Synthesis of High-Performance Photonic Crystal Film for SERS Applications via Drop-Coating Method
Abstract
1. Introduction
2. Materials and Methods
2.1. Material and Substrates
2.2. Synthesis of Monodisperse SiO2 Nanospheres
2.3. Surface Modification of Glass Slides
2.4. Fabrication of Colloidal Crystal Coatings
2.5. Deposition of Au Thin Film
2.6. Characterization
3. Results and Discussion
3.1. Preparation of Uniform SiO2 Nanospheres
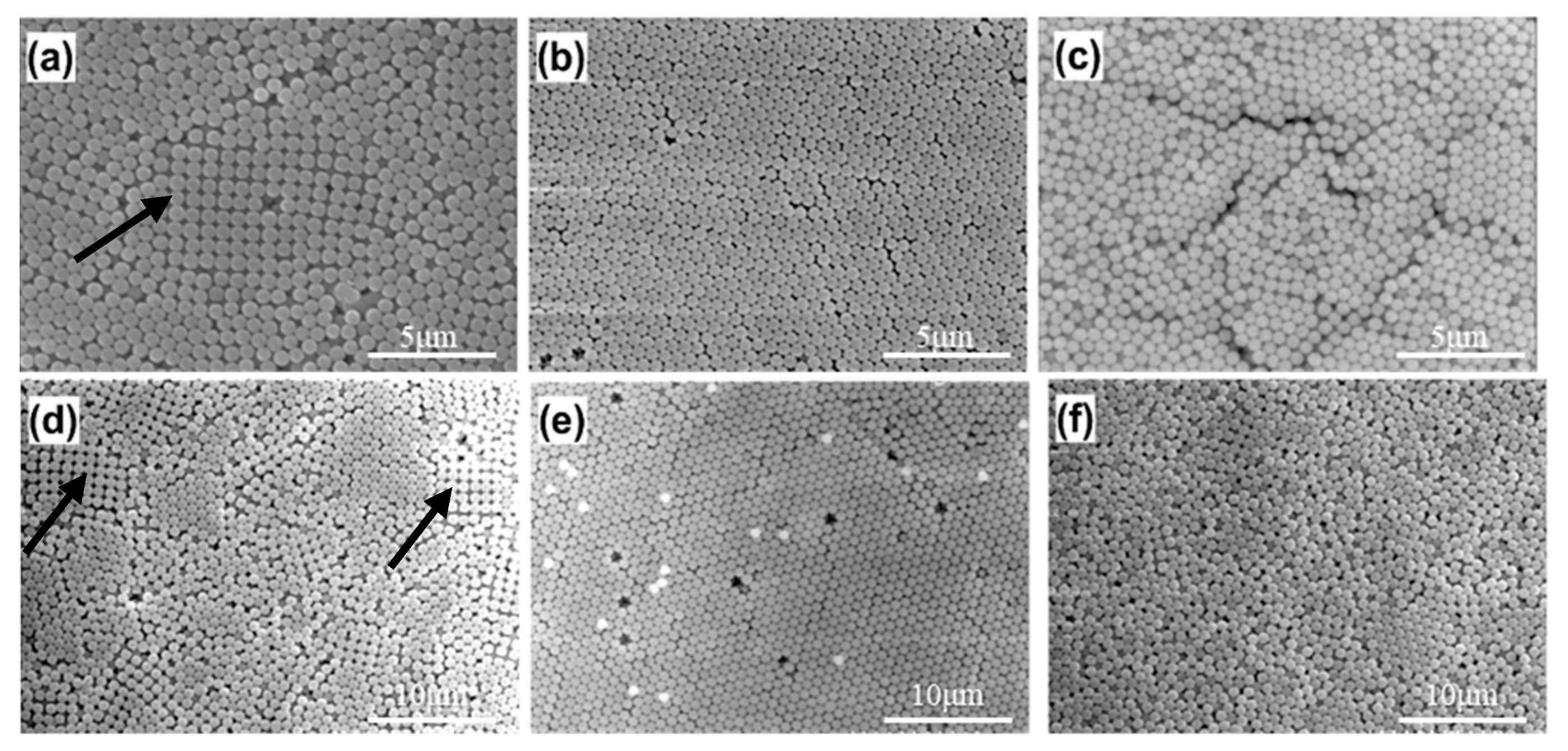
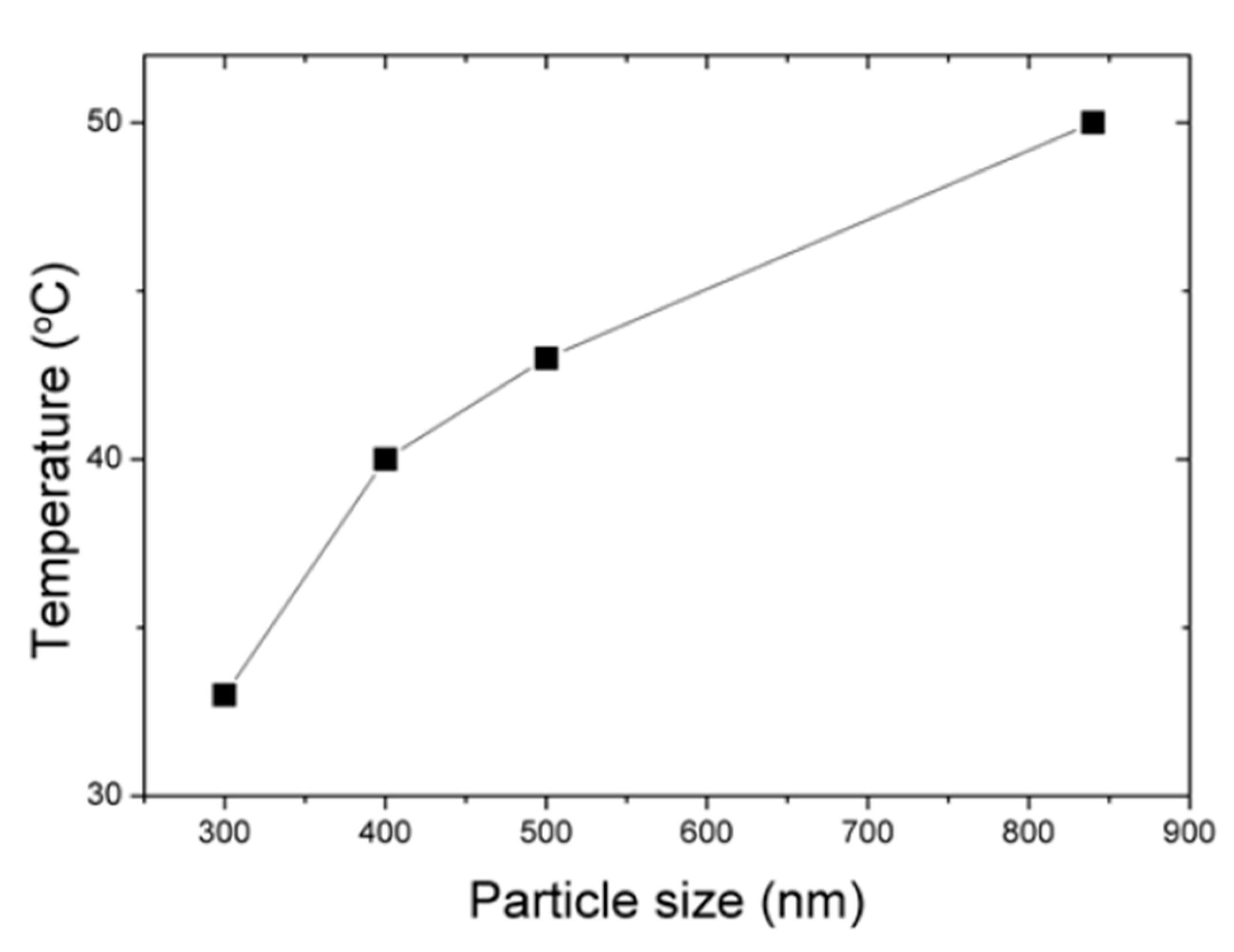
3.2. Modified Drop-Coating Method Using IHEISA Approach
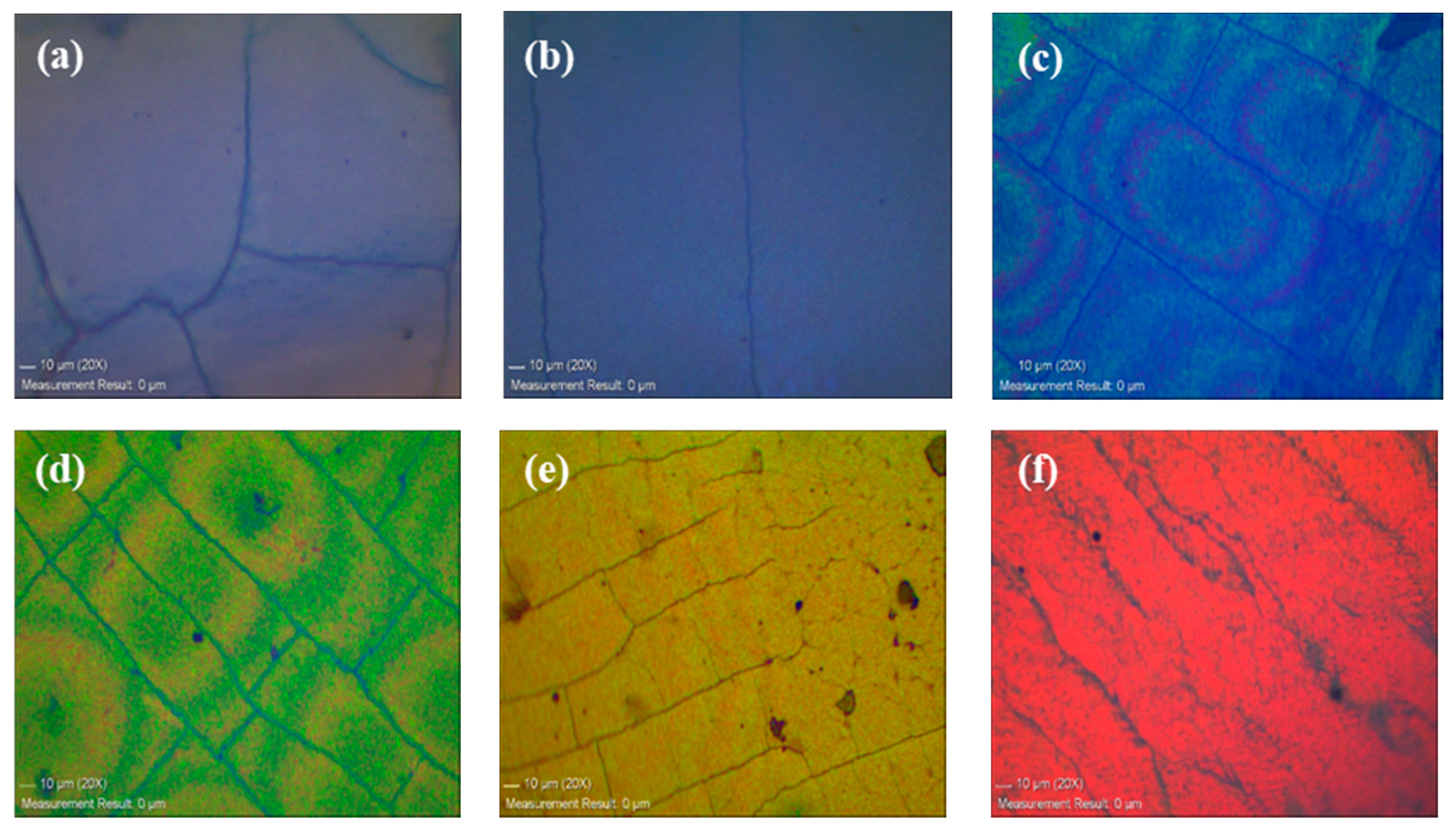
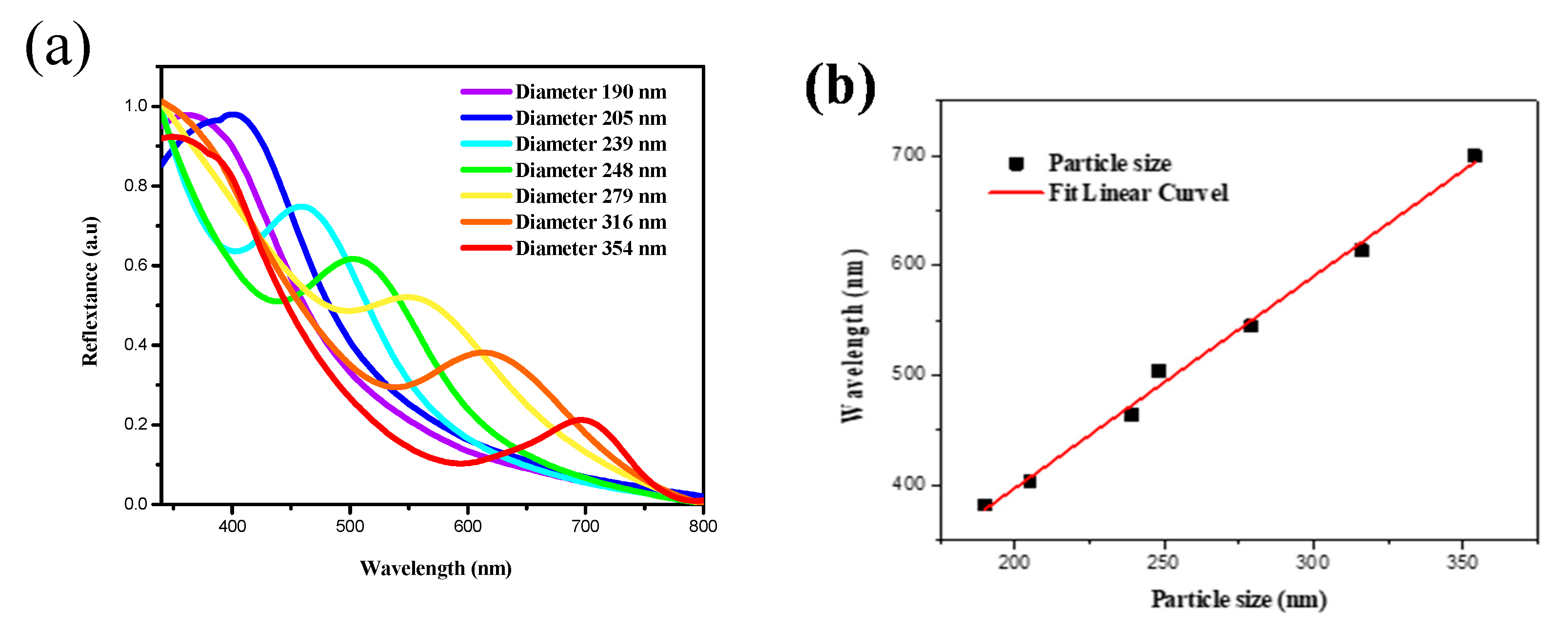
3.3. Optical Properties of the Silica-Based PC Film
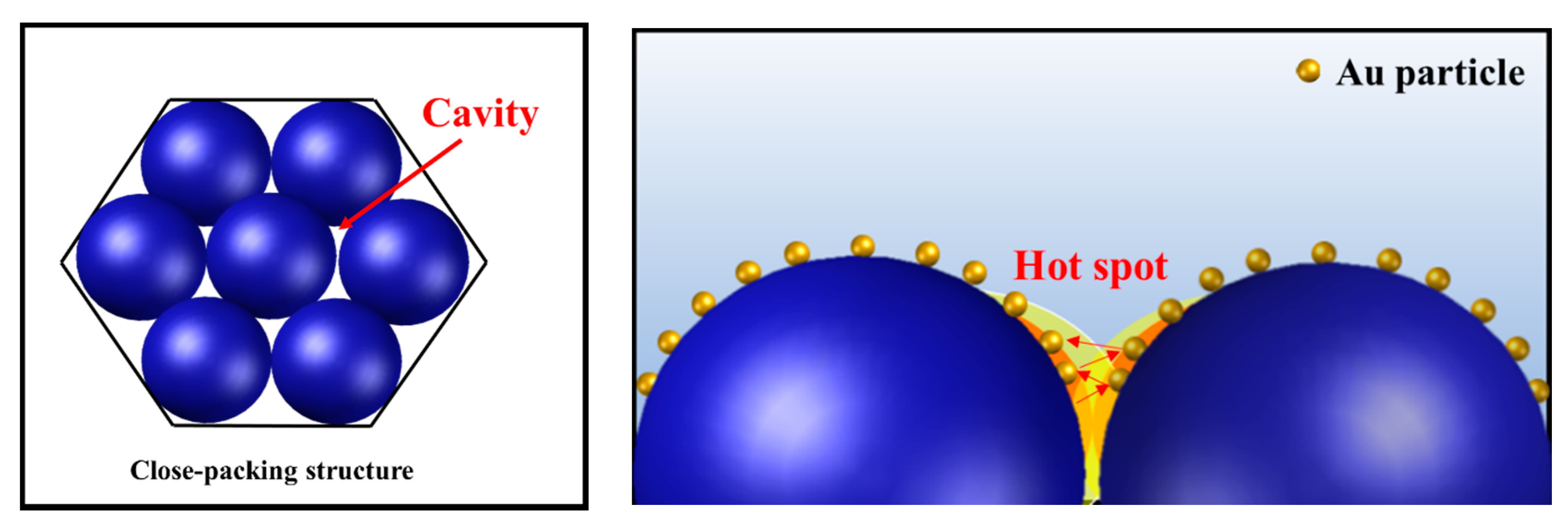
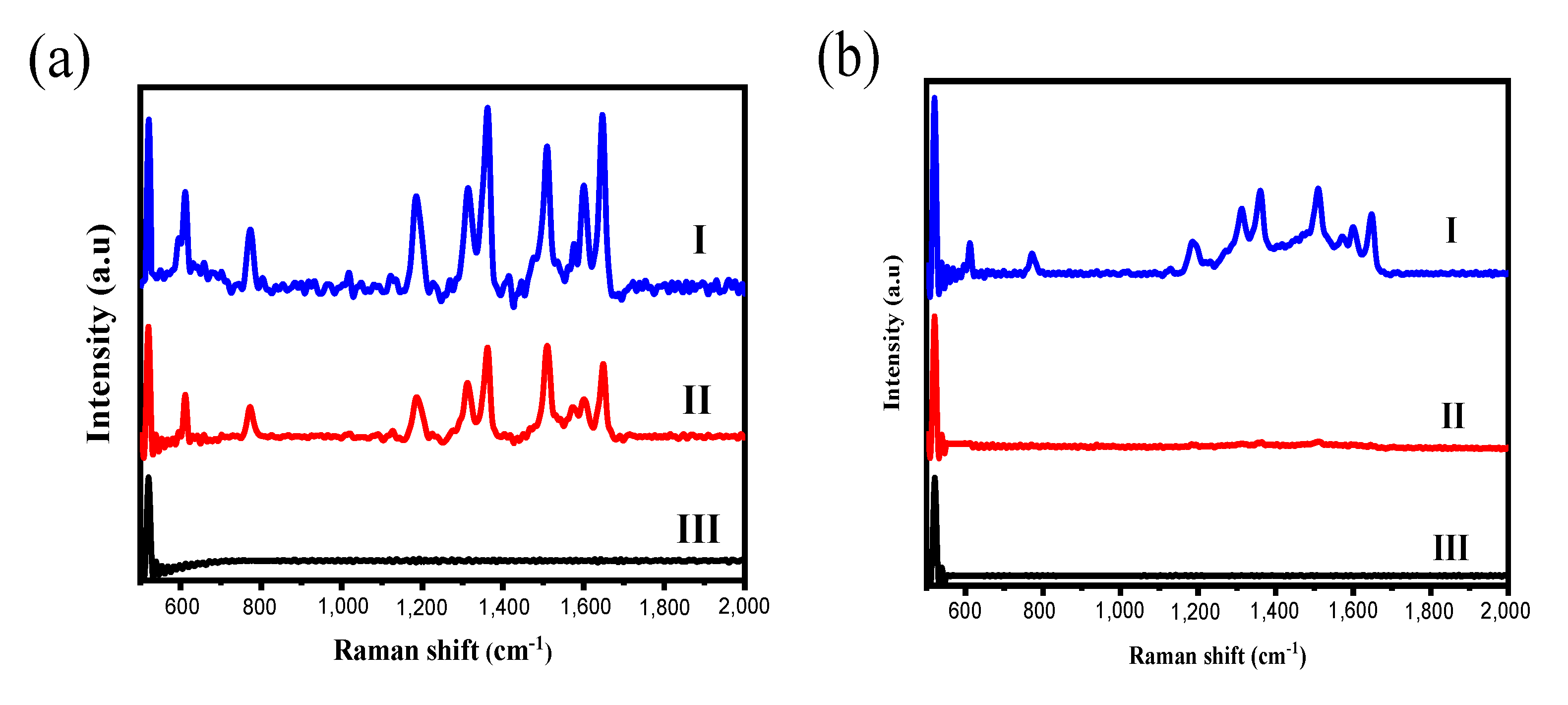
3.4. Application of Silica-Basecd PC Films to SERS Substrates
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Armstrong, E.; O’Dwyer, C. Artificial opal photonic crystals and inverse opal structures-fundamentals and applications from optics to energy storage. J. Mater. Chem. C 2015, 3, 6109–6143. [Google Scholar] [CrossRef]
- Kin, H.N.; Yang, S. Responsive smart windows from nanoparticle-polymer composites. Adv. Funct. Mater. 2020, 30, 1902597. [Google Scholar]
- Gao, W.; Rigout, M.; Owens, H. Self-assembly of silica colloidal crystal thin films with tunable structural colours over a wide visible spectrum. Appl. Surf. Sci. 2016, 380, 12–15. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, K.Q. Photonic crystal structures with tunable structure color as colorimetric sensors. Sensors 2013, 13, 4192–4213. