Surface Functionalization of Mesoporous Carbon for the Enhanced Removal of Strontium and Cesium Radionuclides
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Activated and Mesoporous Carbons
2.2. Functionalization of Mesoporous Carbon
2.3. Characterization of MPC and MMPC
2.4. Adsorption Experiments
3. Results and Discussion
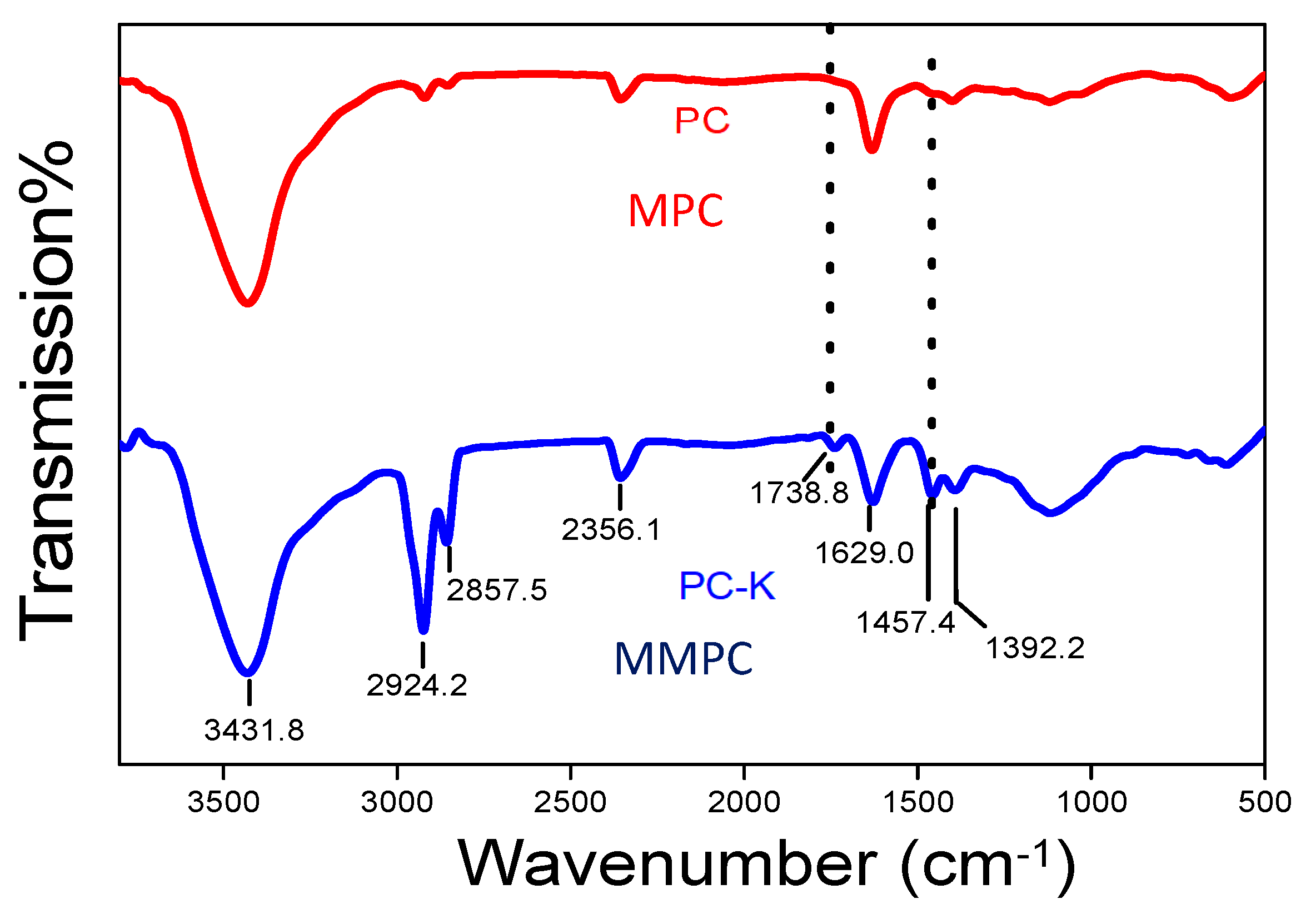
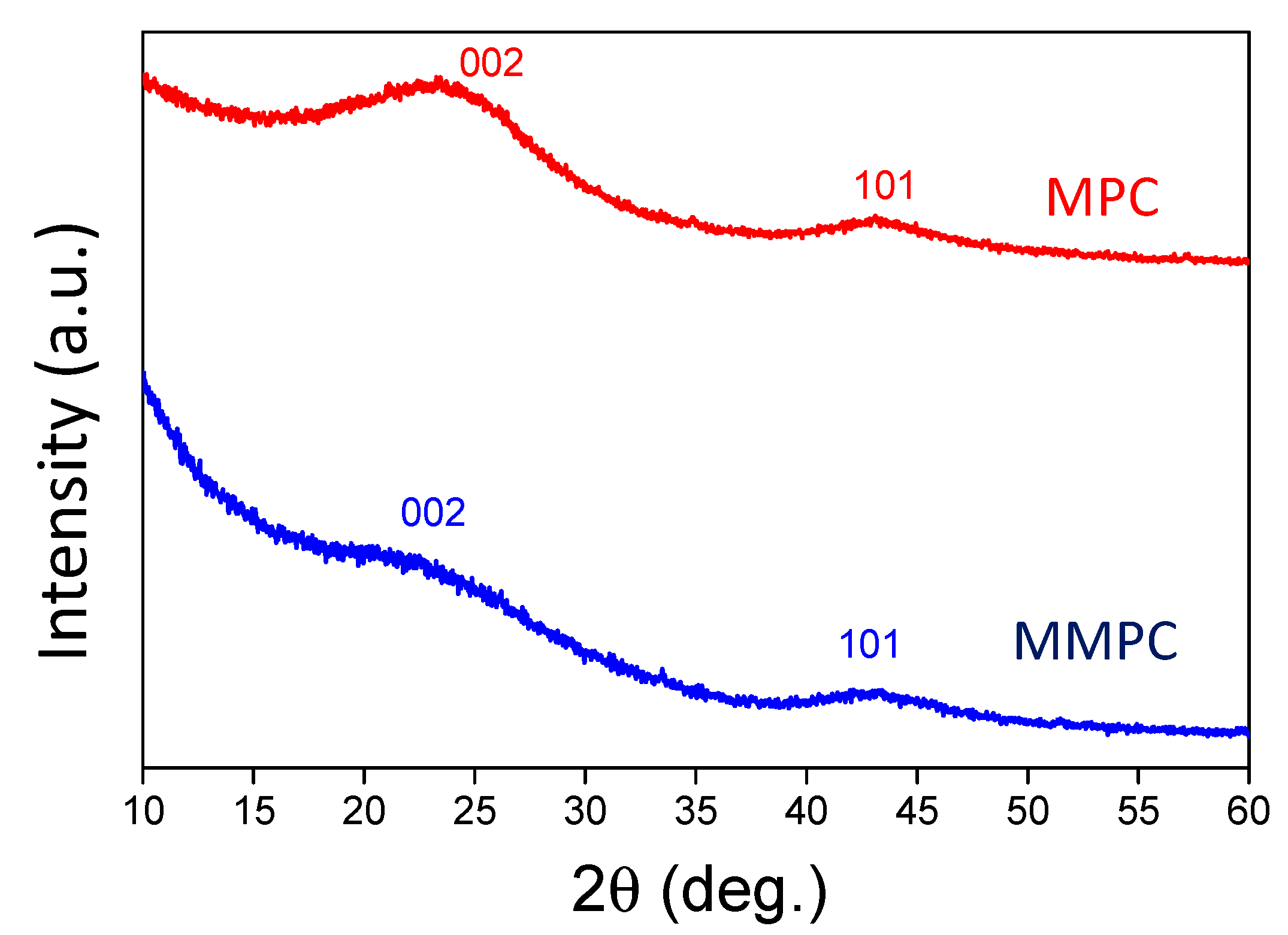
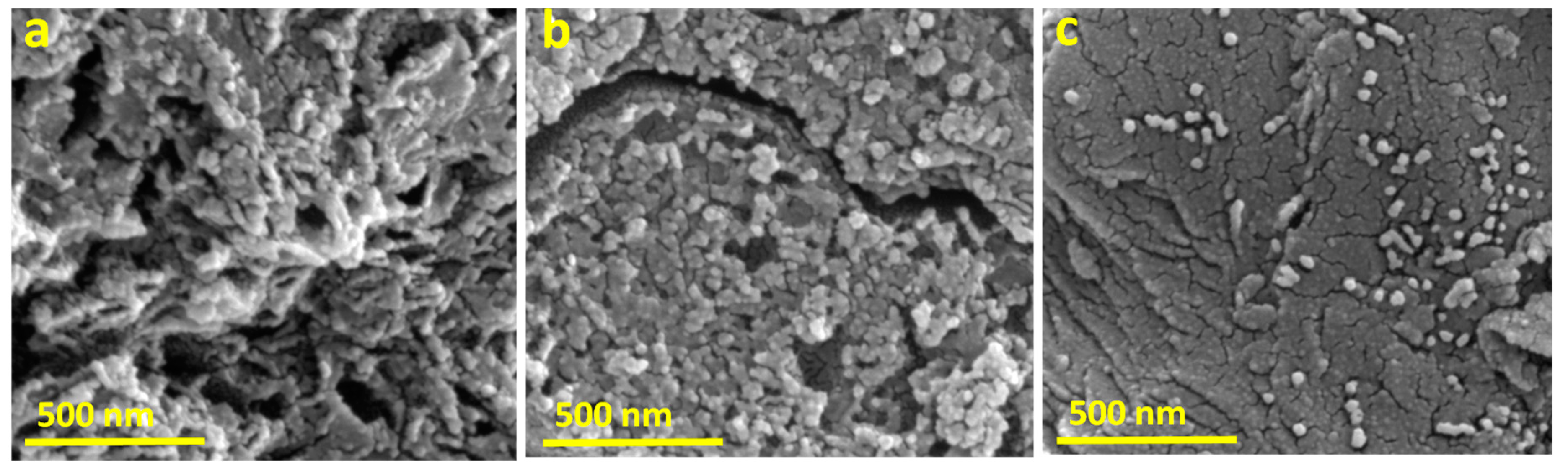
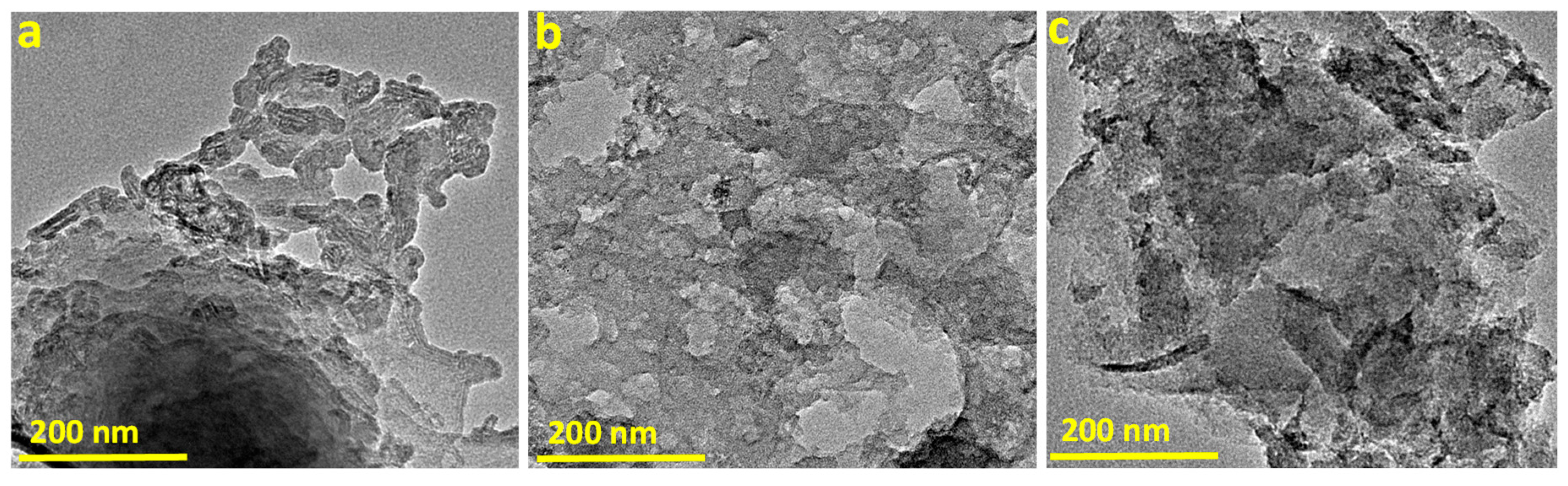
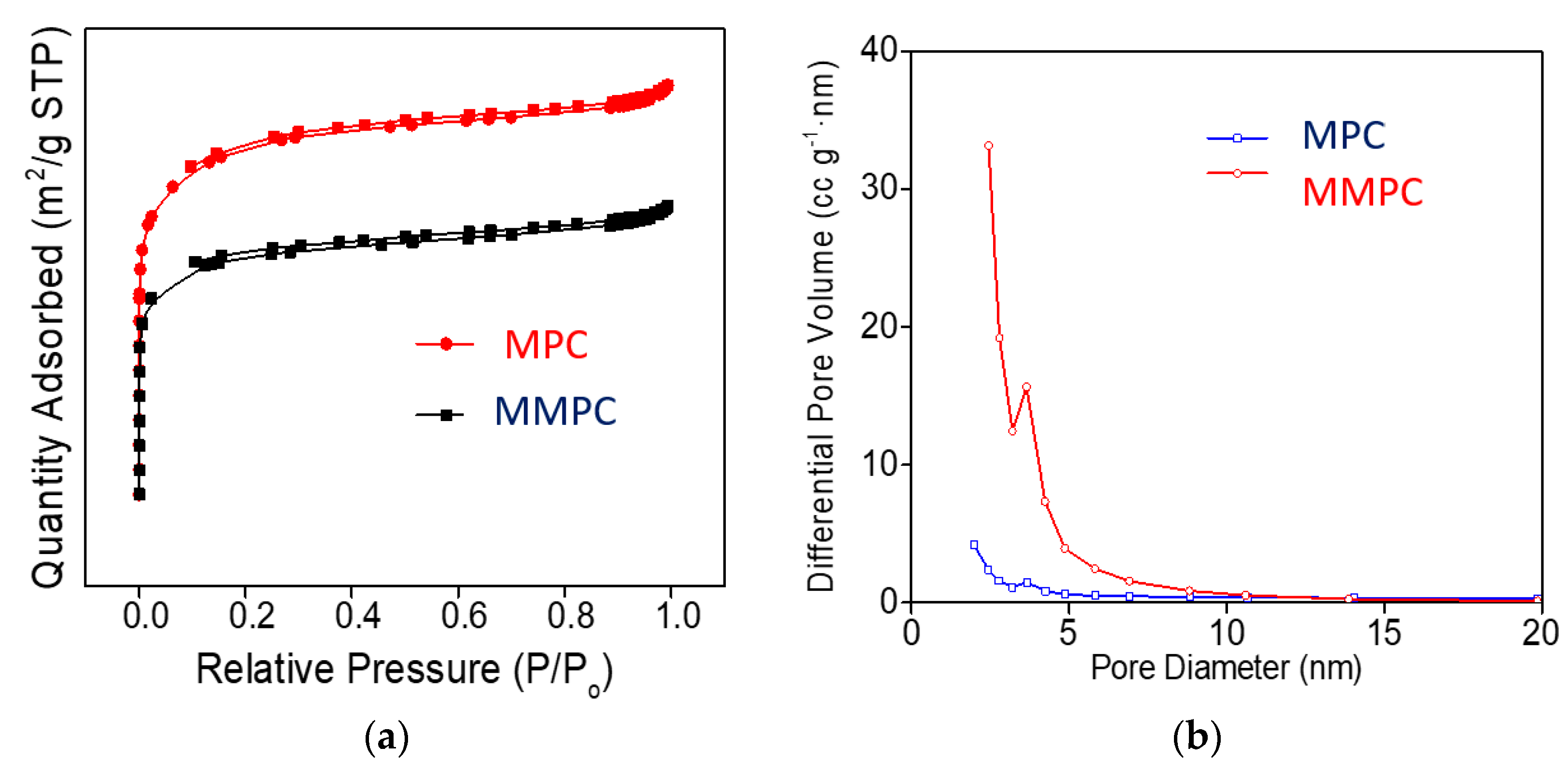
3.1. Characterization of MPC and MMPC
3.2. Optimization of Adsorption Parameters
3.3. Dosage of the Adsorbent
3.4. Optimization of Time
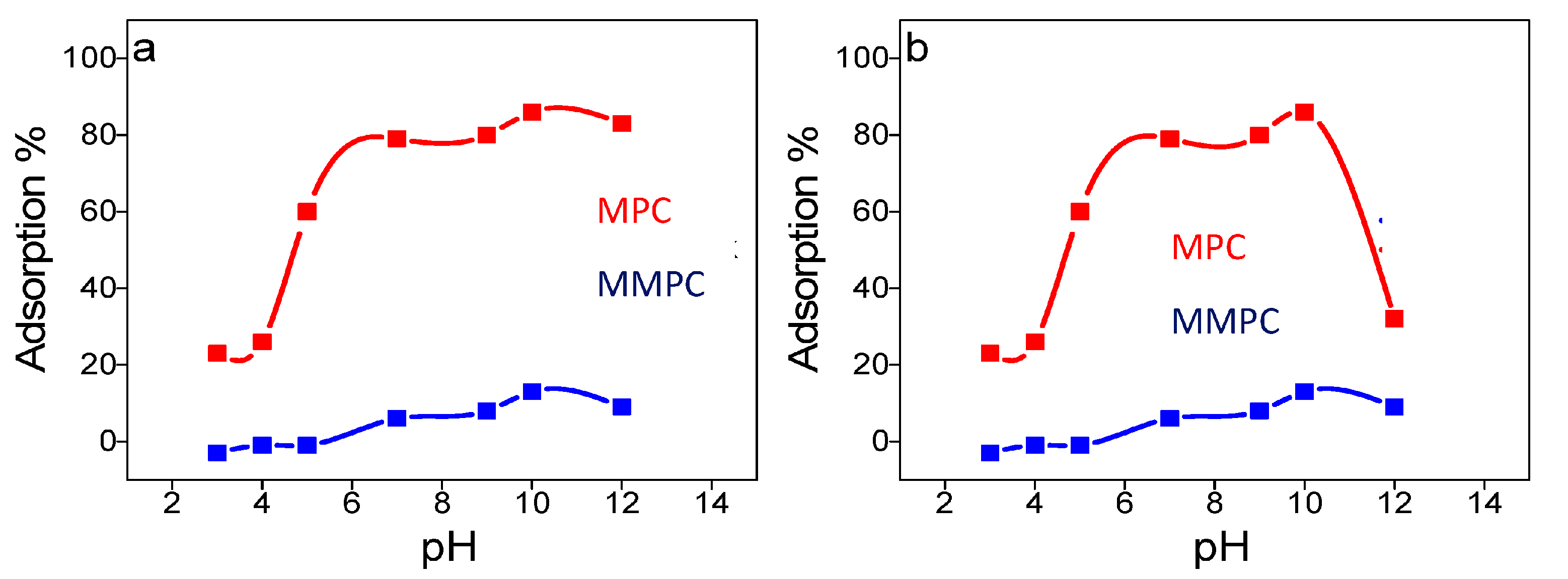
3.5. Optimization of the pH
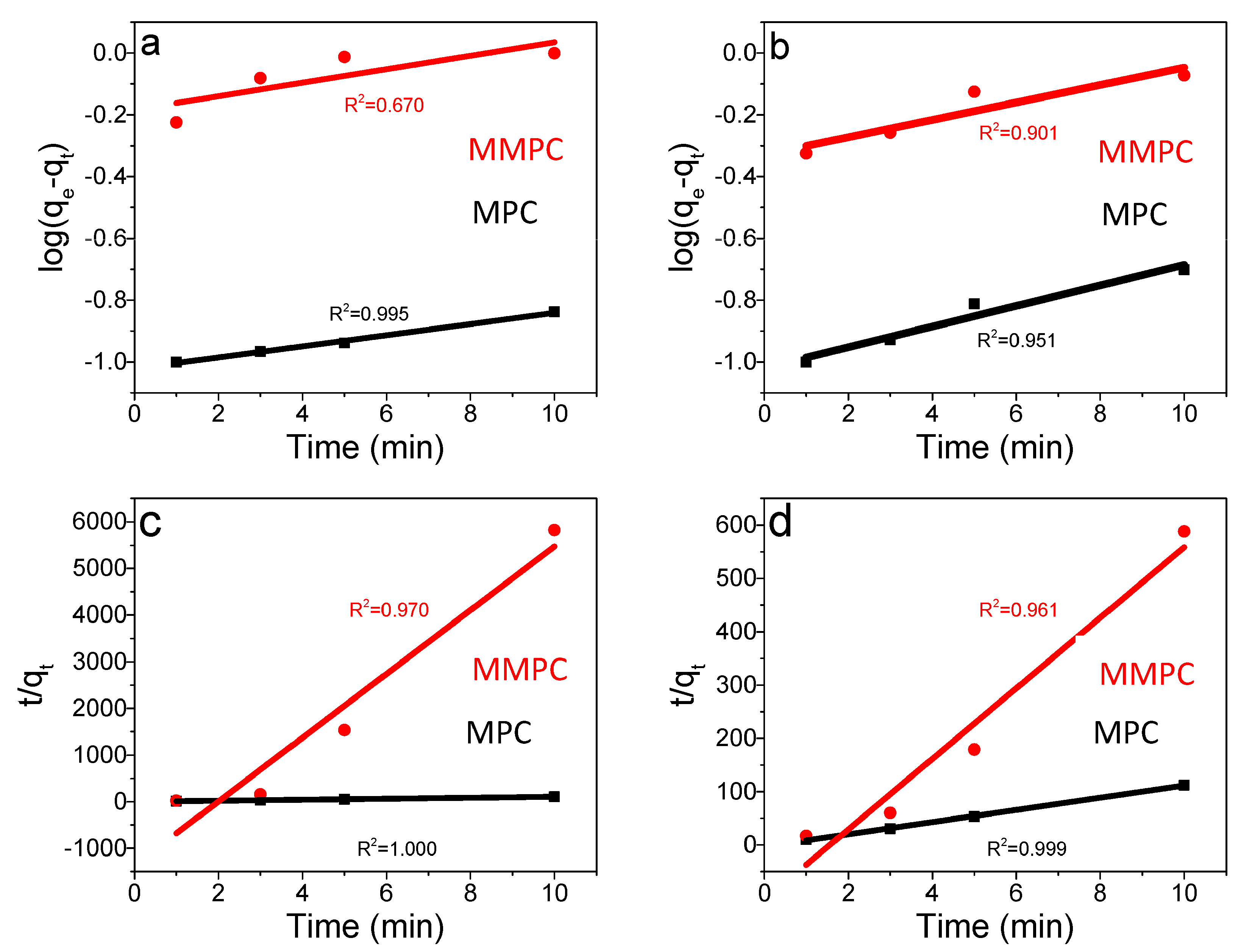
3.6. Adsorption Kinetics
3.7. Adsorption Thermodynamics
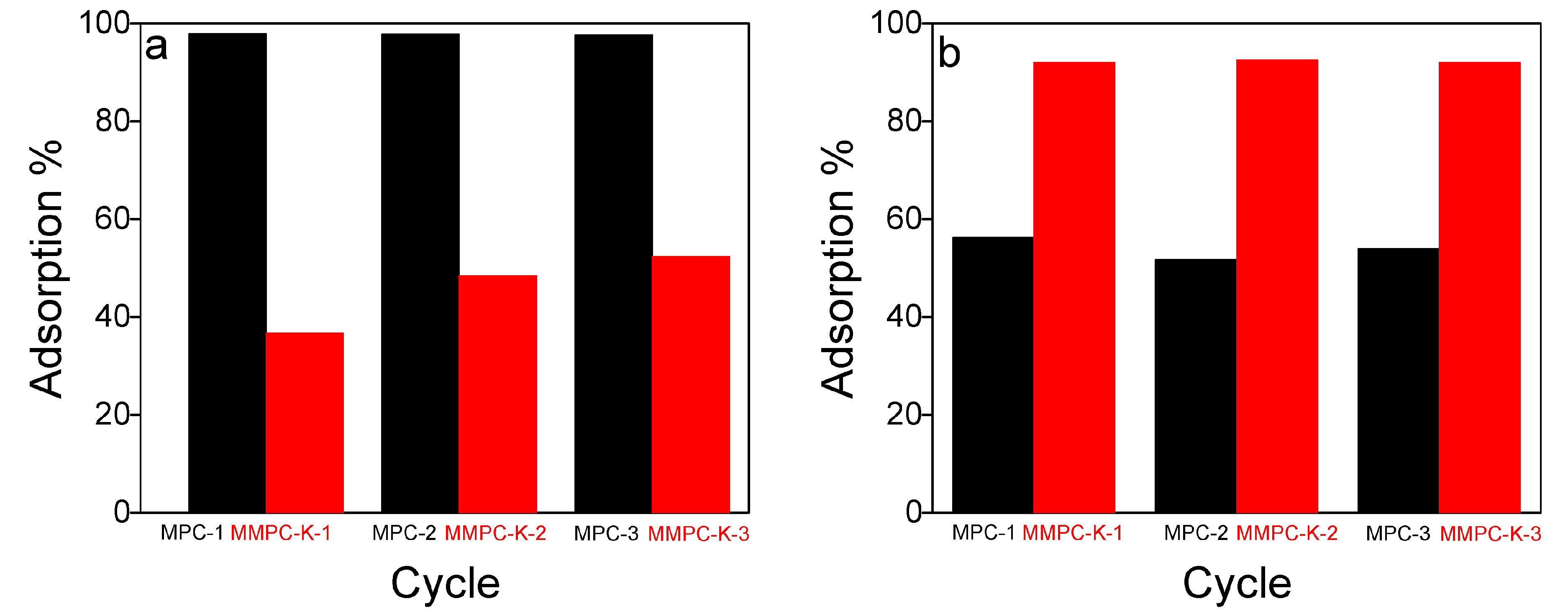
3.8. Regeneration of Adsorbents
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Siddiqui, M.N.; Ali, I.; Asim, M.; Chanbasha, B. Quick removal of nickel metal ions in water using asphalt-based porous carbon. J. Mol. Liq. 2020, 308, 113078. [Google Scholar] [CrossRef]
