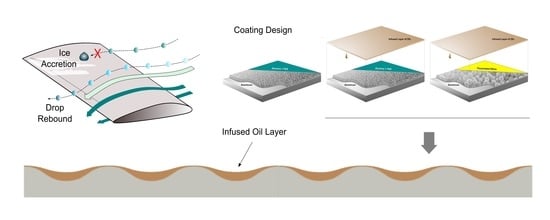
Different Approaches to Low-Wettable Materials for Freezing Environments: Design, Performance and Durability
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Alumina Sol
2.2. Preparation of Silica Nano-Suspension
2.3. Production of LF and SLIPS Samples by Deposition of Hybrid Coatings
2.4. Surface Characterization
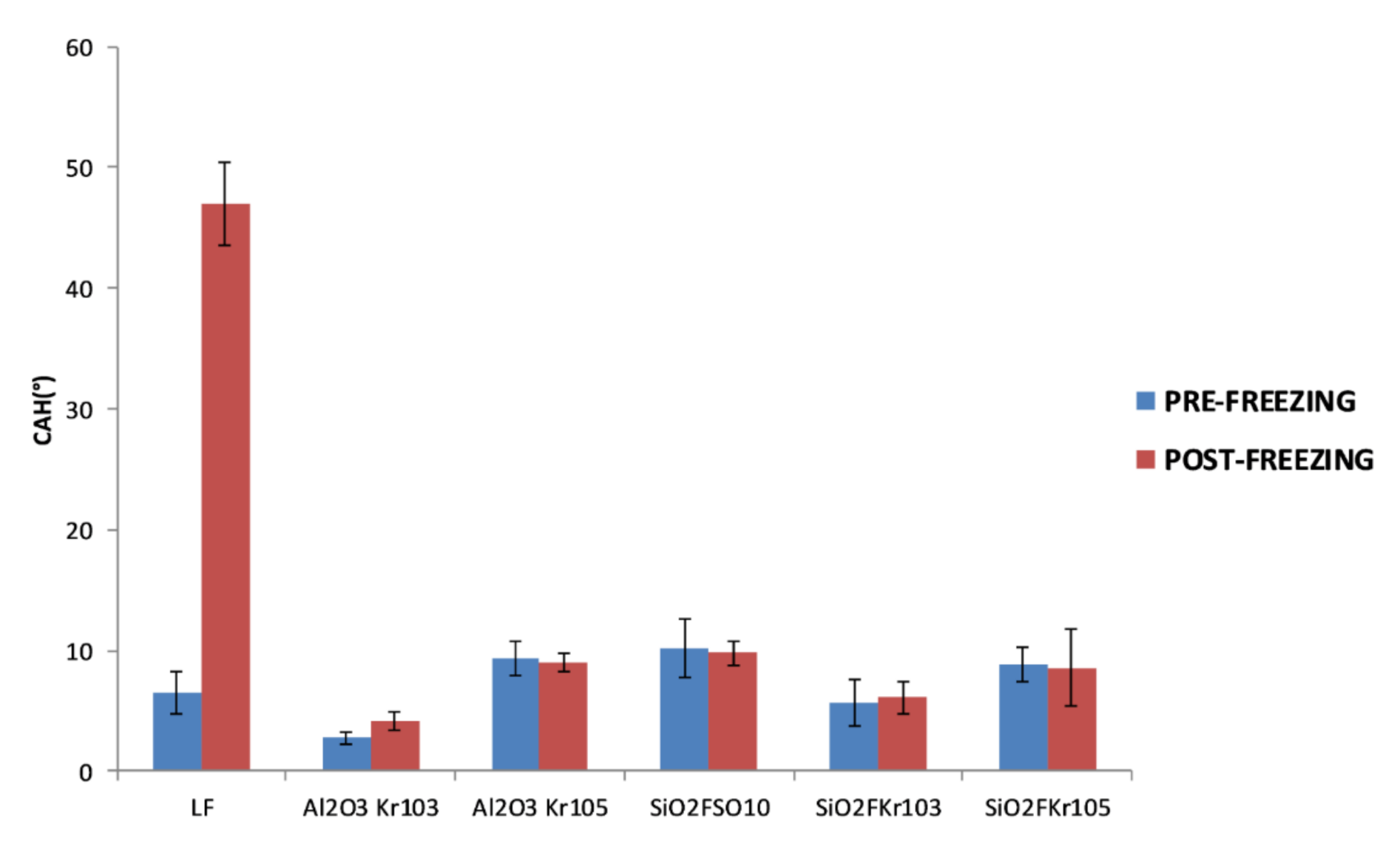
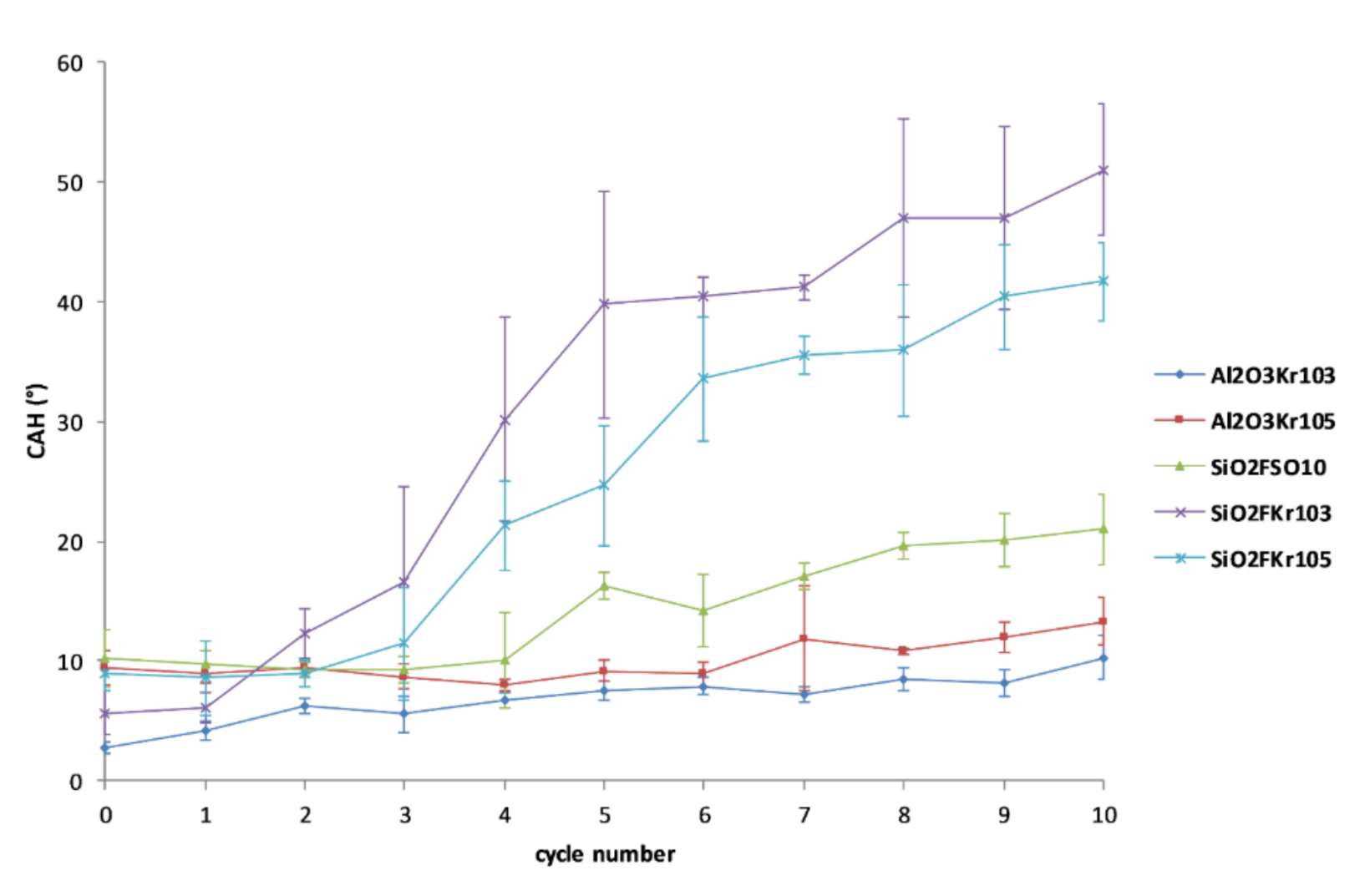
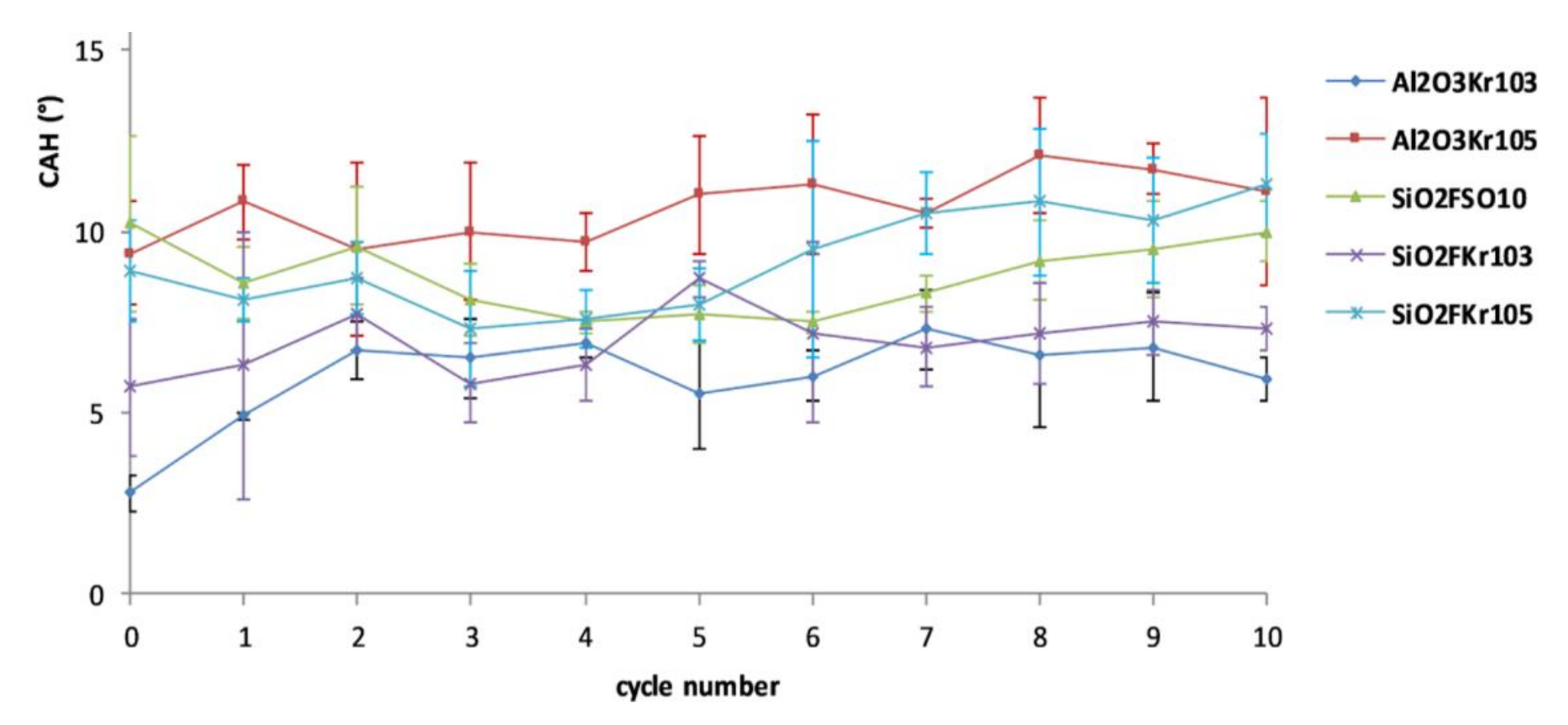
2.5. Assessment of Durability
3. Results and Discussion
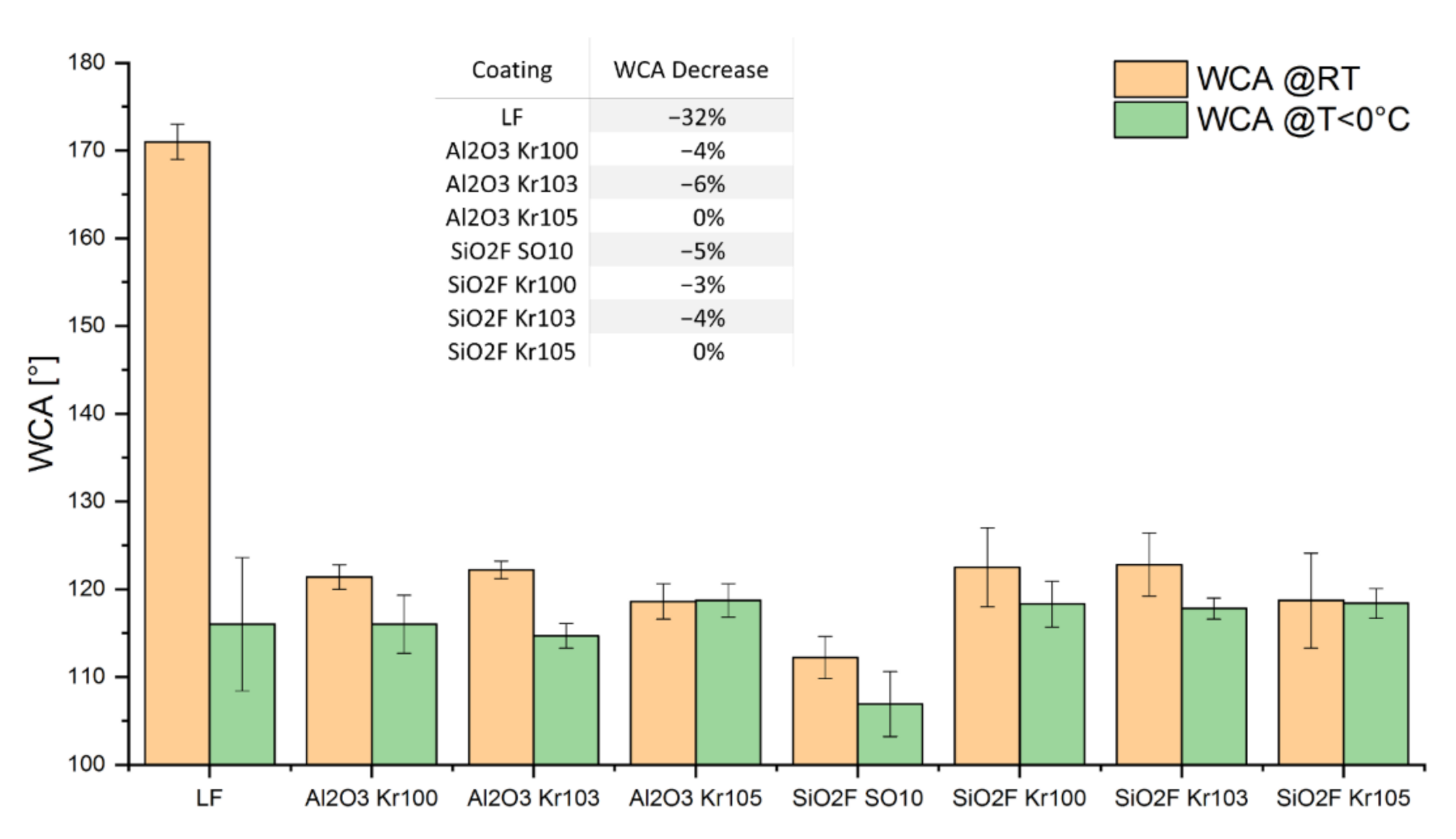
3.1. Wetting Behavior at Room Temperature and Surface Morphology
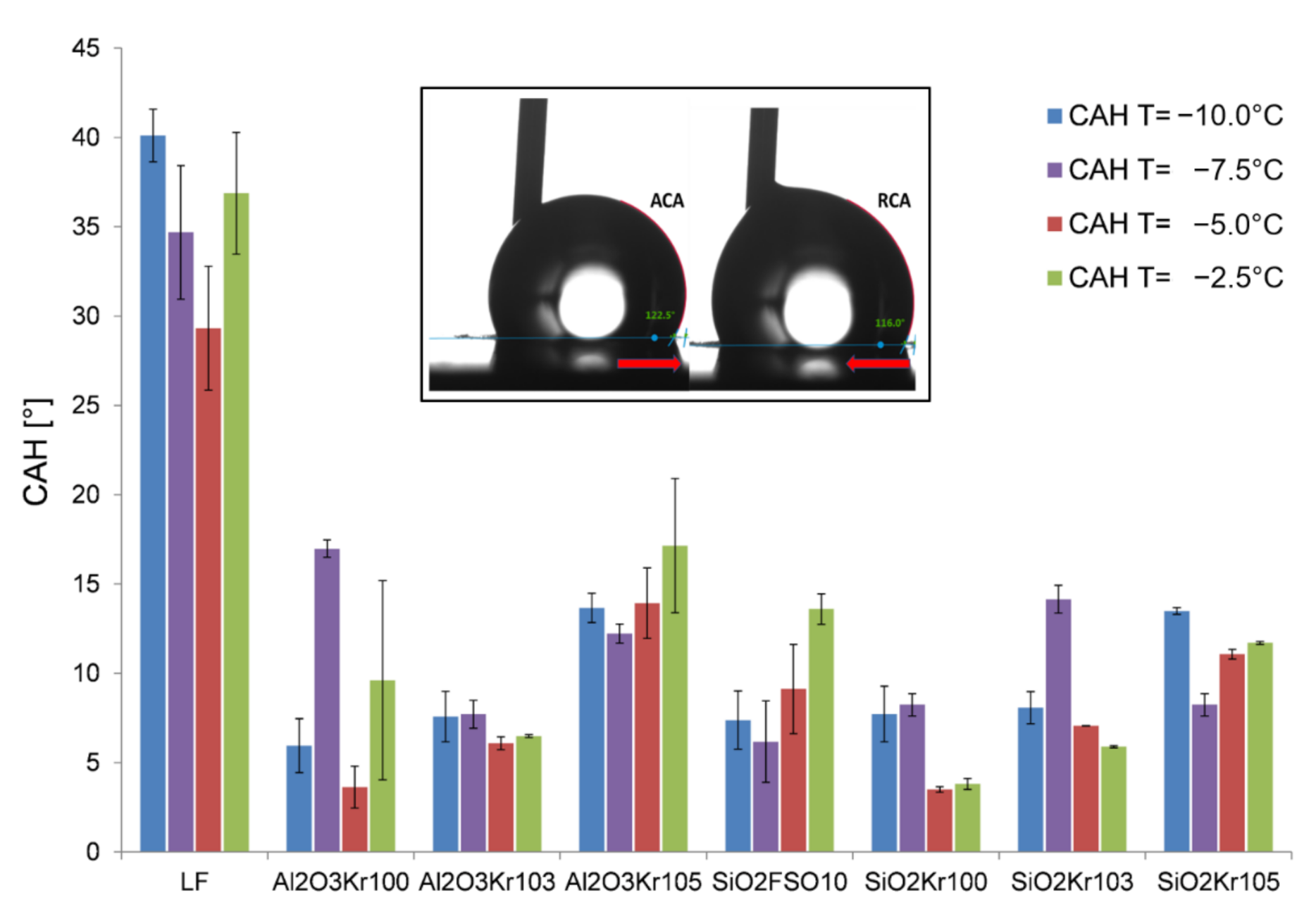
3.2. Wetting Behavior at Low Temperatures
3.3. Durability
- LF (used as reference);
- Al2O3 Kr103 and Al2O3 Kr105;
- SiO2F Kr103 and SiO2F Kr105;
- SiO2F SO10.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Laforte, J.L.; Allaire, M.A.; Laflamme, J. State-of-the-art on power line de-icing. Atmos. Res. 1998, 46, 143–158. [Google Scholar] [CrossRef]
- Farzaneh, M.; Volat, C.; Leblond, A. Anti-icing and de-icing techniques for overhead lines. In Atmospheric Icing of Power Networks; Springer: Dordrecht, The Netherlands, 2008; pp. 229–268. [Google Scholar] [CrossRef]
- Egbert, R.I.; Schrag, R.L.; Bernhart, W.D.; Zumwalt, G.W.; Kendrew, T.J. An investigation of power une de-icing by electro-impulse methods. IEEE Trans. Power Deliv. 1989, 4, 1855–1861. [Google Scholar] [CrossRef]
- Poots, G.; Gent, R.W.; Dart, N.P.; Cansdale, J.T. Aircraft icing. Philos. Trans. R. Soc. London. Ser. A Math. Phys. Eng. Sci. 2000, 358, 2873–2911. [Google Scholar]
