang, F.; Gao, X.; Cheng, S.; Zhang, X.; Dong, H.; Hu, W. Organic field-effect transistor for energy-related applications: Low-Power-consumption devices, near-infrared phototransistors, and organic thermoelectric devices. Adv. Energy Mater. 2018, 8, 1801003. [Google Scholar] [CrossRef]
- Cho, H.; Lee, S.; Cho, N.S.; Jabbour, G.E.; Kwak, J.; Hwang, D.H.; Lee, C. High-mobility pyrene-based semiconductor for organic thin-film transistors. ACS Appl. Mater. Interfaces 2013, 5, 3855–3860. [Google Scholar] [CrossRef] [PubMed]
- Hofmockel, R.; Zschieschang, U.; Kraft, U.; Rödel, R.; Hansen, N.H.; Stolte, M.; Würthner, F.; Takimiya, K.; Kern, K.; Pflaum, J.; et al. High-mobility organic thin-film transistors based on a small-molecule semiconductor deposited in vacuum and by solution shearing. Org. Electron. 2013, 14, 3213–3221. [Google Scholar] [CrossRef]
- Kanimozhi, C.; Yaacobi-Gross, N.; Chou, K.W.; Amassian, A.; Anthopoulos, T.D.; Patil, S. Diketopyrrolopyrrole—diketopyrrolopyrrole-based conjugated copolymer for high-mobility organic field-effect transistors. J. Am. Chem. Soc. 2012, 134, 16532–16535. [Google Scholar] [CrossRef]
- Lee, J.S.; Son, S.K.; Song, S.; Kim, H.; Lee, D.R.; Kim, K.; Ko, M.J.; Choi, D.H.; Kim, B.; Cho, J.H. Importance of solubilizing group and backbone planarity in low band gap polymers for high performance ambipolar field-effect transistors. Chem. Mater. 2012, 24, 1316–1323. [Google Scholar] [CrossRef]
- Mei, Y.; Loth, M.A.; Payne, M.; Zhang, W.; Smith, J.; Day, C.S.; Parkin, S.R.; Heeney, M.; McCulloch, I.; Anthopoulos, T.D.; et al. High mobility field-effect transistors with versatile processing from a small-molecule organic semiconductor. Adv. Mater. 2013, 25, 4352–4357. [Google Scholar] [CrossRef]
- Sakanoue, T.; Sirringhaus, H. Band-like temperature dependence of mobility in a solution-processed organic semiconductor. Nat. Mater. 2010, 9, 736–740. [Google Scholar] [CrossRef] [PubMed]
- Sirringhaus, H. 25th anniversary article: Organic field-effect transistors: The path beyond amorphous silicon. Adv. Mater. 2014, 26, 1319–1335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takimiya, K.; Shinamura, S.; Osaka, I.; Miyazaki, E. Air-stable and high-mobility organic semiconductors based on heteroarenes for field-effect transistors. Heterocycles 2011, 83, 1187–1204. [Google Scholar] [CrossRef]
- Bourass, M.; Touimi Benjelloun, A.; Benzakour, M.; McHarfi, M.; Jhilal, F.; Serein-Spirau, F.; Marc Sotiropoulos, J.; Bouachrine, M. DFT/TD-DFT characterization of conjugational electronic structures and spectral properties of materials based on thieno[3,2-b][1]benzothiophene for organic photovoltaic and solar cell applications. J. Saudi Chem. Soc. 2017, 21, 563–574. [Google Scholar] [CrossRef]
- Chen, H.; Cui, Q.; Yu, G.; Guo, Y.; Huang, J.; Zhu, M.; Guo, X.; Liu, Y. Synthesis and characterization of novel semiconductors based on thieno[3,2-b][1]benzothiophene cores and their applications in the organic thin-film transistors. J. Phys. Chem. C 2011, 115, 23984–23991. [Google Scholar] [CrossRef]