- Alissa, F.M.; Mohammed, D.; Osman, A.M.; Chanbasha, B.; Siddiqui, M.N.; Al-Arfaj, A.A.; Suliman, M.H. Synthesis of highly efficient asphalt-based carbon for adsorption of polycyclic aromatic hydrocarbons and diesel from emulsified aqueous phase. Carbon Lett. 2020, 30, 555–567. [Google Scholar] [CrossRef]
- Basheer, A.A. New generation nano-adsorbents for the removal of emerging contaminants in water. J. Mol. Liq. 2018, 261, 583–593. [Google Scholar] [CrossRef]
- Allen, H.E.; Perdue, E.M.; Brown, D.S. Metals in Groundwater; CRC Press: Boca Raton, FL, USA, 1993. [Google Scholar]
- Todd, T.A.; Todd, T.A.; Law, J.D.; Herbst, R.S. Cesium and Strontium Separation Technologies Literature Review; INEEL/EXT-04-01895; Idaho National Engineering and Environmental Laboratory: Idaho Falls, ID, USA, 2004.
- Aguila, B.; Banerjee, D.; Nie, Z.; Shin, Y.; Ma, S.; Thallapally, P.K. Selective removal of cesium and strontium using porous frameworks from high level nuclear waste. Chem. Commun. 2016, 52, 5940–5942. [Google Scholar] [CrossRef]
- Wilmarth, W.R.; Lumetta, G.J.; Johnson, M.E.; Poirier, M.R.; Thompson, M.C.; Suggs, P.C.; Machara, N.P. Waste-pretreatment technologies for remediation of legacy defense nuclear wastes. Solvent Extr. Ion Exch. 2011, 29, 1–48. [Google Scholar] [CrossRef]