- Susoff, M.; Siegmann, K.; Pfaffenroth, C.; Hirayama, M. Evaluation of icephobic coatings—Screening of different coatings and influence of roughness. Appl. Surf. Sci. 2013, 282, 870–879. [Google Scholar] [CrossRef]
- Guo, P.; Zheng, Y.; Wen, M.; Song, C.; Lin, Y.; Jiang, L. Icephobic/Anti-Icing Properties of Micro/Nanostructured Surfaces. Adv. Mater. 2012, 24, 2642–2648. [Google Scholar] [CrossRef]
- Kraj, A.G.; Bibeau, E.L. Phases of icing on wind turbine blades characterized by ice accumulation. Renew. Energy 2010, 35, 966–972. [Google Scholar] [CrossRef]
- Meuler, A.J.; Smith, J.D.; Varanasi, K.K.; Mabry, J.M.; McKinley, G.H.; Cohen, R.E. Relationships between water wettability and ice adhesion. ACS Appl. Mater. Interfaces 2010, 2, 3100–3110. [Google Scholar] [CrossRef] [PubMed]
- Narhe, R.D.; Beysens, D.A. Growth dynamics of water drops on a square-pattern rough hydrophobic surface. Langmuir 2007, 23, 6486–6489. [Google Scholar] [CrossRef] [PubMed]
- Varanasi, K.K.; Deng, T.; Smith, J.D.; Hsu, M.; Bhate, N. Frost formation and ice adhesion on superhydrophobic surfaces. Appl. Phys. Lett. 2010, 97, 234102. [Google Scholar] [CrossRef]
- Cao, L.; Jones, A.K.; Sikka, V.K.; Wu, J.; Gao, D. Anti-Icing Superhydrophobic Coatings. Langmuir 2009, 25, 12444–12448. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Wang, J.; Li, H.; Song, Y. Super-hydrophobic surfaces to condensed micro-droplets at temperatures below the freezing point retard ice/frost formation. Soft Matter 2011, 7, 3993–4000. [Google Scholar] [CrossRef]
