Electrodeposited Biocoatings, Their Properties and Fabrication Technologies: A Review
Abstract
1. Introduction
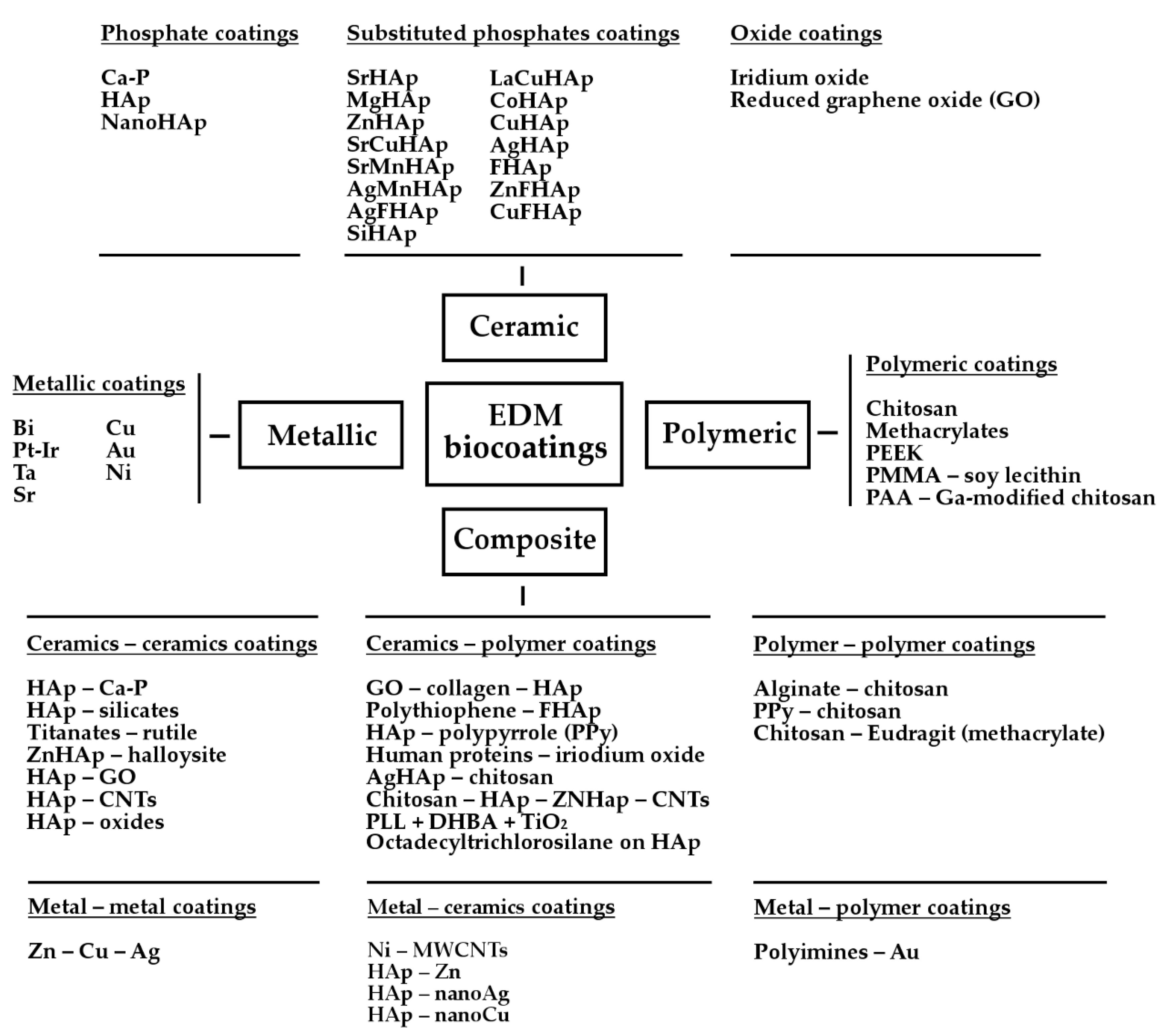
2. Biocoatings
2.1. Metallic Coatings
2.2. Ceramic Coatings
2.2.1. Phosphate Coatings
2.2.2. Substituted Phosphate Coatings
2.2.3. Oxides’ Coatings
2.3. Polymer Coatings
2.4. Composite Coatings
2.4.1. Ceramics-Ceramics Coatings
2.4.2. Ceramics-Polymer Coatings
2.4.3. Metal-Ceramics Coatings
2.4.4. Metal-Polymer Coatings
2.4.5. Polymer-Polymer Coatings
2.5. Effects of Component(s) on Properties of Biocoatings
3. Deposition Technologies
3.1. Electrocathodic Deposition (ECD)
3.1.1. Effect of an Electrolyte Composition
3.1.2. Effect of Deposition Potential
3.1.3. Effect of Deposition Current
3.1.4. Effect of Deposition Time
3.1.5. Effect of Deposition pH
3.1.6. Effect of Deposition Temperature
3.2. Pulse Electrocathodic Deposition (PED)
3.2.1. Effect of an Electrolyte Composition
3.2.2. Effect of Deposition Potential
3.2.3. Effect of Deposition Current
3.2.4. Effect of Deposition pH
3.2.5. Effect of Deposition Temperature
3.3. Electrophoretic Deposition (EPD)
3.3.1. Effect of an Electrolyte Composition
3.3.2. Effect of Deposition Potential
3.3.3. Effect of Deposition Time
3.4. Plasma Electrochemical Oxidation (PEO)
3.5. Electro-Spark Deposition (ESD)
3.6. Electro-Discharge Method (EDM)
3.7. Electropolymerization (EP)
3.8. Effects of Electrodeposition Method on Properties of Coatings
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Nakaya, M.; Uedono, A.; Hotta, A. Recent progress in gas barrier thin film coatings on PET bottles in food and beverage applications. Coatings 2015, 5, 987–1001. [Google Scholar] [CrossRef]
- Aranke, O.; Algenaid, W.; Awe, S.; Joshi, S. Coatings for automotive gray cast iron brake discs: A review. Coatings 2019, 9, 552. [Google Scholar] [CrossRef]
- Ma, L.; Eom, K.; Geringer, J.; Jun, T.S.; Kim, K. Literature review on fretting wear and contact mechanics of tribological coatings. Coatings 2019, 9, 501. [Google Scholar] [CrossRef]
- Verbič, A.; Gorjanc, M.; Simončič, B. Zinc oxide for functional textile coatings: Recent advances. Coatings 2019, 9, 550. [Google Scholar] [CrossRef]
- Bir, F.; Khireddine, H.; Touati, A.; Sidane, D.; Yala, S.; Oudadesse, H. Electrochemical depositions of fluorohydroxyapatite doped by Cu2+, Zn2+, Ag+ on stainless steel substrates. Appl. Surf. Sci. 2012, 258, 7021–7030. [Google Scholar] [CrossRef]
- Tiwari, A.; Seman, S.; Singh, G.; Jayaganthan, R. Nanocrystalline cermet coatings for erosion-corrosion protection. Coatings 2019, 9, 400. [Google Scholar] [CrossRef]
- Mandracci, P.; Mussano, F.; Rivolo, P.; Carossa, S. Surface treatments and functional coatings for biocompatibility improvement and bacterial adhesion reduction in dental implantology. Coatings 2016, 6, 7. [Google Scholar] [CrossRef]
- Hou, N.Y.; Perinpanayagam, H.; Mozumder, M.S.; Zhu, J. Novel development of biocompatible coatings for bone implants. Coatings 2015, 5, 737–757. [Google Scholar] [CrossRef]
- Graziani, G.; Boi, M.; Bianchi, M. A review on ionic substitutions in hydroxyapatite thin films: Towards complete biomimetism. Coatings 2018, 8, 269. [Google Scholar] [CrossRef]
- Cometa, S.; Bonifacio, M.A.; Mattioli-Belmonte, M.; Sabbatini, L.; De Giglio, E. Electrochemical strategies for titanium implant polymeric coatings: The why and how. Coatings 2019, 9, 268. [Google Scholar] [CrossRef]
- Sartori, M.; Maglio, M.; Tschon, M.; Aldini, N.N.; Visani, A.; Fini, M. Functionalization of ceramic coatings for enhancing integration in osteoporotic bone: A systematic review. Coatings 2019, 9, 312. [Google Scholar] [CrossRef]
- Duta, L.; Popescu, A.C. Current status on pulsed laser deposition of coatings from animal-origin calcium phosphate sources. Coatings 2019, 9, 335. [Google Scholar] [CrossRef]
- Paital, S.R.; Dahotre, N.B. Calcium phosphate coatings for bio-implant applications: Materials, performance factors, and methodologies. Mater. Sci. Eng. R Rep. 2009, 66, 1–70. [Google Scholar] [CrossRef]
- Guslitzer-Okner, R.; Mandler, D. Electrochemical coating of medical implants. In Applications of Electrochemistry and Nanotechnology 291 in Biology and Medicine I; Eliaz, N., Ed.; Springer Science & Business Media: Berlin, Germany, 2011; pp. 291–342. ISBN 9781461403470. [Google Scholar]
- Kulkarni, M.; Mazare, A.; Schmuki, P.; Iglič, A. Biomaterial surface modification of titanium and titanium alloys for medical applications. In Nanomedicine; Seifalian, A., de Mel, A., Kalaskar, D.M., Eds.; One Central Press Altrincham: Cheshire, UK, 2014; pp. 111–136. [Google Scholar]
- Asri, R.I.M.; Harun, W.S.W.; Hassan, M.A.; Ghani, S.A.C.; Buyong, Z. A review of hydroxyapatite-based coating techniques: Sol-gel and electrochemical depositions on biocompatible metals. J. Mech. Behav. Biomed. Mater. 2016, 57, 95–108. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Calcium orthophosphates (CaPO4): Occurrence and properties. Prog. Biomater. 2016, 5, 9–70. [Google Scholar] [CrossRef]
- Adeleke, S.A.; Bushroa, A.R.; Sopyan, I. Recent development of calcium phosphate-based coatings on titanium alloy implants. Surf. Eng. Appl. Electrochem. 2017, 53, 419–433. [Google Scholar] [CrossRef]
- Liu, W.; Liu, S.; Wang, L. Surface modification of biomedical titanium alloy: Micromorphology, microstructure evolution and biomedical applications. Coatings 2019, 9, 249. [Google Scholar] [CrossRef]
- Su, Y.; Cockerill, I.; Zheng, Y.; Tang, L.; Qin, Y.X.; Zhu, D. Biofunctionalization of metallic implants by calcium phosphate coatings. Bioact. Mater. 2019, 4, 196–206. [Google Scholar] [CrossRef]
- Yang, J.; Cui, F.; Lee, I.S. Surface modifications of magnesium alloys for biomedical applications. Ann. Biomed. Eng. 2011, 39, 1857–1871. [Google Scholar] [CrossRef]
- Wan, P.; Tan, L.; Yang, K. Surface modification on biodegradable magnesium alloys as orthopedic implant materials to improve the bio-adaptability: A review. J. Mater. Sci. Technol. 2016, 32, 827–834. [Google Scholar] [CrossRef]
- Ananthi, A.; Kumar, S.S.; Phani, K.L. Facile one-step direct electrodeposition of bismuth nanowires on glassy carbon electrode for selective determination of folic acid. Electrochim. Acta 2015, 151, 584–590. [Google Scholar] [CrossRef]
- Cassar, I.R.; Yu, C.; Sambangi, J.; Lee, C.D.; Whalen, J.J.; Petrossians, A.; Grill, W.M. Electrodeposited platinum-iridium coating improves in vivo recording performance of chronically implanted microelectrode arrays. Biomaterials 2019, 205, 120–132. [Google Scholar] [CrossRef] [PubMed]
- Arnould, C.; Delhalle, J.; Mekhalif, Z. Multifunctional hybrid coating on titanium towards hydroxyapatite growth: Electrodeposition of tantalum and its molecular functionalization with organophosphonic acids films. Electrochim. Acta 2008, 53, 5632–5638. [Google Scholar] [CrossRef]
- Frank, M.J.; Walter, M.S.; Tiainen, H.; Rubert, M.; Monjo, M.; Lyngstadaas, S.P.; Haugen, H.J. Coating of metal implant materials with strontium. J. Mater. Sci. Mater. Med. 2013, 24, 2537–2548. [Google Scholar] [CrossRef]
- Mollamahale, Y.B.; Ghorbani, M.; Dolati, A.; Hosseini, D. Electrodeposition of well-defined gold nanowires with uniform ends for developing 3D nanoelectrode ensembles with enhanced sensitivity. Mater. Chem. Phys. 2018, 213, 67–75. [Google Scholar] [CrossRef]
- Nasirpouri, F.; Cheshideh, H.; Samardak, A.Y.; Ognev, A.V.; Zubkov, A.A.; Samardak, A.S. Morphology- and magnetism-controlled electrodeposition of Ni nanostructures on TiO2 nanotubes for hybrid Ni/TiO2 functional applications. Ceram. Int. 2019, 45, 11258–11269. [Google Scholar] [CrossRef]
