Preparation and Characterization of Some Sol-Gel Modified Silica Coatings Deposited on Polyvinyl Chloride (PVC) Substrates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Sol-Gel Silica Materials and of Coatings
2.3. Characterization Techniques and Instrumentation
2.3.1. Photo-Polymerization
2.3.2. Fourier Transforms Infrared Spectroscopy (FTIR)
2.3.3. Thermogravimetric Analysis (TGA)
2.3.4. Dynamic Mechanical Analysis (DMA)
2.3.5. Atomic Force Microscopy (AFM)
2.3.6. Spectroscopic Ellipsometry Measurements
2.3.7. UV–Vis Spectroscopy
2.3.8. Contact Angle (CA) Measurements
3. Results and Discussion
3.1. FTIR Analysis
3.2. TGA Analysis
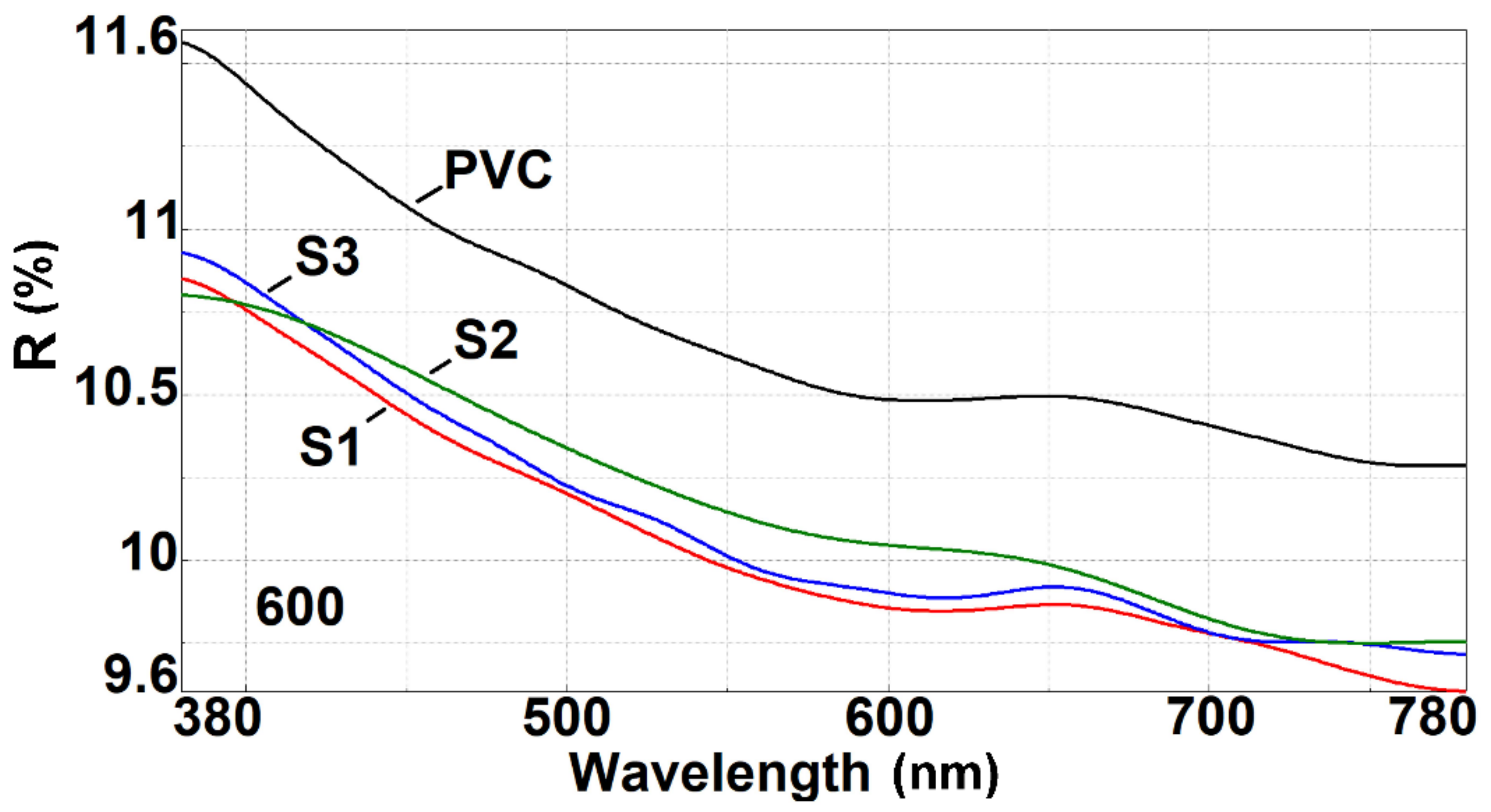
3.3. UV–Vis Spectroscopy
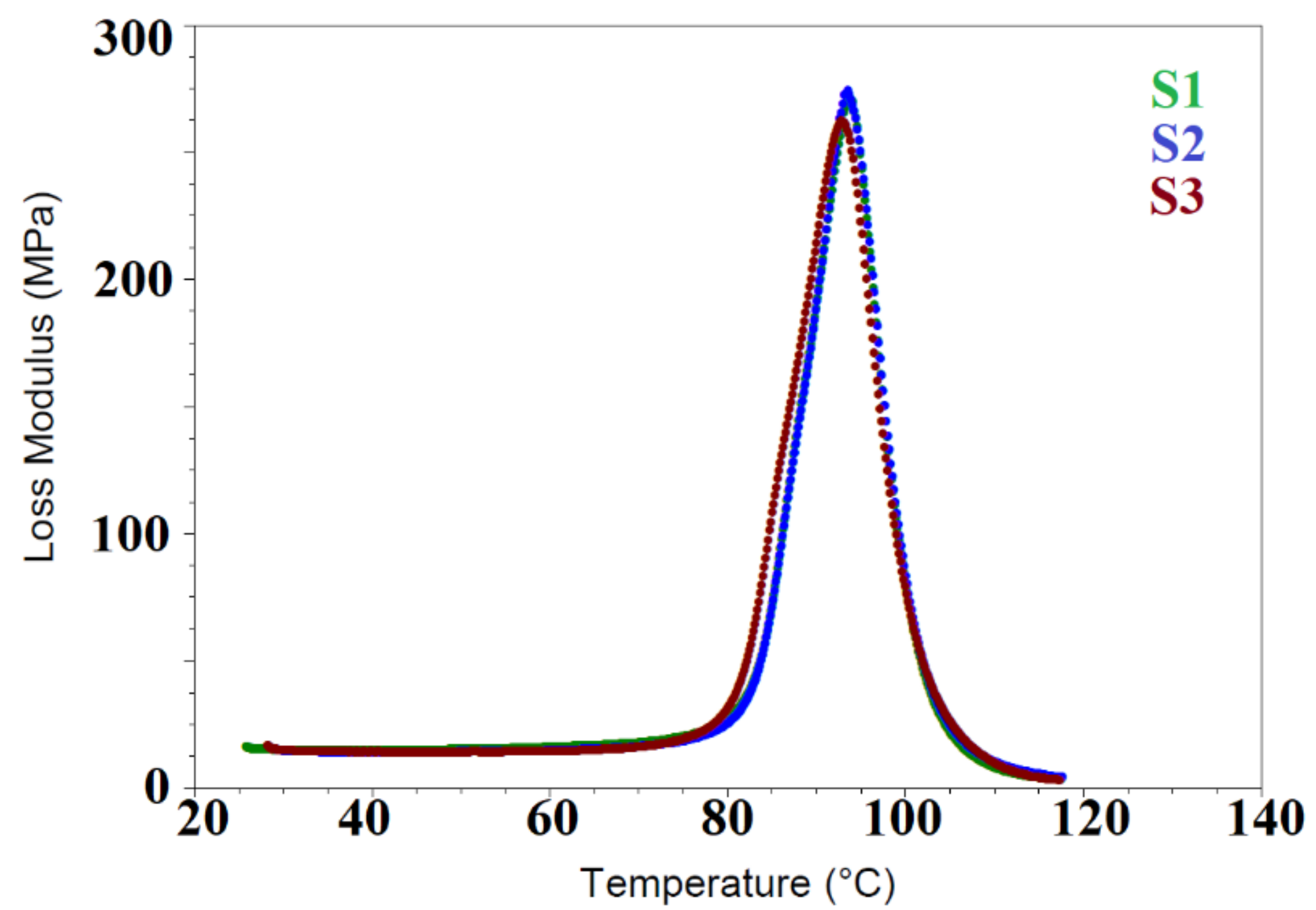
3.4. Dynamic Mechanical Analysis (DMA)
3.5. Atomic Force Microscopy (AFM)
3.6. Contact Angle Measurements
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Matharu, A.S.; Lokesh, K. Green chemistry principles and global drivers for sustainability—An introduction. In Green Chemistry for Surface Coatings, Inks and Adhesives: Sustainable Applications; Höfer, R., Matharu, A.S., Zhang, Z., Eds.; The Royal Society of Chemistry: London, UK, 2019; Volume 1, pp. 1–17. [Google Scholar] [CrossRef]
- Juškevičius, K.; Buzelis, R.; Abromavičius, G.; Samuilovas, R.; Abbas, S.; Belosludtsev, A.; Drazdys, R.; Kičas, S. Argon plasma etching of fused silica substrates for manufacturing high laser damage resistance optical interference coatings. Opt. Mater. Express 2017, 7, 3598–3607. [Google Scholar] [CrossRef]
- Widati, A.A.; Nuryono, N.; Aryanti, D.P.; Wibowo, M.A.; Kunarti, E.S.; Kartini, I.; Rusdiarso, B. Preparation of Water Repellent Layer on Glass Using Hydrophobic Compound Modified Rice Hull Ash Silica. Indones. J. Chem. 2018, 18, 587–593. [Google Scholar] [CrossRef]
