Surface Modification of Aluminum 6061-O Alloy by Plasma Electrolytic Oxidation to Improve Corrosion Resistance Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Coatings Preparation and Process Parameters
2.3. Coatings Characterization Methods
3. Results and Discussion
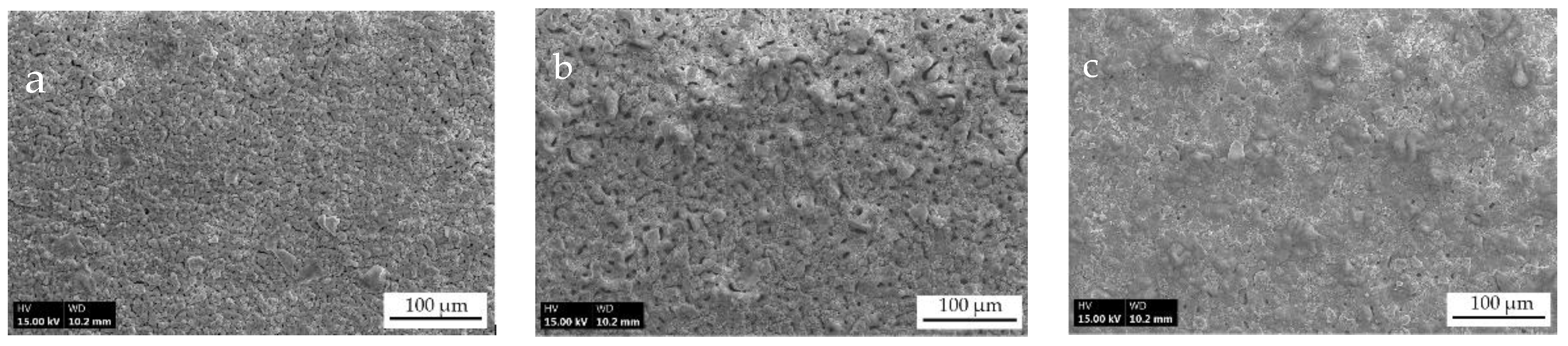
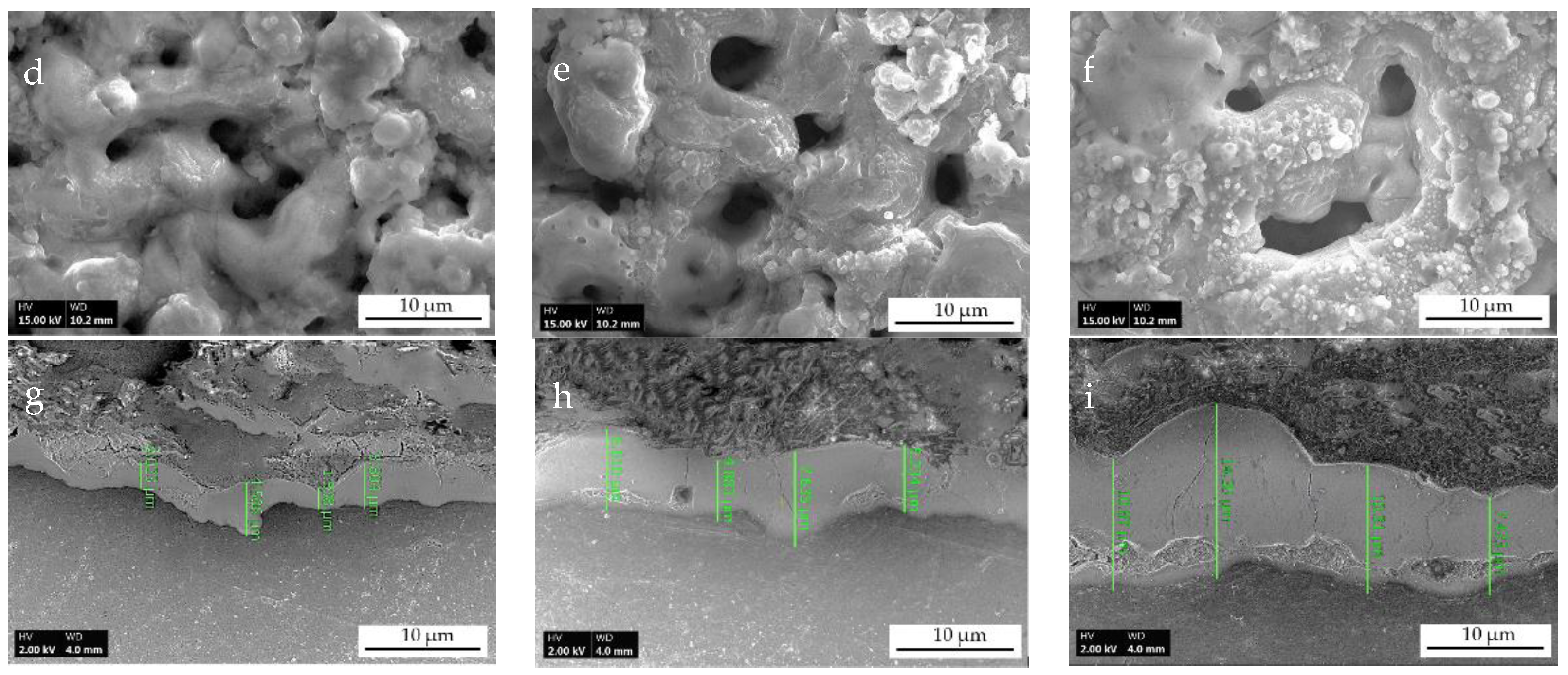
3.1. Visual Surface Appearance and Microstructure Characteristic Features
3.2. Electrochemical Properties
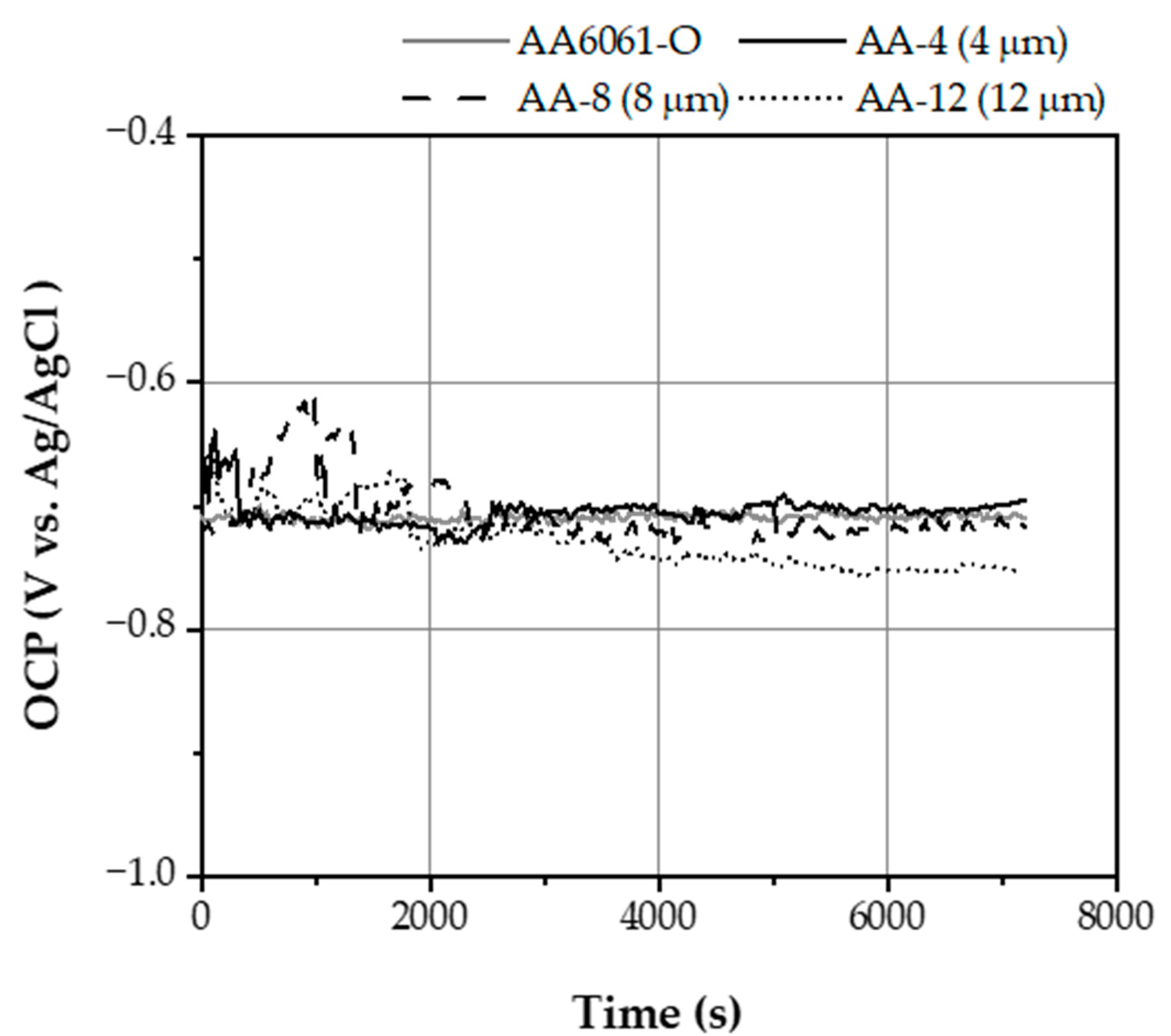
3.2.1. Open Circuit Potential Behavior