- Pavez, J.; Silva, J.F.; Melo, F. Homogeneous calcium carbonate coating obtained by electrodeposition: In situ atomic force microscope observations. Electrochim. Acta 2005, 50, 3488–3494. [Google Scholar] [CrossRef]
- Park, J.H.; Lee, D.Y.; Oh, K.T.; Lee, Y.K.; Kim, K.M.; Kim, K.N. Bioactivity of calcium phosphate coatings prepared by electrodeposition in a modified simulated body fluid. Mater. Lett. 2006, 60, 2573–2577. [Google Scholar] [CrossRef]
- Park, J.H.; Lee, Y.K.; Kim, K.M.; Kim, K.N. Bioactive calcium phosphate coating prepared on H2O2-treated titanium substrate by electrodeposition. Surf. Coat. Technol. 2005, 195, 252–257. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, B.; Lu, J.; Chen, J.; Zhang, X.; Gu, Z. Biomimetic Ca-P coating on pre-calcified Ti plates by electrodeposition method. Appl. Surf. Sci. 2010, 256, 2700–2704. [Google Scholar] [CrossRef]
- Lin, D.Y.; Wang, X.X. Preparation of hydroxyapatite coating on smooth implant surface by electrodeposition. Ceram. Int. 2011, 37, 403–406. [Google Scholar] [CrossRef]
- Mokabber, T.; Zhou, Q.; Vakis, A.I.; van Rijn, P.; Pei, Y.T. Mechanical and biological properties of electrodeposited calcium phosphate coatings. Mater. Sci. Eng. C 2019, 100, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Harun, W.S.W.; Asri, R.I.M.; Alias, J.; Zulkifli, F.H.; Kadirgama, K.; Ghani, S.A.C.; Shariffuddin, J.H.M. A comprehensive review of hydroxyapatite-based coatings adhesion on metallic biomaterials. Ceram. Int. 2018, 44, 1250–1268. [Google Scholar] [CrossRef]
- Gopi, D.; Karthika, A.; Sekar, M.; Kavitha, L.; Pramod, R.; Dwivedi, J. Development of lotus-like hydroxyapatite coating on HELCDEB treated titanium by pulsed electrodeposition. Mater. Lett. 2013, 105, 216–219. [Google Scholar] [CrossRef]
- Drevet, R.; Fauré, J.; Sayen, S.; Marle-Spiess, M.; El Btaouri, H.; Benhayoune, H. Electrodeposition of biphasic calcium phosphate coatings with improved dissolution properties. Mater. Chem. Phys. 2019, 236, 1–7. [Google Scholar] [CrossRef]
- Fathyunes, L.; Khalil-Allafi, J. Effect of employing ultrasonic waves during pulse electrochemical deposition on the characteristics and biocompatibility of calcium phosphate coatings. Ultrason. Sonochem. 2018, 42, 293–302. [Google Scholar] [CrossRef]
- Dudek, K.; Dulski, M.; Goryczka, T.; Gerle, A. Structural changes of hydroxyapatite coating electrophoretically deposited on NiTi shape memory alloy. Ceram. Int. 2018, 44, 11292–11300. [Google Scholar] [CrossRef]
- Bartmanski, M.; Zielinski, A.; Majkowska-Marzec, B.; Strugala, G. Effects of solution composition and electrophoretic deposition voltage on various properties of nanohydroxyapatite coatings on the Ti13Zr13Nb alloy. Ceram. Int. 2018, 44, 19236–19246. [Google Scholar] [CrossRef]
- Jażdżewska, M.; Majkowska-Marzec, B. Hydroxyapatite deposition on the laser modified Ti13Nb13Zr alloy. Adv. Mater. Sci. 2018, 17, 5–13. [Google Scholar] [CrossRef]
- Patel, K.D.; Singh, R.K.; Lee, J.H.; Kim, H.W. Electrophoretic coatings of hydroxyapatite with various nanocrystal shapes. Mater. Lett. 2019, 234, 148–154. [Google Scholar] [CrossRef]
- Araghi, A.; Hadianfard, M.J. Fabrication and characterization of functionally graded hydroxyapatite/TiO2 multilayer coating on Ti-6Al-4V titanium alloy for biomedical applications. Ceram. Int. 2015, 41, 12668–12679. [Google Scholar] [CrossRef]
- Albayrak, O.; El-Atwani, O.; Altintas, S. Hydroxyapatite coating on titanium substrate by electrophoretic deposition method: Effects of titanium dioxide inner layer on adhesion strength and hydroxyapatite decomposition. Surf. Coat. Technol. 2008, 202, 2482–2487. [Google Scholar] [CrossRef]
- Pavlović, M.R.P.; Eraković, S.G.; Pavlović, M.M.; Stevanović, J.S.; Panić, V.V.; Ignjatović, N.L. Anaphoretical/oxidative approach to the in-situ synthesis of adherent hydroxyapatite/titanium oxide composite coatings on titanium. Surf. Coat. Technol. 2019, 358, 688–694. [Google Scholar] [CrossRef]
- Xiong, Y.; Lu, C.; Wang, C.; Song, R. Degradation behavior of n-MAO/EPD bio-ceramic composite coatings on magnesium alloy in simulated body fluid. J. Alloy. Compd. 2015, 625, 258–265. [Google Scholar] [CrossRef]
- Fernando, N.L.; Kottegoda, N.; Jayanetti, S.; Karunaratne, V.; Jayasundara, D.R. Stability of nano-hydroxyapatite thin coatings at liquid/solid interface. Surf. Coat. Technol. 2018, 349, 24–31. [Google Scholar] [CrossRef]
- Yu, J.M.; Choe, H.C. Mg-containing hydroxyapatite coatings on Ti-6Al-4V alloy for dental materials. Appl. Surf. Sci. 2018, 432, 294–299. [Google Scholar] [CrossRef]
- Jeong, Y.H.; Kim, E.J.; Brantley, W.A.; Choe, H.C. Morphology of hydroxyapatite nanoparticles in coatings on nanotube-formed Ti-Nb-Zr alloys for dental implants. Vacuum 2014, 107, 297–303. [Google Scholar] [CrossRef]
- Bucur, A.I.; Linul, E.; Taranu, B.O. Hydroxyapatite coatings on Ti substrates by simultaneous precipitation and electrodeposition. Appl. Surf. Sci. 2020, 527, 146820. [Google Scholar] [CrossRef]
- Sun, X.; Lin, H.; Zhang, C.; Jin, J.; Di, S. Electrochemical studies on CaP Electrodeposition on three dimensional surfaces of selective laser melted titanium scaffold. Coatings 2019, 9, 667. [Google Scholar] [CrossRef]
- Parcharoen, Y.; Kajitvichyanukul, P.; Sirivisoot, S.; Termsuksawad, P. Hydroxyapatite electrodeposition on anodized titanium nanotubes for orthopedic applications. Appl. Surf. Sci. 2014, 311, 54–61. [Google Scholar] [CrossRef]
- Vidal, E.; Buxadera-Palomero, J.; Pierre, C.; Manero, J.M.; Ginebra, M.P.; Cazalbou, S.; Combes, C.; Rupérez, E.; Rodríguez, D. Single-step pulsed electrodeposition of calcium phosphate coatings on titanium for drug delivery. Surf. Coat. Technol. 2019, 358, 266–275. [Google Scholar] [CrossRef]
- Gopi, D.; Indira, J.; Kavitha, L. A comparative study on the direct and pulsed current electrodeposition of hydroxyapatite coatings on surgical grade stainless steel. Surf. Coat. Technol. 2012, 206, 2859–2869. [Google Scholar] [CrossRef]
- Thanh, D.T.M.; Nam, P.T.; Phuong, N.T.; Que, L.X.; Van Anh, N.; Hoang, T.; Lam, T.D. Controlling the electrodeposition, morphology and structure of hydroxyapatite coating on 316L stainless steel. Mater. Sci. Eng. C 2013, 33, 2037–2045. [Google Scholar] [CrossRef] [PubMed]
- Büyüksağiş, A.; Bulut, E.; Kayalı, Y. Corrosion behaviors of hydroxyapatite coated by electrodeposition method of Ti6Al4V, Ti and AISI 316L SS substrates. Prot. Met. Phys. Chem. Surf. 2013, 49, 776–787. [Google Scholar] [CrossRef]
- Chakraborty, R.; Sengupta, S.; Saha, P.; Das, K.; Das, S. Synthesis of calcium hydrogen phosphate and hydroxyapatite coating on SS316 substrate through pulsed electrodeposition. Mater. Sci. Eng. C 2016, 69, 875–883. [Google Scholar] [CrossRef]
- Chakraborty, R.; Saha, P. A comparative study on surface morphology and electrochemical behaviour of hydroacxyapatite-calcium hydrogen phosphate composite coating synthesized in-situ through electro chemical process under various deposition conditions. Surf. Interfaces 2018, 12, 160–167. [Google Scholar] [CrossRef]
- Shibli, S.M.A.; Jayalekshmi, A.C. A novel nano hydroxyapatite-incorporated Ni-P coating as an effective inter layer for biological applications. J. Mater. Sci. Mater. Med. 2009, 20, 711–718. [Google Scholar] [CrossRef]
- Lin, D.Y.; Wang, X.X. Electrodeposition of hydroxyapatite coating on CoNiCrMo substrate in dilute solution. Surf. Coat. Technol. 2010, 204, 3205–3213. [Google Scholar] [CrossRef]