- Soto-Nieto, F.; Farías, R.; Reyes-López, S.Y. Sol-gel and Electrospinning synthesis of silica–hydroxyapatite–silver nanofibers for SEIRAS and SERS. Coatings 2020, 10, 910. [Google Scholar] [CrossRef]
- Gómez, J.M.; Carvajal, J.J.; Bilousov, O.; Díaz, F.; Aguiló, M. Investigation of antireflective and hydrophobic properties in polycrystalline GaN films with dual porosity produced by CVD. Sci. Rep. 2019, 9, 11686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Picolo, N.; Moraes, V.T.D.; Lebrão, G.W.; Lebrão, S.M.G. Sol-Gel processed superhydrophobic plastic surfaces modified with perfluorooctyltriethoxysilane (POTS). Mater. Res. 2019, 22, e20190488. [Google Scholar] [CrossRef]
- Sriboonruang, A.; Kumpika, T.; Sroila, W.; Kantarak, E.; Singjai, P.; Thongsuwan, W. Superhydrophobicity/superhydrophilicity transformation of transparent PS-PMMA-SiO2 nanocomposite films. Ukr. J. Phys. 2018, 63, 226–231. [Google Scholar] [CrossRef]
- Chang, C.C.; Lin, Z.M.; Huang, S.H.; Cheng, L.P. Preparation of highly transparent 13F-modified nano-silica/polymer hydrophobic hard coatings on plastic substrates. J. Appl. Sci. Eng. 2015, 18, 387–394. [Google Scholar] [CrossRef]
- Poirié, T.; Schmitt, T.; Bousser, E.; Vernhes, R.; Martinu, L.; Klemberg-Sapieha, J.E. Hybrid organic/inorganic nanolaminate structures with enhanced tribo-mechanical properties for optical applications. Surf. Coat. Technol. 2017, 315, 399–407. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, L.; Zeng, Z.; Wang, G.; Liu, G.; Zhao, W.; Ren, T.; Xue, Q. Facile fabrication of antifogging, antireflective, and self-cleaning transparent silica thin coatings. Colloids Surf. A Physicochem. Eng. Asp. 2016, 509, 149–157. [Google Scholar] [CrossRef]
- Lin, W.; Sun, Y.; Zheng, J.; Zheng, Y.; Yan, L.; Jiang, B.; Yang, W.; Chen, H.; Zhang, X. Surface modification of Sol-gel silica antireflective coatings by F-PMHS: A simple method for improvement of amphiphobicity. Coatings 2018, 8, 57. [Google Scholar] [CrossRef] [Green Version]