- Kabra, K.; Chaudhary, R.; Sawhney, R.L. Solar photocatalytic removal of metal ions from industrial wastewater. Environ. Prog. 2008, 27, 487–495. [Google Scholar]
- Chen, X.; Huang, G.; Wang, J. Electrochemical reduction/oxidation in the treatment of heavy metal wastewater. J. Metall. Eng. 2013, 2, 161–164. [Google Scholar]
- Lakshtanov, L.Z.; Stipp, S.L.S. Experimental study of nickel (II) interaction with calcite: Adsorption and coprecipitation. Geochim. Cosmochim. Acta 2007, 71, 3686–3697. [Google Scholar]
- Milenkovic, A.; Smiciklas, I.; Bundaleski, N.; Teodoro, O.M.N.D.; Veljovic, D.; Vukelic, N. The role of different minerals from red mud assemblage in Co (II) sorption mechanism. Colloids Surf. A Physicochem. Eng. Asp. 2016, 508, 8–20. [Google Scholar]
- Koshy, N.; Singh, D.N. Fly ash zeolites for water treatment applications. J. Environ. Chem. Eng. 2016, 4, 1460–1472. [Google Scholar]
- Tran, E.L.; Teutsch, N.; Klein-BenDavid, O.; Weisbrod, N. Uranium and cesium sorption to bentonite colloids under carbonate-rich environments: Implications for radionuclide transport. Sci. Total Environ. 