- Coşkun, M.I.; Karahan, I.H.; Yücel, Y. Optimized electrodeposition concentrations for hydroxyapatite coatings on CoCrMo biomedical alloys by computational techniques. Electrochim. Acta 2014, 150, 46–54. [Google Scholar] [CrossRef]
- Etminanfar, M.R.; Khalil-Allafi, J.; Montaseri, A.; Vatankhah-Barenji, R. Endothelialization and the bioactivity of Ca-P coatings of different Ca/P stoichiometry electrodeposited on the Nitinol superelastic alloy. Mater. Sci. Eng. C 2016, 62, 28–35. [Google Scholar] [CrossRef]
- Etminanfar, M.R.; Khalil-Allafi, J.; Parsa, A.B. On the electrocrystallization of pure hydroxyapatite nanowalls on Nitinol alloy using a bipolar pulsed current. J. Alloy. Compd. 2016, 678, 549–555. [Google Scholar] [CrossRef]
- Marashi-Najafi, F.; Khalil-Allafi, J.; Etminanfar, M.R. Biocompatibility of hydroxyapatite coatings deposited by pulse electrodeposition technique on the Nitinol superelastic alloy. Mater. Sci. Eng. C 2017, 76, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Sheykholeslami, S.O.R.; Khalil-Allafi, J.; Fathyunes, L. Preparation, characterization, and corrosion behavior of calcium phosphate coating electrodeposited on the modified nanoporous surface of NiTi alloy for biomedical applications. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2018, 49, 5878–5887. [Google Scholar] [CrossRef]
- Song, Y.W.; Shan, D.Y.; Han, E.H. Electrodeposition of hydroxyapatite coating on AZ91D magnesium alloy for biomaterial application. Mater. Lett. 2008, 62, 3276–3279. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Zeng, R.C.; Chen, R.S.; Liu, C.L.; Gao, J.C. Preparation of calcium phosphate coatings on Mg-1.0Ca alloy. Trans. Nonferrous Met. Soc. China Engl. Ed. 2010, 20, 655–659. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, S.; Li, J.; Zhao, C.; Zhang, X. Electrodeposition of Ca-P coatings on biodegradable Mg alloy: In vitro biomineralization behavior. Acta Biomater. 2010, 6, 1736–1742. [Google Scholar] [CrossRef]
- Saremi, M.; Mohajernia, S.; Hejazi, S. Controlling the degradation rate of AZ31 Magnesium alloy and purity of nano-hydroxyapatit coating by pulse electrodeposition. Mater. Lett. 2014, 129, 111–113. [Google Scholar] [CrossRef]
- Seyedraoufi, Z.S.; Mirdamadi, S. Effects of pulse electrodeposition parameters and alkali treatment on the properties of nano hydroxyapatite coating on porous MgeZn scaffold for bone tissue engineering application. Mater. Chem. Phys. 2014, 148, 519–527. [Google Scholar] [CrossRef]
- Seyedraoufi, Z.S.; Mirdamadi, S. In vitro biodegradability and biocompatibility of porous Mg-Zn scaffolds coated with nano hydroxyapatite via pulse electrodeposition. Trans. Nonferrous Met. Soc. China Engl. Ed. 2015, 25, 4018–4027. [Google Scholar] [CrossRef]
- Monasterio, N.; Ledesma, J.L.; Aranguiz, I.; Garcia-Romero, A.; Zuza, E. Analysis of electrodeposition processes to obtain calcium phosphate layer on AZ31 alloy. Surf. Coat. Technol. 2017, 319, 12–22. [Google Scholar] [CrossRef]
- Han, J.; Blawert, C.; Tang, S.; Yang, J.; Hu, J.; Zheludkevich, M.L. Effect of surface pre-treatments on the formation and degradation behaviour of a calcium phosphate coating on pure magnesium. Coatings 2019, 9, 259. [Google Scholar] [CrossRef]
- Liao, Y.M.; De Feng, Z.; Li, S.W. Preparation and characterization of hydroxyapatite coatings on human enamel by electrodeposition. Thin Solid Film. 2008, 516, 6145–6150. [Google Scholar] [CrossRef]
- Benea, L.; Danaila, E.; Ponthiaux, P. Effect of titania anodic formation and hydroxyapatite electrodeposition on electrochemical behaviour of Ti-6Al-4V alloy under fretting conditions for biomedical applications. Corros. Sci. 2015, 91, 262–271. [Google Scholar] [CrossRef]
- Liu, S.; Li, H.; Zhang, L.; Yin, X.; Guo, Y. In simulated body fluid performance of polymorphic apatite coatings synthesized by pulsed electrodeposition. Mater. Sci. Eng. C 2017, 79, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Chen, X.; Zhang, L.; Liu, Q.; Wang, Y.; Zhang, W.; Zheng, J. Preparation of nano-hydroxyapatite coated carbon nanotube reinforced hydroxyapatite composites. Coatings 2018, 8, 357. [Google Scholar] [CrossRef]
- Li, T.T.; Ling, L.; Lin, M.C.; Jiang, Q.; Lin, Q.; Lou, C.W.; Lin, J.H. Effects of ultrasonic treatment and current density on the properties of hydroxyapatite coating via electrodeposition and its in vitro biomineralization behavior. Mater. Sci. Eng. C 2019, 105, 110062. [Google Scholar] [CrossRef] [PubMed]
- Gopi, D.; Karthika, A.; Nithiya, S.; Kavitha, L. In vitro biological performance of minerals substituted hydroxyapatite coating by pulsed electrodeposition method. Mater. Chem. Phys. 2014, 144, 75–85. [Google Scholar] [CrossRef]
- Drevet, R.; Benhayoune, H. Pulsed electrodeposition for the synthesis of strontium-substituted calcium phosphate coatings with improved dissolution properties. Mater. Sci. Eng. C 2013, 33, 4260–4265. [Google Scholar] [CrossRef]
- Yajing, Y.; Qiongqiong, D.; Yong, H.; Han, S.; Pang, X. Magnesium substituted hydroxyapatite coating on titanium with nanotublar TiO2 intermediate layer via electrochemical deposition. Appl. Surf. Sci. 2014, 305, 77–85. [Google Scholar] [CrossRef]
- Huang, Y.; Hao, M.; Nian, X.; Qiao, H.; Zhang, X.X.; Zhang, X.X.; Song, G.; Guo, J.; Pang, X.; Zhang, H. Strontium and copper co-substituted hydroxyapatite-based coatings with improved antibacterial activity and cytocompatibility fabricated by electrodeposition. Ceram. Int. 2016, 42, 11876–11888. [Google Scholar] [CrossRef]
- Huang, Y.; Qiao, H.; Nian, X.; Zhang, X.; Zhang, X.; Song, G.; Xu, Z.; Zhang, H.; Han, S. Improving the bioactivity and corrosion resistance properties of electrodeposited hydroxyapatite coating by dual doping of bivalent strontium and manganese ion. Surf. Coat. Technol. 2016, 291, 205–215. [Google Scholar] [CrossRef]
- Fu, D.L.; Jiang, Q.H.; He, F.M.; Fu, B.P. Adhesion of bone marrow mesenchymal stem cells on porous titanium surfaces with strontium-doped hydroxyapatite coating. J. Zhejiang Univ. Sci. B 2017, 18, 778–788. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, W.; Zhang, X.; Liu, X.; Xu, Z.; Han, S.; Su, Z.; Liu, H.; Gao, Y.; Yang, H. A prospective material for orthopedic applications: Ti substrates coated with a composite coating of a titania-nanotubes layer and a silver-manganese-doped hydroxyapatite layer. Ceram. Int. 2017, 44, 5528–5542. [Google Scholar] [CrossRef]
- Karthika, A. Aliovalent ions substituted hydroxyapatite coating on titanium for improved medical applications. Mater. Today Proc. 2018, 5, 8768–8774. [Google Scholar] [CrossRef]
- Drevet, R.; Zhukova, Y.; Dubinskiy, S.; Kazakbiev, A.; Naumenko, V.; Abakumov, M.; Fauré, J.; Benhayoune, H.; Prokoshkin, S. Electrodeposition of cobalt-substituted calcium phosphate coatings on Ti22Nb6Zr alloy for bone implant applications. J. Alloys Compd. 2019, 793, 576–582. [Google Scholar] [CrossRef]
- Furko, M.; Della Bella, E.; Fini, M.; Balázsi, C. Corrosion and biocompatibility examination of multi-element modified calcium phosphate bioceramic layers. Mater. Sci. Eng. C 2019, 95, 381–388. [Google Scholar] [CrossRef]
- Meng, E.C.; Guan, S.K.; Wang, H.X.; Wang, L.G.; Zhu, S.J.; Hu, J.H.; Ren, C.X.; Gao, J.H.; Feng, Y.S. Effect of electrodeposition modes on surface characteristics and corrosion properties of fluorine-doped hydroxyapatite coatings on Mg-Zn-Ca alloy. Appl. Surf. Sci. 2011, 257, 4811–4816. [Google Scholar] [CrossRef]
- Qiu, X.; Wan, P.; Tan, L.; Fan, X.; Yang, K. Preliminary research on a novel bioactive silicon doped calcium phosphate coating on AZ31 magnesium alloy via electrodeposition. Mater. Sci. Eng. C 2014, 36, 65–76. [Google Scholar] [CrossRef]
