Phase Transformation and Superstructure Formation in (Ti0.5, Mg0.5)N Thin Films through High-Temperature Annealing
Abstract
:1. Introduction
2. Materials and Methods
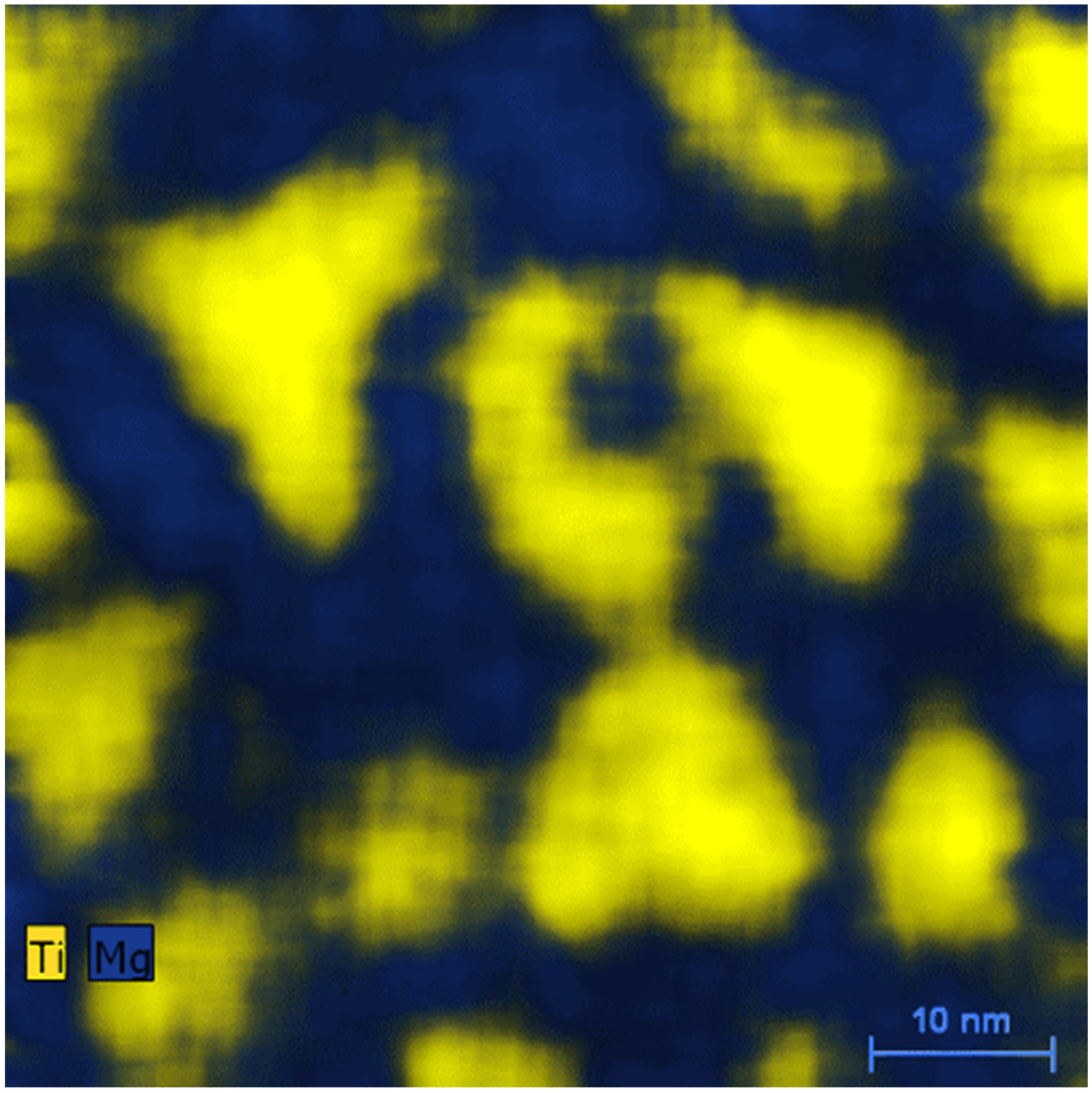
3. Result and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sundgren, J.-E. Structure and properties of TiN coatings. Thin Solid Films 1985, 128, 21–44. [Google Scholar] [CrossRef]
- Hultman, L. Thermal stability of nitride thin films. Vacuum 2000, 57, 1–30. [Google Scholar] [CrossRef]
- Mayrhofer, P.H.; Mitterer, C.; Hultman, L.; Clemens, H. Microstructural design of hard coatings. Prog. Mater. Sci. 2006, 51, 1032–1114. [Google Scholar] [CrossRef]
- Hultman, L.; Bareno, J.; Flink, A.; Söderberg, H.; Larsson, K.; Petrova, V.; Odén, M.; Greene, J.E.; Petrov, I. Interface structure in superhard TiN–SiN nanolaminates and nanocomposites: Film growth experiments and ab initio calculations. Phys. Rev. B 2007, 75, 155437. [Google Scholar] [CrossRef]
- Diserens, M.; Patscheider, J.; Lévy, F. Improving the properties of titanium nitride by incorporation of silicon. Surf. Coat. Technol. 1998, 108–109, 241–246. [Google Scholar] [CrossRef]
- Münz, W.-D. Titanium aluminum nitride films: A new alternative to TiN coatings. J. Vac. Sci. Technol. A 1986, 4, 2717. [Google Scholar] [CrossRef]
- Leyendecker, T.; Lemmer, O.; Esser, S.; Ebberink, J. The development of the PVD coating TiAlN as a commercial coating for cutting tools. Surf. Coat. Technol. 1991, 48, 175–178. [Google Scholar] [CrossRef]