2018, 643, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Metwally, S.S.; Rizk, H.E.; Gasser, M.S. Biosorption of strontium ions from aqueous solution using modified eggshell materials. Radiochim. Acta 2017, 105, 1021–1103. [Google Scholar] [CrossRef]
- Vipin, A.K.; Ling, S.; Fugetsu, B. Sodium cobalt hexacyanoferrate encapsulated in alginate vesicle with CNT for both cesium and strontium removal. Carbohydr. Polym. 2014, 111, 477–484. [Google Scholar] [CrossRef]
- AlSaadi, M.A.; AlMamun, A.; Alam, M.Z.; Amosa, M.K.; Atieh, M.A. Removal of cadmium from water by CNT-PAC composite: Effect of functionalization. Nano 2016, 11, 1650011. [Google Scholar] [CrossRef]
- Ibrahim, R.K.; Hayyan, M.; AlSaadi, M.A.; Hayyan, A.; Ibrahim, S. Environmental application of nanotechnology: Air, soil, and water. Environ. Sci. Pollut. Res. 2016, 23, 13754–13788. [Google Scholar] [CrossRef]
- Thines, R.K.; Mubarak, N.M.; Nizamuddin, S.; Sahu, J.N.; Abdullah, E.C.; Ganesan, P. Application potential of carbon nanomaterials in water and wastewater treatment: A review. J. Taiwan Inst. Chem. Eng. 2017, 72, 116–133. [Google Scholar] [CrossRef]