- Aboudzadeh, N.; Dehghanian, C.; Shokrgozar, M.A. Effect of electrodeposition parameters and substrate on morphology of Si-HA coating. Surf. Coat. Technol. 2019, 375, 341–351. [Google Scholar] [CrossRef]
- Liu, S.J.; Li, H.J.; Zhang, L.L.; Feng, L.; Yao, P. Strontium and magnesium substituted dicalcium phosphate dehydrate coating for carbon/carbon composites prepared by pulsed electrodeposition. Appl. Surf. Sci. 2015, 359, 288–292. [Google Scholar] [CrossRef]
- Peñaflor Galindo, T.G.; Kataoka, T.; Fujii, S.; Okuda, M.; Tagaya, M. Preparation of nanocrystalline zinc-substituted hydroxyapatite films and their biological properties. Colloids Interface Sci. Commun. 2016, 10–11, 15–19. [Google Scholar] [CrossRef]
- Huang, C.N.; Tang, Z.T.; Chan, F.E.; Burnouf, T.; Huang, W.C.; Chen, P.C. Fabrication of co-electrodeposition of plasma proteins/iridium oxide hybrid films. Ceram. Int. 2018, 44, 117–120. [Google Scholar] [CrossRef]
- Gao, X.; Gui, R.; Guo, H.; Wang, Z.; Liu, Q. Creatinine-induced specific signal responses and enzymeless ratiometric electrochemical detection based on copper nanoparticles electrodeposited on reduced graphene oxide-based hybrids. Sens. Actuators B Chem. 2019, 285, 201–208. [Google Scholar] [CrossRef]
- Benea, L.; Celis, J.P. Reactivity of porous titanium oxide film and chitosan layer electrochemically formed on Ti-6Al-4V alloy in biological solution. Surf. Coat. Technol. 2018, 354, 145–152. [Google Scholar] [CrossRef]
- Nakano, K.; Egashira, K.; Masuda, S.; Funakoshi, K.; Zhao, G.; Kimura, S.; Matoba, T.; Sueishi, K.; Endo, Y.; Kawashima, Y.; et al. Formulation of nanoparticle-eluting stents by a cationic electrodeposition coating technology. efficient nano-drug delivery via bioabsorbable polymeric nanoparticle-eluting stents in porcine coronary arteries. JACC Cardiovasc. Interv. 2009, 2, 277–283. [Google Scholar] [CrossRef]
- Jiang, P.L.; Hou, R.Q.; Chen, C.D.; Sun, L.; Dong, S.G.; Pan, J.S.; Lin, C.J. Controllable degradation of medical magnesium by electrodeposited composite films of mussel adhesive protein (Mefp-1) and chitosan. J. Colloid Interface Sci. 2016, 478, 246–255. [Google Scholar] [CrossRef]
- Meng, L.; Li, Y.; Pan, K.; Zhu, Y.; Wei, W.; Li, X.; Liu, X. Colloidal particle based electrodeposition coatings on NiTi alloy: Reduced releasing of nickel ions and improved biocompatibility. Mater. Lett. 2018, 230, 228–231. [Google Scholar] [CrossRef]
- Sak, A.; Moskalewicz, T.; Zimowski, S.; Cieniek, Ł.; Dubiel, B.; Radziszewska, A.; Kot, M.; Łukaszczyk, A. Influence of polyetheretherketone coatings on the Ti-13Nb-13Zr titanium alloy’s bio-tribological properties and corrosion resistance. Mater. Sci. Eng. C 2016, 63, 52–61. [Google Scholar] [CrossRef]
- Chakraborty, R.; Seesala, V.S.; Sengupta, S.; Dhara, S.; Saha, P.; Das, K.; Das, S. Comparison of Osteoconduction, cytocompatibility acand corrosion protection performance of hydroxyapatite-calcium hydrogen phosphate composite coating synthesized in-situ through pulsed electro-deposition with varying amount of phase and crystallinity. Surf. Interfaces 2018, 10, 1–10. [Google Scholar] [CrossRef]
- Shojaee, P.; Afshar, A. Effects of zirconia content on characteristics and corrosion behavior of hydroxyapatite/ZrO2 biocomposite coatings codeposited by electrodeposition. Surf. Coat. Technol. 2015, 262, 166–172. [Google Scholar] [CrossRef]
- Chakraborty, R.; Seesala, V.S.; Sen, M.; Sengupta, S.; Dhara, S.; Saha, P.; Das, K.; Das, S. MWCNT reinforced bone like calcium phosphate—Hydroxyapatite composite coating developed through pulsed electrodeposition with varying amount of apatite phase and crystallinity to promote superior osteoconduction, cytocompatibility and corrosion protection. Surf. Coat. Technol. 2017, 325, 496–514. [Google Scholar] [CrossRef]
- Huang, Y.; Han, S.; Pang, X.; Ding, Q.; Yan, Y. Electrodeposition of porous hydroxyapatite/calcium silicate composite coating on titanium for biomedical applications. Appl. Surf. Sci. 2013, 271, 299–302. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, H.; Qiao, H.; Nian, X.; Zhang, X.; Wang, W.; Zhang, X.; Chang, X.; Han, S.; Pang, X. Anticorrosive effects and in vitro cytocompatibility of calcium silicate/zinc-doped hydroxyapatite composite coatings on titanium. Appl. Surf. Sci. 2015, 357, 1776–1784. [Google Scholar] [CrossRef]
- Mostafa, N.Y.; Montaser, A.; Al-Affray, R.A.; Kamel, M.M.; Alhadhrami, A. Processing and characterization of novel calcium titanate/Na-titanate nanotube/rutile nanocomposite coating on titanium metal. Appl. Phys. A Mater. Sci. Process. 2019, 125, 1–6. [Google Scholar] [CrossRef]
- Chozhanathmisra, M.; Murugan, N.; Karthikeyan, P.; Sathishkumar, S.; Anbarasu, G.; Rajavel, R. Development of antibacterial activity and corrosion resistance properties of electrodeposition of mineralized hydroxyapatite coated on titanium alloy for biomedical applications. Mater. Today Proc. 2017, 4, 12393–12400. [Google Scholar] [CrossRef]
- Chozhanathmisra, M.; Ramya, S.; Kavitha, L.; Gopi, D. Development of zinc-halloysite nanotube/minerals substituted hydroxyapatite bilayer coatings on titanium alloy for orthopedic applications. Colloids Surf. A Physicochem. Eng. Asp. 2016, 511, 357–365. [Google Scholar] [CrossRef]
- Fathyunes, L.; Khalil-Allafi, J. Characterization and corrosion behavior of graphene oxide-hydroxyapatite composite coating applied by ultrasound-assisted pulse electrodeposition. Ceram. Int. 2017, 43, 13885–13894. [Google Scholar] [CrossRef]
- Fathyunes, L.; Khalil-Allafi, J.; Sheykholeslami, S.O.R.; Moosavifar, M. Biocompatibility assessment of graphene oxide-hydroxyapatite coating applied acon TiO2 nanotubes by ultrasound-assisted pulse electrodeposition. Mater. Sci. Eng. C 2018, 87, 10–21. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, F.; Li, H.; Zhao, F.; Li, S. A duplex coating composed of electrophoretic deposited graphene oxide inner-layer and electrodeposited graphene oxide/Mg substituted hydroxyapatite outer-layer on carbon/carbon composites for biomedical application. Ceram. Int. 2018, 44, 21229–21237. [Google Scholar] [CrossRef]
- Gopi, D.; Shinyjoy, E.; Sekar, M.; Surendiran, M.; Kavitha, L.; Sampath Kumar, T.S. Development of carbon nanotubes reinforced hydroxyapatite composite coatings on titanium by electrodeposition method. Corros. Sci. 2013, 73, 321–330. [Google Scholar] [CrossRef]
- Fathyunes, L.; Khalil-Allafi, J.; Moosavifar, M. Development of graphene oxide/calcium phosphate coating by pulse electrodeposition on anodized titanium: Biocorrosion and mechanical behaavior. J. Mech. Behav. Biomed. Mater. 2019, 90, 575–586. [Google Scholar] [CrossRef] [PubMed]
- Khazeni, D.; Saremi, M.; Soltani, R. Development of HA-CNTs composite coating on AZ31 magnesium alloy by cathodic electrodeposition. Part 1: Microstructural and mechanical characterization. Ceram. Int. 2019, 45, 11174–11185. [Google Scholar] [CrossRef]
- Chakraborty, R.; Manna, J.S.; Das, D.; Sen, M.; Saha, P. A comparative outlook of corrosion behaviour and chlorophyll assisted growth kinetics of various carbon nano-structure reinforced hydroxyapatite-calcium orthophosphate coating synthesized in-situ through pulsed electrochemical deposition. Appl. Surf. Sci. 2019, 475, 28–42. [Google Scholar] [CrossRef]
- Długoń, E.; Niemiec, W.; Fra̧czek-Szczypta, A.; Jeleń, P.; Sitarz, M.; Błazewicz, M. Spectroscopic studies of electrophoretically deposited hybrid HAp/CNT coatings on titanium. Spectrochim. Acta Part. A Mol. Biomol. Spectrosc. 2014, 133, 872–875. [Google Scholar] [CrossRef]