- Hörling, A.; Hultman, L.; Odén, M.; Sjölén, J.; Karlsson, L. Mechanical properties and machining performance of Ti1−xAlxN-coated cutting tools. Surf. Coat. Technol. 2005, 191, 384–392. [Google Scholar] [CrossRef]
- Wang, B.; Kerdsongpanya, S.; McGahay, M.E.; Milosevic, E.; Patsalas, P.; Gall, D. Growth and properties of epitaxial Ti1−xMgxN(001) layers. J. Vac. Sci. Technol. A 2018, 36, 061501. [Google Scholar] [CrossRef]
- Baiwei, B.; Gall, D. A new semiconductor: Ti0.5Mg0.5N(001). In Proceedings of the 2018 IEEE Nanotechnology Symposium (ANTS), Albany, NY, USA, 14–15 November 2018. [Google Scholar]
- Sun, W.; Bartel, C.J.; Arca, E.; Bauers, S.R.; Matthews, B.; Orvañanos, B.; Chen, B.-R.; Toney, M.F.; Schelhas, L.T.; Tumas, W.; et al. A map of the inorganic ternary metal nitrides. Nat. Mater. 2019, 18, 732–739. [Google Scholar] [CrossRef] [Green Version]
- Banakh, O.; Balzer, M.; Fenker, M.; Blatter, A. Spectroellipsometric evaluation of colour and oxidation resistance of TiMgN coatings. Thin Solid Films 2004, 455–456, 650–655. [Google Scholar] [CrossRef]
- Fenker, M.; Balzer, M.; Kappl, H.; Banakh, O. Some properties of (Ti,Mg)N thin films deposited by reactive dc magnetron sputtering. Surf. Coat. Technol. 2005, 200, 227–231. [Google Scholar] [CrossRef]
- Fenker, M.; Balzer, M.; Kappl, H. Corrosion behaviour of decorative and wear resistant coatings on steel deposited by reactive magnetron sputtering–Tests and improvements. Thin Solid Films 2006, 515, 27–32. [Google Scholar] [CrossRef]
