One-Step Methods to Fabricate Durable Superhydrophobic Coatings for Flexible Electronic Sensors
Abstract
1. Introduction
2. Experiment
2.1. Materials
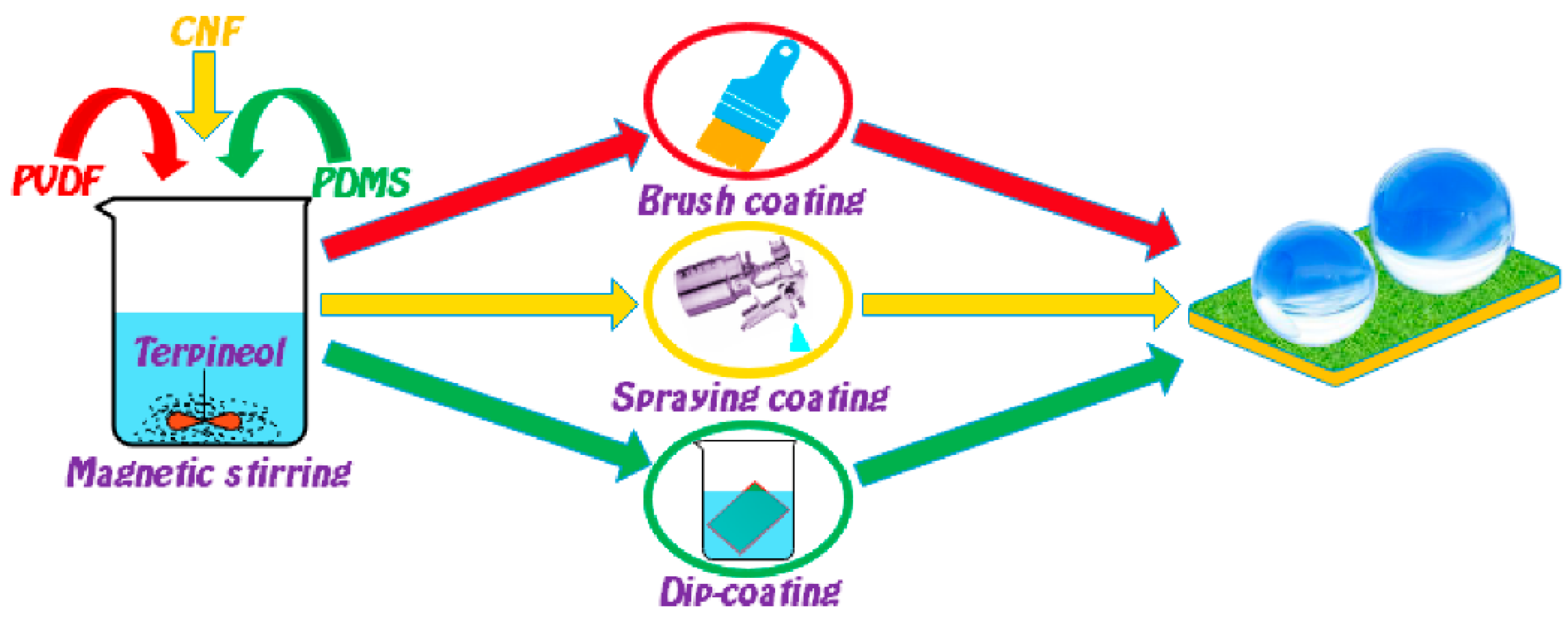
2.2. Preparation of the Flexible Conductive Superhydrophobic Coating
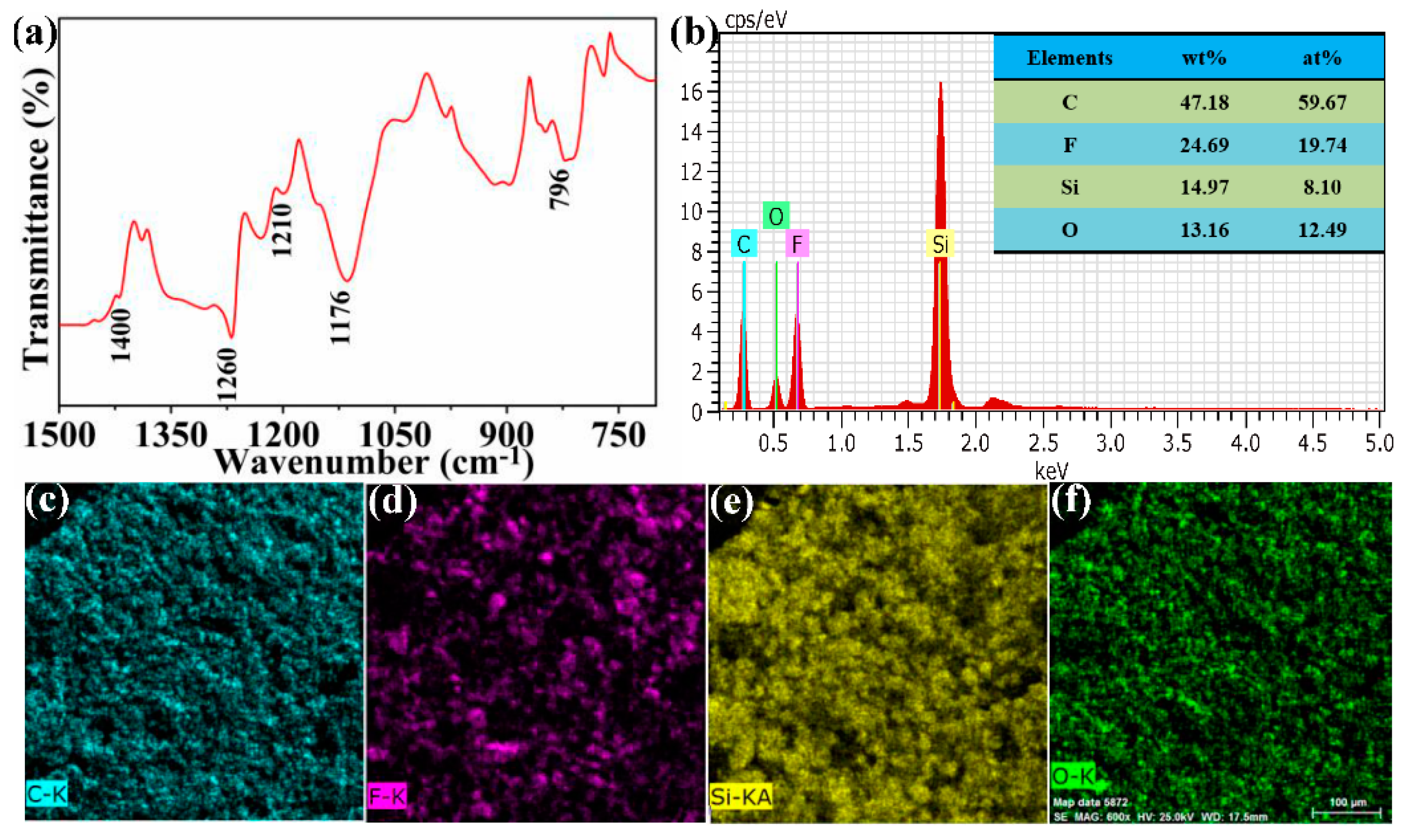
2.3. Characterization
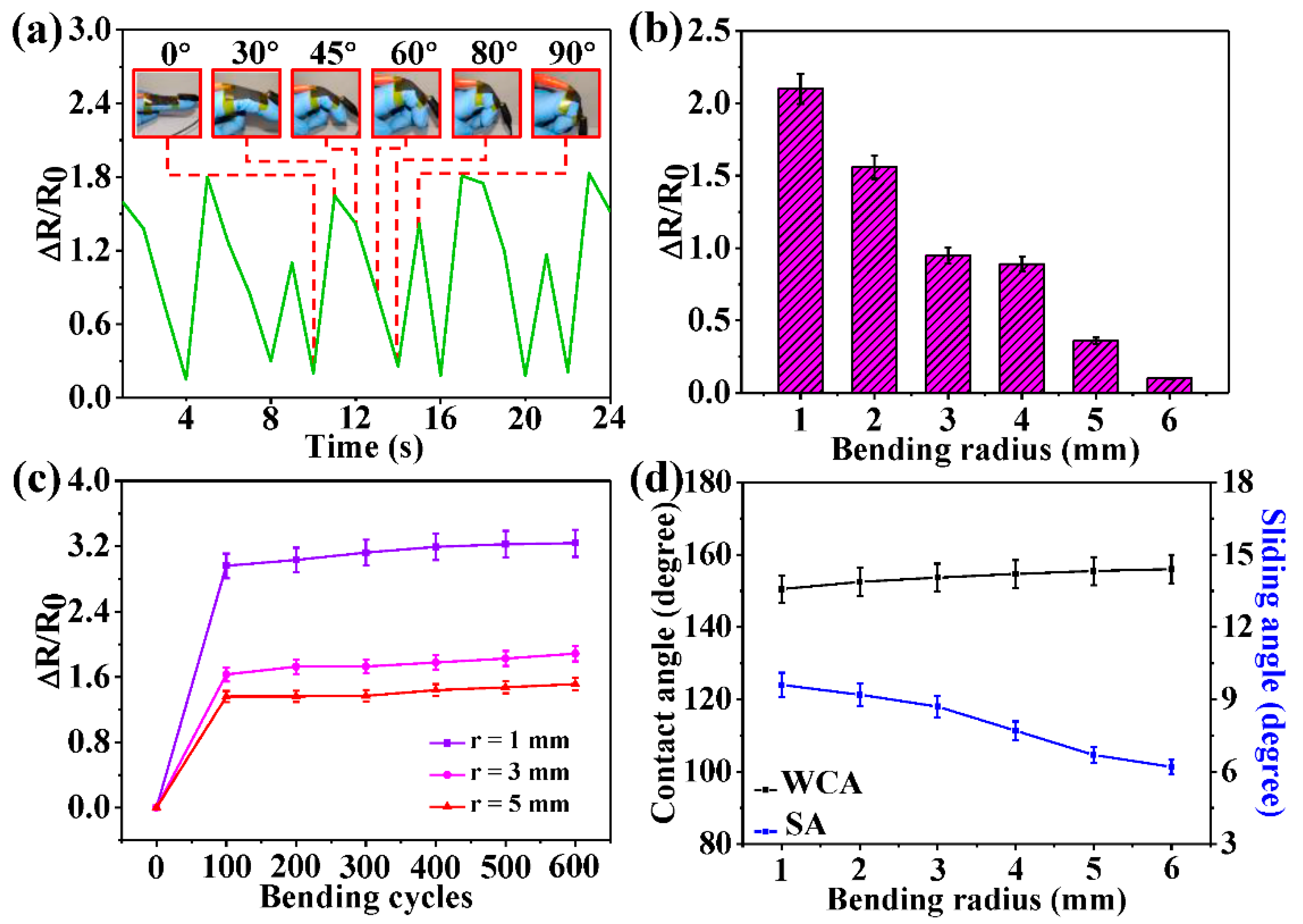
3. Results and Discussion
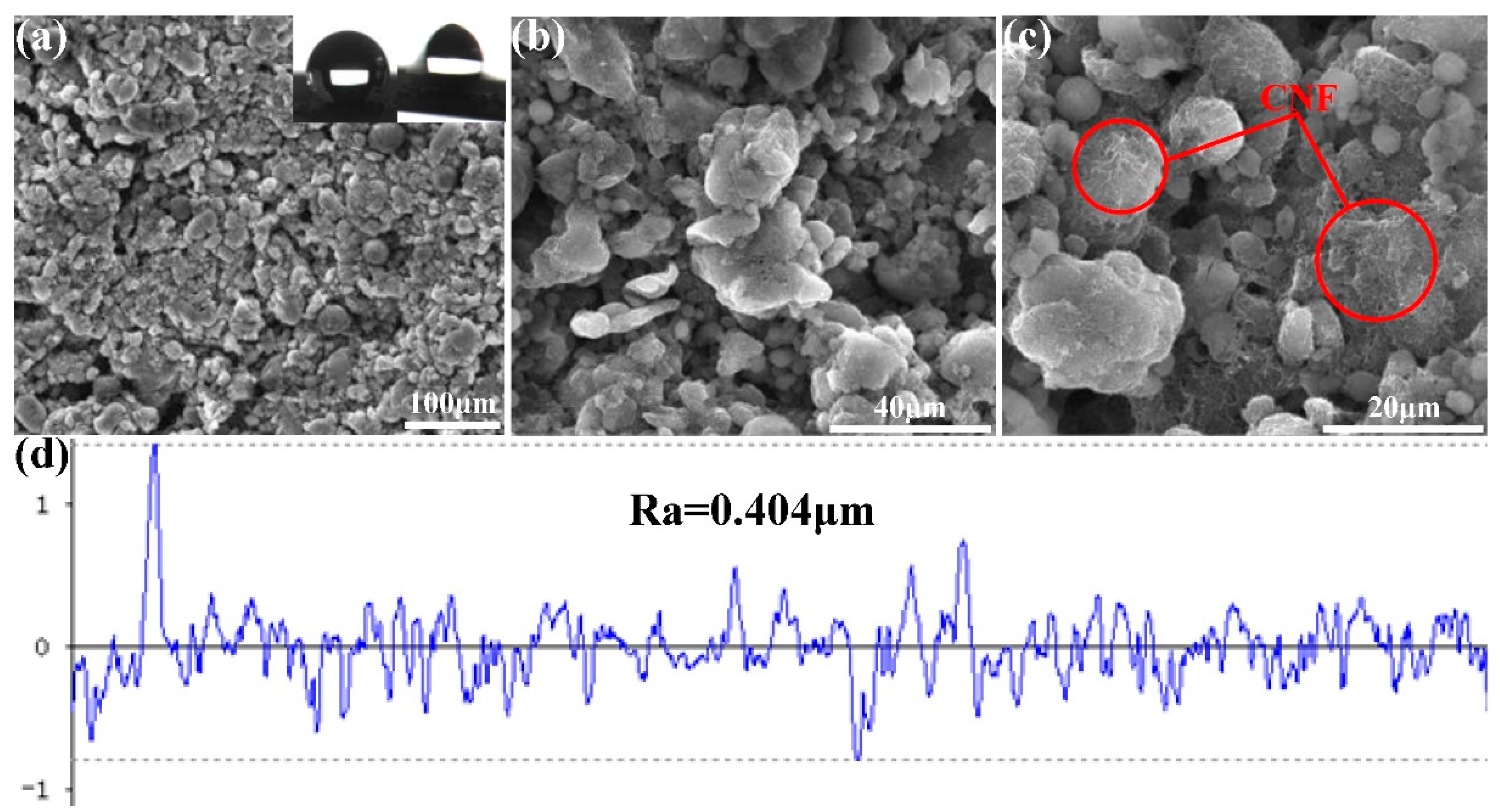
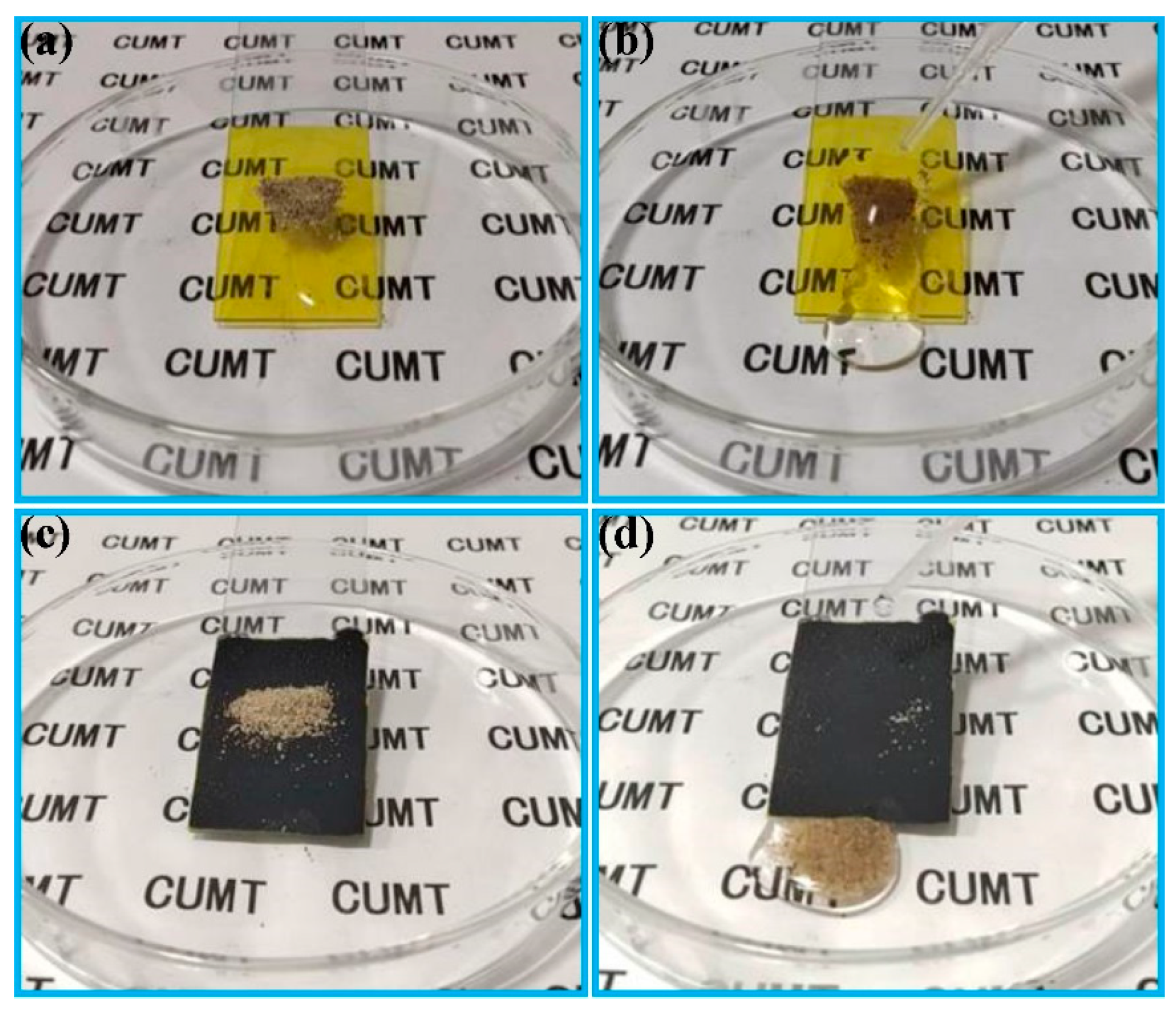
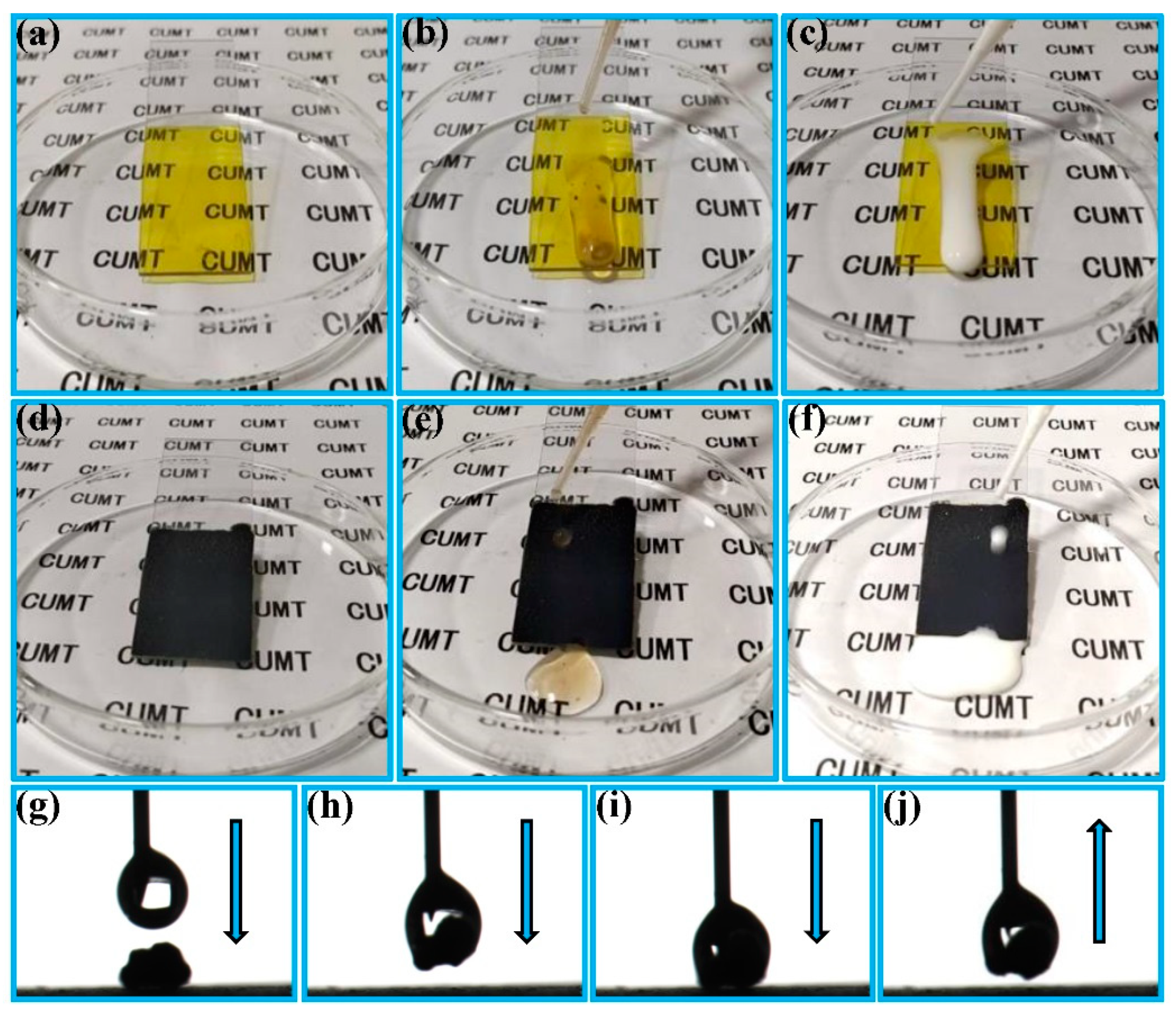
3.1. Fabrication of the FCS Coating
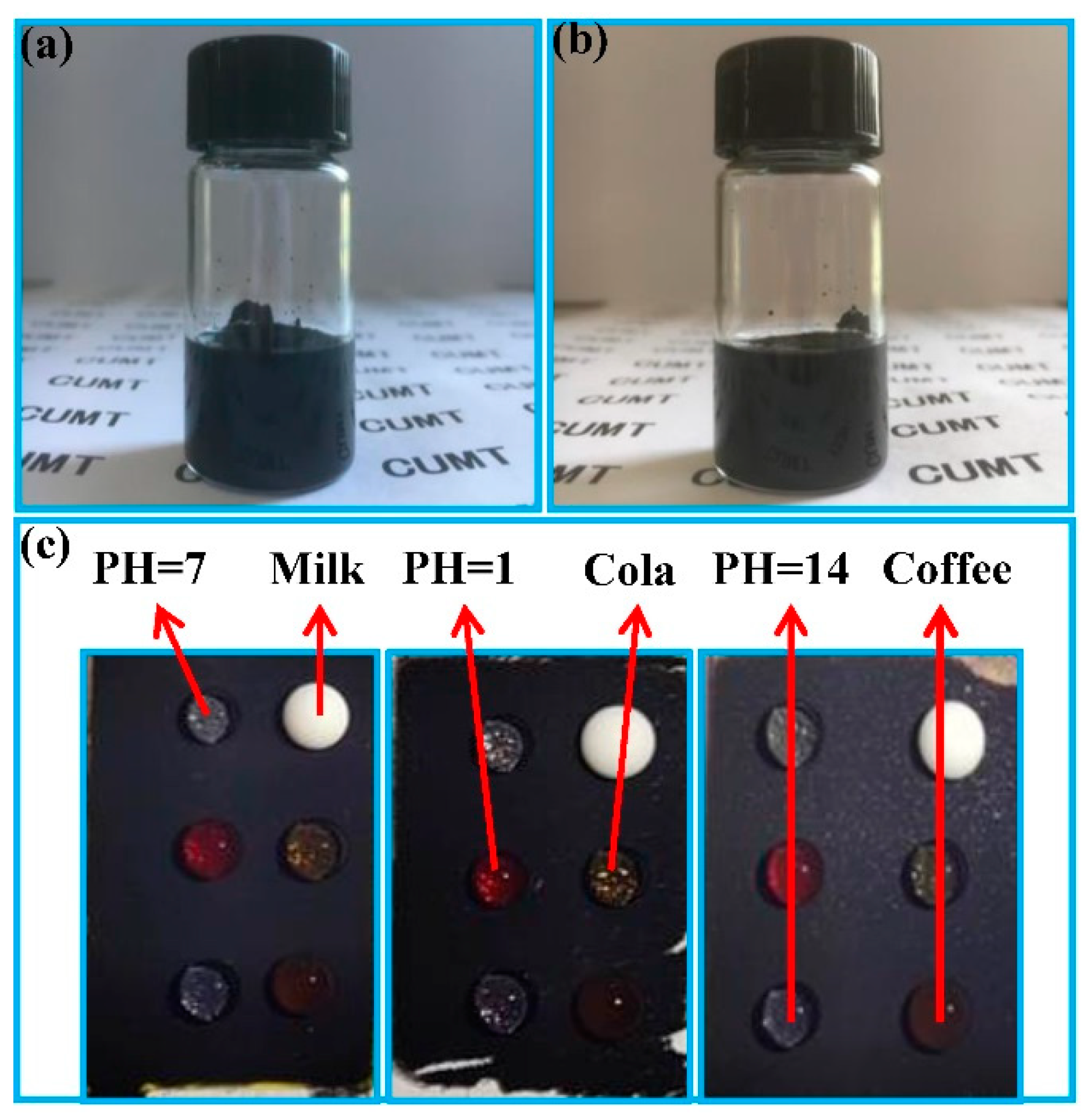
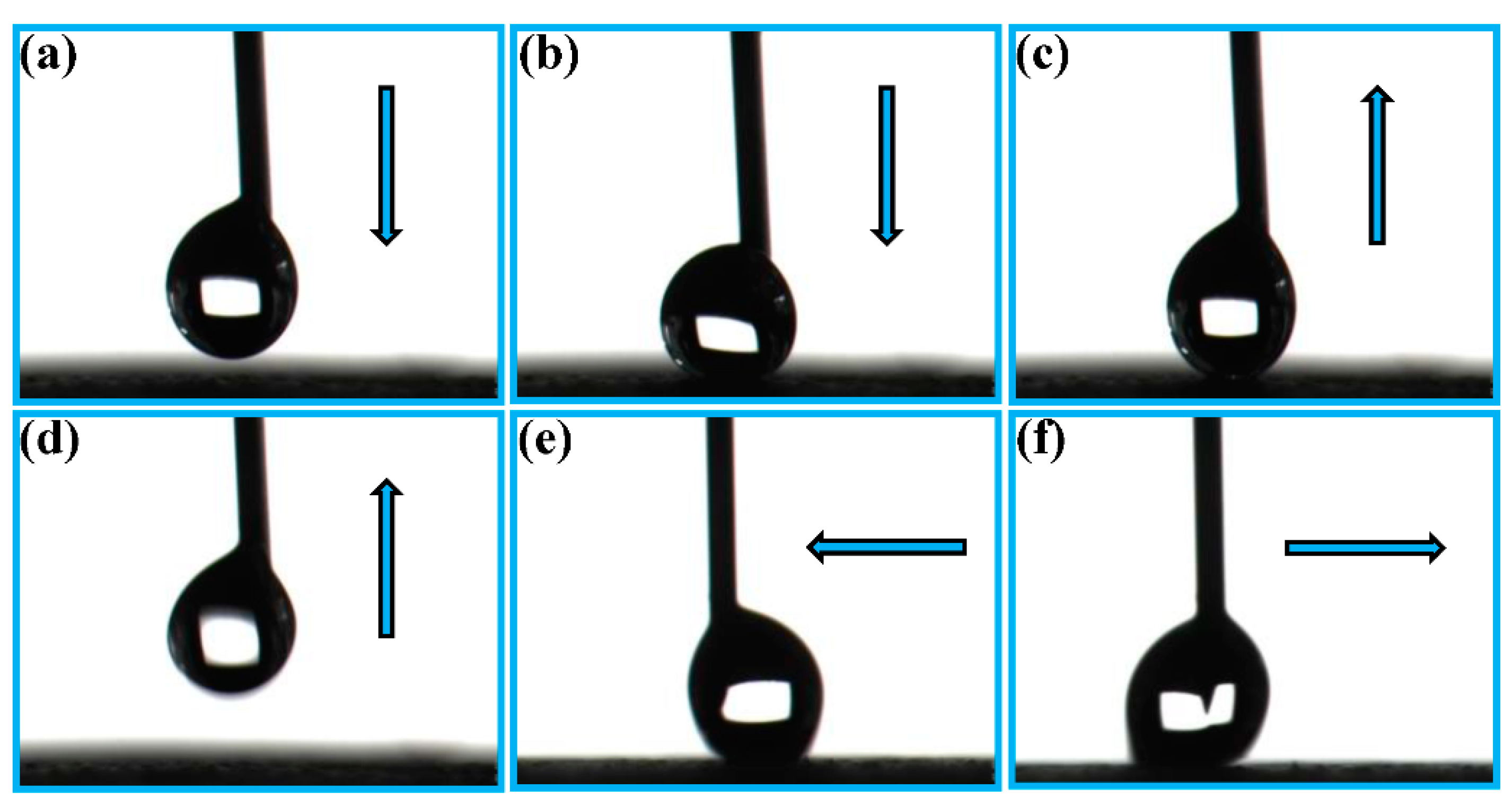
3.2. Water Droplet Adhesion, Self-Cleaning and Anti-Fouling Properties
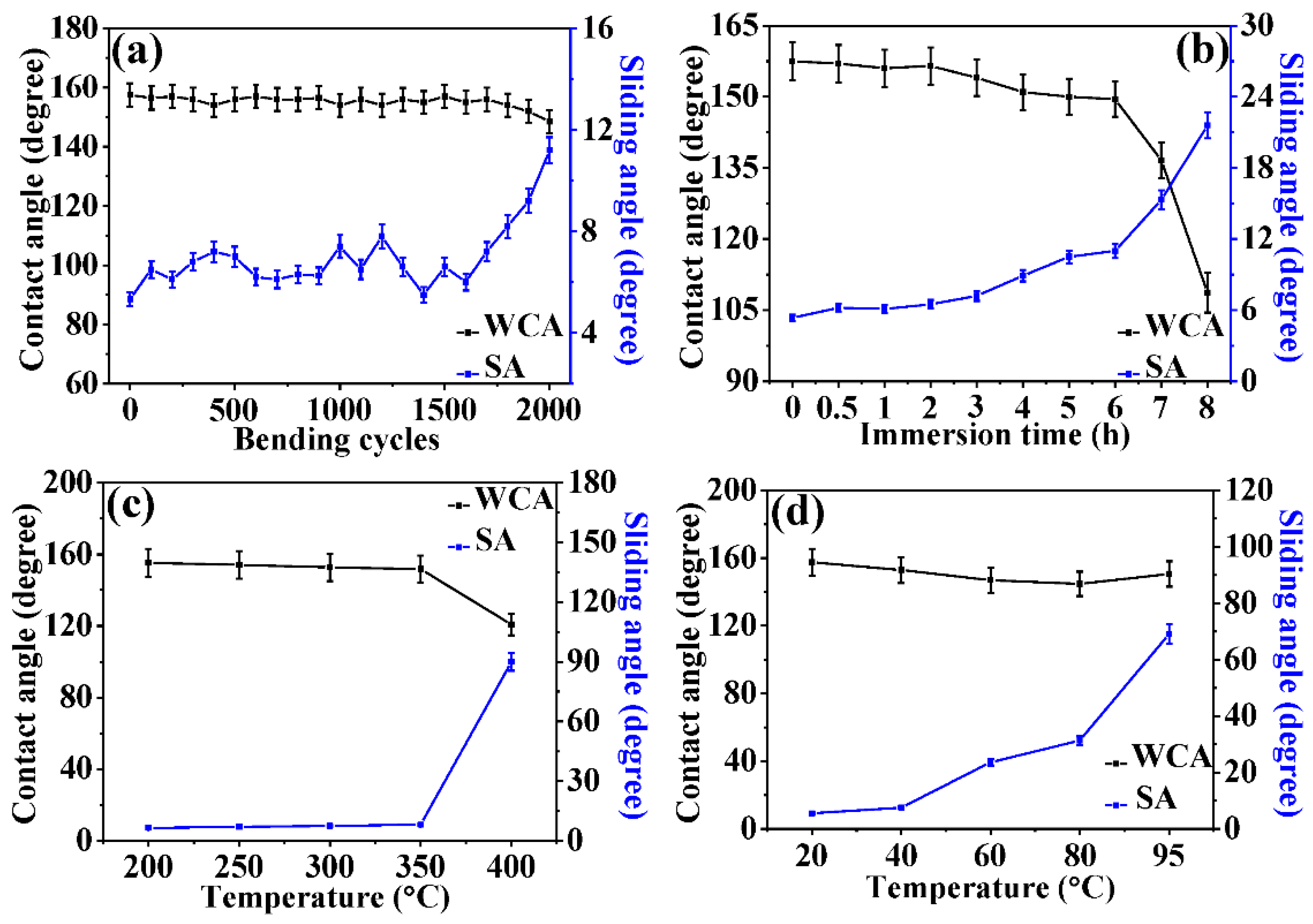
3.3. Durability
3.4. Conductivity and Application
4. Conclusions
Supplementary Materials
Author Contributions
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peng, J.; Zhao, X.; Wang, W.; Gong, X. Durable self-cleaning surfaces with superhydrophobic and highly oleophobic properties. Langmuir 2019, 35, 8404–8412. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Hu, D.; Zheng, X.; Wang, L.; Yu, Z.; An, W.; Na, R.; Li, C.; Li, N.; Lu, Z. Enhancing droplet deposition on wired and curved superhydrophobic leaves. ACS Nano 2019, 13, 7966–7974. [Google Scholar] [CrossRef]
- Ta, M.; Xu, F.; Ahmed, I.; Hou, X. One-step fabrication of superhydrophobic P(VDF-co-HFP) nanofibre membranes using electrospinning technique. J. Appl. Polym. Sci. 2019, 48817, 1–9. [Google Scholar]