- Gao, L.; He, J. A facile dip-coating approach based on three silica sols to fabrication of broadband antireflective superhydrophobic coatings. J. Colloid Interface Sci. 2013, 400, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Ellis-Terrell, C.; Wei, R.; McKnight, R.; Huang, X.; Lin, K. Thermal stability of superhydrophobic and oleophobic silica nanoparticle spray coating. Mater. Today Commun. 2020, 25, 101370. [Google Scholar] [CrossRef]
- Tasleem, S.; Sabah, A.; Cheema, U.A.; Sabir, A. Transparent hydrophobic hybrid silica films by green and chemical surfactants. ACS Omega 2019, 4, 13543–13552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eshaghi, A. Transparent hard self-cleaning nano-hybrid coating on polymeric substrate. Prog. Org. Coat. 2019, 128, 120–126. [Google Scholar] [CrossRef]
- Maghrebi, O.A. Sol-Gel fabrication of polyimide-silica hybrid film and its characterization through TGA and FTIR. Medbiotech J. 2020, 4, 27–29. [Google Scholar] [CrossRef]
- Wang, S.-D.; Luo, S.-S. Fabrication of transparent superhydrophobic silica-based film on a glass substrate. Appl. Surf. Sci. 2015, 258, 5443–5450. [Google Scholar] [CrossRef]
- Islam, S.; Bidin, N.; Haider, Z.; Riaz, S.; Naseem, S.; Saeed, M.A.; Marsin, F.M. Sol-gel-based single and multilayer nanoparticle thin films on low-temperature substrate poly-methyl methacrylate for optical applications. J. Sol. Gel Sci. Technol. 2016, 77, 396–403. [Google Scholar] [CrossRef]
- Ortelli, S.; Costa, A.L. Insulating thermal and water-resistant hybrid coating for fabrics. Coatings 2020, 10, 72. [Google Scholar] [CrossRef] [Green Version]
- Fasce, L.A.; Seltzer, R.; Frontini, P.M. Depth sensing indentation of organic–inorganic hybrid coatings deposited onto a polymeric substrate. Surf. Coat. Technol. 2012, 210, 62–70. [Google Scholar] [CrossRef] [Green Version]