- Hu, C.; Xu, Y.; Duo, S.; Zhang, R.; Li, M. Non-covalent functionalization of carbon nanotubes with surfactants and polymers. J. Chin. Chem. Soc. 2009, 56, 234–239. [Google Scholar] [CrossRef]
- Lu, C.; Chiu, H. Chemical modification of multiwalled carbon nanotubes for sorption of Zn2+ from aqueous solution. Chem. Eng. J. 2008, 139, 462–468. [Google Scholar] [CrossRef]
- Mubarak, N.M.; Sahu, J.N.; Wong, J.R.; Jayakumar, N.S.; Ganesan, P.; Abdullah, E.C. Overview on the Functionalization of Carbon Nanotubes. In Chemical Functionalization of Carbon Nanomaterials; CRC Press: Boca Raton, FL, USA, 2015; pp. 108–127. [Google Scholar]
- Yang, S.; Li, J.; Shao, D.; Hu, J.; Wang, X. Adsorption of Ni (II) on oxidized multi-walled carbon nanotubes: Effect of contact time, pH, foreign ions and PAA. J. Hazard. Mater. 2009, 166, 109–116. [Google Scholar] [CrossRef]
- Mubarak, N.; Daniel, S.; Khalid, M.; Tan, J. Comparative study of functionalize and non-functionalized carbon nanotube for removal of copper from polluted water. Int. J. Chem. Environ. Eng. 2012, 3, 314–317. [Google Scholar]
- Rao, G.P.; Lu, C.; Su, F. Sorption of divalent metal ions from aqueous solution by carbon nanotubes: A review. Sep. Purif. Technol. 2007, 58, 224–231. [Google Scholar]