- Attia, H.; Johnson, D.; Wright, C.; Hilal, N. Robust superhydrophobic electrospun membrane fabricated by combination of electrospinning and electrospraying techniques for air gap membrane distillation. Desalination 2018, 446, 70–82. [Google Scholar] [CrossRef]
- Vidal, K.; Gomez, E.; Goitandia, A.; Angulo-lbanez, A.; Aranzabe, E. The synthesis of a superhydrophobic and thermal stable silica coating via sol-gel process. Coatings 2019, 9, 627. [Google Scholar] [CrossRef]
- Su, X.; Li, H.; Lai, X.; Zhang, L.; Wang, J.; Liao, X.; Zeng, X. Vapor-liquid sol-gel approach to fabricating highly durable and robust superhydrophobic polydimethylsiloxane@silica surface on polyester textile for oil-water separation. ACS Appl. Mater. Interfaces 2017, 9, 28089–28099. [Google Scholar] [CrossRef]
- Wang, S.; Wang, J. Anti-reflective and superhydrophobic films prepared from a sol at different withdrawal speeds. Appl. Surf. Sci. 2019, 476, 1035–1048. [Google Scholar] [CrossRef]
- Qian, X.; Tang, T.; Wang, H.; Chen, C.; Luo, J.; Luo, D.L. Fabrication of hydrophobic Ni surface by chemical etching. Materials 2019, 12, 3546. [Google Scholar] [CrossRef]
- Khodaei, M.; Shadmani, S. Superhydrophobicity on aluminum through reactive-etching and TEOS/GPTMS/nano-Al2O3 silane-based nanocomposite coating. Surf. Coat. Technol. 2019, 374, 1078–1090. [Google Scholar] [CrossRef]
- Tuo, Y.; Zhang, H.; Rong, W.; Jiang, S.; Chen, W.; Liu, X. Drag reduction of anisotropic superhydrophobic surface prepared by laser etching. Langmuir 2019, 35, 11016–11022. [Google Scholar] [CrossRef]