- Hodroj, A.; Chaix-Pluchery, O.; Steyer, P.; Pierson, J.F. Oxidation resistance of decorative (Ti,Mg)N coatings deposited by hybrid cathodic arc evaporation-magnetron sputtering process. Surf. Coat. Technol. 2011, 205, 4547–4553. [Google Scholar] [CrossRef]
- Onder, S.; Kok, F.N.; Kazmanli, K.; Urgen, M. Magnesium substituted hydroxyapatite formation on (Ti,Mg)N coatings produced by cathodic arc PVD technique. Mater. Sci. Eng. C 2013, 33, 4337–4342. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Gall, D. Fully strained epitaxial Ti1−xMgxN (001) layers. Thin Solid Films 2019, 688, 137165. [Google Scholar] [CrossRef]
- Eklund, P.; Kerdsongpanya, S.; Alling, B. Transition-metal-nitride-based thin films as novel energy harvesting materials. J. Mater. Chem. C 2016, 4, 3905–3914. [Google Scholar] [CrossRef] [Green Version]
- Kerdsongpanya, S.; Nong, N.V.; Pryds, N.; Žukauskaitė, A.; Jensen, J.; Birch, J.; Lu, J.; Hultman, L.; Wingqvist, G.; Eklund, P. Anomalously high thermoelectric power factor in epitaxial ScN thin films. Appl. Phys. Lett. 2011, 99, 232113. [Google Scholar] [CrossRef] [Green Version]
- Burmistrova, P.V.; Zakharov, D.N.; Favaloro, T.; Mohammed, A.; Stach, E.A.; Shakouri, A.; Sands, T.D. Effect of deposition pressure on the microstructure and thermoelectric properties of epitaxial ScN(001) thin films sputtered onto MgO(001) substrates. J. Mater. Res. 2015, 30, 626–634. [Google Scholar] [CrossRef] [Green Version]
- Tureson, N.; Marteau, M.; Cabioch, T.; Nong, N.V.; Jensen, J.; Lu, J.; Greczynski, G.; Fournier, D.; Singh, N.; Soni, A.; et al. Effect of ion-implantation-induced defects and Mg dopants on the thermoelectric properties of ScN. Phys. Rev. B 2018, 98, 205307. [Google Scholar] [CrossRef] [Green Version]