- Zhang, Q.; Jin, B.; Wang, B.; Fu, Y.; Zhan, X.; Chen, F. Fabrication of a highly stable superhydrophobic surface with dual-scale structure and its antifrosting properties. Ind. Eng. Chem. Res. 2017, 56, 2754–2763. [Google Scholar] [CrossRef]
- Lynch, F.T.; Khodadoust, A. Effects of ice accretions on aircraft aerodynamics. Prog. Aerosp. Sci. 2001, 37, 669–767. [Google Scholar] [CrossRef]
- Song, J.; Zhao, D.; Han, Z.; Xu, W.; Lu, Y.; Liu, X.; Liu, B.; Carmalt, C.J.; Deng, X.; Parkin, I.P. Super-robust superhydrophobic concrete. J. Mater. Chem. A 2017, 5, 14542–14550. [Google Scholar] [CrossRef]
- Zhan, X.; Yan, Y.; Zhang, Q.; Chen, F. A novel superhydrophobic hybrid nanocomposite material prepared by surface-initiated AGET ATRP and its anti-icing properties. J. Mater. Chem. A 2014, 2, 9390–9399. [Google Scholar] [CrossRef]
- Jung, S.; Dorrestijn, M.; Raps, D.; Das, A.; Megaridis, C.M.; Poulikakos, D. Are superhydrophobic surfaces best for icephobicity? Langmuir 2011, 27, 3059–3066. [Google Scholar] [CrossRef]
- Verho, T.; Bower, C.; Andrew, P.; Franssila, S.; Ikkala, O.; Ras, R.H. Mechanically durable superhydrophobic surfaces. Adv. Mater. 2011, 23, 673–678. [Google Scholar] [CrossRef]
- Cheng, Y.T.; Rodak, D.E. Is the lotus leaf superhydrophobic? Appl. Phys. Lett. 2005, 86, 1–3. [Google Scholar] [CrossRef]
- Wong, T.S.; Kang, S.H.; Tang, S.K.; Smythe, E.J.; Hatton, B.D.; Grinthal, A.; Aizenberg, J. Bioinspired self-repairing slippery surfaces with pressure-stable omniphobicity. Nature 2011, 477, 443–447. [Google Scholar] [CrossRef]