- Peng, C.; Wu, R.; Yang, Y.; Li, C.; Lin, Y.; Chen, S.; Kuai, Z.; Li, L. Hydrothermal formation of controllable hexagonal holes and Er2O3/Er2O3-RGO particles on silicon wafers toward superhydrophobic surfaces. J. Colloid Interface Sci. 2020, 580, 768–775. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, X.; Yi, Z.; Zhou, Z.; Tang, Y.; Yi, Y. Fabriction of ZnO nanorods with strong UV absorption and different hydrophobicity on foamed nickel under different hydrothermal conditions. Micromachines 2019, 10, 164. [Google Scholar] [CrossRef] [PubMed]
- Celik, N.; Torun, I.; Ruzi, M.; Esidir, A.; Onses, M. Fabrication of robust superhydrophobic surfaces by one-step spray coating: Evaporation driven self-assembly of wax and nanoparticles into hierarchical structures. Chem. Eng. J. 2020, 396, 1–11. [Google Scholar] [CrossRef]
- Abd El-Hady, M.; Sharaf, S.; Farouk, A. Highly hydrophobic and UV protective properties of cotton fabric using layer by layer self-assembly technique. Cellulose 2019, 27, 1099–1110. [Google Scholar] [CrossRef]
- Xu, L.; Zhu, D.; Lu, X.; Lu, Q. Transparent, thermally and mechanically stable superhydrophobic coating prepared by an electrochemical template strategy. J. Mater. Chem. A 2015, 3, 3801–3807. [Google Scholar] [CrossRef]
- Sun, J.; Li, Y.; Liu, G.; Chu, F.; Chen, C.; Zhang, Y.; Tian, H.; Song, Y. Patterning superhydrophobic area on a facile fabricated superhydrophilic layer based on inkjet printed water-soluble polymer template. Langmuir 2020, 36, 9952–9959. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Fan, Y.; Han, G.; Guo, Z. Superomniphobic silk fibroin/Ag nanowires membrane for flexible and transparent electronic sensor. ACS Appl. Mater. Interfaces 2020, 12, 10039–10049. [Google Scholar] [CrossRef]