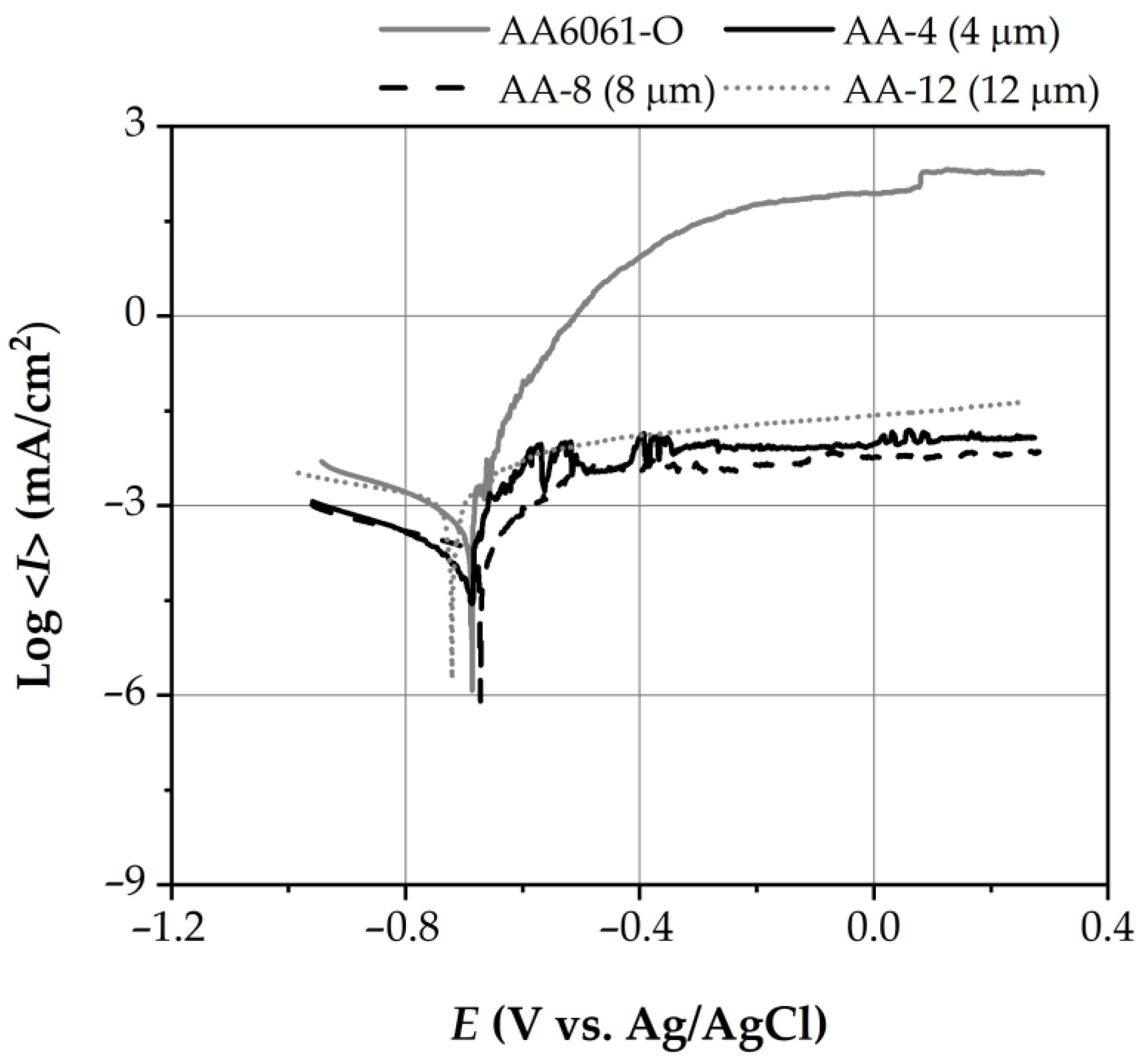
3.2.2. Potentiodynamic Polarization Behavior
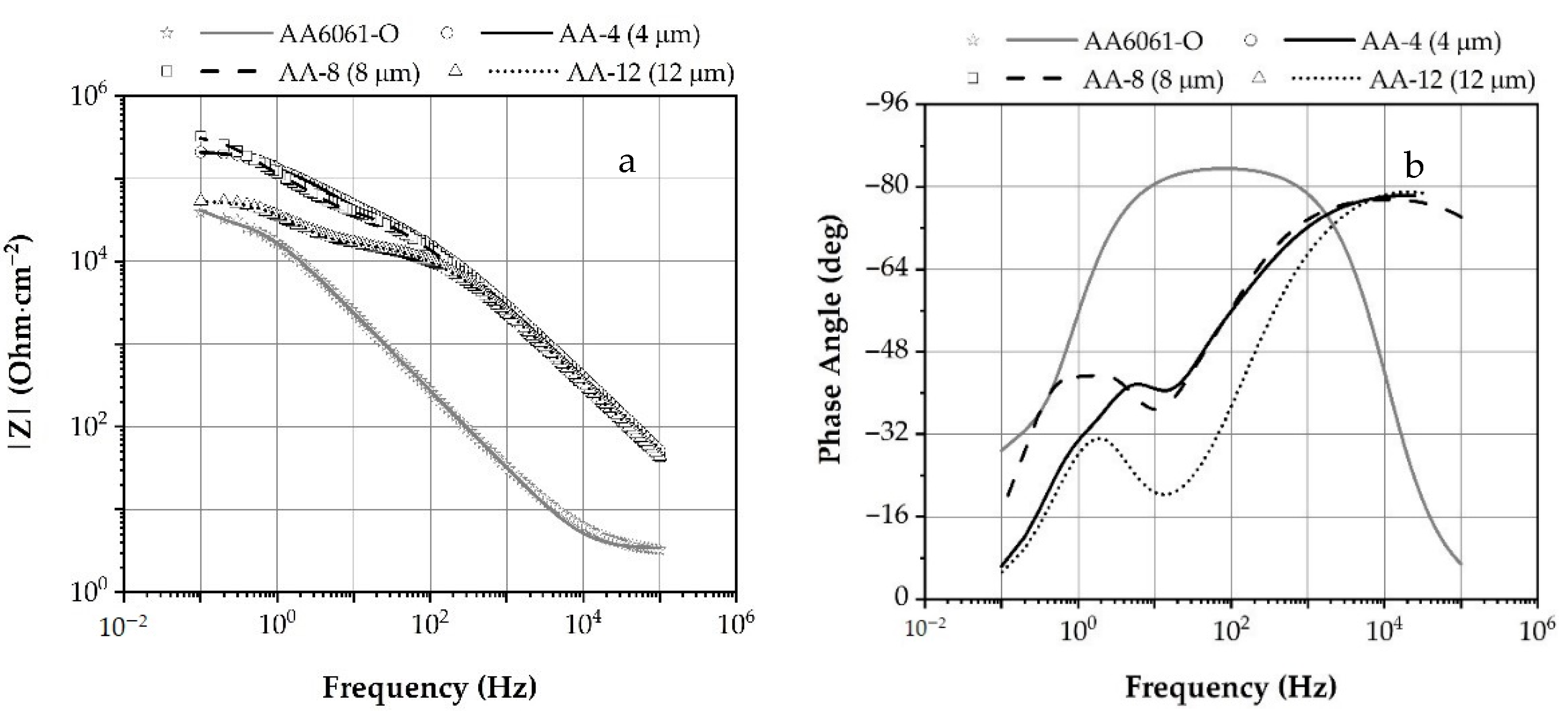
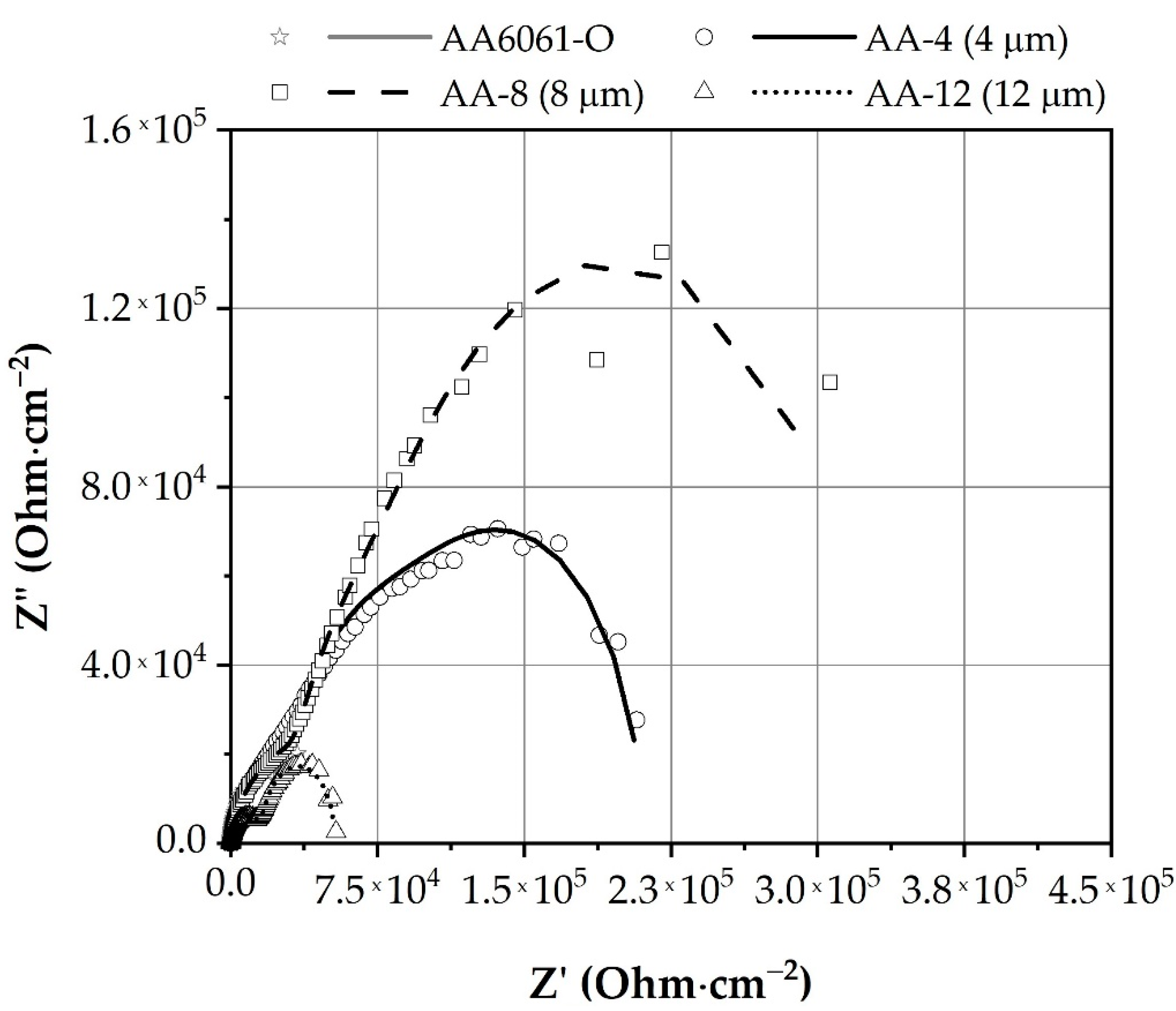
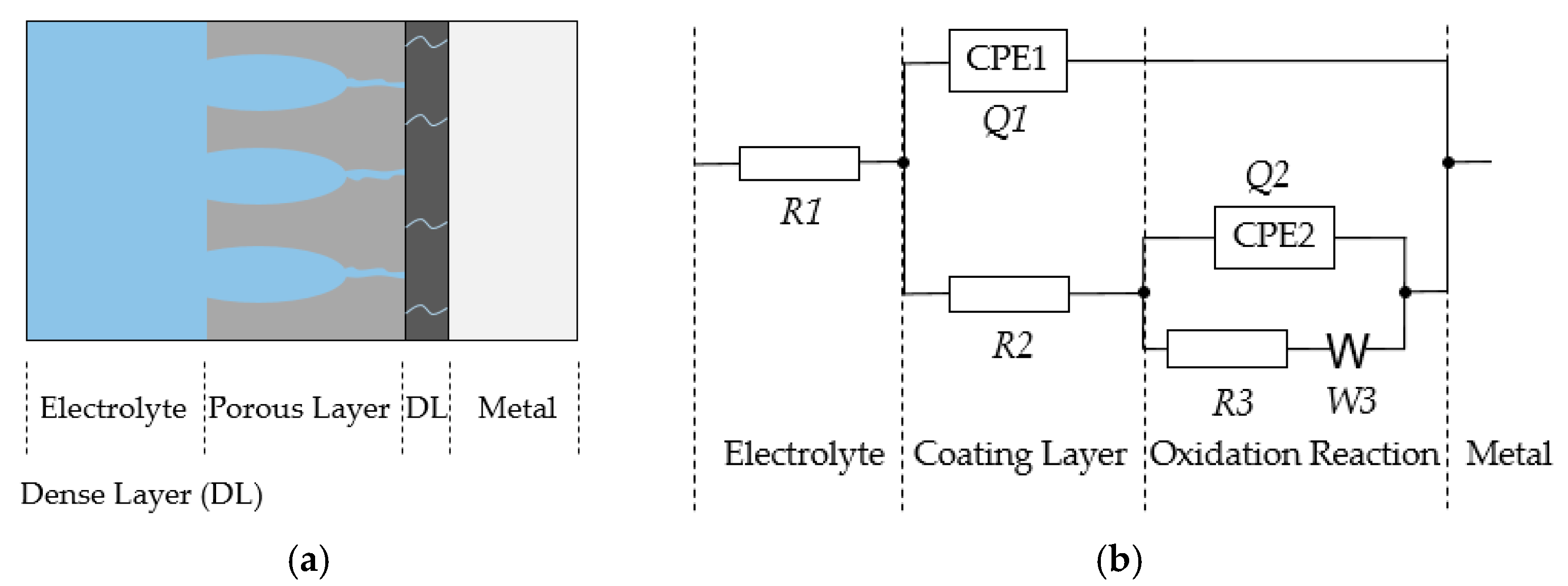
3.2.3. Electrochemical Impedance Spectroscopy
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- ASM Handbook Properties and Selection: Nonferrous Alloys and Special-purpose Materials. ASM International Committee Handbook, 2nd ed.; ASM International: Materials Park, OH, USA, 1990. [Google Scholar]