- Biswas, B.; Saha, B. Development of semiconducting ScN. Phys. Rev. Mater. 2019, 3, 020301. [Google Scholar] [CrossRef]
- Burmistrova, P.V.; Maassen, J.; Favaloro, T.; Saha, B.; Salamat, S.; Koh, Y.R.; Lundstrom, M.S.; Shakouri, A.; Sands, T.D. Thermoelectric properties of epitaxial ScN films deposited by reactive magnetron sputtering onto MgO(001) substrates. J. Appl. Phys. 2013, 113, 153704. [Google Scholar] [CrossRef] [Green Version]
- King, S.W.; Davis, R.F.; Nemanich, R.J. Gas source molecular beam epitaxy of scandium nitride on silicon carbide and gallium nitride surfaces. J. Vac. Sci. Technol. A 2014, 32, 061504. [Google Scholar] [CrossRef]
- Kerdsongpanya, S.; Sun, B.; Eriksson, F.; Jensen, J.; Lu, J.; Koh, Y.K.; Nong, N.V.; Balke, B.; Alling, B.; Eklund, P. Experimental and theoretical investigation of Cr1−xScxN solid solutions for thermoelectrics. J. Appl. Phys. 2016, 120, 215103. [Google Scholar] [CrossRef] [Green Version]
- Kerdsongpanya, S.; Hellman, O.; Sun, B.; Koh, Y.K.; Lu, J.; Nong, N.V.; Simak, S.I.; Alling, B.; Eklund, P. Phonon thermal conductivity of scandium nitride for thermoelectrics from first-principles calculations and thin-film growth. Phys. Rev. B 2017, 96, 195417. [Google Scholar] [CrossRef] [Green Version]
- Alling, B. Metal to semiconductor transition and phase stability of Ti1−xMgxNy alloys investigated by first-principles calculations. Phys. Rev. B 2014, 89, 085112. [Google Scholar] [CrossRef] [Green Version]
