New Antimicrobial Biomaterials for the Reconstruction of Craniofacial Bone Defects
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.1.1. Materials
2.1.2. Preparation of FRC Samples
The Matrices
Silanization of the Woven E-Glass Fibers
The Cured FRCs
2.2. Caracterisation of Experimental Materials
2.2.1. Residual Monomers
2.2.2. Cumulative Gentamicin Release
2.2.3. SEM Investigation of FRC Surface
2.2.4. Antimicrobial Activity in Dynamics
Determination of MIC (Minimum Inhibitory Concentration) and MBC (Minimum Bactericidal Concentration)
3. Results
3.1. Residual Monomers
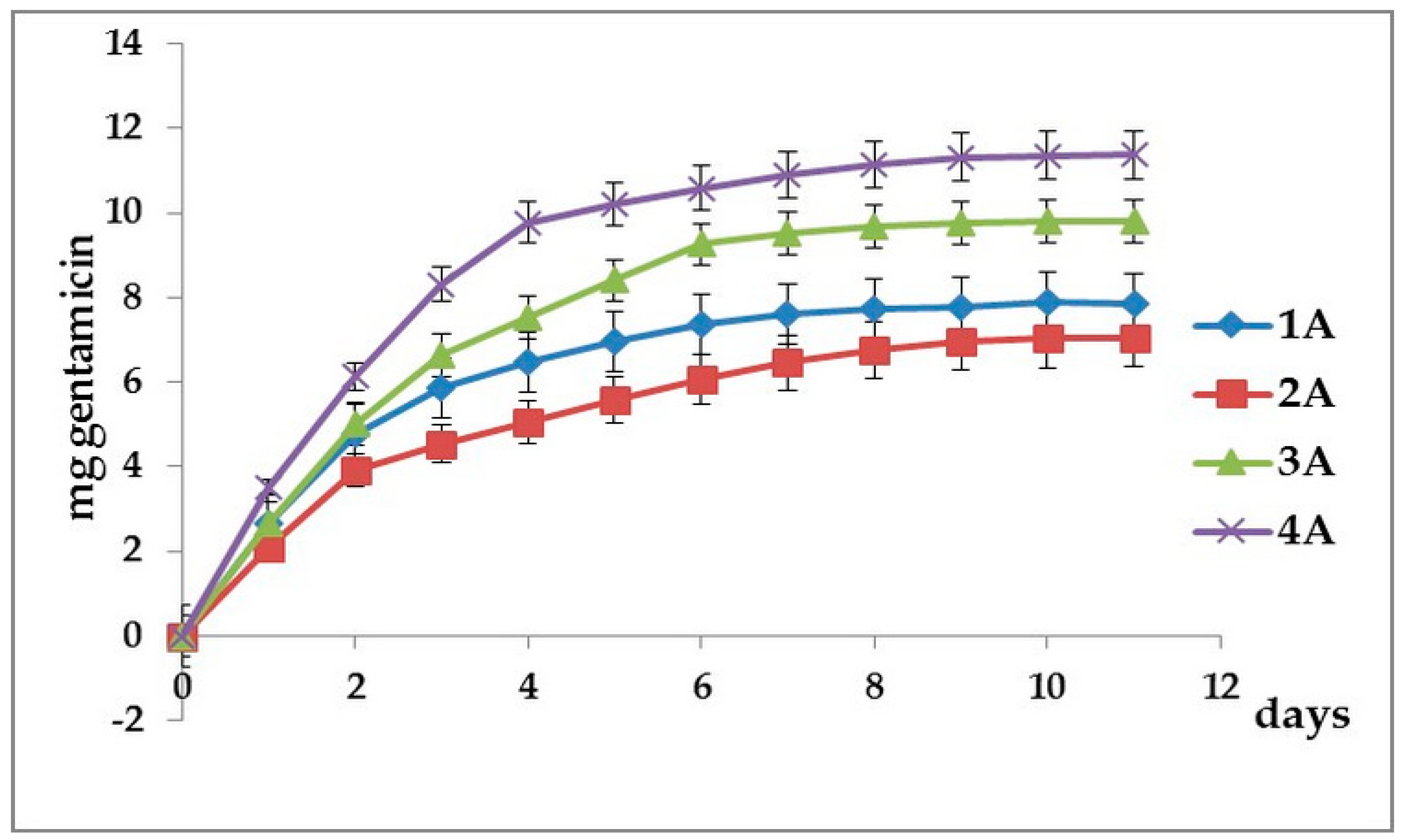
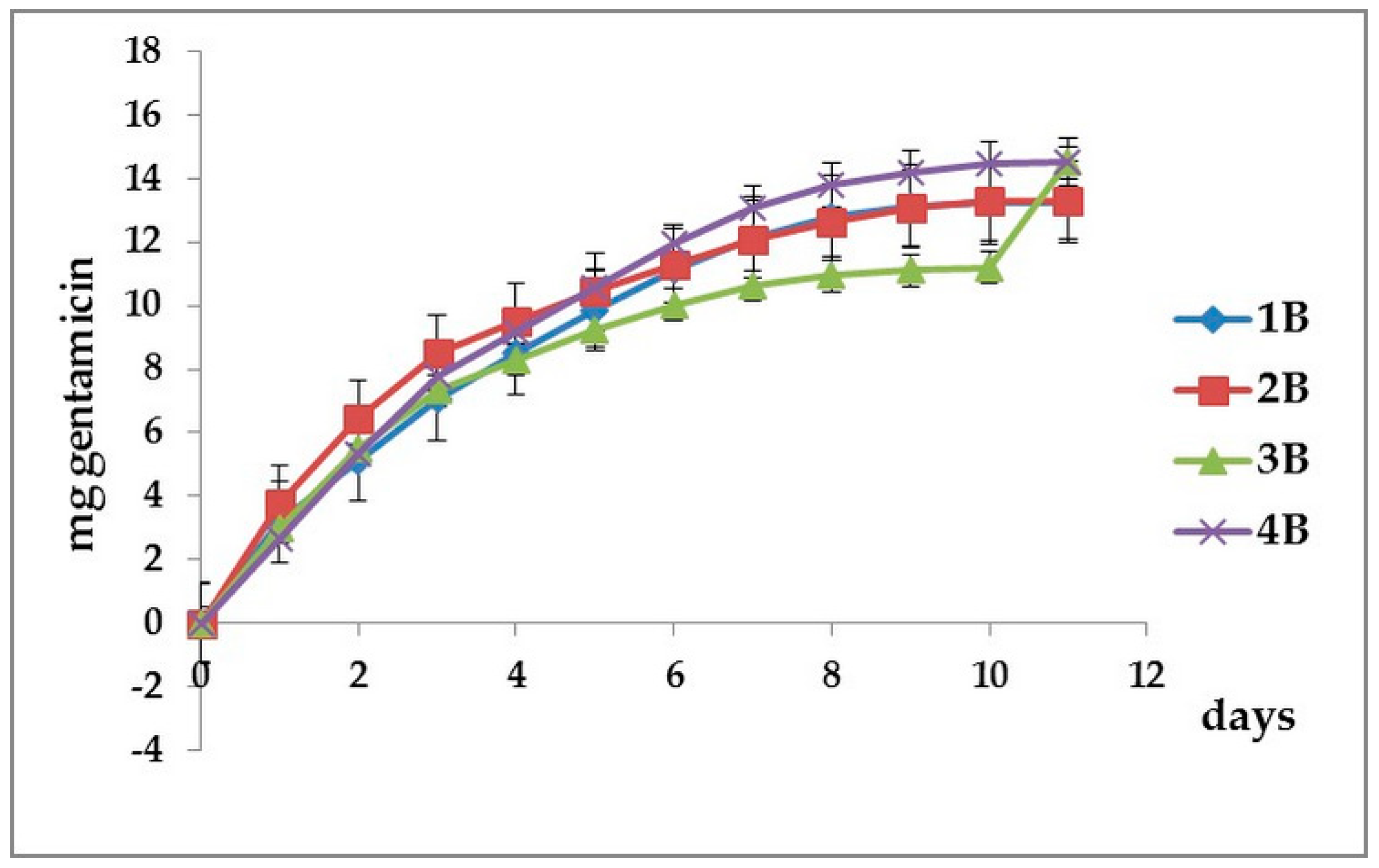
3.2. Cumulative Gentamicin Release
3.3. SEM Investigation of FRC Surface
3.4. Antimicrobial Activity in Dynamics. MIC and MBC Determination
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Abdo Filho, R.C.; Oliveira, T.M.; Lourenço Neto, N.; Gurgel, C.; Abdo, R.C. Reconstruction of bony facial contour deficiencies with polymethylmethacrylate implants: Case report. J. Appl. Oral. Sci. 2011, 19, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Lazar, M.A.; Filip, M.; Vlassa, M.C.; Sorcoi, L.A.; Campian, R.S.; Prejmerean, C. Development and characterization of new fiber-reinforced biocomposites for cranial bone reconstruction. Rev. Rom. Mater. 2016, 46, 142–151. [Google Scholar]
- Yongxi, L.; Luan, X.; Liu, X. Recent advances in periodontal regeneration: A biomaterial perspective. Bioact. Mater. 2020, 5, 297–308. [Google Scholar] [CrossRef]
- Karacan, I.; Ben-Nissan, B.; Wang, H.A.; Juritza, A.; Swain, M.; Mueller, W.; Chu, J.; Stamboulis, A.; Macha, I.; Taraschi, V. Mechanical testing of antimicrobial biocomposite coating on metallic medical implants as drug delivery system. Mater. Sci. Eng. C 2019, 104, 109757. [Google Scholar] [CrossRef]
- Colceriu Burtea, L.; Prejmerean, C.; Prodan, D.; Baldea, I.; Vlassa, M.; Filip, M.; Moldovan, M.; Lazar (Moldovan), M.-A.; Antoniac, A.; Prejmerean, V.; et al. New pre-reacted glass containing dental composites (giomers) with improved fluoride release and biocompatibility. Materials 2019, 12, 4021. [Google Scholar] [CrossRef]
- Lazar, M.A.; Rotaru, H.; Bâldea, I.; Boşca, A.B.; Berce, C.P.; Prejmerean, C.; Prodan, D.; Câmpian, R.S. Evaluation of the biocompatibility of new fiber-reinforced composite materials for craniofacial bone reconstruction. J. Craniofac. Surg. 2016, 27, 1694–1699. [Google Scholar] [CrossRef] [PubMed]