- Cinar, M.E.; Ozturk, T. Thienothiophenes, dithienothiophenes, and thienoacenes: Syntheses, oligomers, polymers, and properties. Chem. Rev. 2015, 115, 3036–3140. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.Y.; Chen, L.H.; Chen, Y.Y.; Chang, W.J.; Wang, J.J.; Lii, K.H.; Yan, J.Y.; Ho, J.C.; Lee, C.C.; Kim, C.; et al. Enhanced performance of benzothieno[3,2-b]thiophene (BTT)-based bottom-contact thin-film transistors. Chem. Eur. J. 2013, 19, 3721–3728. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Zhan, X. High-mobility conjugated polymers based on fused-thiophene building blocks. Macromol. Chem. Phys. 2011, 212, 428–443. [Google Scholar] [CrossRef]
- McCulloch, I.; Heeney, M.; Chabinyc, M.L.; DeLongchamp, D.; Kline, R.J.; Cölle, M.; Duffy, W.; Fischer, D.; Gundlach, D.; Hamadani, B.; et al. Semiconducting thienothiophene copolymers: Design, synthesis, morphology, and performance in thin-film organic transistors. Adv. Mater. 2009, 21, 1091–1109. [Google Scholar] [CrossRef]
- Mori, T.; Nishimura, T.; Yamamoto, T.; Doi, I.; Miyazaki, E.; Osaka, I.; Takimiya, K. Consecutive thiophene-annulation approach to π-extended thienoacene-based organic semiconductors with [1] benzothieno [3,2-b][1] benzothiophene (BTBT) substructure. J. Am. Chem. Soc. 2013, 135, 13900–13913. [Google Scholar] [CrossRef]
- Ozdemir, M.; Choi, D.; Kwon, G.; Zorlu, Y.; Kim, H.; Kim, M.-G.; Seo, S.; Sen, U.; Citir, M.; Kim, C.; et al. Design, synthesis, and characterization of α,ω-disubstituted indeno[1,2-b]fluorene-6,12-dione-thiophene molecular semiconductors. Enhancement of ambipolar charge transport through synthetic tailoring of alkyl substituents. RSC Adv. 2016, 6, 212–226. [Google Scholar] [CrossRef]
- Pandey, M.; Gowda, A.; Nagamatsu, S.; Kumar, S.; Takashima, W.; Hayase, S.; Pandey, S.S. Rapid formation and macroscopic self-assembly of liquid-crystalline, high-mobility, semiconducting thienothiophene. Adv. Mater. Interfaces 2018, 5, 1700875. [Google Scholar] [CrossRef]
- Takimiya, K.; Osaka, I.; Mori, T.; Nakano, M. Organic semiconductors based on [1] benzothieno [3,2-b][1] benzothiophene substructure. Acc. Chem. Res. 2014, 47, 1493–1502. [Google Scholar] [CrossRef]
- Takimiya, K.; Shinamura, S.; Osaka, I.; Miyazaki, E. Thienoacene-based organic semiconductors. Adv. Mater. 2011, 23, 4347–4370. [Google Scholar] [CrossRef]
- Turkoglu, G.; Cinar, M.E.; Ozturk, T. Thiophene-based organic semiconductors. Top. Curr. Chem. 2017, 375, 84. [Google Scholar] [CrossRef]
- Wang, C.; Nakamura, H.; Sugino, H.; Takimiya, K. Methylthionated benzo [1,2-b:4, 5-b′] dithiophenes: A model study to control packing structures and molecular orientation in thienoacene-based organic semiconductors. Chem. Commun. 2017, 53, 9594–9597. [Google Scholar] [CrossRef]
- Xue, Z.; Chen, S.; Gao, N.; Xue, Y.; Lu, B.; Watson, O.A.; Zang, L.; Xu, J. Structural design and applications of stereoregular fused thiophenes and their oligomers and polymers. Polym. Rev. 2019, 60, 318–358. [Google Scholar] [CrossRef]
- Kim, C.; Chen, M.-C.; Chiang, Y.-J.; Guo, Y.-J.; Youn, J.; Huang, H.; Liang, Y.-J.; Lin, Y.-J.; Huang, Y.-W.; Hu, T.-S.; et al. Functionalized dithieno[2,3-b:3′,2′-d]thiophenes (DTTs) for organic thin-film transistors. Org. Electron. 2010, 11, 801–813. [Google Scholar] [CrossRef]
- Sun, Y.M.; Ma, Y.Q.; Liu, Y.Q.; Lin, Y.Y.; Wang, Z.Y.; Wang, Y.; Di, C.A.; Xiao, K.; Chen, X.M.; Qiu, W.F.; et al. High-performance and stable organic thin-film transistors based on fused thiophenes. Adv. Funct. Mater. 2006, 16, 426–432. [Google Scholar] [CrossRef]