- Heyd, J.; Scuseria, G.E.; Ernzerhof, M. Hybrid functionals based on a screened Coulomb potential. J. Chem. Phys. 2003, 118, 8207–8215. [Google Scholar] [CrossRef] [Green Version]
- Irokawa, Y.; Usami, M. First-principles calculations of semiconducting TiMgN2. Jpn. J. Appl. Phys. 2016, 55, 098001. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [Green Version]
- Gharavi, M.A.; Armiento, R.; Alling, B.; Eklund, P. Theoretical study of phase stability, crystal and electronic structure of MeMgN2 (Me = Ti, Zr, Hf) compounds. J. Mater. Sci. 2018, 53, 4294–4305. [Google Scholar] [CrossRef] [Green Version]
- Bauers, S.R.; Hamann, D.M.; Patterson, A.; Perkins, J.D.; Talley, K.R.; Zakutayev, A. Composition, structure, and semiconducting properties of MgxZr2−xN2 thin films. Jpn. J. Appl. Phys. 2019, 58, SC1015. [Google Scholar] [CrossRef]
- Le Febvrier, A.; Landälv, L.; Liersch, T.; Sandmark, D.; Sandström, P.; Eklund, P. An upgraded ultra-high vacuum magnetron-sputtering system for high-versatility and software-controlled deposition. arXiv 2020, arXiv:2010.08411. [Google Scholar]
- Le Febvrier, A.; Jensen, J.; Eklund, P. Wet-cleaning of MgO(001): Modification of surface chemistry and effects on thin film growth investigated by X-ray photoelectron spectroscopy and time-of-flight secondary ion mass spectroscopy. J. Vac. Sci. Technol. A 2017, 35, 021407. [Google Scholar] [CrossRef] [Green Version]