- Liu, H.; Li, Q.; Bu, Y.; Zhang, N.; Wang, C.; Pan, C.; Mi, L.; Guo, Z.; Liu, C.; Shen, C. Stretchable conductive nonwoven fabrics with self-cleaning capability for tunable wearable strain sensor. Nano Energy 2019, 66, 1–9. [Google Scholar] [CrossRef]
- Liu, S.; Zheng, J.; Hao, L.; Yegin, Y.; Bae, M.; Ulugun, B.; Taylor, T.; Scholar, E.; Cisneros-Zevallos, L.; Oh, J.; et al. Dual-functional, superhydrophobic coatings with bacterial anticontact and antimicrobial characteristics. ACS Appl. Mater. Interfaces 2020, 12, 21311–21321. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, D.; Liu, R.; Xie, Y.; Li, J.; Wang, L. A coral reef-like structure fabricated on cellulose paper for simultaneous oil-water separation and electromagnetic shielding protection. ACS Omega 2020, 5, 18105–18113. [Google Scholar] [CrossRef]
- Han, G.; Nguyen, T.; Park, S.; Jung, Y.; Lee, J.; Lim, H. Moth-eye mimicking solid slippery glass surface with icephobicity, transparency, and self-healing. ACS Nano 2020, 14, 10198–10209. [Google Scholar] [CrossRef] [PubMed]
- Sam, E.; Sam, D.; Lv, X.; Liu, B.; Xiao, X.; Gong, S.; Yu, W.; Chen, J.; Liu, J. Recent development in the fabrication of self-healing superhydrophobic surfaces. Chem. Eng. J. 2019, 373, 531–546. [Google Scholar]