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Tang, B.; Ju, B.; Wu, S.; Zhang, S. Multiple colors output on voile through 3D colloidal crystals with robust mechanical properties. ACS Appl. Mater. Interfaces 2017, 9, 3024–3029. [Google Scholar] [CrossRef]
- Li, H.; Xu, Z.; Bao, B.; Sun, N.; Song, Y. Improving the luminescence performance of quantum dot-based photonic crystals for white-light emission. J. Mater. Chem. C 2016, 4, 39–44. [Google Scholar] [CrossRef]
- Tian, Y.; Chen, M.; Zhang, J.; Tong, Y.L.; Wang, C.F.; Wiederrecht, G.P.; Chen, S. Highly enhanced luminescence performance of LEDs via controllable layer-structured 3D photonic crystals and photonic crystal beads. Small Methods 2018, 2, 1800104. [Google Scholar] [CrossRef]
- Hou, J.; Zhang, H.; Yang, Q.; Li, M.; Jiang, L.; Song, Y. Hydrophilic–hydrophobic patterned molecularly imprinted photonic crystal sensors for high-sensitive colorimetric detection of tetracycline. Small 2015, 11, 2738–2742. [Google Scholar] [CrossRef]
- Zhao, X.; Xue, J.; Mu, Z.; Huang, Y.; Lu, M.; Gu, Z. Gold nanoparticle incorporated inverse opal photonic crystal capillaries for optofluidic surface enhanced Raman spectroscopy. Biosens. Bioelectron. 2015, 72, 268–274. [Google Scholar] [CrossRef]
- Kuo, W.K.; Weng, H.P.; Hsu, J.J.; Yu, H.H. Photonic crystal-based sensors for detecting alcohol concentration. Appl. Sci. 2016, 6, 67. [Google Scholar] [CrossRef]