- Tuusa, S.M.; Puska, M.A.; Lassila, L.V.J.; Vallittu, P.K. Residual monomers from glass-fibre-reinforced composite photopolymerised in contact with bone and blood. J. Mater. Sci. Mater. Med. 2005, 16, 15–20. [Google Scholar] [CrossRef]
- Borcherding, K.; Marx, D.; Gätjen, L.; Bormann, N.; Wildemann, B.; Specht, U.; Salz, D.; Thiel, K.; Grunwald, I. Burst release of antibiotics combined with long-term release of silver targeting implant-associated infections: Design, characterization and in vitro evaluation of novel implant hybrid surface. Materials 2019, 12, 3838. [Google Scholar] [CrossRef]
- Dayashankara Rao, J.K.; Malhotra, V.; Batra, R.S.; Kukreja, A. Esthetic correction of depressed frontal bone fracture. Natl. J. Maxillofac. Surg. 2011, 2, 69–72. [Google Scholar] [CrossRef]
- Rupp, M.; Popp, D.; Alt, V. Prevention of infection in open fractures: Where are the pendulums now? Injury 2019, 51 (Suppl. S2), S57–S63. [Google Scholar] [CrossRef]
- Šámal, F.; Ouzký, M.; Strnad, J.; Haninec, P.; Linzer, P.; Filip, M. First experience with cranioplasty using the polyetheretherketone (peek) implant-retrospective five-year follow-up study. Acta Chir. Orthop. Traumatol. Cechoslov. 2019, 86, 431–434. [Google Scholar]
- Gristina, A.G.; Naylor, P.; Myrvik, Q. Infections from biomaterials and implants: A race for the surface. Med. Prog. Technol. 1988, 14, 205–224. [Google Scholar] [PubMed]
- Iaccarino, C.; Mattogno, P.P.; Zanotti, B.; Bellocchi, S.; Verlicchi, A.; Viaroli, E.; Pastorello, G.; Sgulò, F.; Ghadirpour, R.; Servadei, F. Septic complication following porous hydroxyapatite cranioplasty: Prosthesis retention management. J. Neurosurg. Sci. 2018, 62, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Zborníková, E.; Gallo, J.; Večeřová, R.; Bogdanová, K.; Kolář, M.; Vítovská, D.; Do Pham, D.D.; Pačes, O.; Mojr, V.; Šanderová, H.; et al. Evaluation of second-generation lipophosphonoxins as antimicrobial additives in bone cement. ACS Omega 2020, 5, 3165–3171. [Google Scholar] [CrossRef]
- Abu-Ghname, A.; Banuelos, J.; Oliver, J.D.; Vyas, K.; Daniels, D.; Sharaf, B. Outcomes and complications of pediatric cranioplasty: A systematic review. Plast. Reconstr. Surg. 2019, 144, 433e–443e. [Google Scholar] [CrossRef]
- Beuttel, E.; Bormann, N.; Pobloth, A.M.; Duda, G.N.; Wildemann, B. Impact of gentamicin-loaded bone graft on defect healing in a sheep model. Materials 2019, 12, 1116. [Google Scholar] [CrossRef]
