In Vitro Corrosion and Tribocorrosion Performance of Biocompatible Carbide Coatings
Abstract
1. Introduction
2. Materials and Methods
2.1. Coatings Preparation
2.2. Coatings Characterization
3. Results
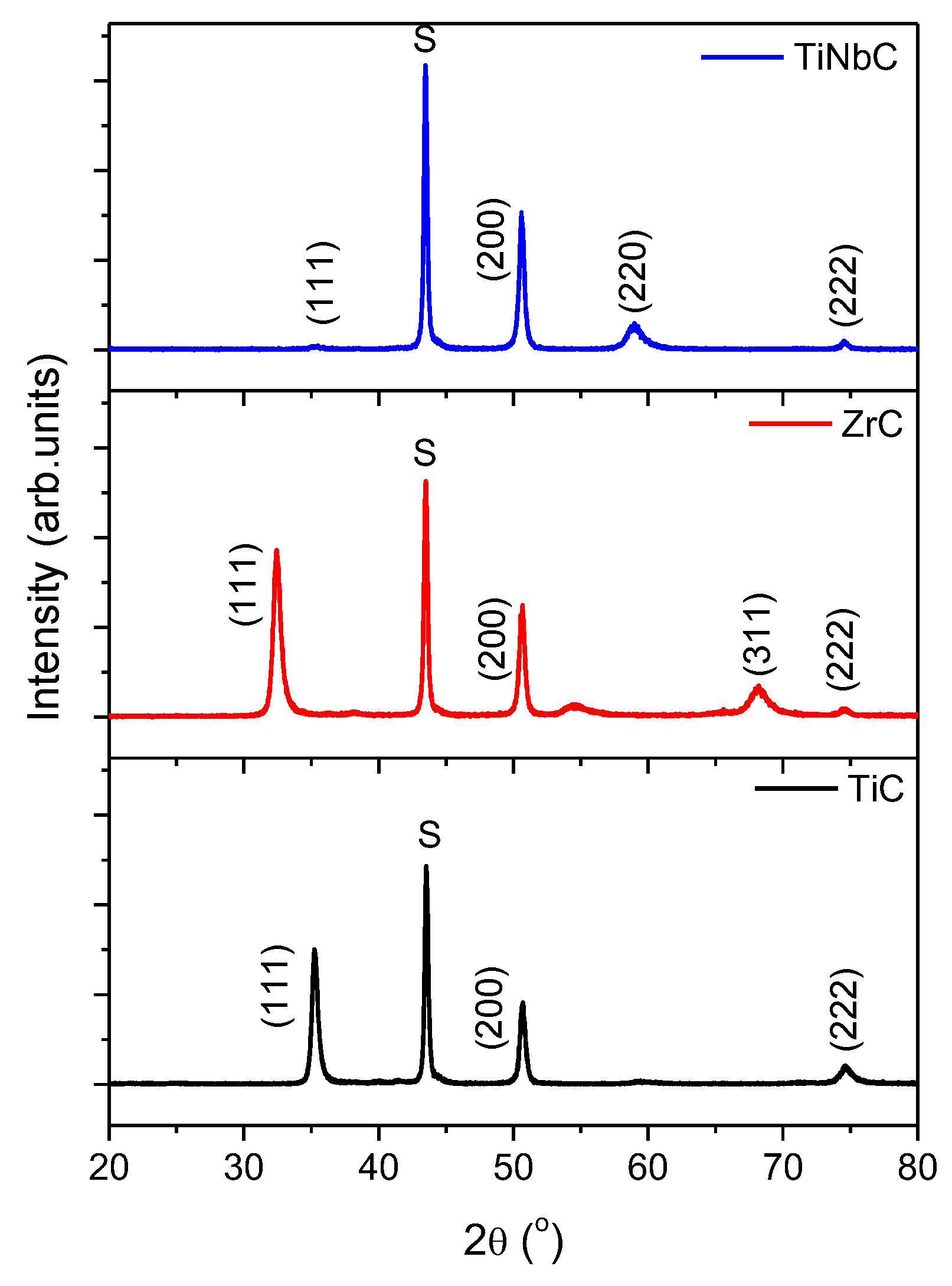
3.1. Elemental and Phase Composition
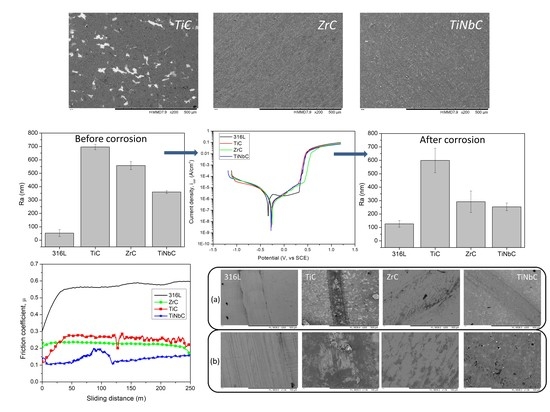
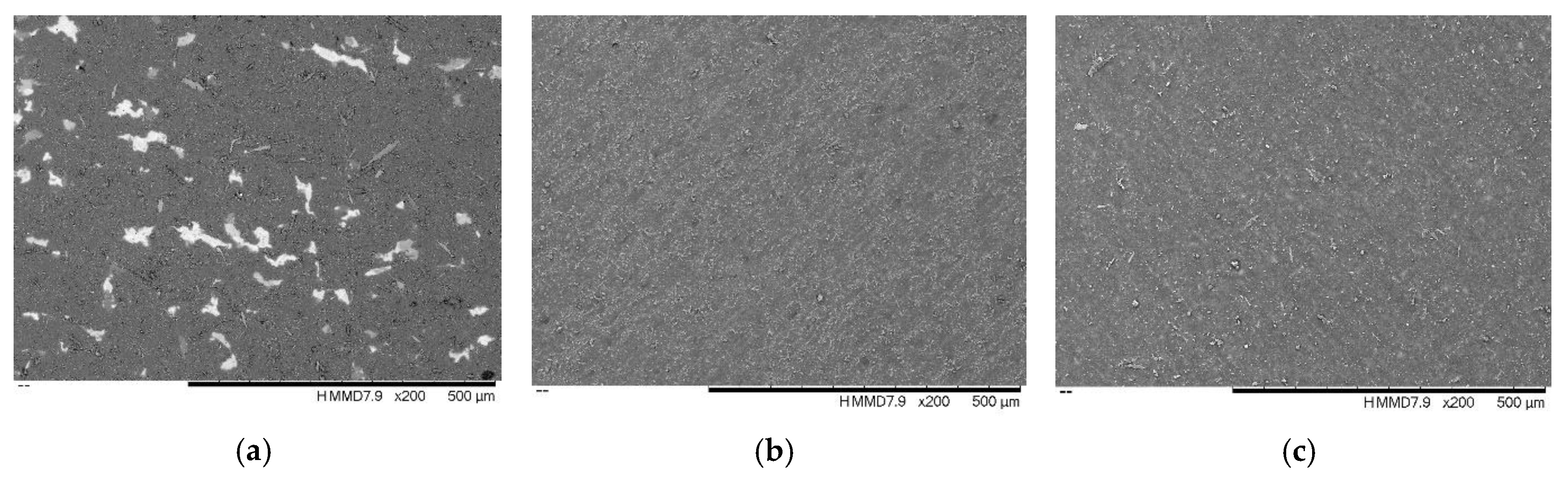