- Maleki-Ghaleh, H.; Khalil-Allafi, J. Characterization, mechanical and in vitro biological behavior of hydroxyapatite-titanium-carbon nanotube composite coatings deposited on NiTi alloy by electrophoretic deposition. Surf. Coat. Technol. 2019, 363, 179–190. [Google Scholar] [CrossRef]
- Poorraeisi, M.; Afshar, A. The study of electrodeposition of hydroxyapatite-ZrO2-TiO2 nanocomposite coatings on 316 stainless steel. Surf. Coat. Technol. 2018, 339, 199–207. [Google Scholar] [CrossRef]
- Pei, L.; Zhang, B.; Luo, H.; Wu, X.; Li, G.; Sheng, H.; Zhang, L. Electrodeposition of ZnO Nanoprism-Zn substituted hydroxyapatite duplex layer coating for carbon fiber. Ceram. Int. 2019, 45, 14278–14286. [Google Scholar] [CrossRef]
- Göncü, Y.; Geçgin, M.; Bakan, F.; Ay, N. Electrophoretic deposition of hydroxyapatite-hexagonal boron nitride composite coatings on Ti substrate. Mater. Sci. Eng. C 2017, 79, 343–353. [Google Scholar] [CrossRef]
- Khanmohammadi, S.; Ilkhchi, M.O.; Khalil-Allafi, J. Electrophoretic deposition and characterization of bioglass-whisker hydroxyapatite nanocomposite coatings on titanium substrate. Surf. Coat. Technol. 2019, 378, 124949. [Google Scholar] [CrossRef]
- Yılmaz, E.; Çakıroğlu, B.; Gökçe, A.; Findik, F.; Gulsoy, H.O.; Gulsoy, N.; Mutlu, Ö.; Özacar, M. Novel hydroxyapatite/graphene oxide/collagen bioactive composite coating on Ti16Nb alloys by electrodeposition. Mater. Sci. Eng. C 2019, 101, 292–305. [Google Scholar] [CrossRef]
- Kumar, A.M.; Adesina, A.Y.; Hussein, M.A.; Ramakrishna, S.; Al-Aqeeli, N.; Akhtar, S.; Saravanan, S. PEDOT/FHA nanocomposite coatings on newly developed Ti-Nb-Zr implants: Biocompatibility and surface protection against corrosion and bacterial infections. Mater. Sci. Eng. C 2019, 98, 482–495. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, R.; Seesala, V.S.; Manna, J.S.; Saha, P.; Dhara, S. Synthesis, characterization and cytocompatibility assessment of hydroxyapatite-polypyrrole composite coating synthesized through pulsed reverse electrochemical deposition. Mater. Sci. Eng. C 2019, 94, 597–607. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Zhitomirsky, I. Electrodeposition of hydroxyapatite-silver-chitosan nanocomposite coatings. Surf. Coat. Technol. 2008, 202, 3815–3821. [Google Scholar] [CrossRef]
- Yan, Y.; Zhang, X.; Li, C.; Huang, Y.; Ding, Q.; Pang, X. Preparation and characterization of chitosan-silver/hydroxyapatite composite coatings onTiO2 nanotube for biomedical applications. Appl. Surf. Sci. 2015, 332, 62–69. [Google Scholar] [CrossRef]
- Zhao, P.; Zhao, Y.; Xiao, L.; Deng, H.; Du, Y.; Chen, Y.; Shi, X. Electrodeposition to construct free-standing chitosan/layered double hydroxides hydro-membrane for electrically triggered protein release. Colloids Surf. B Biointerfaces 2017, 158, 474–479. [Google Scholar] [CrossRef]
- Zhong, Z.; Qin, J.; Ma, J. Electrophoretic deposition of biomimetic zinc substituted hydroxyapatite coatings with chitosan and carbon nanotubes on titanium. Ceram. Int. 2015, 41, 8878–8884. [Google Scholar] [CrossRef]
- Clifford, A.; Lee, B.E.J.; Grandfield, K.; Zhitomirsky, I. Biomimetic modification of poly-L-lysine and electrodeposition of nanocomposite coatings for orthopaedic applications. Colloids Surf. B Biointerfaces 2019, 176, 115–121. [Google Scholar] [CrossRef]
- Baştan, F.E.; Atiq Ur Rehman, M.; Avcu, Y.Y.; Avcu, E.; Üstel, F.; Boccaccini, A.R. Electrophoretic co-deposition of PEEK-hydroxyapatite composite coatings for biomedical applications. Colloids Surf. B Biointerfaces 2018, 169, 176–182. [Google Scholar] [CrossRef]
- Patiño-Herrera, R.; González-Alatorre, G.; Estrada-Baltazar, A.; Escoto-Chavéz, S.E.; Pérez, E. Hydrophobic coatings for prevention of dental enamel erosion. Surf. Coat. Technol. 2015, 275, 148–154. [Google Scholar] [CrossRef]
- Khabazian, S.; Sanjabi, S. The effect of multi-walled carbon nanotube pretreatments on the electrodeposition of Ni-MWCNTs coatings. Appl. Surf. Sci. 2011, 257, 5850–5856. [Google Scholar] [CrossRef]
- El-Wassefy, N.A.; Reicha, F.M.; Aref, N.S. Electro-chemical deposition of nano hydroxyapatite-zinc coating on titanium metal substrate. Int. J. Implant. Dent. 2017, 3, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Zhang, X.; Savino, K.; Gabrys, P.; Gao, Y.; Chaimayo, W.; Miller, B.L.; Yates, M.Z. Antimicrobial silver-hydroxyapatite composite coatings through two-stage electrochemical synthesis. Surf. Coat. Technol. 2016, 301, 13–19. [Google Scholar] [CrossRef]
- Bartmanski, M.; Cieslik, B.; Glodowska, J.; Kalka, P.; Pawlowski, L.; Pieper, M.; Zielinski, A. Electrophoretic deposition (EPD) of nanohydroxyapatite-nanosilver coatings on Ti13Zr13Nb alloy. Ceram. Int. 2017, 43, 11820–11829. [Google Scholar] [CrossRef]
- Bartmanski, M. The properties of nanosilver—Doped nanohydroxyapatite coating on the Ti13zr13Nb alloy. Adv. Mater. Sci. 2017, 17, 18–28. [Google Scholar] [CrossRef]
- Bartmanski, M.; Zielinski, A.; Jazdzewska, M.; Głodowska, J.; Kalka, P. Effects of electrophoretic deposition times and nanotubular oxide surfaces on properties of the nanohydroxyapatite/nanocopper coating on the Ti13Zr13Nb alloy. Ceram. Int. 2019, 45, 20002–20010. [Google Scholar] [CrossRef]
- Majkowska-Marzec, B.; Rogala-Wielgus, D.; Bartmański, M.; Bartosewicz, B.; Zieliński, A.S. Comparison of Properties of the Hybrid and Bilayer MWCNTs—Hydroxyapatite Coatings on Ti Alloy. Coatings 2019, 9, 643. [Google Scholar] [CrossRef]
- Jang, J.M.; Kim, S.D.; Park, T.E.; Choe, H.C. Ultra-fine structures of Pd-Ag-HAp nanoparticle deposition on protruded TiO2 barrier layer for dental implant. Appl. Surf. Sci. 2018, 432, 285–293. [Google Scholar] [CrossRef]
- Silva-Ichante, M.; Reyes-Vidal, Y.; Bácame-Valenzuela, F.J.; Ballesteros, J.C.; Arciga, E.; Ţălu, Ş.; Méndez-Albores, A.; Trejo, G. Electrodeposition of antibacterial Zn-Cu/silver nanoparticle (AgNP) composite coatings from an alkaline solution containing glycine and AgNPs. J. Electroanal. Chem. 2018, 823, 328–334. [Google Scholar] [CrossRef]
- Kumar, A.M.; Nagarajan, S.; Ramakrishna, S.; Sudhagar, P.; Kang, Y.S.; Kim, H.; Gasem, Z.M.; Rajendran, N. Electrochemical and in vitro bioactivity of polypyrrole/ceramic nanocomposite coatings on 316L SS bio-implants. Mater. Sci. Eng. C 2014, 43, 76–85. [Google Scholar] [CrossRef]
- Lázaro-Martínez, J.M.; Byrne, A.J.; Rodríguez-Castellón, E.; Manrique, J.M.; Jones, L.R.; Dall’Orto, V.C. Linear polyethylenimine-decorated gold nanoparticles: One-step electrodeposition and studies of interaction with viral and animal proteins. Electrochim. Acta 2019, 301, 126–135. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, X.; Gu, J.; Yang, H.; Nie, J.; Ma, G. Electrodeposition of alginate/chitosan layer-by-layer composite coatings on titanium substrates. Carbohydr. Polym. 2014, 103, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.M.; Suresh, B.; Das, S.; Obot, I.B.; Adesina, A.Y.; Ramakrishna, S. Promising bio-composites of polypyrrole and chitosan: Surface protective and in vitro biocompatibility performance on 316L SS implants. Carbohydr. Polym. 2017, 173, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Pawłowski, Ł.; Bartmański, M.; Strugała, G.; Mielewczyk-Gryń, A.; Jażdżewska, M.; Zieliński, A. Electrophoretic deposition and characterization of Chitosan/Eudragit E 100 coatings on titanium substrate. Coatings 2020, 10, 607. [Google Scholar] [CrossRef]