- Chen, M.-C.; Vegiraju, S.; Huang, C.-M.; Huang, P.-Y.; Prabakaran, K.; Yau, S.L.; Chen, W.-C.; Peng, W.-T.; Chao, I.; Kim, C.; et al. Asymmetric fused thiophenes for field-effect transistors: Crystal structure–film microstructure–transistor performance correlations. J. Mater. Chem. C 2014, 2, 8892–8902. [Google Scholar] [CrossRef]
- Li, P.; Cui, Y.; Song, C.; Zhang, H. Electronic and charge transport properties of dimers of dithienothiophenes: Effect of structural symmetry and linking mode. RSC Adv. 2015, 5, 50212–50222. [Google Scholar] [CrossRef]
- Liu, Y.; Di, C.A.; Du, C.; Liu, Y.; Lu, K.; Qiu, W.; Yu, G. Synthesis, structures, and properties of fused thiophenes for organic field-effect transistors. Chem. Eur. J. 2010, 16, 2231–2239. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.C.; Chiang, Y.J.; Kim, C.; Guo, Y.J.; Chen, S.Y.; Liang, Y.J.; Huang, Y.W.; Hu, T.S.; Lee, G.H.; Facchetti, A.; et al. One-pot [1 + 1 + 1] synthesis of dithieno[2,3-b:3′,2′-d]thiophene (DTT) and their functionalized derivatives for organic thin-film transistors. Chem. Commun. 2009, 1846–1848. [Google Scholar] [CrossRef]
- Tsuzuki, S.; Orita, H.; Sato, N. Intermolecular interactions of oligothienoacenes: Do S⋯S interactions positively contribute to crystal structures of sulfur-containing aromatic molecules? J. Chem. Phys. 2016, 145, 174503. [Google Scholar] [CrossRef] [PubMed]
- Youn, J.; Vegiraju, S.; Emery, J.D.; Leever, B.J.; Kewalramani, S.; Lou, S.J.; Zhang, S.; Prabakaran, K.; Ezhumalai, Y.; Kim, C.; et al. Diperfluorophenyl fused thiophene semiconductors for n-type organic thin film transistors (OTFTs). Adv. Electron. Mater. 2015, 1, 1500098. [Google Scholar] [CrossRef]
- Zhang, H.; Dong, H.; Li, Y.; Jiang, W.; Zhen, Y.; Jiang, L.; Wang, Z.; Chen, W.; Wittmann, A.; Hu, W. Novel air stable organic radical semiconductor of dimers of dithienothiophene, single crystals, and field-effect transistors. Adv. Mater. 2016, 28, 7466–7471. [Google Scholar] [CrossRef]
- Zhang, L.; Tan, L.; Hu, W.; Wang, Z. Synthesis, packing arrangement and transistor performance of dimers of dithienothiophenes. J. Mater. Chem. 2009, 19, 8216–8222. [Google Scholar] [CrossRef]
- Zhu, M.; Luo, H.; Wang, L.; Guo, Y.; Zhang, W.; Liu, Y.; Yu, G. The synthesis of 2,6-dialkylphenyldithieno[3,2-b:2′,3′-d]thiophene derivatives and their applications in organic field-effect transistors. Dye. Pigment. 2013, 98, 17–24. [Google Scholar] [CrossRef]
- Shi, J.; Li, Y.; Jia, M.; Xu, L.; Wang, H. Organic semiconductors based on annelated β-oligothiophenes and its application for organic field-effect transistors. J. Mater. Chem. 2011, 21, 17612–17614. [Google Scholar] [CrossRef]
- Ho, D.; Jeon, M.; Kim, H.; Gidron, O.; Kim, C.; Seo, S. Solution-processable dithieno[3,2-b:2′,3′-d]thiophene derivatives for organic thin-film transistors and complementary-like inverters. Org. Electron. 2018, 52, 356–363. [Google Scholar] [CrossRef]
- Vegiraju, S.; Luo, X.-L.; Li, L.-H.; Afraj, S.N.; Lee, C.; Zheng, D.; Hsieh, H.-C.; Lin, C.-C.; Hong, S.-H.; Tsai, H.-C.; et al. Solution processable pseudo n-thienoacenes via intramolecular S···S lock for high performance organic field effect transistors. Chem. Mater. 2020, 32, 1422–1429. [Google Scholar] [CrossRef]
- Zhang, L.; Tan, L.; Wang, Z.; Hu, W.; Zhu, D. High-performance, stable organic field-effect transistors based on trans-1, 2-(dithieno [2,3-b:3′,2′-d] thiophene) ethene. Chem. Mater. 2009, 21, 1993–1999. [Google Scholar] [CrossRef]