- Xing, H.; Li, J.; Shi, Y.; Guo, J.; Wei, J. Thermally driven photonic actuator based on silica opal photonic crystal with liquid crystal elastomer. ACS Appl. Mater. Interfaces 2016, 8, 9440–9445. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Moon, J.H. Monolithic multiscale bilayer inverse opal electrodes for dye-sensitized solar cell applications. Nanoscale 2015, 7, 5164–5168. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Lei, J.; Wang, L.; Zhang, J.; Liu, Y. TiO2 inverse opal photonic crystals: Synthesis, modification, and applications—A review. J. Alloy. Compd. 2018, 769, 740–757. [Google Scholar] [CrossRef]
- Zhao, D.; Liu, S.; Yang, H.; Ma, Z.; Reuterskiöld-Hedlund, C.; Hammar, M.; Zhou, W. Printed large-area single-mode photonic crystal bandedge surface-emitting lasers on silicon. Sci. Rep. 2016, 6, 18860. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Zhou, L.; Fan, Q.; Chai, L.; Shao, J. The vertical deposition self-assembly process and the formation mechanism of poly (styrene-co-methacrylic acid) photonic crystals on polyester fabrics. J. Mater. Sci. 2016, 51, 2859–2868. [Google Scholar] [CrossRef]
- Gao, W.; Rigout, M.; Owens, H. The structural coloration of textile materials using self-assembled silica nanoparticles. J. Nanopart. Res. 2017, 19, 303. [Google Scholar] [CrossRef]
- O’Dwyer, C. Color-coded batteries-electro-photonic inverse opal materials for enhanced electrochemical energy storage and optically encoded diagnostics. Adv. Mater. 2016, 28, 5681–5688. [Google Scholar] [CrossRef]
- Collins, G.; Armstrong, E.; McNulty, D.; O’Hanlon, S.; Geaney, H.; O’Dwyer, C. 2D and 3D photonic crystal materials for photocatalysis and electrochemical energy storage and conversion. Sci. Technol. Adv. Mater. 2016, 17, 563–582. [Google Scholar] [CrossRef]