- Buchheit, R.G.; Grant, R.P.; Hlava, P.F.; McKenzie, B.; Zender, G.L. Local dissolution phenomena associated with s-phase (Al2CuMg) particles in aluminum alloy. J. Electrochem. Soc. 1997, 144, 2621–2628. [Google Scholar] [CrossRef]
- Guillaumin, V.; Mankowski, G. Localized corrosion of AA 2024 T351 aluminum alloy in chloride media. Corros. Sci. 1998, 41, 421–438. [Google Scholar] [CrossRef]
- Blanc, C.; Gastaud, S.; Mankowski, G. Mechanistic studies of the corrosion of 2024 aluminum alloy in nitrate solutions. J. Electrochem. Soc. 2003, 150, 396–404. [Google Scholar] [CrossRef]
- Ilevbare, G.O.; Schneider, O.; Kelly, R.G.; Scully, J.R. In Situ confocal laser scanning microscopy of AA 2024-T3 corrosion metrology I: Localized corrosion of particles. J. Electrochem. Soc. 2004, 151, 453–464. [Google Scholar] [CrossRef]
- Buchheit, R.G.; Montes, L.P.; Martinez, M.A.; Hlava, P.F. The electrochemical characteristics of bulk-synthesized materials. J. Electrochem. Soc. 1999, 146, 4424–4428. [Google Scholar] [CrossRef]
- Andreatta, F.; Terryn, H.; Wit, J.H.W. Corrosion behaviour of different tempers of aluminium alloy. Electrochim. Acta 2004, 49, 2851–2862. [Google Scholar] [CrossRef]
- Cerchier, P.; Pezzato, L.; Gennari, C.; Moschin, E.; Moro, I.; Dabala, M. PEO coating containing copper: A promising anticorrosive and antifouling coating for seawater application of AA 7075. Surf. Coat. Technol. 2020, 393, 125774. [Google Scholar] [CrossRef]
- Csokan, P.; Sc, C.C. Hard anodizing: Studies of the relation between anodizing conditions and the growth and properties of hard anodic oxide coatings. Electroplat. Met. Finish. 1962, 15, 75–82. [Google Scholar]
- Dzhurinskiy, D.; Maeva, E.; Leshchinsky, E. Corrosion protection of light alloys using low pressure cold spray. J. Term. Spray Technol. 2012, 21, 304–313. [Google Scholar] [CrossRef]
- Zhang, P.; Nie, X.; Hu, H. Wear protection of Al383/SiO2 metal matrix composites by plasma electrolytic oxidation (PEO) process. SAE Int. J. Mater. Manuf. 2010, 3, 55–62. [Google Scholar] [CrossRef]
- MAGPULS Bipolar-Pulsed Power Supplies. Available online: https://magpuls.de/en/puls-plasma-generators-unipolar.html (accessed on 1 November 2020).