- Suliman, M.H.; Adam, A.; Siddiqui, M.N.; Yamani, Z.H.; Qamar, M. Facile synthesis of ultrathin interconnected carbon nanosheets as a robust support for small and uniformly-dispersed iron phosphide for the hydrogen evolution reaction. Carbon 2019, 144, 764–771. [Google Scholar] [CrossRef]
- Gutierrez, M.; Fuentes, H.R. A mechanistic modeling of montomorillonite contamination by cesium sorption. Appl. Clay Sci. 1996, 11, 11–24. [Google Scholar] [CrossRef]
- Giannakopoulou, F.; Haidouti, C.; Chronopoulou, A.; Gasparatos, D. Sorption behavior of cesium on various soils under different pH levels. J. Hazard. Mater. 2007, 49, 553–556. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.K.; Singh, D.K.; Sinha, S. Adsorptive removal of Hg (II) from synthetic and real aqueous solutions using modified papaya seed. J. Dispers. Sci. Technol. 2016, 37, 1613–1622. [Google Scholar] [CrossRef]
- Ghasemi, M.; Naushad, M.; Ghasemi, N.; Khosravi-Fard, Y. A novel agricultural waste based adsorbent for the removal of Pb (II) from aqueous solution: Kinetics, equilibrium and thermodynamic studies. J. Ind. Eng. Chem. 2014, 20, 454–461. [Google Scholar] [CrossRef]