- Likodimos, V. Photonic crystal-assisted visible light activated TiO2 photocatalysis. Appl. Catal. B Environ. 2018, 230, 269–303. [Google Scholar] [CrossRef]
- Chen, H.; Lou, R.; Chen, Y.; Chen, L.; Lu, J.; Dong, Q. Photonic crystal materials and their application in biomedicine. Drug Deliv. 2017, 24, 775–780. [Google Scholar] [CrossRef]
- Kitano, K.; Suzuki, K.; Ishizaki, K.; Noda, S. Three-dimensional photonic crystals fabricated by simultaneous multidirectional etching. Phys. Rev. B 2015, 91, 155308. [Google Scholar] [CrossRef]
- Grishina, D.A.; Harteveld, C.A.; Woldering, L.A.; Vos, W.L. Method for making a single-step etch mask for 3D monolithic nanostructures. Nanotechnology 2015, 26, 505302. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wan, Y.; Li, Y.; Cai, Z.; Li, H.L.; Zhao, X.S.; Li, Q. Binary colloidal crystals fabricated with a horizontal deposition method. Langmuir 2009, 25, 6753–6759. [Google Scholar] [CrossRef]
- Russell, J.L.; Noel, G.H.; Warren, J.M.; Tran, N.L.L.; Mallouk, T.E. Binary colloidal crystal films grown by vertical evaporation of silica nanoparticle suspensions. Langmuir 2017, 33, 10366–10373. [Google Scholar] [CrossRef]
- Liu, G.; Zhou, L.; Wu, Y.; Wang, C.; Fan, Q.; Shao, J. The fabrication of full color P (S t-MAA) photonic crystal structure on polyester fabrics by vertical deposition self-assembly. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, X. Rapid preparation of hexagonal and square array colloidal crystals via electrophoresis-assisted self-assembly method. Mater. Lett. 2015, 142, 109–111. [Google Scholar] [CrossRef]