- Linder, L.K.B.; Birgersson, U.; Lundgren, K.; Illies, C.; Engstrand, T. Patient-specific titanium-reinforced calcium phosphate implant for the repair and healing of complex cranial defects. World Neurosurg. 2019, 122, e399–e407. [Google Scholar] [CrossRef]
- Lazar, M.A.; Vodnar, D.; Prodan, D.; Rotaru, H.; Roman, C.R.; Sorcoi, L.A.; Baciut, G.; Campian, R.S. Antibacterial coating on biocomposites for cranio-facial reconstruction. Clujul Med. 2016, 89, 430–434. [Google Scholar] [CrossRef]
- Dhal, C.; Mishra, R. In vitro and in vivo evaluation of gentamicin sulphate-loaded PLGA nanoparticle-based film for the treatment of surgical site infection. Drug Deliv. Transl. Res. 2020. [Google Scholar] [CrossRef]
- Maczynska, B.; Secewicz, A.; Smutnicka, D.; Szymczyk, P.; Dudek-Wicher, R.; Junka, A.; Bartoszewicz, M. In vitro efficacy of gentamicin released from collagen sponge in eradication of bacterial biofilm preformed on hydroxyapatite surface. PLoS ONE. 2019, 14, e0217769. [Google Scholar] [CrossRef]
- Neut, D.; Dijkstra, R.J.; Thompson, J.I.; Kavanagh, C.; van der Mei, H.C.; Busscher, H.J. A biodegradable gentamicin-hydroxyapatite-coating for infection prophylaxis in cementless hip prostheses. Eur. Cell. Mater. 2015, 29, 42–55. [Google Scholar] [CrossRef] [PubMed]
- Lazar, M.A.; Rotaru, H.; Prodan, D.; Armencea, G.; Bere, P.; Roman, R.; Campian, R. Evaluation of the morphology and structure of e glass fiber-reinforced composites for cranio-facial bone reconstruction. Stud. UBB Chem. 2016, 2, 249–260. [Google Scholar]
- Prejmerean, C.; Moldovan, M.; Silaghi-Dumitrescu, L.; Prodan, D.; Furtos, G.; Trif, M.; Popescu, V.; Pascalau, V.; Damian, C.; Silaghi-Dumitrescu, R. Composition versus physico-mechanical properties of some dental experimental polymers. Mater. Plast. 2011, 48, 27–32. [Google Scholar]
- Prodan, D.; Molea, A.; Moldovan, M.; Popescu, V.; Silaghi-Dumitrescu, L.; Prejmerean, C.; Boboia, S.; Sarosi, C. Synthesis and characterization of the hydroxyapatite and TiO2 doped hydroxyapatite powders. J. Optoelectron. Adv. Mater. 2014, 16, 1300–1305. [Google Scholar]
- Oshima, S.; Sato, T.; Honda, M.; Suetsugu, Y.; Ozeki, K.; Kikuchi, M. Fabrication of gentamicin-loaded hydroxyapatite/collagen bone-like nanocomposite for anti-infection bone void fillers. Int. J. Mol. Sci. 2020, 21, 551. [Google Scholar] [CrossRef]