- Chen, Y.; Wang, L.; Wu, Z.; Luo, J.; Li, B.; Huang, X.; Xue, H.; Gao, J. Super-hydrophobic, durable and cost-effective carbon black/rubber composites for high performance strain sensors. Compos. Part B-Eng. 2019, 176, 1–10. [Google Scholar] [CrossRef]
- Gao, J.; Wang, L.; Guo, Z.; Li, B.; Wang, H.; Luo, J.; Huang, X.; Xue, H. Flexible, superhydrophobic, and electrically conductive polymer nanofiber composite for multifunctional sensing applications. Chem. Eng. J. 2020, 381, 1–12. [Google Scholar] [CrossRef]
- Ho, D.; Cheon, S.; Hong, P.; Park, J.; Suk, J.; Kim, D.; Han, J. Multifunctional smart textronics with blowlogpun nonwoven fabrics. Adv. Funct. Mater. 2019, 29, 1–9. [Google Scholar] [CrossRef]
- Su, X.; Li, H.; Lai, X.; Chen, Z.; Zeng, X. 3D porous superhydrophobic CNT/EVA composites for recoverable shape reconfiguration and underwater vibration detection. Adv. Funct. Mater. 2019, 29, 1–11. [Google Scholar] [CrossRef]
- Xu, R.; Zhang, K.; Xu, X.; He, M.; Lu, F.; Su, B. Superhydrophobic WS2-nanosheet-wrapped sponges for underwater detection of tiny vibration. Adv. Sci. 2018, 5, 1–10. [Google Scholar] [CrossRef]
- Das, S.; Srinivasan, S.; Stromberg, L.; He, Q.; Garland, N.; Straszheim, W.; Ajayan, P.; Balasubramanian, G.; Claussen, J. Superhydrophobic inkjet printed flexible graphene circuits via direct-pulsed laser writing. Nanoscale 2017, 9, 19058–19065. [Google Scholar] [CrossRef]
- Gustafsson, L.; Jansson, R.; Hedhammar, M.; van der Wijngaart, W. Structuring of functional spider silk wires, coatings, and sheets by self-assembly on superhydrophobic pillar surfaces. Adv. Mater. 2017, 30, 1–8. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, D.; Guo, Z. A facile modifier-free approach to fabricate antistatic superhydrophobic composite coatings with remarkable thermal stability and corrosion resistance. J. Bionic Eng. 2020, 17, 421–435. [Google Scholar] [CrossRef]