- Bonifacio, M.A.; Cometa, S.; Dicarlo, M.; Baruzzi, F.; de Candia, S.; Gloria, A.; Giangregorio, M.M.; Mattioli-Belmonte, M.; De Giglio, E. Gallium-modified chitosan/poly(acrylic acid) bilayer coatings for improved titanium implant performances. Carbohydr. Polym. 2017, 166, 348–357. [Google Scholar] [CrossRef]
- Trzaskowska, P.A.; Poniatowska, A.; Tokarska, K.; Wiśniewski, C.; Ciach, T.; Malinowska, E. Promising electrodeposited biocompatible coatings for steel obtained from polymerized microemulsions. Colloids Surf. A Physicochem. Eng. Asp. 2020, 591, 124555. [Google Scholar] [CrossRef]
- Liu, L.; Qi, W.; Gao, X.; Wang, C.; Wang, G. Synergistic effect of metal ion additives on graphitic carbon nitride nanosheet-templated electrodeposition of Cu@CuO for enzyme-free glucose detection. J. Alloys Compd. 2018, 745, 155–163. [Google Scholar] [CrossRef]
- Zhao, Z.W.; Zhang, G.; Li, H.G. Preparation of calcium phosphate coating on pure titanium substrate by electrodeposition method. J. Cent. South. Univ. Technol. Engl. Ed. 2004, 11, 147–151. [Google Scholar] [CrossRef]
- Hou, X.; Liu, X.; Xu, J.; Shen, J.; Liu, X. A self-optimizing electrodeposition process for fabrication of calcium phosphate coatings. Mater. Lett. 2001, 50, 103–107. [Google Scholar] [CrossRef]
- Manso, M.; Jiménez, C.; Morant, C.; Herrero, P.; Martínez-Duart, J. Electrodeposition of hydroxyapatite coatings in basic conditions. Biomaterials 2000, 21, 1755–1761. [Google Scholar] [CrossRef]
- Beaufils, S.; Rouillon, T.; Millet, P.; Le Bideau, J.; Weiss, P.; Chopart, J.P.; Daltin, A.L. Synthesis of calcium-deficient hydroxyapatite nanowires and nanotubes performed by template-assisted electrodeposition. Mater. Sci. Eng. C 2019, 98, 333–346. [Google Scholar] [CrossRef]
- Khalili, V.; Khalil-Allafi, J.; Frenzel, J.; Eggeler, G. Bioactivity and electrochemical behavior of hydroxyapatite-silicon-multi walled carbon nano-tubes composite coatings synthesized by EPD on NiTi alloys in simulated body fluid. Mater. Sci. Eng. C 2017, 71, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Pawlik, A.; Rehman, M.A.U.; Nawaz, Q.; Bastan, F.E.; Sulka, G.D.; Boccaccini, A.R. Fabrication and characterization of electrophoretically deposited chitosan-hydroxyapatite composite coatings on anodic titanium dioxide layers. Electrochim. Acta 2019, 307, 465–473. [Google Scholar] [CrossRef]
- Molaei, A.; Yari, M.; Afshar, M.R. Modification of electrophoretic deposition of chitosan-bioactive glass-hydroxyapatite nanocomposite coatings for orthopedic applications by changing voltage and deposition time. Ceram. Int. 2015, 41, 14537–14544. [Google Scholar] [CrossRef]
- Durdu, S.; Usta, M.; Berkem, A.S. Bioactive coatings on Ti6Al4V alloy formed by plasma electrolytic oxidation. Surf. Coat. Technol. 2016, 301, 85–93. [Google Scholar] [CrossRef]
- Liu, S.; Li, B.; Liang, C.; Wang, H.; Qiao, Z. Formation mechanism and adhesive strength of a hydroxyapatite/TiO2composite coating on a titanium surface prepared by micro-arc oxidation. Appl. Surf. Sci. 2016, 362, 109–114. [Google Scholar] [CrossRef]
- Shi, M.; Li, H. The morphology, structure and composition of microarc oxidation (MAO) ceramic coating in Ca-P electrolyte with complexing agent EDTMPS and interpretation hypothesis of MAO process. Surf. Eng. Appl. Electrochem. 2016, 52, 32–42. [Google Scholar] [CrossRef]
- Mao, Y.; Yan, J.; Wang, L.; Dong, W.; Jia, Y.; Hu, X.; Wang, X. Formation and properties of bioactive barium titanate coatings produced by plasma electrolytic oxidation. Ceram. Int. 2018, 44, 12978–12986. [Google Scholar] [CrossRef]
- Luo, S.; Wang, Q.; Ye, R.; Ramachandran, C.S. Effects of electrolyte concentration on the microstructure and properties of plasma electrolytic oxidation coatings on Ti-6Al-4V alloy. Surf. Coat. Technol. 2019, 375, 864–876. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, Y.; Jiang, D.; Jiao, Y.; Wu, Y.; Peng, Z.; Zhou, J.; Wu, J.; Dong, Z. Synthesis and characterization of a bi-functional hydroxyapatite/Cu-doped TiO2 composite coating. Ceram. Int. 2019, 45, 6693–6701. [Google Scholar] [CrossRef]
- Dziaduszewska, M.; Shimabukuro, M.; Seramak, T.; Zielinski, A. Effects of micro-arc oxidation process parameters on characteristics of calcium-phosphate containing oxide layers on the selective laser melted Ti13Zr13Nb alloy. Coatings 2020, 10, 745. [Google Scholar] [CrossRef]
- Antonio, R.F.; Rangel, E.C.; Mas, B.A.; Duek, E.A.R.; Cruz, N.C. Growth of hydroxyapatite coatings on tantalum by plasma electrolytic oxidation in a single step. Surf. Coat. Technol. 2019, 357, 698–705. [Google Scholar] [CrossRef]
- Cengiz, S.; Azakli, Y.; Tarakci, M.; Stanciu, L.; Gencel, Y. Microarc oxidation type discharge types and bio properties of the coating synthesized on zirconium. Mater. Sci. Eng. C. 2017, 77, 374–383. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.K.; Jang, Y.S.; Kim, S.Y.; Lee, M.H. Functions achieved by the hyaluronic acid derivatives coating and hydroxide film on bio-absorbed Mg. Appl. Surf. Sci. 2019, 473, 31–39. [Google Scholar] [CrossRef]
- Durdu, S.; Aktuǧ, S.L.; Korkmaz, K. Characterization and mechanical properties of the duplex coatings produced on steel by electro-spark deposition and micro-arc oxidation. Surf. Coat. Technol. 2013, 236, 303–308. [Google Scholar] [CrossRef]
- Durdu, S.; Korkmaz, K.; Aktuğ, S.L.; Çakır, A. Characterization and bioactivity of hydroxyapatite-based coatings formed on steel by electro-spark deposition and micro-arc oxidation. Surf. Coat. Technol. 2017, 326, 111–120. [Google Scholar] [CrossRef]
- Aliyu, A.A.; Abdul-Rani, A.M.; Rao, T.V.V.L.N.; Axinte, E.; Hastuty, S.; Parameswari, R.P.; Subramaniam, J.R.; Thyagarajan, S.P. Characterization, adhesion strength and in-vitro cytotoxicity investigation of hydroxyapatite coating synthesized on Zr-based BMG by electro discharge process. Surf. Coat. Technol. 2019, 370, 213–226. [Google Scholar] [CrossRef]
- Devgan, S.; Sidhu, S.S. Surface modification of β-type titanium with multi-walled CNTs/ μ-HAp powder mixed electro discharge treatment process. Mater. Chem. Phys. 2020, 239, 122005. [Google Scholar] [CrossRef]
- Momeni, M.M.; Nazari, Z. Pt/PANI-MWCNTs nanocomposite coating prepared by electropolymerisation-electrodeposition for glycerol electro-oxidation. Surf. Eng. 2015, 31, 472–479. [Google Scholar] [CrossRef]
- Rikhari, B.; Mani, S.P.; Rajendran, N. Electrochemical behavior of polypyrrole/chitosan composite coating on Ti metal for biomedical applications. Carbohydr. Polym. 2018, 189, 126–137. [Google Scholar] [CrossRef]
- Almeida, L.C.; Correia, R.D.; Squillaci, G.; Morana, A.; La Cara, F.; Correia, J.P.; Viana, A.S. Electrochemical deposition of bio-inspired laccase-polydopamine films for phenolic sensors. Electrochim. Acta 2019, 319, 462–471. [Google Scholar] [CrossRef]
| Coating; Substrate | Solution and pH | Current Density and/or Voltage Control | Deposition Time and Temperature | Reference |
|---|---|---|---|---|
| Metallic Coatings | ||||
| Bismuth | Bismuth nitrate in acetate pH 4.5 | −1.0 V | 100 s | [23] |
| Cu-Cu2O; graphitic carbon nitride | H2SO4, Cu2SO4, Ce and Ni salts | −0.8 V | - | [148] |
| Ni; Ti | nickel sulfate, nickel chloride, boric acid | 50 mA/cm2 | RT 15–60 s | [28] |
| Ni/Au; PC | NiSO4, H3BO3, KCl (for Ni) Au(CN)2 for Ag | 1.4 V | 1 min (for Ni) | [27] |
| Sr; Ti and TiZr | strontium-acetate and acetic acid, Sr-NaF, Sr-NaCl. pH 5 | 1.3 mA/cm2 | 21 °C 60 s | [26] |
| Pt-Ir | - | - | - | [24] |
| Ceramic Coatings | ||||
| Calcium phosphate (Ca-P); Ti | CaCl2, Ca(H2PO4)2, H2O2. pH 2.5–6.0 | −0.8 V vs. SCE. | 1.67–60 min 45–65 °C | [149] |