- Niemelä-Anttonen, H.; Koivuluoto, H.; Tuominen, M.; Teisala, H.; Juuti, P.; Haapanen, J.; Harra, J.; Stenroos, C.; Lahti, J.; Kuusipalo, J.; et al. Icephobicity of slippery liquid infused porous surfaces under multiple freeze—thaw and ice accretion—detachment cycles. Adv. Mater. Interfaces 2018, 5, 1800828. [Google Scholar]
- Kim, P.; Wong, T.S.; Alvarenga, J.; Kreder, M.J.; Adorno-Martinez, W.E.; Aizenberg, J. Liquid-infused nanostructured surfaces with extreme anti-ice and anti-frost performance. ACS Nano 2012, 6, 6569–6577. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Jin, B.; Zhang, Q.; Zhan, X.; Chen, F. Anti-icing performance of super-wetting surfaces from icing-resistance to ice-phobic aspects: Robust hydrophobic or slippery surfaces. J. Alloys Compd. 2018, 765, 721–730. [Google Scholar] [CrossRef]
- Wexler, J.S.; Jacobi, I.; Stone, H.A. Shear-driven failure of liquid-infused surfaces. Phys. Rev. Lett. 2015, 114, 168301. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Alcaraz, M.L.; Rubner, M.F.; Cohen, R.E. Zwitter-wettability and antifogging coatings with frost-resisting capabilities. ACS Nano 2013, 7, 2172–2185. [Google Scholar] [CrossRef]
- Mishchenko, L.; Hatton, B.; Bahadur, V.; Taylor, J.A.; Krupenkin, T.; Aizenberg, J. Design of ice-free nanostructured surfaces based on repulsion of impacting water droplets. ACS Nano 2010, 4, 7699–7707. [Google Scholar] [CrossRef]