- Al-Bataineh, Q.M.; Alssad, A.M.; Ahmad, A.A.; Telfah, A. A novel optical model of the experimental transmission spectra of nanocomposite PVC-PS hybrid thin films doped with silica nanoparticles. Heliyon 2020, 6, e04177. [Google Scholar] [CrossRef]
- Abdel-Baset, T.; Elzayat, M.; Mahrous, S. Characterization and optical and dielectric properties of polyvinyl chloride/silica nanocomposites films. Int. J. Polym. Sci. 2016, 1, 1707018. [Google Scholar] [CrossRef] [Green Version]
- Sutar, R.S.; Kalel, P.J.; Latthe, S.S.; Kumbhar, D.A.; Mahajan, S.S.; Chikode, P.P.; Patil, S.S.; Kadam, S.S.; Gaikwad, V.H.; Bhosale, A.K.; et al. Superhydrophobic PVC/SiO2 coating for self-cleaning application. Macromol. Symp. 2020, 393, 2000034–2000039. [Google Scholar] [CrossRef]
- Purcar, V.; Stamatin, I.; Cinteza, O.; Petcu, C.; Raditoiu, V.; Ghiurea, M.; Miclaus, T.; Andronie, A. Fabrication of hydrophobic and antireflective coatings based on hybrid silica films by sol-gel process. Surf. Coat. Technol. 2012, 206, 4449–4454. [Google Scholar] [CrossRef]
- Ul-Hamid, A.; Soufi, K.Y.; Al-Hadhrami, L.M.; Shemsi, A.M. Failure investigation of an underground low voltage XLPE insulated cable. Anti Corros. Method Mater. 2015, 62, 281–287. [Google Scholar] [CrossRef]
- Jung, M.R.; Horgen, F.D.; Orski, S.V.; Rodriguez, V.C.; Beers, K.L.; Balazs, G.H.; Jones, T.T.; Work, T.M.; Brignac, K.C.; Royer, S.-J.; et al. Validation of ATR FT-IR to identify polymers of plastic marine debris, including those ingested by marine organisms. Mar. Pollut. Bull. 2018, 127, 707–716. [Google Scholar] [CrossRef]
- Rădiţoiu, V.; Purcar, V.; Rădiţoiu, A.; Raduly, M.F.; Frone, A.N.; Anastasescu, M.; Stoica, M.; Alexandrescu, E.; Şomoghi, R.; Manea, R.; et al. Sol-gel hybrid films based on organosilanes with long alkyl chains. J. Coat. Technol. Res. 2020, 17, 1389–1399. [Google Scholar] [CrossRef]