- Gharavi, M.A.; Greczynski, G.; Eriksson, F.; Lu, J.; Balke, B.; Fournier, D.; le Febvrier, A.; Pallier, C.; Eklund, P. Synthesis and characterization of single-phase epitaxial Cr2N thin films by reactive magnetron sputtering. J. Mater. Sci. 2019, 54, 1434–1442. [Google Scholar] [CrossRef] [Green Version]
- Haasch, R.T.; Lee, T.-Y.; Gall, D.; Greene, J.E.; Petrov, I. Epitaxial TiN(001) grown and analyzed in situ by XPS and UPS. II. Analysis of Ar+ Sputter Etched Layers. Sur. Sci. Spectra 2000, 7, 204–212. [Google Scholar] [CrossRef]
- Le Febvrier, A.; Tureson, N.; Stilkerich, N.; Greczynski, G.; Eklund, P. Effect of impurities on morphology, growth mode, and thermoelectric properties of (111) and (001) epitaxial-like ScN films. J. Phys. D Appl. Phys. 2018, 52, 035302. [Google Scholar] [CrossRef]
- Hohenberg, P.; Kohn, W. Inhomogeneous electron gas. Phys. Rev. 1964, 136, B864–B871. [Google Scholar] [CrossRef] [Green Version]
- Kohn, W.; Sham, L.J. Self-consistent equations including exchange and correlation effects. Phys. Rev. 1965, 140, A1133–A1138. [Google Scholar] [CrossRef] [Green Version]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef] [Green Version]
- Kresse, G.; Hafner, J. Ab initio molecular dynamics for open-shell transition metals. Phys. Rev. B 1993, 48, 13115–13118. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for Ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Björkman, T. CIF2Cell: Generating geometries for electronic structure programs. Comput. Phys. Commun. 2011, 182, 1183–1186. [Google Scholar] [CrossRef]