- Richelly, L.N.; Ferreira, M.; Clodomiro, A. Plasma species and coating compositions in aluminum treated by PEO using shot square pulse. Mater. Res. 2020, 23. [Google Scholar] [CrossRef]
- Clyne, T.W.; Troughton, S.C. A review of recent work on discharge characteristics during plasma electrolytic oxidation of various metals. Int. Mater. Rev. 2019, 64, 127–162. [Google Scholar] [CrossRef] [Green Version]
- Pezzato, L.; Cerchier, P.; Brunelli, K.; Bartolozzi, A.; Bertani, R.; Dabala, M. Electrolytic oxidation coatings with fungicidal properties. Surf. Eng. 2018, 35, 325–333. [Google Scholar] [CrossRef]
- Wang, K.; Koo, B.-H.; Lee, C.-G.; Kim, Y.-J.; Lee, S.-H.; Byon, E. Effects of electrolytes variation on formation of oxide layers of 6061 Al alloys by plasma electrolytic oxidation. Trans. Nonferrous Met. Soc. China 2009, 19, 866–870. [Google Scholar] [CrossRef]
- Yerokhin, A.; Nie, X.; Leyland, A.; Matthews, A.; Dowey, S. Plasma electrolysis for surface engineering. Surf. Coat. Technol. 1999, 122, 73–93. [Google Scholar] [CrossRef]
- Gnedenkov, S.; Khrisanfova, O.; Zavidnaya, A.; Sinebrukhov, S.; Kovryanov, A.; Scorobogatova, T.; Gordienko, P. Production of hard and heat-resistant coatings on aluminium using a plasma micro-discharge. Surf. Coat. Technol. 2000, 123, 24–28. [Google Scholar] [CrossRef]
- Dehnavi, V.; Luan, B.L.; Liu, X.Y.; Shoesmith, D.W.; Rohani, S. Correlation between plasma electrolytic oxidation treatment stages and coating microstructure on aluminum. Surf. Coat. Technol. 2015, 269, 91–99. [Google Scholar] [CrossRef]
- Gulden, F.; Reinhold, B.; Gramstat, S.; Stich, A.; Tetzlaff, U.; Höppel, H.W. Investigation of the run-in and corrosion behavior of a PEO-coated aluminum brake disc. In Proceedings of the 10th International Munich Chassis Symposium 2019, Wiesbaden, Germany, 2 November 2019. [Google Scholar]
- Patel, V.K.; Bhowmik, S. Plasma processing of aluminum alloys to promote adhesion: A critical review. Rev. Adhes. Adhes. 2017, 5, 79–104. [Google Scholar] [CrossRef]
- Curran, J.A.; Clyne, T.W. Thermo-physical properties of plasma electrolytic oxide coatings on aluminium. Surf. Coat. Technol. 2005, 199, 168–176. [Google Scholar] [CrossRef]
- Dahm, K.; Black, A.; Shrestha, S. Dearnley, plasma electrolytic oxidation treatment of aluminium alloys for lightweight disc brake rotors. In Proceedings of the IMechE Conference on Braking, New York, NY, USA, 9–10 June 2009; pp. 53–60. [Google Scholar]
- ASTM E1588-20, Standard Practice for Gunshot Residue Analysis by Scanning Electron Microscopy/Energy Dispersive X-ray Spectrometry; ASTM International: West Conshohocken, PA, USA, 2020.
- Chiesa, R.; Sandrini, E.; Santin, M.; Rondelli, G.; Cigada, A. Osseointegration of titanium and its alloys by anodic spark deposition and other electrochemical techniques: A review. J. Appl. Biomater. 2003, 1, 91–107. [Google Scholar]
- Dicu, M.; Abrudeanu, M.; Millet, J.; Moga, S.; Rizea, V.; Ducu, C. The influence of electrochemistry parameters on some physico-chemical properties of microarc oxidation biocompatible coatings on titanium. Sci. Bull. 2012, 74, 193–202. [Google Scholar]
- Ye, Z.; Liu, D.; Zhang, X.; Wu, Z.; Long, F. Influence of combined shot peening and PEO treatment on corrosion fatigue behavior of 7A85 aluminum alloy. Appl. Surf. Sci. 2019, 486, 72–79. [Google Scholar] [CrossRef]