- Hung, P.S.; Liao, C.H.; Chou, Y.S.; Wang, G.R.; Wang, C.J.; Chung, W.A.; Wu, P.W. High throughput fabrication of large-area colloidal crystals via a two-stage electrophoretic deposition method. Electrochim. Acta 2019, 317, 52–60. [Google Scholar] [CrossRef]
- Katagiri, K.; Tanaka, Y.; Uemura, K.; Inumaru, K.; Seki, T.; Takeoka, Y. Structural color coating films composed of an amorphous array of colloidal particles via electrophoretic deposition. NPG Asia Mater. 2017, 9, e355. [Google Scholar] [CrossRef]
- Koegler, P.; Dunn, M.; Wang, P.Y.; Thissen, H.; Kingshott, P. A novel approach to quantitatively assess the uniformity of binary colloidal crystal assemblies. Crystals 2016, 6, 84. [Google Scholar] [CrossRef]
- Huang, D.; Zeng, M.; Wang, L.; Zhang, L.; Cheng, Z. Biomimetic colloidal photonic crystals by coassembly of polystyrene nanoparticles and graphene quantum dots. RSC Adv. 2016, 8, 34839–34847. [Google Scholar] [CrossRef]
- Nagao, D.; Kameyama, R.; Matsumoto, H.; Kobayashi, Y.; Konno, M. Single- and multi-layered patterns of polystyrene and silica particles assembled with a simple dip-coating. Colloids Surf. A Physicochem. Eng. Asp. 2008, 317, 722–729. [Google Scholar] [CrossRef]
- Jiang, Q.; Li, C.; Shi, S.; Zhao, D.; Xiong, L.; Wei, H.; Yi, L. Assembling ultra-thick and crack-free colloidal crystals via an isothermal heating evaporation induced self-assembly method. J. Non Cryst. Solids 2012, 358, 1611–1616. [Google Scholar] [CrossRef]
- Dalmis, R.; Birlik, I.; Azem NF, A.; Çelik, E. Modification of the sedimentation method for PMMA photonic crystal coatings. Colloids Surf. A Physicochem. Eng. Asp. 2019, 577, 194–201. [Google Scholar] [CrossRef]