- Kim, B.H.; Lee, S.C.; Lee, H.J.; Ok, J.H. Reversed-phase liquid chromatographic method for the analysis of aminoglycoside antibiotics using pre-column derivatization with phenylisocyanate. Biomed. Chromatogr. 2003, 17, 396–403. [Google Scholar] [CrossRef]
- Jayol, A.; Nordmann, P.; André, C.; Poirel, L.; Dubois, V. Evaluation of three broth microdilution systems to determine colistin susceptibility of Gram-negative bacilli. J. Antimicrob. Chemother. 2018, 73, 1272–1278. [Google Scholar] [CrossRef]
- Khan, Z.A.; Siddiqui, M.F.; Park, S. Current and emerging methods of antibiotic susceptibility testing. Diagnostics 2019, 9, 49. [Google Scholar] [CrossRef]
- Vallittu, P.K. Fiber-Reinforced Composites for Implant Applications. Curr. Oral Health Rep. 2018, 5, 194–201. [Google Scholar] [CrossRef]
- Lethaus, B.; Ter Laak, M.P.; Laeven, P.; Laeven, P.; Beerens, M.; Koper, D.; Poukens, J.; Kessler, P. A treatment algorithm for patients with large skull bone defects and first results. J. Craniomaxillofac. Surg. 2011, 39, 435–440. [Google Scholar] [CrossRef]
- Engstrand, T. Biomaterials and biologics in craniofacial reconstruction. J. Craniofac. Surg. 2012, 23, 239–242. [Google Scholar] [CrossRef] [PubMed]
- Tuusa, S.M.; Peltola, M.J.; Tirri, T.; Lassila, L.V.J.; Vallittu, P.K. Frontal bone defect repair with experimental glass-fiber-reinforced composite with bioactive glass granule coating. J. Biomed. Mater. Res. Part B 2007, 82, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Aitasalo, K.M.; Piitulainen, J.M.; Rekola, J.; Vallittu, P.K. Craniofacial bone reconstruction with bioactive fibre-reinforced composite implant. Head Neck 2014, 36, 722–728. [Google Scholar] [CrossRef]
- Tuusa, S.M.; Peltola, M.J.; Tirri, T.; Puska, M.A.; Röyttä, M.; Aho, H.; Sandholm, J.; Lassila, L.V.J.; Vallittu, P.K. Reconstruction of critical size calvarial bone defects in rabbits with glass-fiber-reinforced composite with bioactive glass granule coating. J. Biomed. Mater. Res. B 2008, 84, 510–519. [Google Scholar] [CrossRef] [PubMed]
- Falland-Cheung, L.; Waddell, J.N.; Chun Li, K.; Tong, D.; Brunton, P. Investigation of the elastic modulus, tensile and flexural strength of five skull simulant materials for impact testing of a forensic skin/skull/brain model. J. Mech. Behav. Biomed. Mater. 2017, 68, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Gezgin, O.; Korkut, E.; Tulumbacı, F.; Özer, H.; Sener, Y. HPLC analysis of eluted monomers released from dental composites containing bioactive glass. J. Adhes. Sci. Technol. 2018, 32, 1724–1731. [Google Scholar] [CrossRef]