| Ca-P; Ti | NH4H2PO4, Ca(NO3)2. | - | 37 °C | [150] |
| Ca-P; Ti | NH4H2PO4, Ca(NO3)2, NaNO3. pH = 7.4 | 3.33 mA/cm2 | 159 min 24 °C | [106] |
| Ca-P; Ti | Modified SBF: NaCl, NaHCO3, KH2PO4, pH 7.4 | from −1.5 V to −2.5 V vs. SCE | 1 h 60 °C | [30,31] |
| Ca-P; precalcified (NaOH) Ti | Ca(NO3)2, NH4H2PO4. pH = 6 | 2.5 mA/cm2 | 60 min RT | [32] |
| Ca-P; Ti scaffold | CaCl2, NH4H2PO4 | - | - | [51] |
| Ca-P; Ti-Ni | Three different conditions; (i) Ca(NO3)2, NH4H2PO4, H2O2. pH = 4.3 (ii) and (iii) as above, pH = 6 | (i) −0.6 mA/cm2 (ii) −0.5 mA/cm2 (iii) −3 mA/cm2 for 1 s and reverse current 0.1 mA/cm2 for 2 s | (i) 70 °C (ii) and (iii) 65 °C | [63] |
| Ca-P; Mg | Ca(NO3)2 KH2PO4 pH = 4.6 | 3.5 V | 90 min 47 °C | [73] |
| Ca-P; Mg alloy | NaNO3, NH4H2PO4, Ca(NO3)2, H2O2. pH 5 | 2.5–20 V | 40–60 min 20 °C | [72] |
| Ca-P; Mg-1Ca | Ca(NO3)2, NH4H2PO4. pH = 5 | −2.5 V vs. SCE | 20–240 min | [67] |
| HAp; Ti | NH4H2PO4, Ca(NO3)2 pH = 7.2 | −2.5 V | 10 min 80 °C | [52] |
| HAp; Ti | Ca(NO3)2, NH4H2PO4. | −1.8 V vs. Ag/AgCl | 5 s 80 °C | [33] |
| HAp; Ti | (i) calcium acetate, acetic acid. (ii) Na3PO4, NaOH pH 9.1 | 2–4 V | 1 h | [151] |
| HAp | Supersaturated solution of Ca(NO3)2 and NH4H2PO4. | 1.5 V | 80 °C 1 and 4 h | [50] |
| HAp; Ti-6Al-4V | Ca(NO3)2, NH4(H2PO4), NaNO3. pH 4.2 | 0.6 mA/cm2 | 45 min RT | [75] |
| HAp; Ti, Ti6Al4V, stainless steels | Ca(NO3)2, NH4(H2PO4), NaNO3, H2O2. pH 5.5 | 3 V | 1 h 85 °C | [56] |
| HAp; CoCrMo | Ca(NO3)2, NH4H2PO4, H2O2 pH = 4.5 | 3 mA/cm2 | 30 min 20 °C | [61] |
| HAp; CoNiCrMo | Ca(NO3)2, NH4H2PO4. | From −1.4 to −2.2 V versus Ag/AgCl) | 80 °C | [60] |
| HAp; Mg | Ca(NO3)2, NH4H2PO4, H2O2. pH = 4.3. | 4 V | 2 h RT | [66] |
| HAp; Au, PC, stainless steels | Ca(NO3)2, NH4(H2PO4), H2O2. pH 4.5 or 6 | 120–250 mA/cm2 or −1.6 V vs. Ag/AgCl | 3–10 min 70 °C | [152] |
| HAp; enamel | Ca(NO3)2, NH4H2PO4, NaNO3 | 0.5 mA/cm2 | 1 h 55 °C | [74] |
| HAp; PVA/PLA | H2O2, CaCl2, KH2PO4 | 2.5–7.5 mA/cm2 | 1 h | [78] |
| FHAp; stainless steels | CaCl2, NH4(H2PO4), NH4F, H2O2 pH = 4.6 | 1 mA/cm2 | 1 min 20–65 °C | [5] |
| Brushite (DCPD) or FHAp; Mg | Ca(NO3)2, NH4H2PO4. For FHAp, NaNO3 and NaF added. pH = 4.4 | 5 (DCPD) or 0.5 (FHAp) mA/cm2 | RT (DCPD) or 60 °C (FHAp) | [68] |
| AgHAp; Ti | NaCl, tris(hydroxymethyl)aminomethane, CaCl2, KH2PO4. pH = 7.2 | 12.5 mA/cm2 | 95 °C | [134] |
| AgMnHAp; Ti | Ca(NO3)2, NH4H2PO4, Mn(NO3)2, AgNO3 | 0.09 mA/cm2 | 65 °C | [85] |
| (La/Cu)HAp | Ca(NO3)2, La(NO3)2, Cu(CH3COO)2, NH4H2PO4, H2O2. pH = 4.5 | 1.0 mA/cm2 | 1 h 65 °C | [86] |
| MgHAp; nanotubular TiO2 | Ca(NO3)2, NH4H2PO4, Mg(NO3)2. pH = 4.2 | 0.85 mA/cm2 | 35 min 65 °C | [81] |
| SrHAp; Ti | CaCl2, NH4(H2PO4), SrCl2, NaNO3 | 3.0 V | 1 h 85 °C | [84] |
| SrCuHAp | Ca(NO3)2, Sr(NO3)2, CuNO3)2, NH4H2PO4. pH = 4.4 | 0.85 mA/cm2 | 30 min 65 °C | [82] |
| SrMnHAp | Ca(NO3)2, Sr(NO3)2, Mn(NO3)2, NH4H2PO4. pH = 4.3 | 0.85 mA/cm2 | 30 min 65 °C | [83] |
| HAp + CNTs | Ca(NO3)2, NH4H2PO4, H2O2 MWCNTs | 3 V | pH 4.7 | [114] |
| ZnHAp; Ti | Ca(NO3)2 NH4H2PO NaNO3, H2O2. | 2.5 V | 2 h 85 °C | [133] |
| ZnHAp; stainless steel | Ca(NO3)2, NH4H2PO4, H2O2. pH = 4.5 | 0.5–3 mA/cm2 | 1 h 65 °C | [54] |
| CaCO3; indium tin oxide | CaCl2, NaHCO3, NaCl. pH = 8.25 | −0.86 V | 25 °C | [29] |
| Polymer Coatings | ||||
| Chitosan; Ti-6Al-4V | CH3COOH, Chitosan, NaOH pH = 4.75 | 0.6 mA/cm2 | 10 min RT | [96] |
| Poly (DL-lactide-co-glycolide) (PLGA); stainless steel | PLGA solution | 2 mA | - | [97] |
| Composite Coatings | ||||
| Ni-MWCNTs | NiSO4, NiCl2, H3BO3, saccharine | 80 mA/cm2 | - | [132] |
| Pd/Ag/HAp | NH4H2PO4, NH4F, HAp, Pd, Ag | 23 V | 1 h | [139] |
| NanoHAp-CNTs | CNTs, NH4H2PO4, Ca(NO3)2, NaNO3. pH = 7.4 | 5 mA | 15–30 min 100 °C | [77] |
| CNTs-HAp | Ca(NO3)2, K2HPO4, CNTs | −1.4 V vs. SCE | 1 h | [112] |
| HAp-CaSiO3 | nano-SiO2, Ca(NO3)2, NH4H2PO4. pH = 4.2 | 0.8 mA/cm2 | 30 min 65 °C | [104] |
| HAp-CaHPO4; stainless steels | CaCl2; NH4H2PO4 | 5 or 10 mA/cm2; 1 V, 2 V or3 V | RT | [58] |
| ZnHAp-CaSiO3 | Ca(NO3)2, NH4H2PO4, Zn(NO3)2. pH = 4.2 | 0.8 mA/cm2 | 30 min 65 °C | [105] |
| ZnO/ZnHAp hybrid coating; carbon fiber | Zn(NO3)2, Ca(NO3)2, NH4H2PO4. | 1st stage: 0.6 mA/cm2 (ECD). 2nd stage: 3 V (EPD). | 1st stage: 30 min, 343 K 2nd stage: 60 min | [119] |
| Zn-halloysite nanotubes (HNT)/SrSmHAp hybride coating; Ti6Al4V | Ca(NO3)2, Sr(NO3)2, Sm(NO3)2, NH4H2PO4 | 1.0 mA/cm2 | 30 min RT | [108] |
| Halloysite nanotubes (HNT)-CeHAp; Ti alloy | Ca(NO3)2, NH4H2PO4, Ce(NO3)2, halloysite nanoclay, HCl. pH = 4.5 | - | - | [107] |
| HAp-Ag-chitosan; Pt, graphite or stainless steel | chitosan solutions containing HAp nanoparticles and dissolved AgNO3 | 0.1 mA/cm2 | - | [125] |
| HAp-ZrO2; Ti | Ca(NO3)2, NH4H2PO4, NaNO3, H2O2, ZrO2 particles. pH = 4.5 | 1 mA/cm2 | 45 min 65 °C | [102] |
| HAp-ZrO2-TiO2 | Ca(NO3)2, NH4H2PO4, NaNO3, ZrO2, TiO2. pH = 4.2 | Constant direct current | 85 °C 2 h | [118] |
| HAp-GO-collagen; Ti-Nb | Ca(NO3)2, NH4H2PO4, GO, collagen in SBF. pH = 4.1–4.3 | 2 mA/cm2 | 60 min 33 °C | [122] |
| GO (an inner layer)/GO-MgHAp (an outer layer); C/C composites | 1st stage (GO inner-layer): Go water suspension. 2nd stage (GO-MGHAp): NH4H2PO4, Ca(NO3)2, Mg(NO3)2, GO. | 1st stage: 30–70 V (EPD). 2nd stage: 3 mA (ECD). | 1st stage: 1–7 min (EPD). 2nd stage: 1 h, 50 °C (ECD). | [111] |
| Chitosan-AgHAp on nanotubular TiO2 | Ca(NO3)2, NH4H2PO4, AgNO3. | 0.85 mA/cm2 35 min at 50◦C | 35 min 50 °C | [126] |
| Poliethyleneimine (PEI)-Ag | Hydrogen tetrachlorate (III). | −1.2 V vs. (Ag/AgCl) | 45 s | [142] |
| Polypyrrole-chitosan; stainless steel | Pyrrole in oxalic acid, with and without the addition of chitosan. | 15 mA | 1 h | [144] |
| Polyacrylic acid (PAA) followed by Ga-modified chitosan; Ti | PAA water solution | For Ga-modified chitosan: 1.5 V | 15–60 min | [146] |
| Alginate/chitosan (layer-by-layer coating) | Chitosan water solution Alginate dissolved in acetic acid | 20 V | 20 min | [143] |
| Chitosan-protein; Mg | Chitosan in acetic acid Proteins in citric acid | 1 mA/cm2 | 10 min | [98] |
| Coating; Substrate | Solution | Voltage/Current for Pulsed Mode Deposition | Deposition Time and Temperature | Reference |
|---|---|---|---|---|
| Metallic Coatings | ||||
| Ni; Ti | NiSO4, H3BO3. pH 2 or 5 | From 0 to −1.5 V at the scan rates of 20 and 50 mV/s | - | [28] |
| Tantalum | LiF, TaF5 | −2.6 V to 1.6 V | 30 s to 2 h | [25] |
| Ceramic Coatings | ||||
| Ca-P; Ti | Ca(NO3)2, NH4H2PO4, H2O2, GO. pH 6 | Pulsed mode 15 mA/cm2 duty cycle 0.1 | 65 °C | [38] |
| Ca-P; Ti | Ca(NO3)2 NH4H2PO4, H2O2. pH 4.3 | Pulse mode −1.4 V Duty cycle 0.5 | 1–30 min | [34] |
| Ca-P; Ti | Ca(NO3)2, NH4H2PO4, chlorhexidine digluconate. pH 4.2 | Pulse mode 2–5 mA/cm2 | 40–60 °C 30 min | [53] |
| Ca-P; Mg alloy | NaNO3, NH4H2PO4, Ca(NO3)2, H2O2. pH 5.0 | Pulse mode (i) Constant voltage 2.5–20 V or (ii) Constant current density 10–200 mA/cm2 Duty cycle 0.25–0.75 | 20 °C (i) 40–60 min (ii) 2–6 h | [72] |
| Ca-P; NiTi | Ca(NO3)2, NH4H2PO4, H2O2. pH 4.3 | 5, 10, 15, and 20 mA/cm2 Duty cycle 0.1 | 25 min | [65] |
| Ca-P; Ti-Ni | Three different conditions; (i) Ca(NO3)2, NH4H2PO4, H2O2. pH 4.3 (ii) and (iii) as above, pH 6 | Constant mode (i) 0.6 mA/cm2, or (ii) −0.5 mA/cm2 (iii) pulsed mode-3 mA/cm2 duty cycle 0.33 | (i) 70 °C (ii) and (iii) 65 °C | [62] |
| Ca-P and HAp; stainless steel | CaCl2, NH4H2PO4, NaCl. | Pulsed mode 5, 10 and 20 mA/cm2 duty cycle 0.2 | 1 h room temperature (RT) | [57] |
| Polymorphic apatites; C/C composites | Ca(NO3)2, NH4H2PO4. | Pulse mode 3, 5 and 10 V Duty cycle 0.2 | 60 °C 3 h | [76] |