- Prado, L.A.D.A.; Radovanovic, E.; Pastore, H.O.; Yoshida, I.V.P.; Torriani, I.L. Poly (phenylsilsesquioxane)s: Structural and morphological characterization. J. Polym. Sci. A Polym. Chem. 2000, 38, 1580–1958. [Google Scholar] [CrossRef]
- Capeletti, B.L.; Zimnoch, J.H. Fourier Transform Infrared and Raman Characterization of Silica-Based Materials. Mat. Sci. 2016. [Google Scholar] [CrossRef] [Green Version]
- Sriramulu, D.; Reed, E.L.; Annamalai, M.; Venkatesan, T.V.; Valiyaveettil, S. Synthesis and characterization of superhydrophobic, self-cleaning NIR-reflective silica nanoparticles. Sci. Rep. 2016, 6, 35993. [Google Scholar] [CrossRef] [Green Version]
- Lu, Z.; Xu, L.; He, Y.; Zhou, J. One-step facile route to fabricate functionalized nano-silica and silicone sealant based transparent superhydrophobic coating. Thin Solid Film. 2019, 692, 137560. [Google Scholar] [CrossRef]
- Zhang, X.P.; Lan, P.J.; Lu, Y.H.; Li, J.; Xu, H.; Zhang, J.; Lee, Y.; Rhee, J.Y.; Choy, K.L.; Song, W.J. Multifunctional antireflection coatings based on novel hollow silica-silica nanocomposites. ACS Appl. Mater. Interfaces 2014, 6, 1415–1423. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhao, H.; Su, Y.; Zhang, C.; Feng, C.; Liu, Q.; Shen, J. Low-Temperature Preparation of Mechanically Robust and Contamination-Resistant Antireflective Coatings for Flexible Polymeric Glasses via Embedding of Silica Nanoparticles and HMDS Modification. ACS Appl. Mater. Interfaces 2019, 11, 37084–37093. [Google Scholar] [CrossRef] [PubMed]
- Alam, K.; Saher, S.; Ali, S.; Mujtaba, A.; Qamar, A. Super hydrophilic nano particulate coating for solar PV module. In Proceedings of the 16th International Bhurban Conference on Applied Sciences and Technology (IBCAST-2019), Islamabad, Pakistan, 8–12 January 2019. [Google Scholar] [CrossRef]