- DeForce, B.; Eden, T.; Potter, J.; Champagne, V.; Leyman, P.; Helfritch, D. Application of aluminum coatings for the corrosion, protection of magnesium by cold spray. In Proceedings of theTri-Service Corrosion Conference, Denver, DC, USA, 17–21 November 2007. [Google Scholar]
- Curioni, M.; Scenini, F. The mechanism of hydrogen evolution during anodic polarization of aluminium. Electrochim. Acta 2015, 180, 712–721. [Google Scholar] [CrossRef]
- Kaseem, M.; Yang, H.W.; Ko, Y.G. Toward a nearly defect-free coating via high-energy plasma sparks. Sci. Rep. 2017, 7, 2378. [Google Scholar] [CrossRef] [PubMed]
- Ryu, H.S.; Mun, S.-J.; Lim, T.S.; Kim, H.-C.; Shin, K.-S.; Hong, S.-H.; Tanasini, P.; Diethelm, S.; Van Herle, J.; Favrat, D.; et al. Microstructure evolution during plasma electrolytic oxidation and its effects on the electrochemical properties of AZ91D Mg alloy. J. Electrochem. Soc. 2011, 158, 266–273. [Google Scholar] [CrossRef]
- Macdonald, D. Reflections on the history of electrochemical impedance spectroscopy. Electrochim. Acta 2006, 51, 1376–1388. [Google Scholar] [CrossRef]
| Al | Si | Fe | Cu | Mn | Mg | Cr | Zn | Ti |
|---|---|---|---|---|---|---|---|---|
| balance | 0.40–0.80 | Max 0.70 | 0.15–0.40 | 0.15 | 0.80–1.20 | 0.04–0.35 | 0.25 | 0.15 |
| Specimen Designation | ton (µs) | toff (µs) | Treatment Time (min) | Actual Current * (A) | Voltage Reached (V) |
|---|---|---|---|---|---|
| AA-4 | 400 | 200 | 15 | 1.5 | 435 |
| AA-8 | 400 | 200 | 30 | 1.5 | 450 |
| AA-12 | 400 | 200 | 45 | 1.5 | 470 |
| Specimen Designation | Eb (V) | Ecorr (V) | Icorr (µA/cm2) | OCP (V) | Roughness, Ra (µm) | Coating Thickness * (µm) |
|---|---|---|---|---|---|---|
| AA6061-O | −0.66 | −0.67 | 0.23 | −0.70 | 0.44 | N/A |
| AA-4 | −0.64 | −0.68 | 0.06 | −0.70 | 0.86 | 4 ± 1 |
| AA-8 | −0.62 | −0.68 | 0.29 | −0.71 | 1.08 | 8 ± 1 |
| AA-12 | −0.65 | −0.72 | 0.44 | −0.75 | 1.09 | 12 ± 1 |
| Sample Designation | R1 (Ω·cm2) | CPE1 | R2 (Ω·cm2) | CPE2 | R3 (Ω·cm2) | W3 (Ω·s−1/2·cm−2) | Rtotal (Ω·cm2) | ||
|---|---|---|---|---|---|---|---|---|---|
| Q1 (F·sa1·cm−2) | a1 | Q2 (F·sa2·cm−2) | a2 | ||||||
| AA-4 | 2.48 | 1.43 × 10−7 | 0.88 | 21,059 | 1.91 × 10−6 | 0.57 | 274,766 | −24,656 | 295,825 |
| AA-8 | 1.56 | 2.18 × 10−7 | 0.86 | 34,245 | 2.19 × 10−6 | 0.71 | 425,000 | −32,954 | 459,245 |
| AA-12 | 1.25 | 2.05 × 10−7 | 086 | 14,410 | 7.36 × 10−6 | 0.77 | 51,568 | −5993 | 65,996 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dzhurinskiy, D.V.; Dautov, S.S.; Shornikov, P.G.; Akhatov, I.S. Surface Modification of Aluminum 6061-O Alloy by Plasma Electrolytic Oxidation to Improve Corrosion Resistance Properties. Coatings 2021, 11, 4. https://doi.org/10.3390/coatings11010004
Dzhurinskiy DV, Dautov SS, Shornikov PG, Akhatov IS. Surface Modification of Aluminum 6061-O Alloy by Plasma Electrolytic Oxidation to Improve Corrosion Resistance Properties. Coatings. 2021; 11(1):4. https://doi.org/10.3390/coatings11010004
Chicago/Turabian StyleDzhurinskiy, Dmitry V., Stanislav S. Dautov, Petr G. Shornikov, and Iskander Sh. Akhatov. 2021. "Surface Modification of Aluminum 6061-O Alloy by Plasma Electrolytic Oxidation to Improve Corrosion Resistance Properties" Coatings 11, no. 1: 4. https://doi.org/10.3390/coatings11010004
APA StyleDzhurinskiy, D. V., Dautov, S. S., Shornikov, P. G., & Akhatov, I. S. (2021). Surface Modification of Aluminum 6061-O Alloy by Plasma Electrolytic Oxidation to Improve Corrosion Resistance Properties. Coatings, 11(1), 4. https://doi.org/10.3390/coatings11010004