- Sandu, I.; Dumitru, M.; Fleaca, C.T.; Dumitrache, F. Hanging colloidal drop: A new photonic crystal synthesis route. Photonics Nanostruct. 2018, 29, 42–48. [Google Scholar] [CrossRef]
- Yan, J.; Bloom, M.; Bae, S.C.; Luijten, E.; Granick, S. Linking synchronization to self-assembly using magnetic Janus colloids. Nature 2012, 491, 578–581. [Google Scholar] [CrossRef]
- Wong, S.; Kitaev, V.; Ozin, G.A. Colloidal crystal films: Advances in universality and perfection. J. Am. Chem. Soc. 2003, 125, 15589–15598. [Google Scholar] [CrossRef]
- Jiang, P.; McFarland, M.J. Large-scale fabrication of wafer-size colloidal crystals, macroporous polymers and nanocomposites by spin-coating. J. Am. Chem. Soc. 2004, 126, 13778–13786. [Google Scholar] [CrossRef]
- You, B.; Wen, N.; Shi, L.; Wu, L.; Zi, J. Facile fabrication of a three-dimensional colloidal crystal film with large-area and robust mechanical properties. J. Mater. Chem. 2009, 19, 3594–3597. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, M.; Ge, X.; Wu, M.; Wu, Q.; Yang, J.; Liu, N. Facile fabrication of free-standing colloidal-crystal films by interfacial self-assembly. J. Colloid Interface Sci. 2011, 353, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wen, Y.; Feng, X.; Song, Y.; Jiang, L. Control over the wettability of colloidal crystal films by assembly temperature. Macromol. Rapid Commun. 2006, 27, 188–192. [Google Scholar] [CrossRef]
- Jiang, Y.; Sun, D.W.; Pu, H.; Wei, Q. Surface enhanced Raman spectroscopy (SERS): A novel reliable technique for rapid detection of common harmful chemical residues. Trends Food Sci. Technol. 2018, 75, 10–22. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Z.; Wu, L.; Pei, Y.; Chen, P.; Cui, Y. Rapid simultaneous detection of multi-pesticide residues on apple using SERS technique. Analyst 2014, 20, 5148–5154. [Google Scholar] [CrossRef] [PubMed]