| HAp; Ti | CaCl2, K2HPO4, H2O2. | 1 mA/cm2 duty cycle 0.2 and 0.8 | 1 h | [36] |
| HAp; stainless steel | Ca(NO3)2, NH4H2PO4, NaNO3. pH = 5.77 | Pulse mode −1.6 V/SCE, scanning rate 5 mV/s | 25 °C 26.667 min | [55] |
| HAp; NiTi | Ca(NO3)2, NH4H2PO4, NaNO3, H2O2. pH 6.0 | Pulsed mode 3.0 mA/cm2 | 25 min 65 °C | [63] |
| HAp; NiTi | Ca(NO3)2, NH4H2PO4, NaNO3, H2O2. pH 4.3 | Pulse mode 1.5–15 mA/cm2 duty cycle 0.2 | 25 min 70 °C | [64] |
| HAp; Mg alloys | Ca(NO3)2, NH4H2PO4, Na2SiO3, NaNO3. pH 4, 5 or 6 | Pulsed mode 40 and 60 mA/cm2 duty cycle 0.1, 0.2 | 30 min 25–100 °C | [91] |
| NanoHAp; Mg alloy | Ca(NO3)2, NH4H2PO4, H2O2. pH = 4.5 | Pulse mode −3V Duty cycle 0.2 | RT | [69] |
| NanoHAp; Mg-Zn scaffold | Ca(NO3)2, NH4H2PO4, NaNO3 | 20–40 mA/cm2 Duty cycle 0.1 and 0.2 temperature, | 55, 70, 85 and 100 °C 1 h | [70] |
| NanoHAp; Mg-Zn scaffolds | Ca(NO3)2, NH4H2PO4, NaNO3. pH = 5.0 | 40 mA/cm2 duty cycle 0.1 | 85 °C 1 h | [71] |
| HAp; Au | Ca(NO3)2. NH4H2PO4, H2O2, pH 4.5 or 6 | Constant mode: 1.6 V vs. Ag/AgCl followed by a pulsed mode duty cycle 0.33 | 45 min 70°C | [152] |
| HAp-Ca3(PO4)2; Ti6Al4V | Ca(NO3)2, NH4H2PO4, with or without H2O2. pH = 4.4 | Pulsed mode 8 mA/cm2 | 21 min 50 °C | [37] |
| HAp; nanoTiO2 | Ca(NO3)2, NH4H2PO4 | 2.5 mA/cm2 Duty cycle 0.5 | 20–120 s | [49] |
| CoCa-P; Ti22Nb6Zr | Ca(NO3)2, NH4H2PO4, Co(NO3)2, H2O2. | Pulsed mode 15mA/cm2 | 15 min | [87] |
| FHAp; Mg-Zn-Ca | NaNO3, NH4H2PO4 Ca(NO3)2, NaF, H2O2. pH 5.0 | Pulse mode 1 mA/cm2 | 65 °C | [89] |
| SiHAp; Mg alloy | Ca(NO3)2, NH4H2PO4, NaNO3, tetraethoxysilane. | Pulse mode 0.4–0.6 V Duty cycle 0.3 | 40–80°C 40 min | [90] |
| SrCa-P; Ti6Al4V | Ca(NO3)2, NH4H2PO4, Sr(NO3)2. | Pulsed mode 15 mA/cm2 | 15 min 60 °C | [80] |
| (Sr,Mg,Zn)HAp; Ti-6Al-4V | CaCl2, SrCl2, MgCl2, ZnCl2, NH4H2PO4, H2O2. pH 4.5 | Pulsed mode 1 mA/cm2 duty cycle 0.2 and 0.8 | 1 h 65 °C | [79] |
| (Sr,Mg)Ca3(PO4)2; C/C composite | Ca(NO3)2 Sr(NO3)2, Mg(NO3)2 NH4H2PO4. | Pulse mode 2.5 V Duty cycle 0.4. | 3 h 50 °C | [92] |
| (Zn,Mg,Sr,Ag)Ca-P; Ti6Al4V | Ca(NO3)2, NH4H2PO4, AgNO3, Zn(NO3)2, Sr(NO3)2, Mg(NO3)2, H2O2. | Pulsed mode 400 mA/cm2 duty cycle 0.2 | 70 °C | [88] |
| ZnHAp | Ca(NO3)2, NH4H2PO4, H2O2. pH 4.5 | Pulsed mode 0.5–3 mA/cm2 | 1 h 65 °C | [95] |
| HAp + CNTs | Ca(NO3)2, NH4H2PO4, H2O2 MWCNTs | 3 V | pH 4.7 | [95] |
| Composite Coatings | ||||
| Reduced graphene oxide (rGO)-polydopamine-CuNPs-Nil blue; glass carbon | Cu(NO3)2, phosphate-buffered saline (PBS). | Pulse mode from −0.5 V to 0.8 V, a scan rate of 100 mV/s | - | [95] |
| MCWNT–HAp; stainless steel | CaCl2, NH4H2PO4, NaCl. | Pulsed mode 5, 10, and 20 mA/cm2 duty cycle 0.2 | 1 h RT | [103] |
| HAp-CaHPO4; stainless steel | CaCl2, NH4H2PO4. | Pulsed mode Either constant current 5 and 10 mA/cm2, or constant voltage 1, 2 and 3 V | RT | [58] |
| Reduced graphene oxide (rGO) and MWCNT/HAp–calcium orthophosphate phases; stainless steel | CaCl2, NH4H2PO4. | Pulsed mode 10 mA/cm2 | 900 s RT | [115] |
| HAp-polypyrrole; stainless steel | Ca(NO3)2, NH4H2PO4, KNO3, pyrrole monomer. | Pulsed mode 5, 10, and 20 mA/cm2 | 1500 s RT | [124] |
| Graphene oxide (GO)-HAp; Ti | Ca(NO3)2, NH4H2PO4, H2O2, GO. pH 4.2 | Constant or pulsed mode 15 mA/cm2 duty cycle 0.1 | 65 °C | [109] |
| Graphene oxide (GO)-HAp; Ti | Ca(NO3)2, NH4H2PO4, H2O2, GO. pH 4.5 | Pulsed mode 15 mA/cm2 duty cycle 0.1 | 50 s 65 °C | [110] |
| GO-calcium phosphate; Ti | Ca(NO3)2, NH4H2PO4, NaNO3, H2O2, GO. pH 6 | Pulsed mode 15 mA/cm2 duty cycle 0.1 | 65 °C | [113] |
| HAp-CNTs; Mg alloy | Ca(NO3)2, NH4H2PO4, H2O2.pH 4.7 | −3 V duty cycle 0.2 | RT | [114] |
| Iridium oxide/human plasma proteins | Iridium chloride, oxalic acid, human plasma pH = 10.2 | Cyclic voltammetry from −0.6 to 0.8 V vs. Ag/AgCl scan rate 10 mV/s | - | [94] |
| Polypyrrole/Nb2O5; stainless steel | Pyrrole in oxalic acid, with the addition of Nb2O5 | Cyclic voltammetry −0.6 V to +0.7 V vs. SCE the scan rate of 50 mV/s | - | [141] |
| Polyacrylic acid (PAA) followed by Ga-modified chitosan; Ti | PAA water solution | For PAA only: from 0 to −1.2 V | 4 min | [146] |
| Poly (3,4-ethylenedioxythiophene) (PEDOT)/FHAp; Ti-Nb-Zr | LiClO4, ACN (acetonitrile), monomer EDOT, FHAp | Sweeping the potential from −600 to 1600 mV sweeping rate of 0.05 V/s | - | [123] |
| ZnO/ZnHAp hybrid coating; carbon fiber | Zn(NO3)2, Ca(NO3)2, NH4H2PO4. | 1st stage: 0.6 mA/cm2 (ECD). 2nd stage: 3 V EPD). | 1st stage: 30 min, 343 K 2nd stage: 60 min | [119] |
| Zn,Cu/AgNPs | CuCl2, ZnCl2, glycine, cetyltrimethylammonium bromide (CTAB), AgNPs. pH = 10 | Cyclic voltammetry from 0.1 to −1.6 V vs.SCE | - | [140] |
| Coating; Substrate | Electrolyte | Voltage/Current | Deposition Time and Temperature | Reference |
|---|---|---|---|---|
| HAp; NiTi | ethanol | 40 V | 20 s | [39] |
| HAp; TiO2 | - | 200 V | 1 min | [44] |
| HAp; Ti | ethanol | 10–45 V | 1–8 min | [42] |
| HAp; TiO2 | ethanol | 60 V | 45 s RT | [45] |
| HAp; Au | Ethanol and octadecyltri- chlorosilane | 70 V/cm2 | 1 h | [131] |
| Hap + TiO2 | acetylacetone | 20 V | 30–120 s | [43] |
| HAp + CNTs; NiTi | ethanol | 30 V | 30 s | [116] |
| Hap + MWCNTs | butanol | 60 V | 2 min | [117] |
| Hap + MNWCNTs + nanoAg + nanoCu | ethanol, isopropanol | 11 and 30 V | 2 min RT | [138] |
| nanoHAp; Ag | ethanol | 10 V | [47] | |
| Nano(Zn/Ca)HAp; (Si)Ti | - | 10, 50, and 100 V/cm | 1 min pH 12 | [93] |
| nanoHAp; Ti | ethanol | 10 V | 10 min | [41] |
| nanoHAp; (Mg,Zr,Ce) oxides | water | 380 V | 10 min | [46] |
| nanoHAp | 0.1, 0.2 or 0.5 g nanoHAp | 15, 30, and 50 V | 1 min | [40] |
| nanoHAp + nanoAg | ethanol | 15 and 30 V for nanoHAp 60 V for nanoAg | 1 min for nanoHAp 5 min for nanoAg | [135] |
| nanoHAp + nanoAg | ethanol | 50 V | 1 min | [136] |
| nanoHAp + nanoCu; TiO2 | ethanol | 30 V | 1 and 2 min RT | [137] |
| nanoHAp + borium nitride; Ti | ethanol | 100 and 150 V | 5–20 s pH 4 | [120] |
| PEEK + HAp | ethanol | 75 V | 45 s pH 5.5 | [130] |
| HAp+Si + MWCNTs | - | cathodic | - | [153] |
| Chitosan + HAp; TiO2 | acetic acid, etanol, water | 10–15 V | 3–9 min | [154] |
| Hap + ZNHAp + MWCNTs + chitosan | - | - | - | [128] |
| Bioglass + HAp (whiskers) | isopropanol | 40 V | 1 min | [121] |
| Chitosan + bioglass + HAp | acetic acid, etanol, water | 20 and 30 V | 5 and 15 min pH 3.3, 4, 5 | [155] |
| GO (graphene oxide) + MgHAp; C/C composites | - | - | - | [111] |
| The poly-l-lysine (PLL) + 3,4-dihydroxybenzylaldehyde (DHBA) + HAp + TiO2 | ethanol-water | 50 V | - | [129] |
| methacrylates | dioxan | 15 V | 5 min | [99] |
| PEEK | ethanol | 70–115 V | 1 min | [100] |
| Chitosan + Eudragit | acetic acid | 10 and 30 V | 1 and 3 min | [145] |
| PMMA + soy lecithin | the microemulsion of coconut oil and water | 1 mA (4–15 V) | 30 min RT | [147] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zielinski, A.; Bartmanski, M. Electrodeposited Biocoatings, Their Properties and Fabrication Technologies: A Review. Coatings 2020, 10, 782. https://doi.org/10.3390/coatings10080782
Zielinski A, Bartmanski M. Electrodeposited Biocoatings, Their Properties and Fabrication Technologies: A Review. Coatings. 2020; 10(8):782. https://doi.org/10.3390/coatings10080782
Chicago/Turabian StyleZielinski, Andrzej, and Michal Bartmanski. 2020. "Electrodeposited Biocoatings, Their Properties and Fabrication Technologies: A Review" Coatings 10, no. 8: 782. https://doi.org/10.3390/coatings10080782
APA StyleZielinski, A., & Bartmanski, M. (2020). Electrodeposited Biocoatings, Their Properties and Fabrication Technologies: A Review. Coatings, 10(8), 782. https://doi.org/10.3390/coatings10080782