- Armiento, R.; Tholander, C.; Björkman, T.; Armiento, R.; Steneteg, P.; Mogyasinz, I. The High-Throughput Toolkit (httk). Available online: http://httk.openmaterialsdb.se/ (accessed on 12 January 2021).
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Wang, L.; Maxisch, T.; Ceder, G. Oxidation energies of transition metal oxides within the GGA + U framework. Phys. Rev. B 2006, 73, 195107. [Google Scholar] [CrossRef] [Green Version]
- Jain, A.; Hautier, G.; Moore, C.J.; Ong, S.P.; Fischer, C.C.; Mueller, T.; Persson, K.A.; Ceder, G. A high-throughput infrastructure for density functional theory calculations. Comp. Mater. Sci. 2011, 50, 2295–2310. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Droghetti, A.; Baadji, N.; Sanvito, S. MgN: A possible material for spintronic applications. Phys. Rev. B 2009, 80, 235310. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Nutt, S.R.; Kirby, K.W. Surface modification of sapphire by magnesium-ion implantation. J. Am. Ceram. Soc. 1999, 82, 3260–3262. [Google Scholar] [CrossRef]
- Ningthoujam, R.S.; Gajbhiye, N.S. Synthesis, electron transport properties of transition metal nitrides and applications. Prog. Mater. Sci. 2015, 70, 50–154. [Google Scholar] [CrossRef]
- Greczynski, G.; Hultman, L. Compromising science by ignorant instrument calibration—Need to revisit half a century of published XPS data. Angew. Chem. Int. Ed. 2020, 59, 5002–5006. [Google Scholar] [CrossRef] [PubMed]
- Cava, R.J.; Murphy, D.W.; Zahurak, S.M.; Santoro, A.; Roth, R.S. The crystal structures of the lithium-inserted metal oxides Li0.5TiO2 anatase, LiTi2O4 spinel, and Li2Ti2O. J. Solid State Chem. 1984, 53, 64–75. [Google Scholar] [CrossRef]