- Khedir, K.R.; Kannarpady, G.K.; Ishihara, H.; Woo, J.; Asar, M.P.; Ryerson, C.; Biris, A.S. Temperature-dependent bouncing of super-cooled water on teflon-coated superhydrophobic tungsten nanorods. Appl. Surf. Sci. 2013, 279, 76–84. [Google Scholar] [CrossRef]
- Schutzius, T.M.; Jung, S.; Maitra, T.; Eberle, P.; Antonini, C.; Stamatopoulos, C.; Poulikakos, D. Physics of icing and rational design of surfaces with extraordinary icephobicity. Langmuir 2015, 31, 4807–4821. [Google Scholar] [CrossRef]
- Sarshar, M.A.; Swarctz, C.; Hunter, S.; Simpson, J.; Choi, C.-H. Effects of contact angle hysteresis on ice adhesion and growth on superhydrophobic surfaces under dynamic flow conditions. Colloid Polym. Sci. 2013, 291, 427–435. [Google Scholar] [CrossRef]
- Raimondo, M.; Blosi, M.; Caldarelli, A.; Guarini, G.; Veronesi, F. Wetting behavior and remarkable durability of amphiphobic aluminum alloys surfaces in a wide range of environmental conditions. Chem. Eng. J. 2014, 258, 101–109. [Google Scholar] [CrossRef]
- Kleingartner, J.A.; Srinivasan, S.; Mabry, J.M.; Cohen, R.E.; McKinley, G.H. Utilizing dynamic tensiometry to quantify contact angle hysteresis and wetting state transitions on nonwetting surfaces. Langmuir 2013, 29, 13396–13406. [Google Scholar] [CrossRef]
- Motta, A.; Cannelli, O.; Boccia, A.; Zanoni, R.; Raimondo, M.; Caldarelli, A.; Veronesi, F. A mechanistic explanation of the peculiar amphiphobic properties of hybrid organic-inorganic coatings by combining XPS characterization and DFT modeling. ACS Appl. Mater. Interfaces 2015, 7, 19941–19947. [Google Scholar] [CrossRef] [PubMed]