- Kim, C.; Huang, P.-Y.; Jhuang, J.-W.; Chen, M.-C.; Ho, J.-C.; Hu, T.-S.; Yan, J.-Y.; Chen, L.-H.; Lee, G.-H.; Facchetti, A.; et al. Novel soluble pentacene and anthradithiophene derivatives for organic thin-film transistors. Org. Electron. 2010, 11, 1363–1375. [Google Scholar] [CrossRef]
- Lee, J.; Yun, C.; Reddy, M.R.; Ho, D.; Kim, C.; Seo, S. Synthesis and characterization of fluorenone-based donor-acceptor small molecule organic semiconductors for organic field-effect transistors. Macromol. Res. 2020, 28, 654–659. [Google Scholar] [CrossRef]
- Hu, T.; Han, L.; Xiao, M.; Bao, X.; Wang, T.; Sun, M.; Yang, R. Enhancement of photovoltaic performance by increasing conjugation of the acceptor unit in benzodithiophene and quinoxaline copolymers. J. Mater. Chem. C 2014, 2, 8047–8053. [Google Scholar] [CrossRef]
- Park, K.H.; Park, J.B.; Zong, K. A convenient synthesis of dithieno [3,2-b:2′,3′-d] thiophenes from thiophene. Synthesis 2016, 48, 4126–4130. [Google Scholar]
- Sánchez-Carrera, R.S.; Odom, S.A.; Kinnibrugh, T.L.; Sajoto, T.; Kim, E.-G.; Timofeeva, T.V.; Barlow, S.; Coropceanu, V.; Marder, S.R.; Bredas, J.-L. Electronic properties of the 2, 6-diiododithieno [3,2-b:2′,3′-d] thiophene molecule and crystal: A joint experimental and theoretical study. J. Phys. Chem. B 2010, 114, 749–755. [Google Scholar] [CrossRef]
- Lu, Z.Z.; Peng, J.D.; Wu, A.K.; Lin, C.H.; Wu, C.G.; Ho, K.C.; Lin, Y.C.; Lu, K.L. Heteroleptic ruthenium sensitizers with hydrophobic fused-thiophenes for use in efficient dye-sensitized solar cells. Eur. J. Inorg. Chem. 2016, 2016, 1214–1224. [Google Scholar] [CrossRef]
- Navacchia, M.L.; Melucci, M.; Favaretto, L.; Zanelli, A.; Gazzano, M.; Bongini, A.; Barbarella, G. Alkylenesulfanyl-bridged Bithienyl cores for simultaneous tuning of electronic, filming, and thermal properties of oligothiophenes. Org. Lett. 2008, 10, 3665–3668. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.H.; Cho, K.; Frisbie, C.D.; Sirringhaus, H.; Podzorov, V. Critical assessment of charge mobility extraction in FETs. Nat. Mater. 2017, 17, 2–7. [Google Scholar] [CrossRef]
- McCulloch, I.; Salleo, A.; Chabinyc, M. Avoid the kinks when measuring mobility. Science 2016, 352, 1521–1522. [Google Scholar] [CrossRef] [PubMed]
- Tamayo, A.B.; Tantiwiwat, M.; Walker, B.; Nguyen, T.-Q. Design, synthesis, and self-assembly of oligothiophene derivatives with a diketopyrrolopyrrole core. J. Phys. Chem. C 2008, 112, 15543–15552. [Google Scholar] [CrossRef]
- Jung, M.; Yoon, Y.; Park, J.H.; Cha, W.; Kim, A.; Kang, J.; Gautam, S.; Seo, D.; Cho, J.H.; Kim, H.; et al. Nanoscopic management of molecular packing and orientation of small molecules by a combination of linear and branched alkyl side chains. ACS Nano 2014, 8, 5988–6003. [Google Scholar] [CrossRef]
- Rancatore, B.J.; Kim, B.; Mauldin, C.E.; Fréchet, J.M.J.; Xu, T. Organic semiconductor-containing supramolecules: Effect of small molecule crystallization and molecular packing. Macromolecules 2016, 49, 833–843. [Google Scholar] [CrossRef]
- Wu, Y.; Li, Y.; Gardner, S.; Ong, B.S. Indolo [3,2-b] carbazole-based thin-film transistors with high mobility and stability. J. Am. Chem. Soc. 2005, 127, 614–618. [Google Scholar] [CrossRef]