- Kandah, M.I.; Shawabkeh, R.; Al-Zboon, M.A. Synthesis and characterization of activated carbon from asphalt. Appl. Surf. Sci. 2006, 253, 821–826. [Google Scholar] [CrossRef]
- Zhan, X.; Zhong, C.; Lin, Q.; Luo, S.; Zhang, X.; Fang, C. Facile fabrication of graphitic mesoporous carbon with ultrathin walls from petroleum asphalt. J. Anal. Appl. Pyrolysis 2017, 126, 154–157. [Google Scholar] [CrossRef]
- Zhang, X.; Lin, Q.; Luo, Q.; Ruan, K.; Peng, K. Preparation of novel oxidized mesoporous carbon with excellent adsorption performance for removal of malachite green and lead ion. Appl. Surf. Sci. 2018, 442, 322–331. [Google Scholar] [CrossRef]
- Pan, L.; Wang, Y.; Hu, H.; Li, X.; Liu, J.; Guan, L.; Tian, W.; Wang, X.; Li, W.; Wu, M. 3D self-assembly synthesis of hierarchical porous carbon from petroleum asphalt for supercapacitors. Carbon 2018, 134, 345–353. [Google Scholar] [CrossRef]


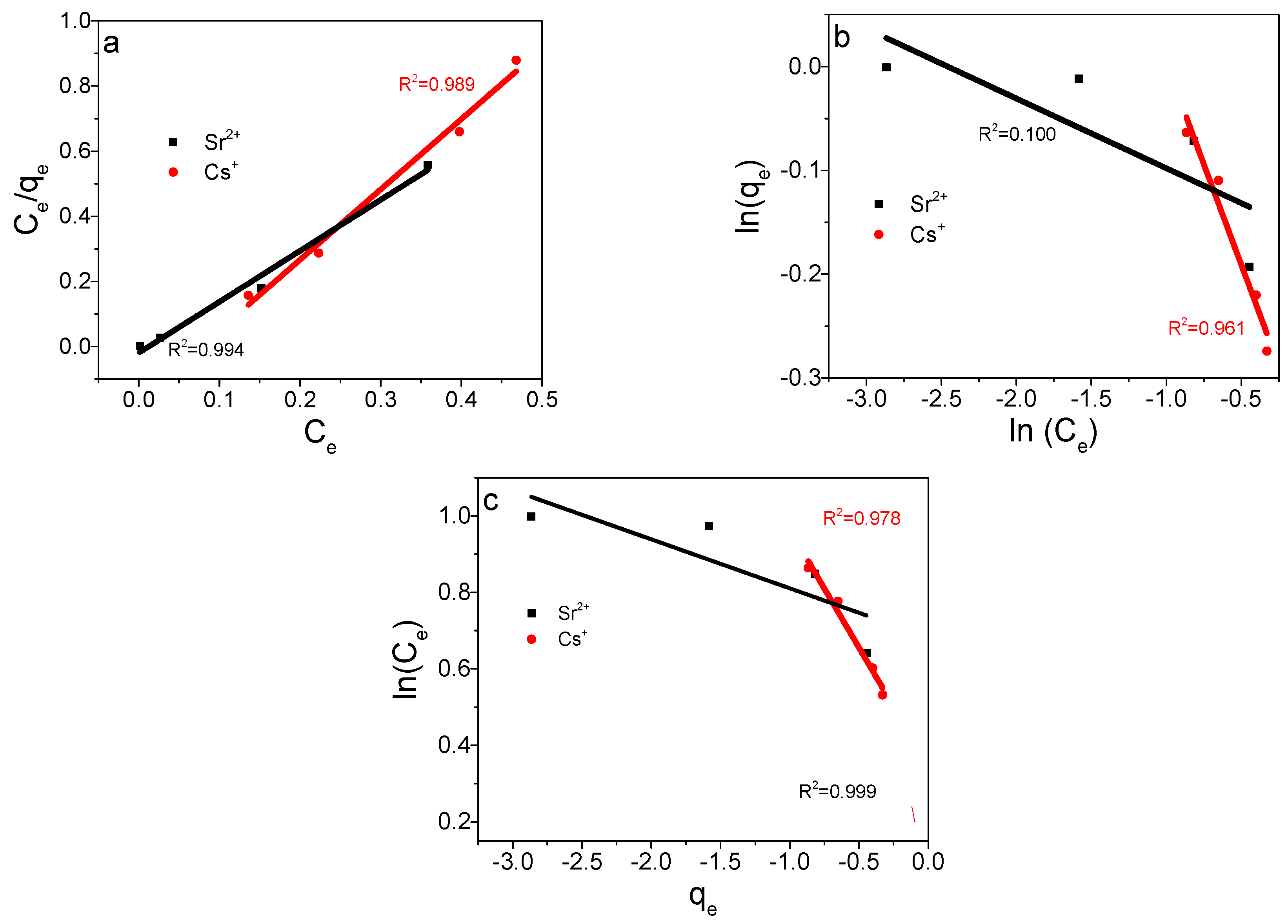

| Parameters | ∆H (kJ·mol−1) | ∆S (kJ K−1·mol−1) | ∆G (kJ·mol−1) |
|---|---|---|---|
| Cs+ | 5.87 | 0.031 | −3.37 |
| Sr2+ | 4.45 | 0.029 | −4.19 |
| Carbon | Surface Area (m2·g−1) | Pore Size (nm) | Ref. |
|---|---|---|---|
| Activated carbon (Jordan Asphalt) | - | - | [30] |
| Graphitic mesoporous carbon | 479 | 7.53 | [31] |