- Li, L.; Bai, Y.; Li, L.; Wang, S.; Zhang, T. A superhydrophobic smart coating for flexible and wearable sensing electronics. Adv. Mater. 2017, 19, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Li, H.; Lai, X.; Chen, Z.; Zeng, X. Highly stretchable and conductive superhydrophobic coating for flexible electronics. ACS Appl. Mater. Interfaces 2018, 10, 10587–10597. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Fan, S.; Lu, Y.; Feng, H.; Qiu, J. Proposal and verification of a novel superhydrophobic-conductive anti-corrosion polyaniline-silica coating. Bull. Chem. Soc. Jpn. 2020, 93, 1114–1120. [Google Scholar] [CrossRef]
- Song, B.; Li, J.; Wu, F.; Patel, S.; Hah, J.; Wang, X.; Moon, K.; Wong, C. Processing and characterization of silver-filled conductive polysulfide sealants for aerospace applications. Soft Matter 2018, 14, 9036–9043. [Google Scholar] [CrossRef]
- Saddiqi, N.; Seeger, S. Chemically resistant, electric conductive, and superhydrophobic coatings. Adv. Mater. Interfaces 2019, 9, 1–9. [Google Scholar] [CrossRef]
- Hong, T.; Jeong, S.; Choi, Y.; Lim, T.; Ju, S. Superhydrophobic, elastic, and conducting polyurethane-carbon nanotube-silane-aerogel composite microfiber. Polymers 2020, 12, 1772. [Google Scholar] [CrossRef]