- Bahadur, V.; Mishchenko, L.; Hatton, B.; Taylor, J.A.; Aizenberg, J.; Krupenkin, T. Predictive model for ice formation on superhydrophobic surfaces. Langmuir 2011, 27, 14143–14150. [Google Scholar] [CrossRef] [PubMed]
- Tsai, P.; Lammertink RG, H.; Wessling, M.; Lohse, D. Evaporation-triggered wetting transition for water droplets upon hydrophobic microstructures. Phys. Rev. Lett. 2010, 104, 2–3. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, M.; Gerasopoulos, K.; Enright, R.; Culver, J.N.; Ghodssi, R.; Wang, E.N. Biotemplated hierarchical surfaces and the role of dual length scales on the repellency of impacting droplets. Appl. Phys. Lett. 2012, 100, 1–6. [Google Scholar] [CrossRef]
- Papadopoulos, P.; Mammen, L.; Deng, X.; Vollmer, D.; Butt, H.J. How superhydrophobicity breaks down. Proc. Natl. Acad. Sci. USA 2013, 110, 3254–3258. [Google Scholar] [CrossRef]
- Bormashenko, E.; Pogreb, R.; Whyman, G.; Erlich, M. Cassie-Wenzel wetting transition in vibrating drops deposited on rough surfaces: Is the dynamic Cassie-Wenzel wetting transition a 2D or 1D affair? Langmuir 2007, 23, 6501–6503. [Google Scholar] [CrossRef]
- Lafuma, A.; Quéré, D. Superhydrophobic states. Nat. Mater. 2003, 2, 457–460. [Google Scholar] [CrossRef]
- Bartolo, D.; Bouamrirene, F.; Verneuil, E.; Buguin, A.; Silberzan, P.; Moulinet, S. Bouncing or sticky droplets: Impalement transitions on superhydrophobic micropatterned surfaces. Europhys. Lett. 2006, 74, 299–305. [Google Scholar] [CrossRef]