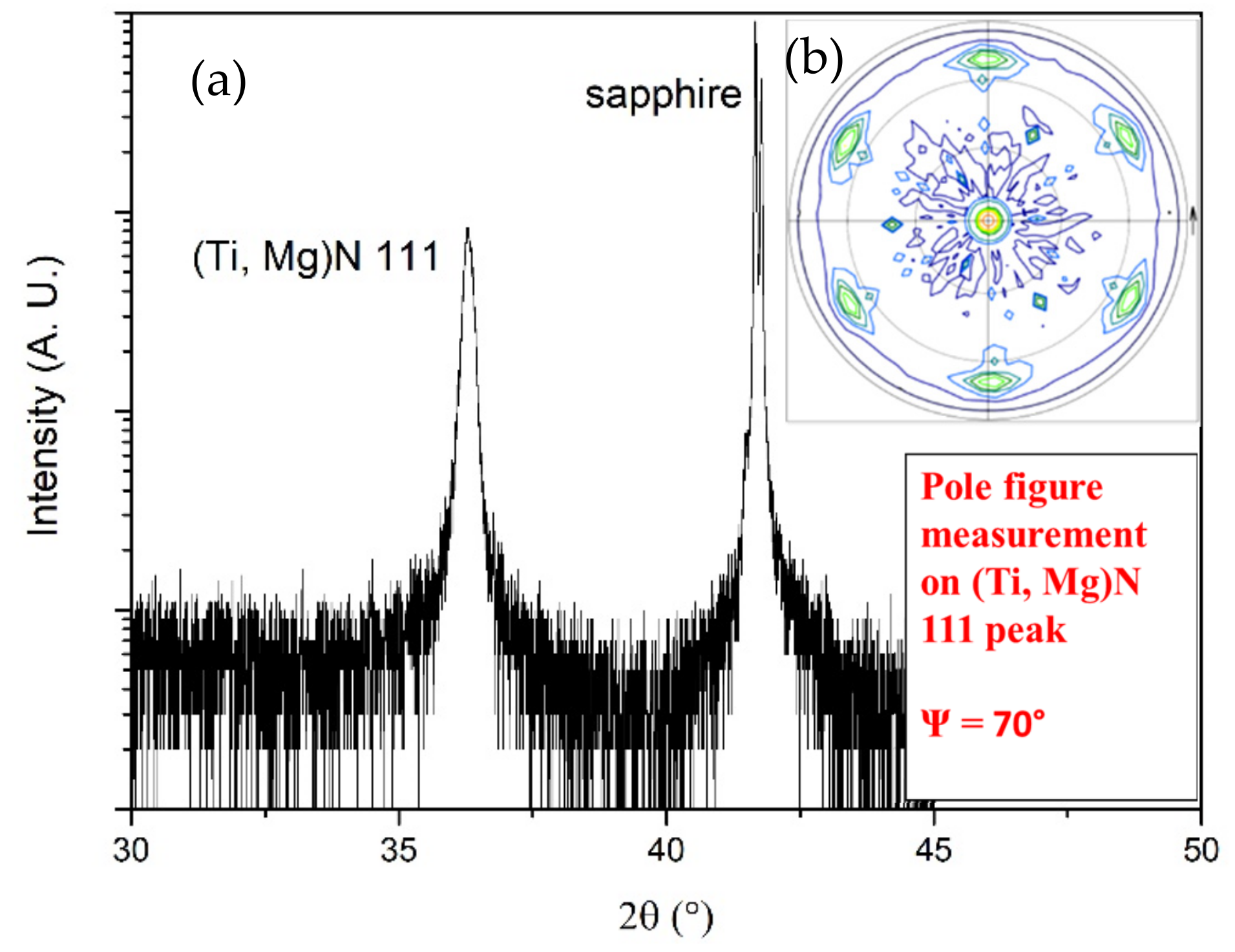


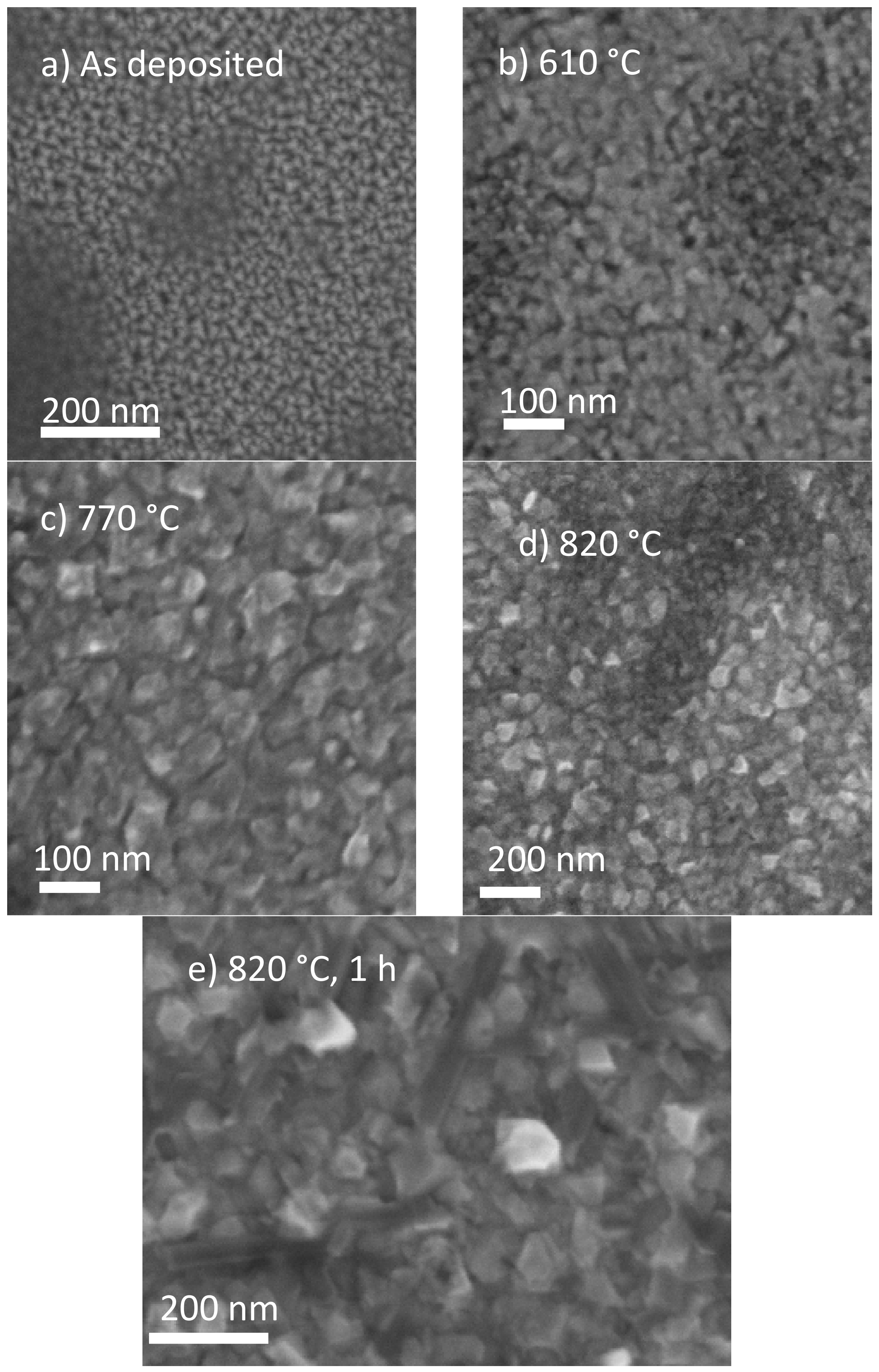
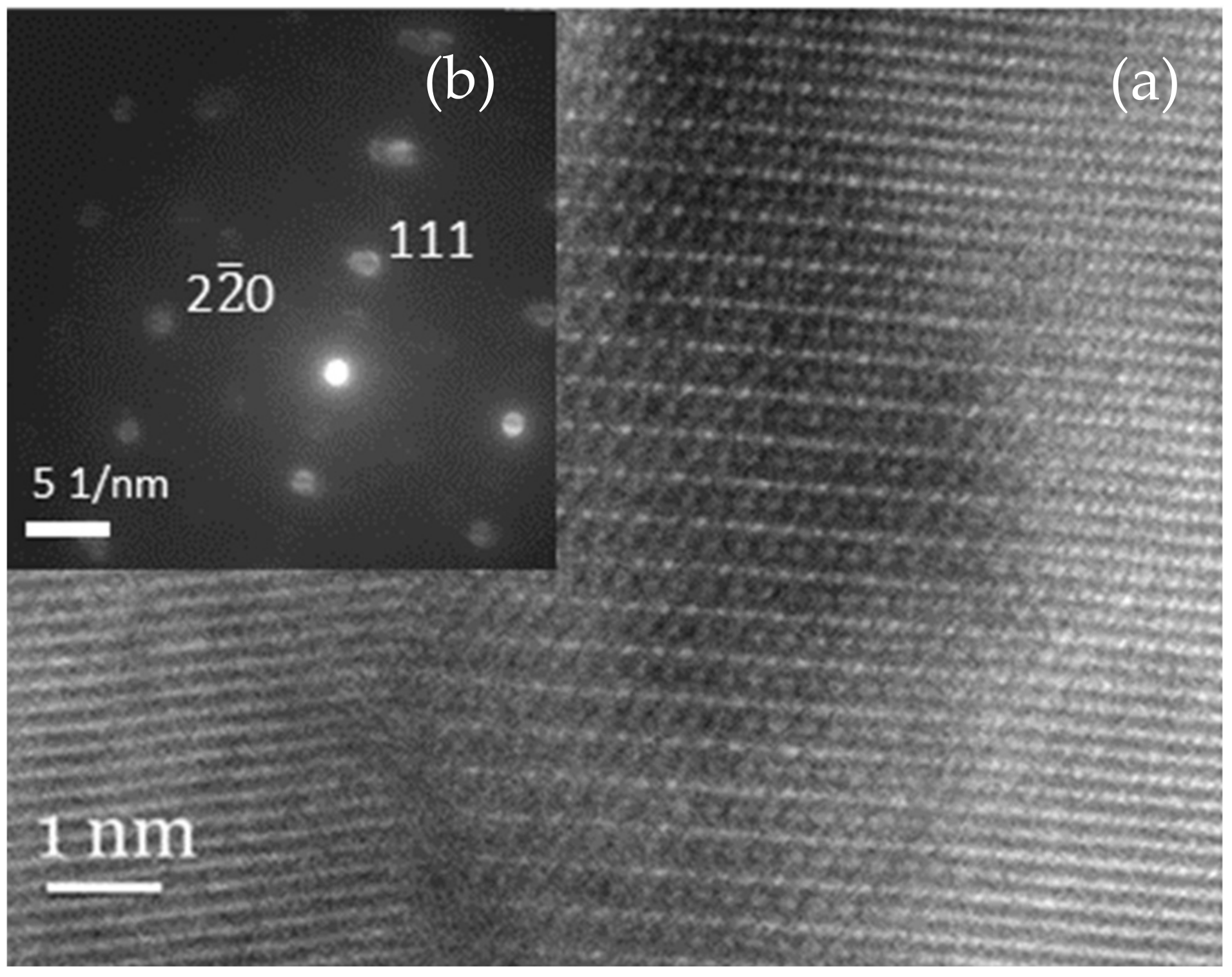
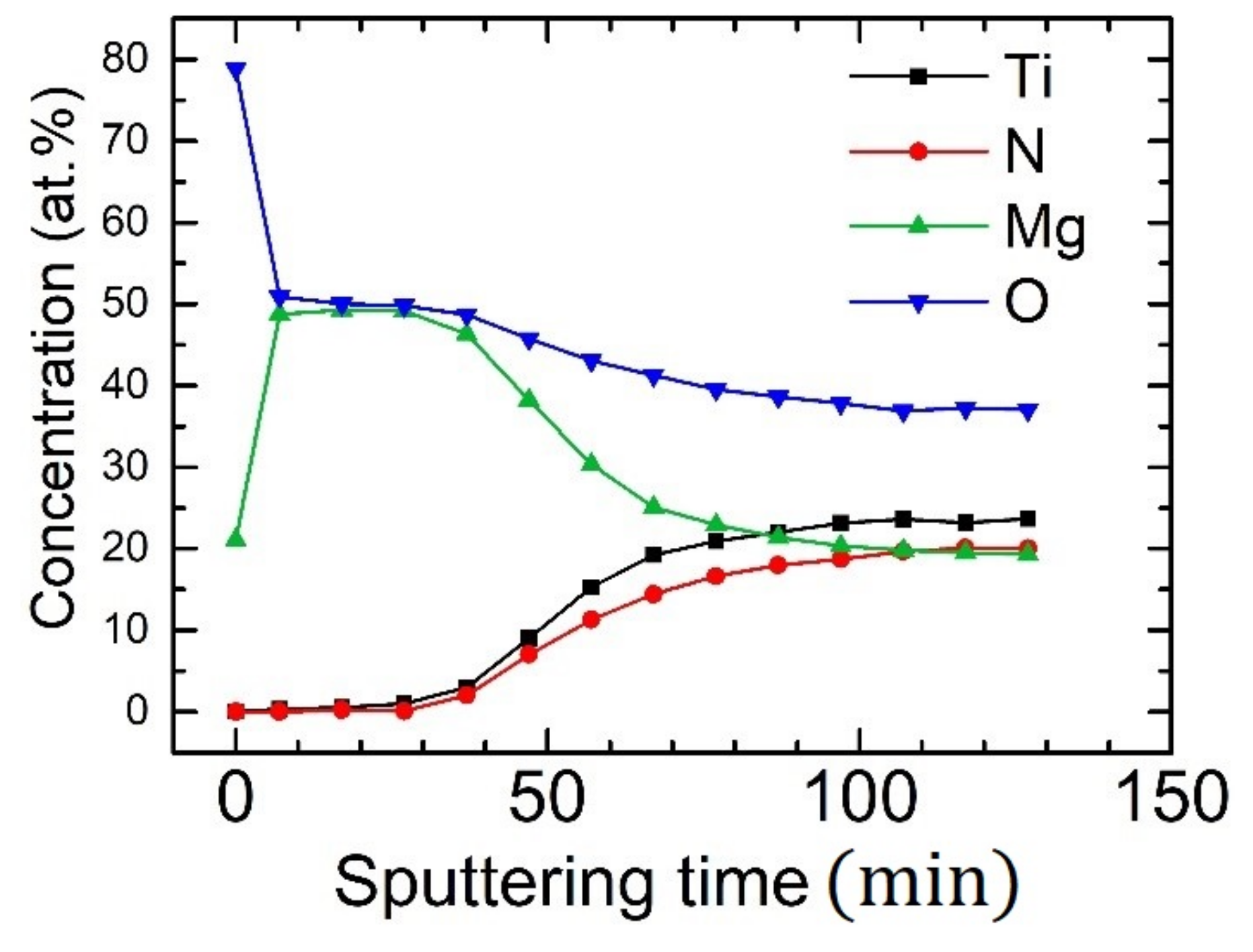

| 2θ (°) | d-Spacing (Å) | Multiple |
|---|---|---|
| 18.25 | 4.86 | 1 |
| 36.88 | 2.44 | 1/2 |
| 56.75 | 1.62 | 1/3 |
| 78.53 | 1.22 | 1/4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gharavi, M.A.; le Febvrier, A.; Lu, J.; Greczynski, G.; Alling, B.; Armiento, R.; Eklund, P. Phase Transformation and Superstructure Formation in (Ti0.5, Mg0.5)N Thin Films through High-Temperature Annealing. Coatings 2021, 11, 89. https://doi.org/10.3390/coatings11010089
Gharavi MA, le Febvrier A, Lu J, Greczynski G, Alling B, Armiento R, Eklund P. Phase Transformation and Superstructure Formation in (Ti0.5, Mg0.5)N Thin Films through High-Temperature Annealing. Coatings. 2021; 11(1):89. https://doi.org/10.3390/coatings11010089
Chicago/Turabian StyleGharavi, Mohammad Amin, Arnaud le Febvrier, Jun Lu, Grzegorz Greczynski, Björn Alling, Rickard Armiento, and Per Eklund. 2021. "Phase Transformation and Superstructure Formation in (Ti0.5, Mg0.5)N Thin Films through High-Temperature Annealing" Coatings 11, no. 1: 89. https://doi.org/10.3390/coatings11010089
APA StyleGharavi, M. A., le Febvrier, A., Lu, J., Greczynski, G., Alling, B., Armiento, R., & Eklund, P. (2021). Phase Transformation and Superstructure Formation in (Ti0.5, Mg0.5)N Thin Films through High-Temperature Annealing. Coatings, 11(1), 89. https://doi.org/10.3390/coatings11010089