- Lu, Y.; Sathasivam, S.; Song, J.; Crick, C.R.; Carmalt, C.J.; Parkin, I. Robust self-cleaning surfaces that function when exposed to either air or oil. Science 2015, 347, 1132–1135. [Google Scholar] [CrossRef]
- Das, A.; Megaridis, C.; Liu, L.; Wang, T.; Biswas, A. Design and synthesis of superhydrophobic carbon nanofiber composite coatings for terahertz frequency shielding and attenuation. Appl. Phys. Lett. 2011, 98, 1–3. [Google Scholar] [CrossRef]
- Baldelli, A.; Ou, J.; Barona, D.; Li, W.; Amirfazli, A. Sprayable, superhydrophobic, electrically, and thermally conductive coating. Adv. Mater. Interfaces 2020, 19, 1–9. [Google Scholar] [CrossRef]
- Liao, X.; Li, H.; Lai, X.; Chen, W.; Zeng, X. Facile fabrication of superhydrophobic conductive polydimethylsiloxane@silver nanowires cotton fabric via dipping-thermal curing method. Mater. Lett. 2019, 255, 1–4. [Google Scholar] [CrossRef]
- Chen, K.; Gou, W.; Xu, L.; Zhao, Y. Low cost and facile preparation of robust multifunctional coatings with self-healing superhydrophobicity and high conductivity. Compos. Sci. Technol. 2018, 156, 177–185. [Google Scholar] [CrossRef]
- Yin, X.; Yu, S.; Bi, X.; Liu, E.; Zhao, Y. Robust superhydrophobic 1D Ni3S2 nanorods coating for self-cleaning and anti-scaling. Ceram. Int. 2019, 45, 24618–24624. [Google Scholar] [CrossRef]
- Zhong, A.; Li, J.; Zhang, Y.; Zhang, F.; Wang, T.; Zhang, G.; Sun, R.; Wong, C. Low temperature microwave fabrication of three-dimensional graphene/polyimide foams with flexibility strain responsivity. Compos. Part A-Appl. Sci. Manuf. 2020, 137, 1–10. [Google Scholar] [CrossRef]