- Wilson, P.W.; Lu, W.; Xu, H.; Kim, P.; Kreder, M.J.; Alvarenga, J.; Aizenberg, J. Inhibition of ice nucleation by slippery liquid-infused porous surfaces (SLIPS). Phys. Chem. Chem. Phys. 2013, 15, 581–585. [Google Scholar] [CrossRef]
- Kulinich, S.A.; Farhadi, S.; Nose, K.; Du, X.W. Superhydrophobic surfaces: Are they really ice-repellent? Langmuir 2011, 27, 25–29. [Google Scholar] [CrossRef]
- Karmouch, R.; Ross, G.G. Experimental study on the evolution of contact angles with temperature near the freezing point. J. Phys. Chem. C 2010, 114, 4063–4066. [Google Scholar] [CrossRef]
- Stamatopoulos, C.; Hemrle, J.; Wang, D.; Poulikakos, D. Exceptional anti-icing performance of self-impregnating slippery surfaces. ACS Appl. Mater. Interf. 2017, 9, 10233–10242. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.D.; Dhiman, R.; Anand, S.; Reza-Garduno, E.; Cohen, R.E.; McKinley, G.H.; Varanasi, K.K. Droplet mobility on lubricant-impregnated surfaces. Soft Matter 2013, 9, 1772–1780. [Google Scholar] [CrossRef]
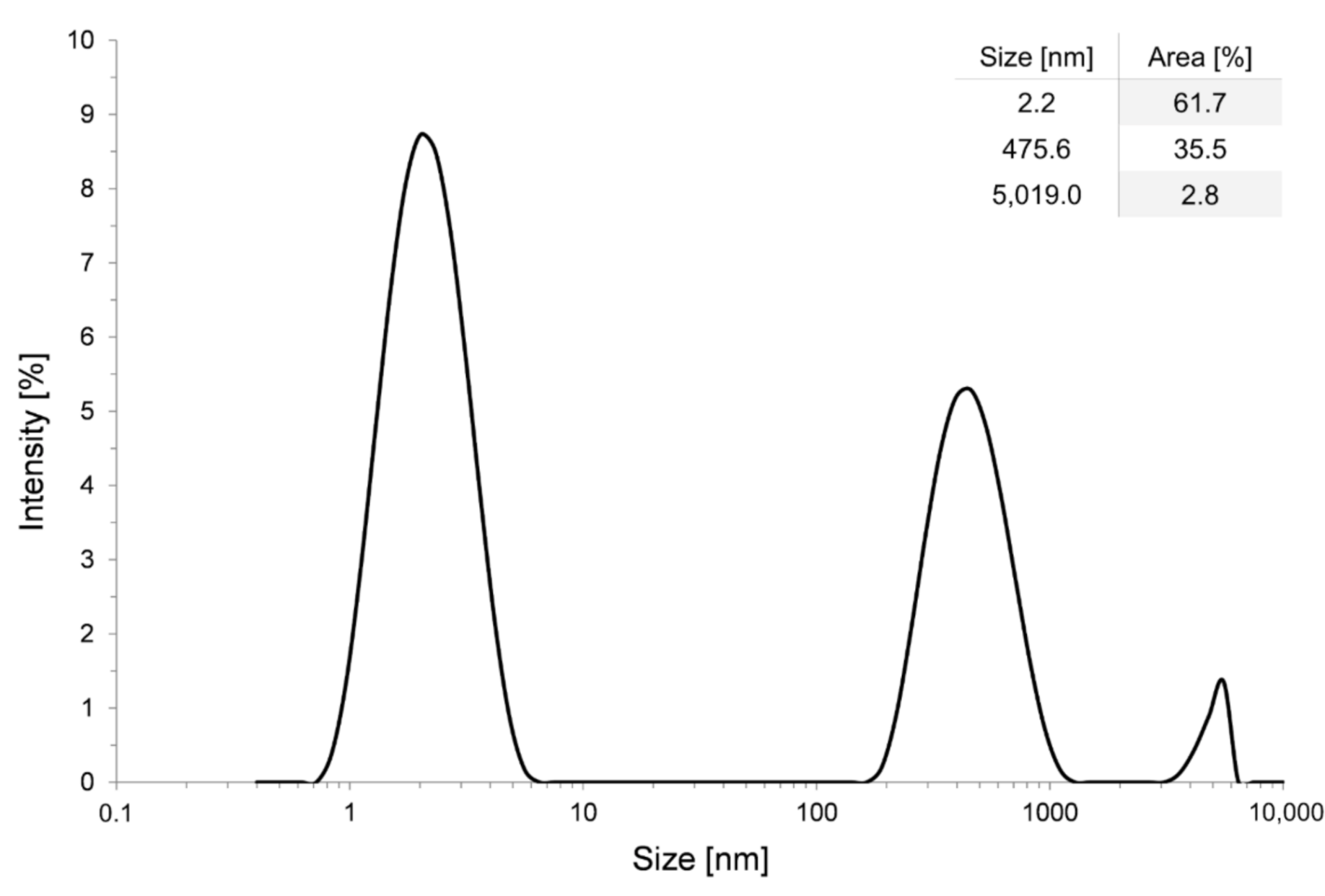
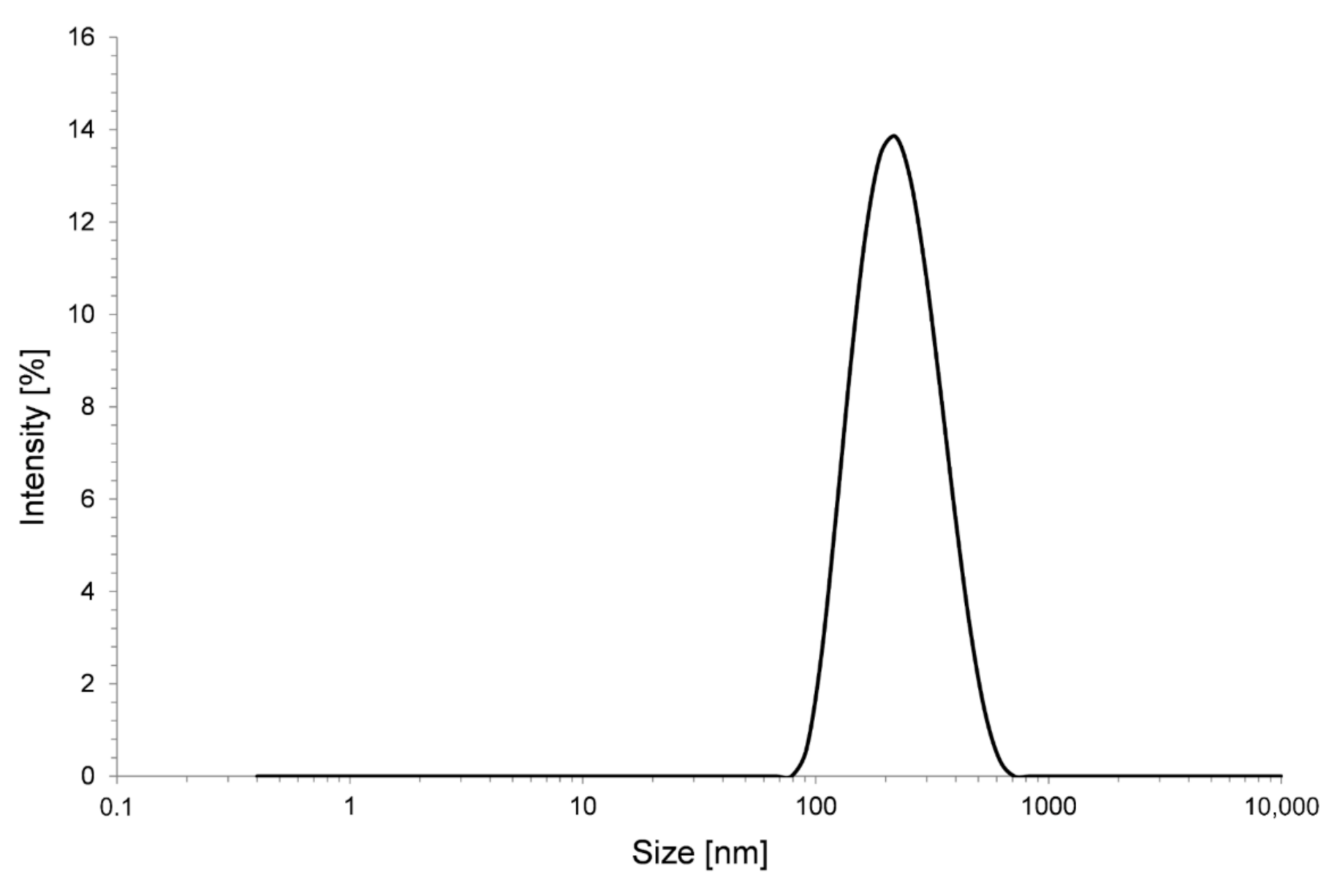




| Sample | Inorganic Layer | Organic Layer | Lubricant |
|---|---|---|---|
| LF | Alumina | FAS | / |
| Al2O3Kr100 | Alumina | FAS | Krytox™ GPL 100 |
| Al2O3Kr103 | Alumina | FAS | Krytox™ GPL 103 |
| Al2O3Kr105 | Alumina | FAS | Krytox™ GPL 105 |
| SiO2F SO10 | Fluorinated Silica | / | Silicone oil 10cSt |
| SiO2FKr100 | Fluorinated Silica | / | Krytox™ GPL 100 |
| SiO2FKr103 | Fluorinated Silica | / | Krytox™ GPL 103 |
| SiO2FKr105 | Fluorinated Silica | / | Krytox™ GPL 105 |
| Sample | WCA (°) ± ⌠ (°) | CAH (°) ± ⌠ (°) |
|---|---|---|