- Hughes, J.M.; Budd, P.M.; Tiede, K.; Lewis, J. Polymerized high internal phase emulsion monoliths for the chromatographic separation of engineered nanoparticles. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Nemtsec Ivan, V.; Tambasov Igor, A.; Ivanenko Alexander, A.; Zyryanov Victor, Y.A. Angle-resolved reflection spectroscopy of high-quality PMMA opal crystal. Photonics Nanostruct. 2018, 28, 37–44. [Google Scholar]
- Lai, C.F.; Wang, Y.C. Colloidal photonic crystals containing silver nanoparticles with tunable structural colors. Crystals 2016, 6, 61. [Google Scholar] [CrossRef]
- Padmanabhan, S.C.; McGrath, J.; Bardosova, M.; Pemble, M.E. A facile method for the synthesis of highly monodisperse silica@gold@silica core-shell-shell particles and their use in the fabrication of three-dimensional metallodielectric photonic crystals. J. Mater. Chem. 2012, 22, 11978–11987. [Google Scholar] [CrossRef]
- Rastogi, V.; Melle, S.; Calderon, O.G.; García, A.A.; Marquez, M.; Velev, O.D. Synthesis of light-diffracting assemblies from microspheres and nanoparticles in droplets on a superhydrophobic surface. Adv. Mater. 2008, 20, 4263–4268. [Google Scholar] [CrossRef]
- Sperling, M.; Gradzielski, M. Droplets, evaporation and a superhydrophobic surface: Simple tools for guiding colloidal particles into complex materials. Gels 2017, 3, 15. [Google Scholar] [CrossRef]