- Owens, D.; Wendt, R. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Aktij, S.; Taghipour, A.; Rahimpour, A.; Mollahosseini, A.; Tiraferri, A. A critical review on ultrasonic-assisted fouling control and cleaning of fouled membranes. Ultrasonics 2020, 108, 1–20. [Google Scholar]
- Li, J.; Wei, Y.; Huang, Z.; Wang, F.; Yan, X.; Wu, Z. Electrohydrodynamic behavior of water droplets on a horizontal super hydrophobic surface and its self-cleaning application. Appl. Surf. Sci. 2017, 403, 133–140. [Google Scholar] [CrossRef]
- Qi, W.; Li, J.; Weisensee, P. Evaporation of sessile water droplets on horizontal and vertical bi-philic patterned surfaces. Langmuir 2019, 35, 17185–17192. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Chen, K.; Zhang, D.; Guo, Z. One-Step Methods to Fabricate Durable Superhydrophobic Coatings for Flexible Electronic Sensors. Coatings 2021, 11, 95. https://doi.org/10.3390/coatings11010095
Liu X, Chen K, Zhang D, Guo Z. One-Step Methods to Fabricate Durable Superhydrophobic Coatings for Flexible Electronic Sensors. Coatings. 2021; 11(1):95. https://doi.org/10.3390/coatings11010095
Chicago/Turabian StyleLiu, Xiang, Kai Chen, Dekun Zhang, and Zhiguang Guo. 2021. "One-Step Methods to Fabricate Durable Superhydrophobic Coatings for Flexible Electronic Sensors" Coatings 11, no. 1: 95. https://doi.org/10.3390/coatings11010095
APA StyleLiu, X., Chen, K., Zhang, D., & Guo, Z. (2021). One-Step Methods to Fabricate Durable Superhydrophobic Coatings for Flexible Electronic Sensors. Coatings, 11(1), 95. https://doi.org/10.3390/coatings11010095