- Łagocka, R.; Mazurek-Mochol, M.; Jakubowska, K.; Bendyk-Szeffer, M.; Chlubek, D.; Buczkowska-Radlińska, J. Analysis of base monomer elution from 3 flowable bulk-fill composite resins using high performance liquid chromatography (HPLC). Med. Sci. Monit. 2018, 24, 4679–4690. [Google Scholar] [CrossRef]
- Moharamzadeh, K.; Van Noort, R.; Brook, I.M.; Scutt, A.M. HPLC analysis of components released from dental composites with different resin compositions using different extraction media. J. Mater. Sci. Mater. Med. 2007, 18, 133–137. [Google Scholar] [CrossRef]
- Ferracane, J.L.; Condon, J.R. Rate of elution of leachable components from composite. Dent. Mater. 1990, 6, 282–287. [Google Scholar] [CrossRef]
- Van Landuyt, K.L.; Nawrot, T.; Geebelen, B.; De Munck, J.; Snauwaert, J.; Yoshihara, K.; Scheers, H.; Godderis, L.; Hoet, P.; Van Meerbeek, B. How much do resin-based dental materials release? A meta-analytical approach (Review). Dent. Mater. 2011, 27, 723–747. [Google Scholar] [CrossRef]
- Kurt, A.; Altintas, S.H.; Kiziltas, M.V.; Tekkeli, S.E.; Guler, E.M.; Kocyigit, A.; Usumez, A. Evaluation of residual monomer release and toxicity of self-adhesive resin cements. Dent. Mater. J. 2018, 37, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Alshali, R.Z.; Salim, N.A.; Sung, R.; Satterthwaite, J.D.; Silikas, N. Analysis of long-term monomer elution from bulk-fill and conventional resin-composites using high performance liquid chromatography. Dent. Mater. 2015, 31, 1587–1598. [Google Scholar] [CrossRef] [PubMed]
- Rashid, H.; Sheikh, Z.; Vohra, F. Allergic effects of the residual monomer used in denture base acrylic resins. Eur. J. Dent. 2015, 9, 614–619. [Google Scholar] [CrossRef]
- Moldovan, M.; Balazsi, R.; Soanca, A.; Roman, A.; Sarosi, C.; Prodan, D.; Vlassa, M.; Cojocaru, I.; Saceleanu, V.; Cristescu, I. Evaluation of the degree of conversion, residual monomers and mechanical properties of some light-cured dental resin composites. Materials 2019, 12, 2109. [Google Scholar] [CrossRef] [PubMed]