- Lin, H.; Song, L.P.; Huang, Y.J.; Cheng, Q.; Yang, Y.P.; Guo, Z.Y.; Su, F.M.; Chen, T. Macroscopic Au@Pani core/shell nanoparticle superlattice monolayer film with dual-responsive plasmonic switches. ACS Appl. Mater. Interfaces 2020, 12, 11296–11304. [Google Scholar] [CrossRef]
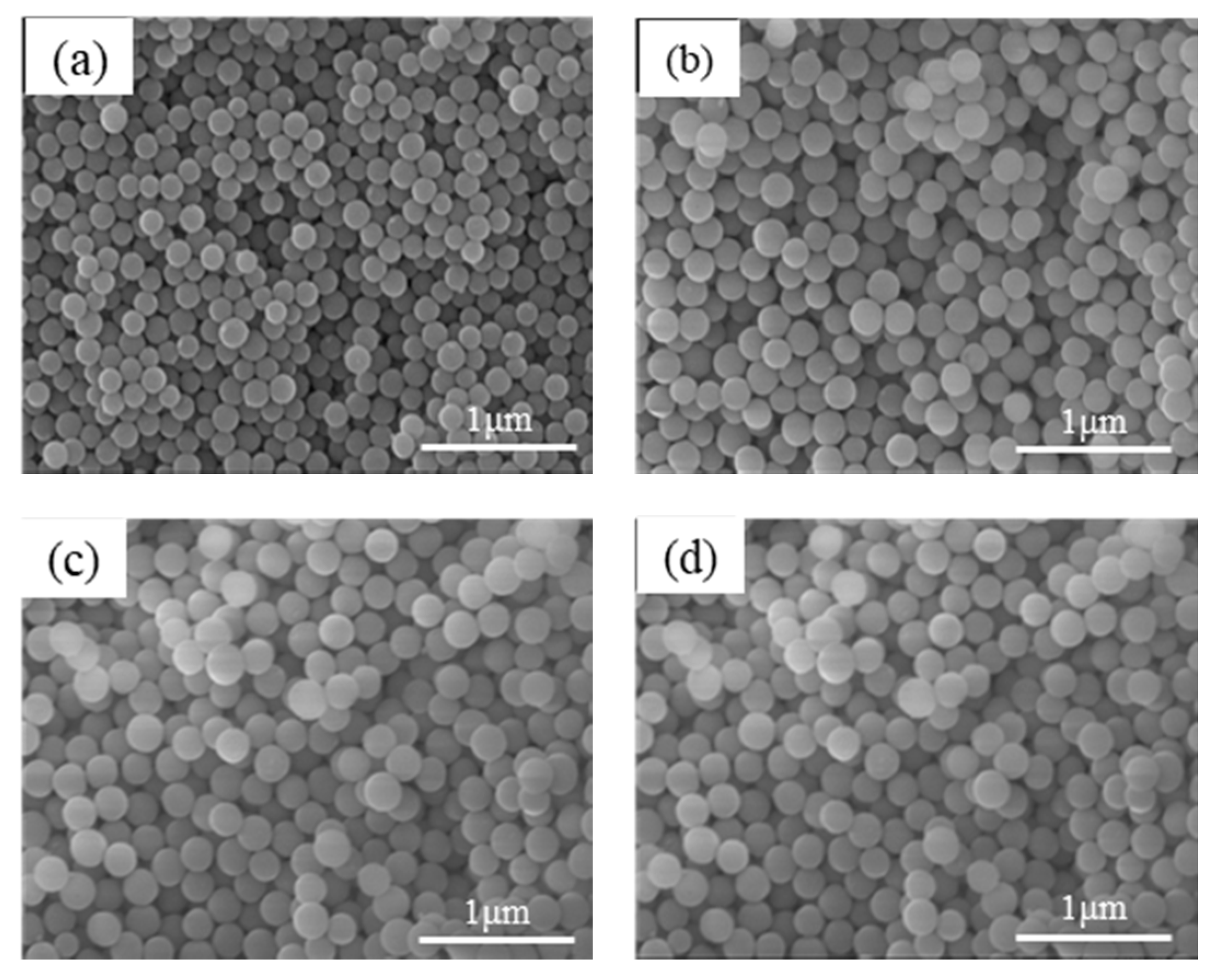
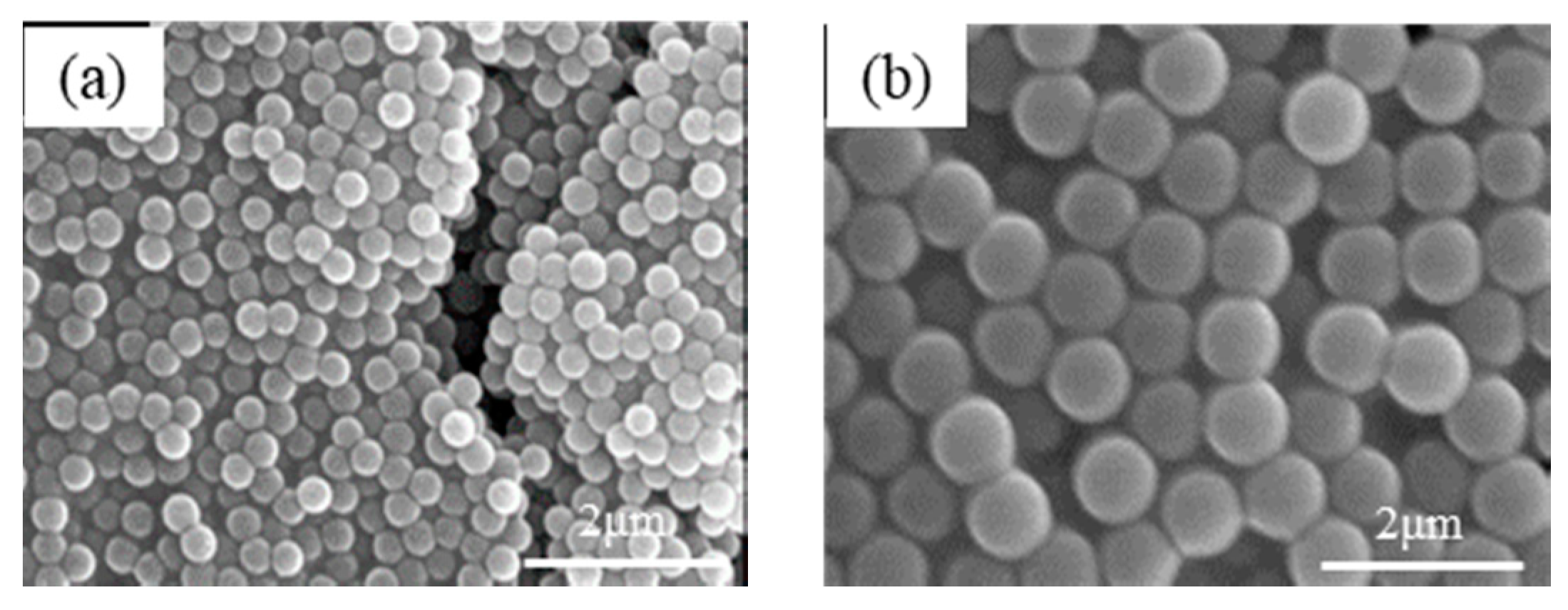
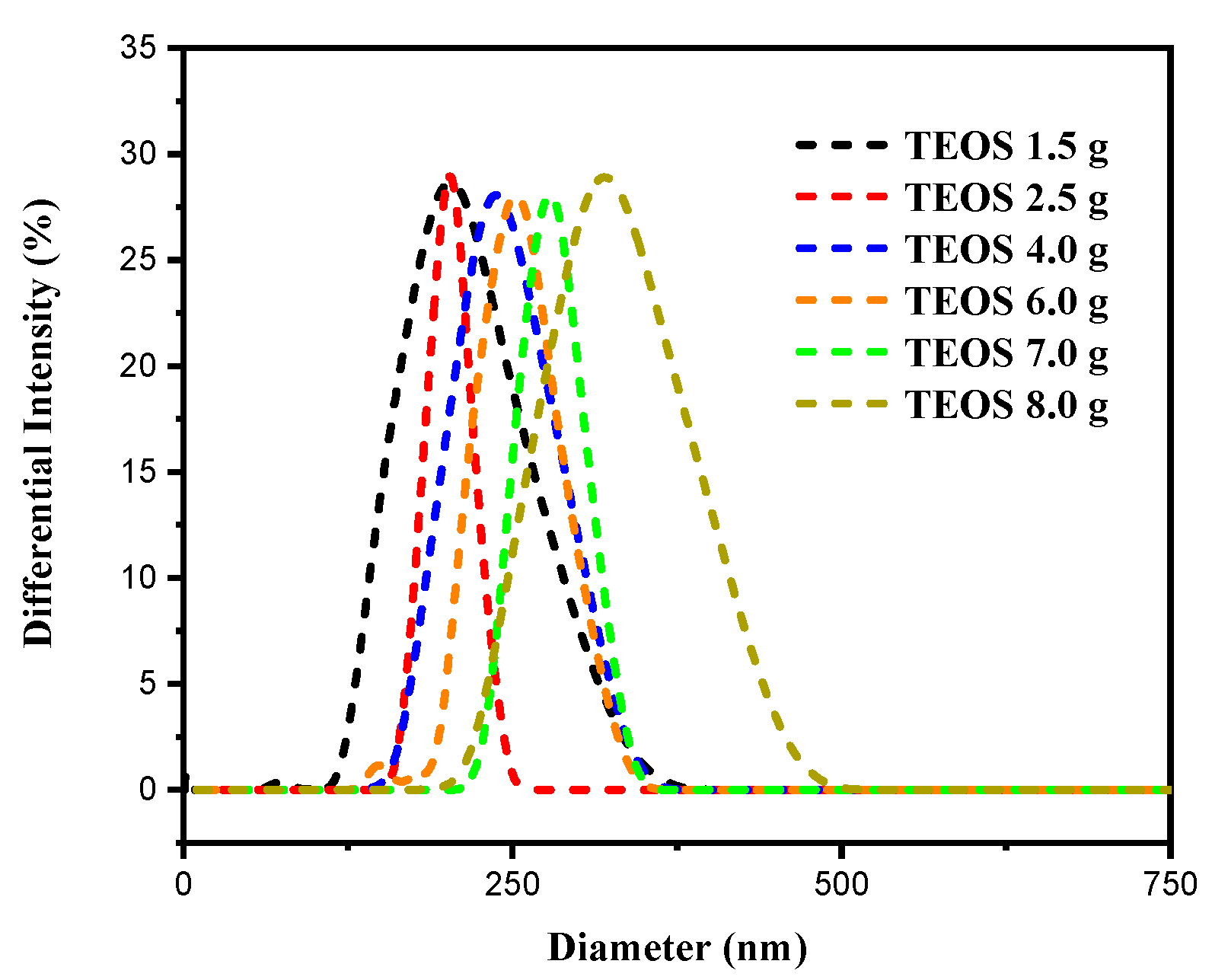
| TEOS (g) | PDI | Particle Size (nm) |
|---|---|---|
| 1.5 | 0.068 | 190 |
| 2.5 | 0.001 | 205 |
| 4.0 | 0.057 | 239 |
| 6.0 | 0.007 | 247 |
| 7.0 | 0.002 | 279 |
| 8.0 | 0.040 | 316 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, M.-X.; Liu, C.-H.; Lee, H.; Lee, B.-W.; Hsu, C.-H.; Lin, H.-P.; Wu, Y.-C. Synthesis of High-Performance Photonic Crystal Film for SERS Applications via Drop-Coating Method. Coatings 2020, 10, 679. https://doi.org/10.3390/coatings10070679
Wei M-X, Liu C-H, Lee H, Lee B-W, Hsu C-H, Lin H-P, Wu Y-C. Synthesis of High-Performance Photonic Crystal Film for SERS Applications via Drop-Coating Method. Coatings. 2020; 10(7):679. https://doi.org/10.3390/coatings10070679
Chicago/Turabian StyleWei, Ming-Xue, Chao-Hui Liu, Han Lee, Bo-Wei Lee, Chun-Han Hsu, Hong-Ping Lin, and Yu-Chun Wu. 2020. "Synthesis of High-Performance Photonic Crystal Film for SERS Applications via Drop-Coating Method" Coatings 10, no. 7: 679. https://doi.org/10.3390/coatings10070679
APA StyleWei, M.-X., Liu, C.-H., Lee, H., Lee, B.-W., Hsu, C.-H., Lin, H.-P., & Wu, Y.-C. (2020). Synthesis of High-Performance Photonic Crystal Film for SERS Applications via Drop-Coating Method. Coatings, 10(7), 679. https://doi.org/10.3390/coatings10070679