- Purcar, V.; Rădiţoiu, V.; Dumitru, A.; Nicolae, C.A.; Frone, A.N.; Anastasescu, M.; Rădiţoiu, A.; Raduly, M.F.; Gabor, R.A.; Căprărescu, S. Antireflective coating based on TiO2 nanoparticles modified with coupling agents via acid-catalyzed sol-gel method. Appl. Surf. Sci. 2019, 487, 819–824. [Google Scholar] [CrossRef]
- Da Silva, D.G.; Costa, V.C.; Nunes, R.A.X. Preparation of antireflective silica coating by the sol-gel method for heliothermic power plants. Mat. Res. 2018, 21, e20170970. [Google Scholar] [CrossRef] [Green Version]
- Zou, X.; Tao, C.; Yang, K.; Yang, F.; Lv, H.; Yan, L.; Yan, H.; Li, Y.; Xie, Y.; Yuan, X.; et al. Rational design and fabrication of highly transparent, flexible, and thermally stable superhydrophobic coatings from raspberry-like hollow silica nanoparticles. Appl. Surf. Sci. 2018, 440, 700–711. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, Z.; Li, Y.; Xu, C. Highly transparent and durable superhydrophobic hybrid nanoporous coatings fabricated from polysiloxane. ACS Appl. Mater. Interfaces 2014, 6, 10014–10021. [Google Scholar] [CrossRef] [PubMed]


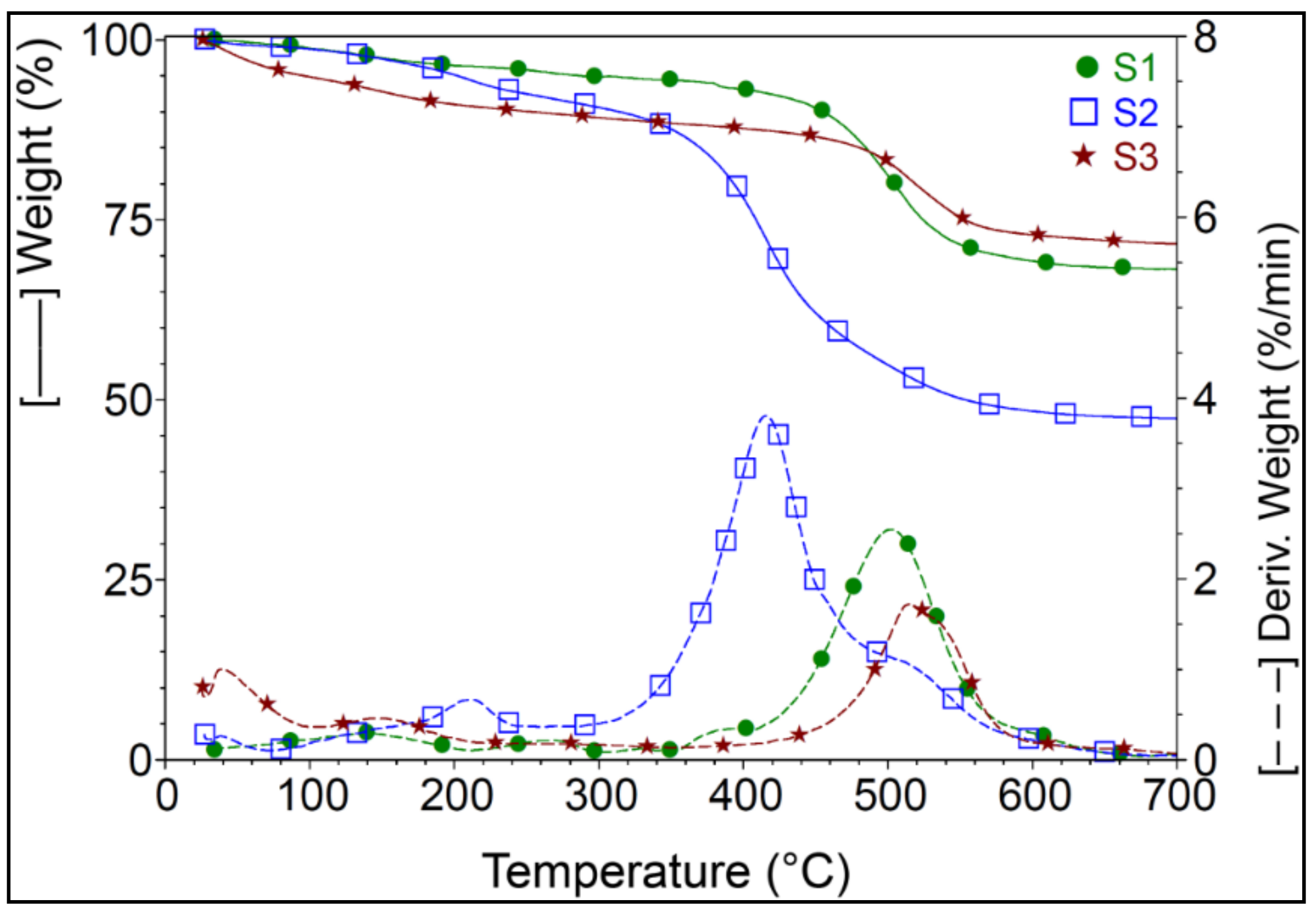


| Sample | 30–90 °C | 90–290 °C | 290–700 °C | Residue | |||
|---|---|---|---|---|---|---|---|
| Wt. Loss | Tmax1 | Wt. Loss | Tmax | Wt. Loss | Tmax | 700 °C | |
| % | °C | % | °C | % | °C | % | |
| S1 | 0.88 | 142.2 | 4.15 | 269.2 | 26.82 | 501.8 | 68.14 |
| S2 | 1.09 | - | 7.88 | 212.0 | 43.60 | 415.8 | 47.41 |
| S3 | 4.76 | 149.9 | 5.87 | - | 17.71 | 515.3 | 71.66 |
| Sample | Storage Modulus (SM), E′ | Loss Modulus, E″ | Loss Factor, Tanδ | Stiffness | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| T | E′ | Onset Point-I | T | E″ Peak Max. | T | Tanδ Peak Max | T | Stiffness | ||
| notation | °C | MPa | °C | E′, MPa | °C | MPa | °C | Peak Max. | °C | N/m |
| PVC | 30 | 1901 | 80.58 | 1834 | 94.00 | 283.8 | 100.39 | 1.252 | 30 | 144,058 ± 0.12% |