- Denis, A.B.; Diagone, C.A.; Plepis, A.M.; Viana, R.B. The effect of the polymerization initiator and light source on the elution of residual Bis-GMA and TEGDMA monomers: A study using liquid chromatography with UV detection. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 151, 908–915. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Liu, X.; Tan, L.; Cui, Z.; Yang, X.; Liang, Y.; Li, Z.; Zheng, Y.; Yeung, K.W.K.; Wu, S. Enhancing the antibacterial efficacy of low-dose gentamicin with 5 minute assistance of photothermy at 50 °C. Biomater. Sci. 2019, 7, 1437–1447. [Google Scholar] [CrossRef] [PubMed]
- Akers, J.A.; Johnson, T.M.; Hill, R.B.; Kawaguchi, S. Rational Prophylactic Antibiotic Selection for Sinus Elevation Surgery. Clin. Adv. Periodontics 2020, 10, 42–55. [Google Scholar] [CrossRef] [PubMed]
- Decker, B.K.; Nagrebetsky, A.; Lipsett, P.A.; Wiener-Kronish, J.P.; O’Grady, N.P. Controversies in perioperative antimicrobial prophylaxis. Anesthesiology 2020, 132, 586–597. [Google Scholar] [CrossRef]
- Kuruvilla, M.; Sexton, M.; Wiley, Z.; Langfitt, T.; Lynde, G.C.; Wolf, F. A streamlined approach to optimize perioperative antibiotic prophylaxis in the setting of penicillin allergy labels. J. Allerg. Clin. Immunol. Pract. 2020, 8, 1316–1322. [Google Scholar] [CrossRef]
- Vander Poorten, V.; Uyttebroek, S.; Robbins, K.T.; Rodrigo, J.P.; de Bree, R.; Laenen, A.; Saba, N.F.; Suarez, C.; Mäkitie, A.; Rinaldo, A.; et al. Perioperative Antibiotics in Clean-Contaminated Head and Neck Surgery: A Systematic Review and Meta-Analysis. Adv. Ther. 2020, 37, 1360–1380. [Google Scholar] [CrossRef]
- Davidson, D.J.; Spratt, D.; Liddle, A.D. Implant materials and prosthetic joint infection: The battle with the biofilm. EFORT Open Rev. 2019, 4, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Ge, Z.; Ma, R.; Xu, G.; Chen, Z.; Zhang, D.; Wang, Q.; Hei, L.; Ma, W. Development and in vitro release of isoniazid and rifampicin-loaded bovine serum albumin nanoparticles. Med. Sci. Monit. 2018, 24, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Lane, D.D.; Fessler, A.K.; Goo, S.; Williams, D.L.; Stewart, R.J. Sustained tobramycin release from polyphosphate double network hydrogels. Acta Biomater. 2017, 50, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Nastri, L.; De Rosa, A.; De Gregorio, V.; Grassia, V.; Donnarumma, G. A new controlled-release material containing metronidazole and doxycycline for the treatment of periodontal and peri-implant diseases: Formulation and in vitro testing. Int. J. Dent. 2019, 2019. [Google Scholar] [CrossRef]