| Uncoated sample (Ref.) | 89 ± 2 | 59 ± 7 |
| LF | 171 ± 2 | 7 ± 2 |
| Al2O3 Kr100 | 121 ± 1 | 2 ± 1 |
| Al2O3 Kr103 | 122 ± 1 | 3 ± 1 |
| Al2O3 Kr105 | 119 ± 2 | 9 ± 1 |
| SiO2F SO10 | 112 ± 2 | 10 ± 2 |
| SiO2F Kr100 | 123 ± 4 | 6 ± 2 |
| SiO2F Kr103 | 123 ± 4 | 6 ± 2 |
| SiO2F Kr105 | 119 ± 5 | 9 ± 1 |
| Lubricant Oil | γ (mN/m) ± ⌠ (mN/m) | μ (cSt) |
|---|---|---|
| Krytox™100 | 16.60 ± 0.04 | 12.4 1 |
| Krytox™103 | 17.41 ± 0.01 | 82 1 |
| Krytox™105 | 18.35 ± 0.08 | 522 1 |
| Silicone Oil 10cst | 20.01 ± 0.30 | 9–11 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boveri, G.; Corozzi, A.; Veronesi, F.; Raimondo, M. Different Approaches to Low-Wettable Materials for Freezing Environments: Design, Performance and Durability. Coatings 2021, 11, 77. https://doi.org/10.3390/coatings11010077
Boveri G, Corozzi A, Veronesi F, Raimondo M. Different Approaches to Low-Wettable Materials for Freezing Environments: Design, Performance and Durability. Coatings. 2021; 11(1):77. https://doi.org/10.3390/coatings11010077
Chicago/Turabian StyleBoveri, Giulio, Alessandro Corozzi, Federico Veronesi, and Mariarosa Raimondo. 2021. "Different Approaches to Low-Wettable Materials for Freezing Environments: Design, Performance and Durability" Coatings 11, no. 1: 77. https://doi.org/10.3390/coatings11010077
APA StyleBoveri, G., Corozzi, A., Veronesi, F., & Raimondo, M. (2021). Different Approaches to Low-Wettable Materials for Freezing Environments: Design, Performance and Durability. Coatings, 11(1), 77. https://doi.org/10.3390/coatings11010077