| Oxidized meoporous carbon | 333.6 | 3.93 | [32] |
| Hierarchical porous carbon | 3581 | 2.20 | [33] |
| MMPC | 667 | 3.60 | This work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamedelniel Suliman, M.; Nahid Siddiqui, M.; Basheer, C. Surface Functionalization of Mesoporous Carbon for the Enhanced Removal of Strontium and Cesium Radionuclides. Coatings 2020, 10, 923. https://doi.org/10.3390/coatings10100923
Hamedelniel Suliman M, Nahid Siddiqui M, Basheer C. Surface Functionalization of Mesoporous Carbon for the Enhanced Removal of Strontium and Cesium Radionuclides. Coatings. 2020; 10(10):923. https://doi.org/10.3390/coatings10100923
Chicago/Turabian StyleHamedelniel Suliman, Munzir, Mohammad Nahid Siddiqui, and Chanbasha Basheer. 2020. "Surface Functionalization of Mesoporous Carbon for the Enhanced Removal of Strontium and Cesium Radionuclides" Coatings 10, no. 10: 923. https://doi.org/10.3390/coatings10100923
APA StyleHamedelniel Suliman, M., Nahid Siddiqui, M., & Basheer, C. (2020). Surface Functionalization of Mesoporous Carbon for the Enhanced Removal of Strontium and Cesium Radionuclides. Coatings, 10(10), 923. https://doi.org/10.3390/coatings10100923