- Duewelhenke, N.; Krut, O.; Eysel, P. Influence on mitochondria and cytotoxicity of different antibiotics administered in high concentrations on primary human osteoblasts and cell lines. Antimicrob. Agents Chemother. 2007, 51, 54–63. [Google Scholar] [CrossRef]
- Wahlig, H.; Dingeldein, E.; Bergmann, R.; Reuss, K. The release of gentamicin from polymethylmethacrylate beads. An experimental and pharmacokinetic study. J. Bone. Jt. Surg. Br. 1978, 60, 270–275. [Google Scholar] [CrossRef]
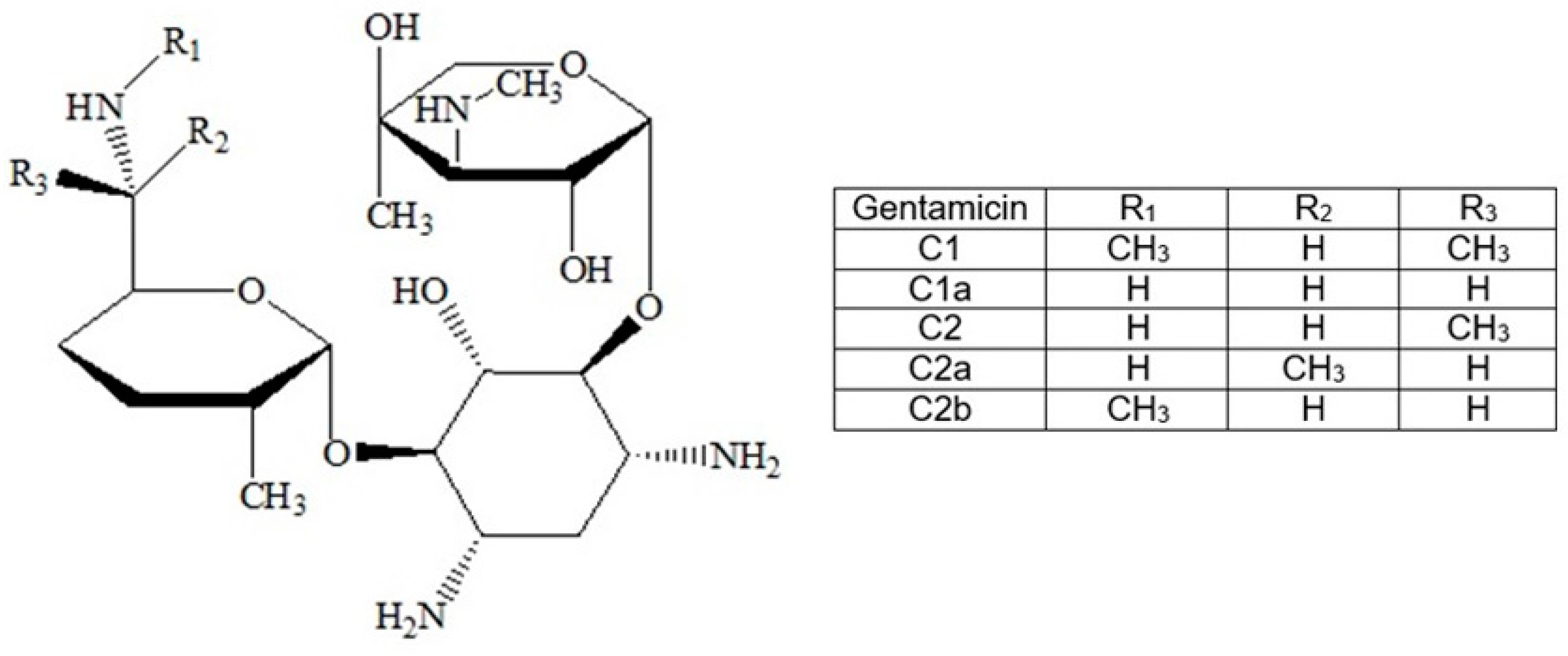
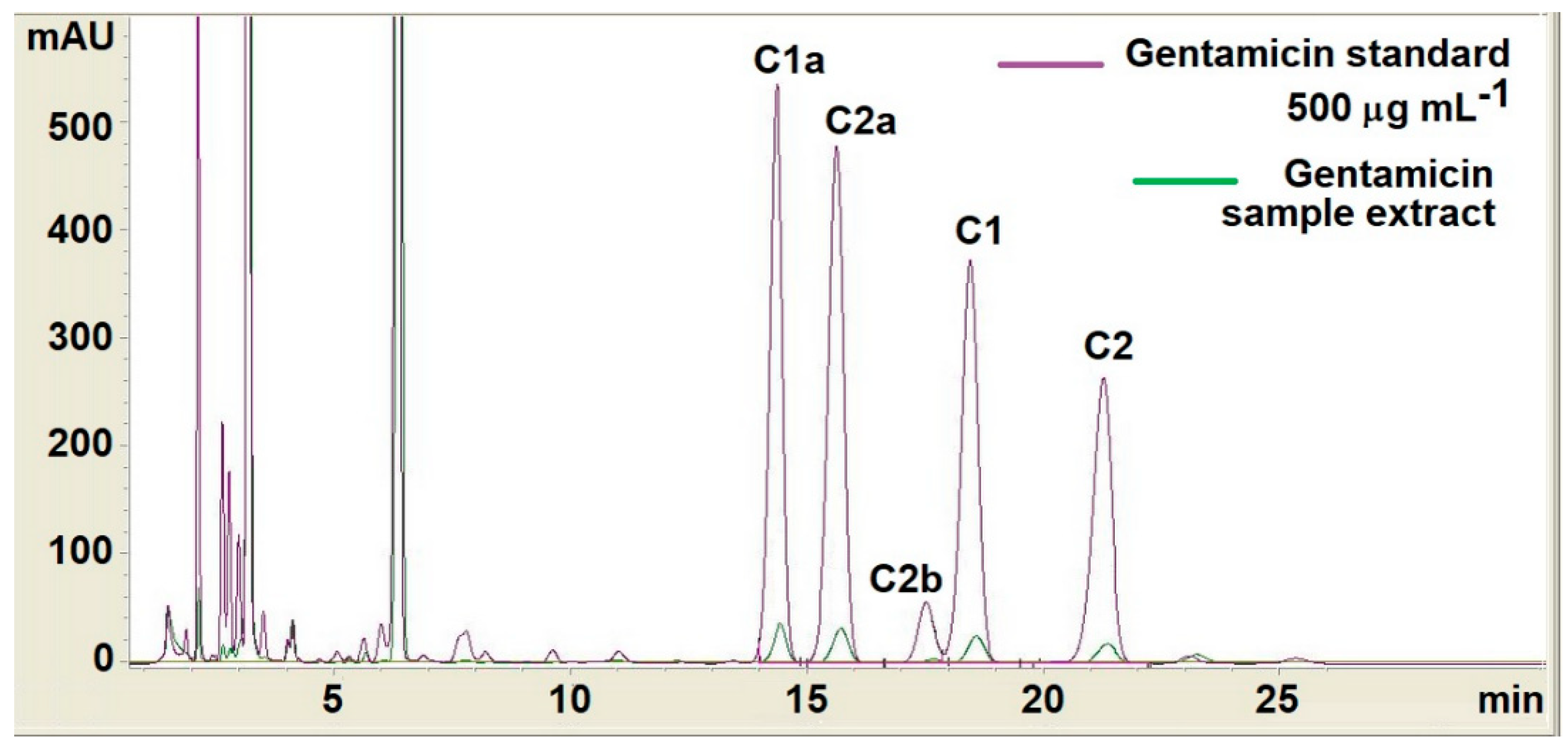
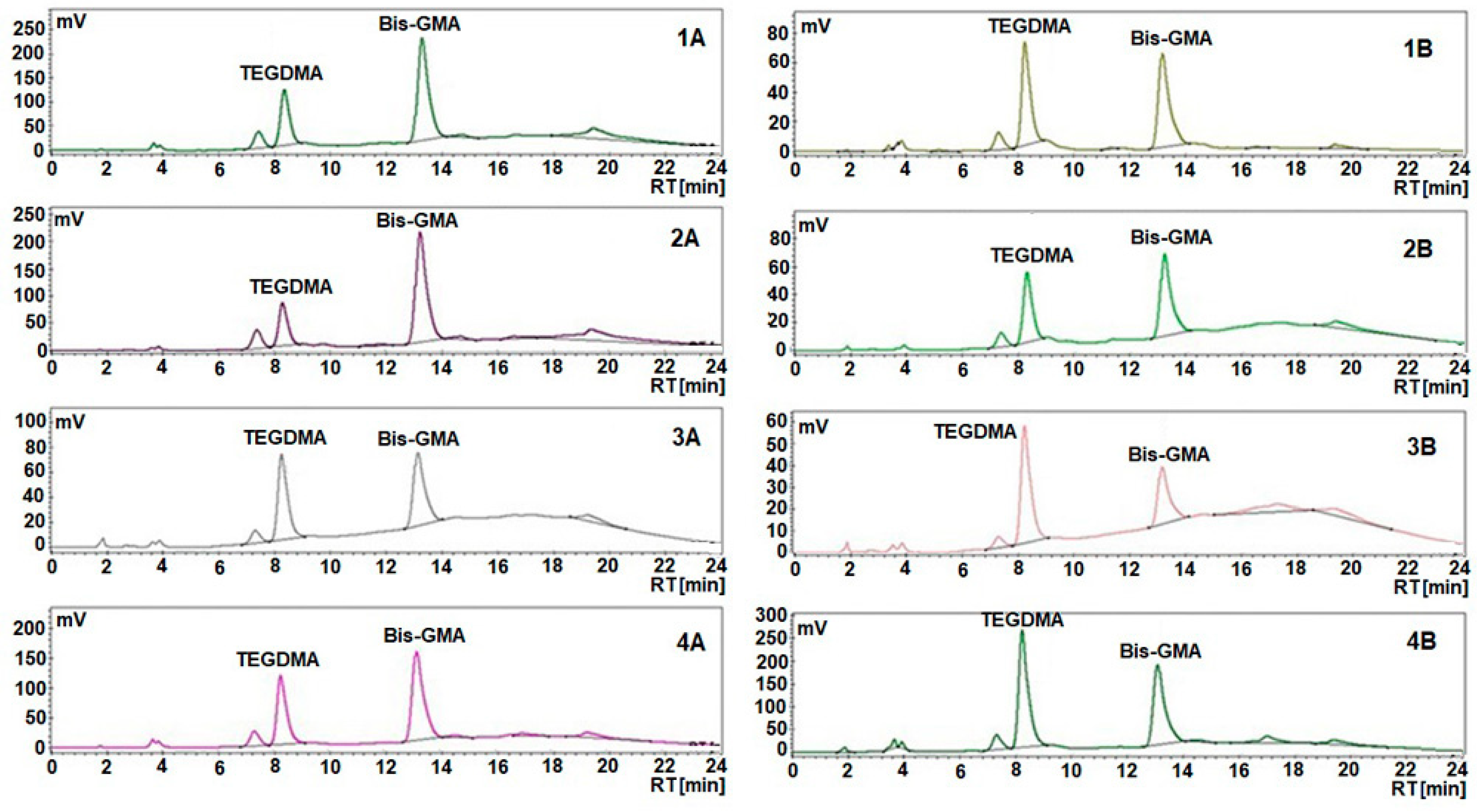

| Samples Code | Bis-GMA (%) | TEGDMA (%) | PMMA (%) | HA (%) | ZrO2 (%) | Gentamicin (%) | Thermic Treatment | Solvent |
|---|---|---|---|---|---|---|---|---|
| 1A | 35.64 | 23.76 | - | 6.6 | 12 | 22 | - | chloroform |
| 2A | 35.64 | 23.76 | - | 6.6 | 12 | 22 | 100 °C, 2 h | chloroform |
| 3A | 35.64 | 23.76 | - | 6.6 | 12 | 22 | - | ethyl alcohol |
| 4A | 35.64 | 23.76 | - | 6.6 | 12 | 22 | - | acetone |
| 1B | 17.82 | 23.76 | 17.82 | 6.6 | 12 | 22 | - | chloroform |
| 2B | 17.82 | 23.76 | 17.82 | 6.6 | 12 | 22 | 100 °C, 2 h | chloroform |
| 3B | 17.82 | 23.76 | 17.82 | 6.6 | 12 | 22 | - | ethyl alcohol |
| 4B | 17.82 | 23.76 | 17.82 | 6.6 | 12 | 22 | - | acetone |
| Sample Code | Initial Bis-GMA (g) | Extracted Bis-GMA (mg/2 mL) | Extracted Bis-GMA (%) | Initial TEGDMA (g) | Extracted TEGDMA (mg/2 mL) | Extracted TEGDMA (%) |
|---|---|---|---|---|---|---|
| 1A | 0.068 | 1.168 | 1.7 | 0.045 | 0.709 | 1.6 |
| 2A | 0.068 | 1.120 | 1.6 | 0.045 | 0.466 | 1 |
| 3A | 0.058 | 0.258 | 0.4 | 0.039 | 0.431 | 1.1 |
| 4A | 0.062 | 0.812 | 1.3 | 0.041 | 0.753 | 1.8 |
| 1B | 0.030 | 0.363 | 1.2 | 0.030 | 0.430 | 1.4 |
| 2B | 0.030 | 0.240 | 0.8 | 0.030 | 0.276 | 0.9 |
| 3B | 0.028 | 0.056 | 0.2 | 0.028 | 0.325 | 1.2 |
| 4B | 0.039 | 0.985 | 2.5 | 0.039 | 1.724 | 4.4 |
| Day | Released Quantity of Gentamicin (µg/mL) | MIC (µg/mL) | MBC (µg/mL) | ||
|---|---|---|---|---|---|
| S. aureus | P. aeruginosa | S. aureus | P. aeruginosa | ||
| 1 | 160.00 | 0.15 | 0.62 | 0.30 | 1.24 |
| 2 | 22.00 | 0.17 | 0.68 | 0.34 | 1.36 |
| 3 | 11.02 | 0.16 | 0.68 | 0.32 | 1.36 |
| 4 | 9.66 | 0.15 | 0.6 | 0.30 | 1.2 |
| 5 | 5.60 | 0.16 | 0.7 | 0.32 | 1.4 |
| 6 | 5.42 | 0.16 | 0.66 | 0.32 | 1.32 |
| 7 | 5.02 | 0.15 | 0.62 | 0.30 | 1.24 |