- Zschieschang, U.; Ante, F.; Yamamoto, T.; Takimiya, K.; Kuwabara, H.; Ikeda, M.; Sekitani, T.; Someya, T.; Kern, K.; Klauk, H. Flexible low-voltage organic transistors and circuits based on a high-mobility organic semiconductor with good air stability. Adv. Mater. 2010, 22, 982–985. [Google Scholar] [CrossRef] [PubMed]
- Khatun, M.N.; Dey, A.; Meher, N.; Iyer, P.K. Long alkyl chain induced OFET characteristic with low threshold voltage in an n-type perylene monoimide semiconductor. ACS Appl. Electron. Mater. 2021, 3, 3575–3587. [Google Scholar] [CrossRef]
- Galindo, S.; Tamayo, A.; Leonardi, F.; Mas-Torrent, M. Control of polymorphism and morphology in solution sheared organic field-effect transistors. Adv. Funct. Mater. 2017, 27, 1700526. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Becerril, H.A.; Roberts, M.E.; Nishi, Y.; Bao, Z. Experimental study and statistical analysis of solution-shearing processed organic transistors based on an asymmetric small-molecule semiconductor. IEEE Trans. Electron. Devices 2009, 56, 176–185. [Google Scholar] [CrossRef]
- Possanner, S.K.; Zojer, K.; Pacher, P.; Zojer, E.; Schürrer, F. Threshold voltage shifts in organic thin-film transistors due to self-assembled monolayers at the dielectric surface. Adv. Funct. Mater. 2009, 19, 958–967. [Google Scholar] [CrossRef]
- Okachi, T. Mobility overestimation due to minority carrier injection and trapping in organic field-effect transistors. Org. Electron. 2018, 57, 34–44. [Google Scholar] [CrossRef]





| DSC Tm (°C) a | TGA Td (°C) b | UV-Vis λmax (nm) c | Egap (eV) d | Eoxonset (eV) e | HOMO (eV) e | LUMO (eV) f | |
|---|---|---|---|---|---|---|---|
| 1 | 42 | 292 | 262 | 3.75 | 1.14 | −5.42 | −1.67 |
| 2 | 139 | 396 | 400 | 2.78 | 0.81 | −5.09 | −2.31 |
| 3 | 127 | 392 | 399 | 2.73 | 0.90 | −5.19 | −2.46 |
| Compound | µmax (cm2/Vs) (µavg (cm2/Vs)) | Ion/Ioff | VTH (V) |
|---|---|---|---|
| 1 | 0.000028 ± 0.000002 (0.000030) | (4.6 ± 2.0) × 103 | −13 ± 4 |
| 2 | 0.067 ± 0.033 (0.10) | (1.4 ± 0.8) × 107 | −23 ± 4 |
| 3 | 0.0091 ± 0.0019 (0.011) | (7.6 ± 1.3) × 106 | −22 ± 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, E.; Jang, Y.; Ho, D.; Chae, W.; Earmme, T.; Kim, C.; Seo, S. Development of Dithieno[3,2-b:2′,3′-d]thiophene (DTT) Derivatives as Solution-Processable Small Molecular Semiconductors for Organic Thin Film Transistors. Coatings 2021, 11, 1222. https://doi.org/10.3390/coatings11101222
Choi E, Jang Y, Ho D, Chae W, Earmme T, Kim C, Seo S. Development of Dithieno[3,2-b:2′,3′-d]thiophene (DTT) Derivatives as Solution-Processable Small Molecular Semiconductors for Organic Thin Film Transistors. Coatings. 2021; 11(10):1222. https://doi.org/10.3390/coatings11101222
Chicago/Turabian StyleChoi, Eunjin, Yuhyeon Jang, Dongil Ho, Wookil Chae, Taeshik Earmme, Choongik Kim, and SungYong Seo. 2021. "Development of Dithieno[3,2-b:2′,3′-d]thiophene (DTT) Derivatives as Solution-Processable Small Molecular Semiconductors for Organic Thin Film Transistors" Coatings 11, no. 10: 1222. https://doi.org/10.3390/coatings11101222
APA StyleChoi, E., Jang, Y., Ho, D., Chae, W., Earmme, T., Kim, C., & Seo, S. (2021). Development of Dithieno[3,2-b:2′,3′-d]thiophene (DTT) Derivatives as Solution-Processable Small Molecular Semiconductors for Organic Thin Film Transistors. Coatings, 11(10), 1222. https://doi.org/10.3390/coatings11101222