| S1 | 30 | 1957 | 80.26 | 1890 | 93.72 | 271.3 | 99.97 | 1.049 | 30 | 147,921 ± 0.42% |
| S2 | 30 | 1901 | 80.05 | 1885 | 93.72 | 277.2 | 100.07 | 0.997 | 30 | 144,058 ± 1.58% |
| S3 | 30 | 1898 | 78.84 | 1838 | 92.81 | 262.2 | 100.07 | 0.931 | 30 | 151,884 ± 1.31% |
| Sample | Film Thickness (nm) | MSE |
|---|---|---|
| S1 | 3522.8 | 2.94 |
| S2 | 1288.0 | 3.43 |
| S3 | 1584.9 | 2.62 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Purcar, V.; Rădițoiu, V.; Rădițoiu, A.; Manea, R.; Raduly, F.M.; Ispas, G.C.; Frone, A.N.; Nicolae, C.A.; Gabor, R.A.; Anastasescu, M.; et al. Preparation and Characterization of Some Sol-Gel Modified Silica Coatings Deposited on Polyvinyl Chloride (PVC) Substrates. Coatings 2021, 11, 11. https://doi.org/10.3390/coatings11010011
Purcar V, Rădițoiu V, Rădițoiu A, Manea R, Raduly FM, Ispas GC, Frone AN, Nicolae CA, Gabor RA, Anastasescu M, et al. Preparation and Characterization of Some Sol-Gel Modified Silica Coatings Deposited on Polyvinyl Chloride (PVC) Substrates. Coatings. 2021; 11(1):11. https://doi.org/10.3390/coatings11010011
Chicago/Turabian StylePurcar, Violeta, Valentin Rădițoiu, Alina Rădițoiu, Raluca Manea, Florentina Monica Raduly, Georgiana Cornelia Ispas, Adriana Nicoleta Frone, Cristian Andi Nicolae, Raluca Augusta Gabor, Mihai Anastasescu, and et al. 2021. "Preparation and Characterization of Some Sol-Gel Modified Silica Coatings Deposited on Polyvinyl Chloride (PVC) Substrates" Coatings 11, no. 1: 11. https://doi.org/10.3390/coatings11010011
APA StylePurcar, V., Rădițoiu, V., Rădițoiu, A., Manea, R., Raduly, F. M., Ispas, G. C., Frone, A. N., Nicolae, C. A., Gabor, R. A., Anastasescu, M., Stroescu, H., & Căprărescu, S. (2021). Preparation and Characterization of Some Sol-Gel Modified Silica Coatings Deposited on Polyvinyl Chloride (PVC) Substrates. Coatings, 11(1), 11. https://doi.org/10.3390/coatings11010011