| 8 | 4.90 | 0.15 | 0.6 | 0.30 | 1.2 |
| 9 | 3.58 | 0.11 | 0.88 | 0.22 | 1.76 |
| 10 | 4.42 | 0.13 | 0.55 | 0.26 | 1.1 |
| 11 | 4.00 | 0.12 | 0.5 | 0.24 | 1 |
| 12 | 3.88 | 0.18 | 0.72 | 0.36 | 1.14 |
| 13 | 3.24 | 0.17 | 0.64 | 0.34 | 1.28 |
| 14 | 2.63 | 0.16 | 0.62 | 0.32 | 1.24 |
| Mean and standard deviation | 0.151 ± 0.019 | 0.652 ± 0.088 | 0.301 ± 0.040 | 1.276 ± 0.183 | |
| Day | Released Quantity of Gentamicin (µg/mL) | MIC (µg/mL) | MBC (µg/mL) | ||
|---|---|---|---|---|---|
| P. aeruginosa | S. aureus | P. aeruginosa | S. aureus | ||
| 1 | 250.00 | 0.12 | 0.48 | 0.24 | 0.96 |
| 2 | 34.66 | 0.14 | 0.54 | 0.28 | 1.18 |
| 3 | 15.70 | 0.12 | 0.49 | 0.24 | 0.98 |
| 4 | 10.32 | 0.16 | 0.64 | 0.32 | 1.28 |
| 5 | 7.52 | 0.13 | 0.47 | 0.26 | 0.94 |
| 6 | 6.72 | 0.21 | 0.84 | 0.42 | 1.68 |
| 7 | 5.32 | 0.16 | 0.66 | 0.32 | 1.32 |
| 8 | 3.38 | 0.11 | 0.84 | 0.22 | 1.68 |
| 9 | 3.48 | 0.2 | 0.86 | 0.40 | 1.72 |
| 10 | 3.06 | 0.18 | 0.76 | 0.32 | 1.52 |
| 11 | 3.46 | 0.2 | 0.86 | 0.40 | 1.72 |
| 12 | 3.08 | 0.12 | 0.51 | 0.24 | 1.02 |
| 13 | 2.6 | 0.16 | 0.64 | 0.32 | 1.28 |
| 14 | 2.4 | 0.18 | 0.46 | 0.36 | 0.92 |
| Mean and standard deviation | 0.156 ± 0.033 | 0.646 ± 0.158 | 0.306 ± 0.067 | 1.329 ± 0.305 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miron, A.E.; Moldovan, M.; Prejmerean, C.A.; Prodan, D.; Vlassa, M.; Filip, M.; Badea, M.E.; Moldovan, M.A. New Antimicrobial Biomaterials for the Reconstruction of Craniofacial Bone Defects. Coatings 2020, 10, 678. https://doi.org/10.3390/coatings10070678
Miron AE, Moldovan M, Prejmerean CA, Prodan D, Vlassa M, Filip M, Badea ME, Moldovan MA. New Antimicrobial Biomaterials for the Reconstruction of Craniofacial Bone Defects. Coatings. 2020; 10(7):678. https://doi.org/10.3390/coatings10070678
Chicago/Turabian StyleMiron (Lungu), Andreea Elena, Marioara Moldovan, Cristina Alexandra Prejmerean, Doina Prodan, Mihaela Vlassa, Miuța Filip, Mîndra Eugenia Badea, and Mădălina Anca Moldovan. 2020. "New Antimicrobial Biomaterials for the Reconstruction of Craniofacial Bone Defects" Coatings 10, no. 7: 678. https://doi.org/10.3390/coatings10070678
APA StyleMiron, A. E., Moldovan, M., Prejmerean, C. A., Prodan, D., Vlassa, M., Filip, M., Badea, M. E., & Moldovan, M. A. (2020). New Antimicrobial Biomaterials for the Reconstruction of Craniofacial Bone Defects. Coatings, 10(7), 678. https://doi.org/10.3390/coatings10070678