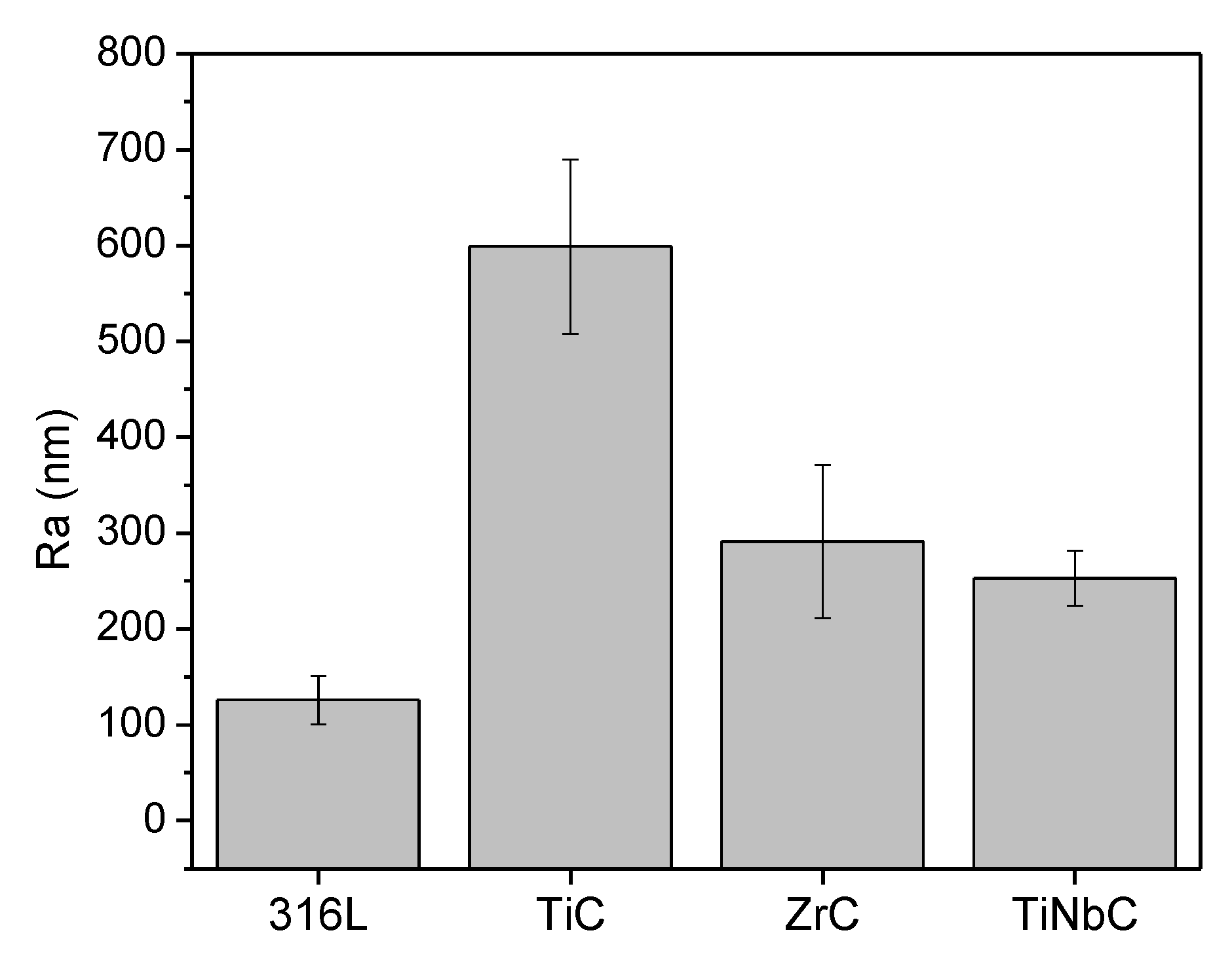
3.2. Morphology and Roughness
3.3. Hardness and Adhesion
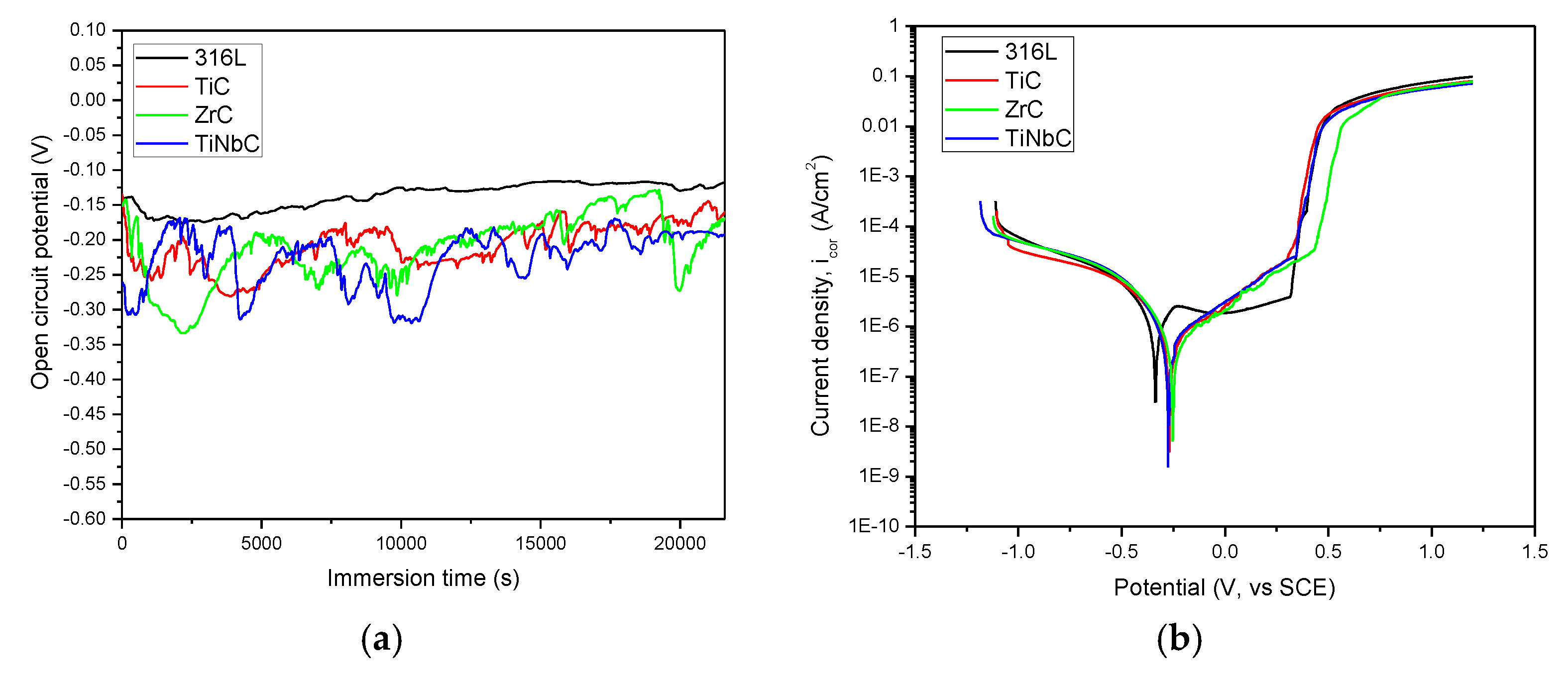
3.4. In Vitro Corrosion Resistance
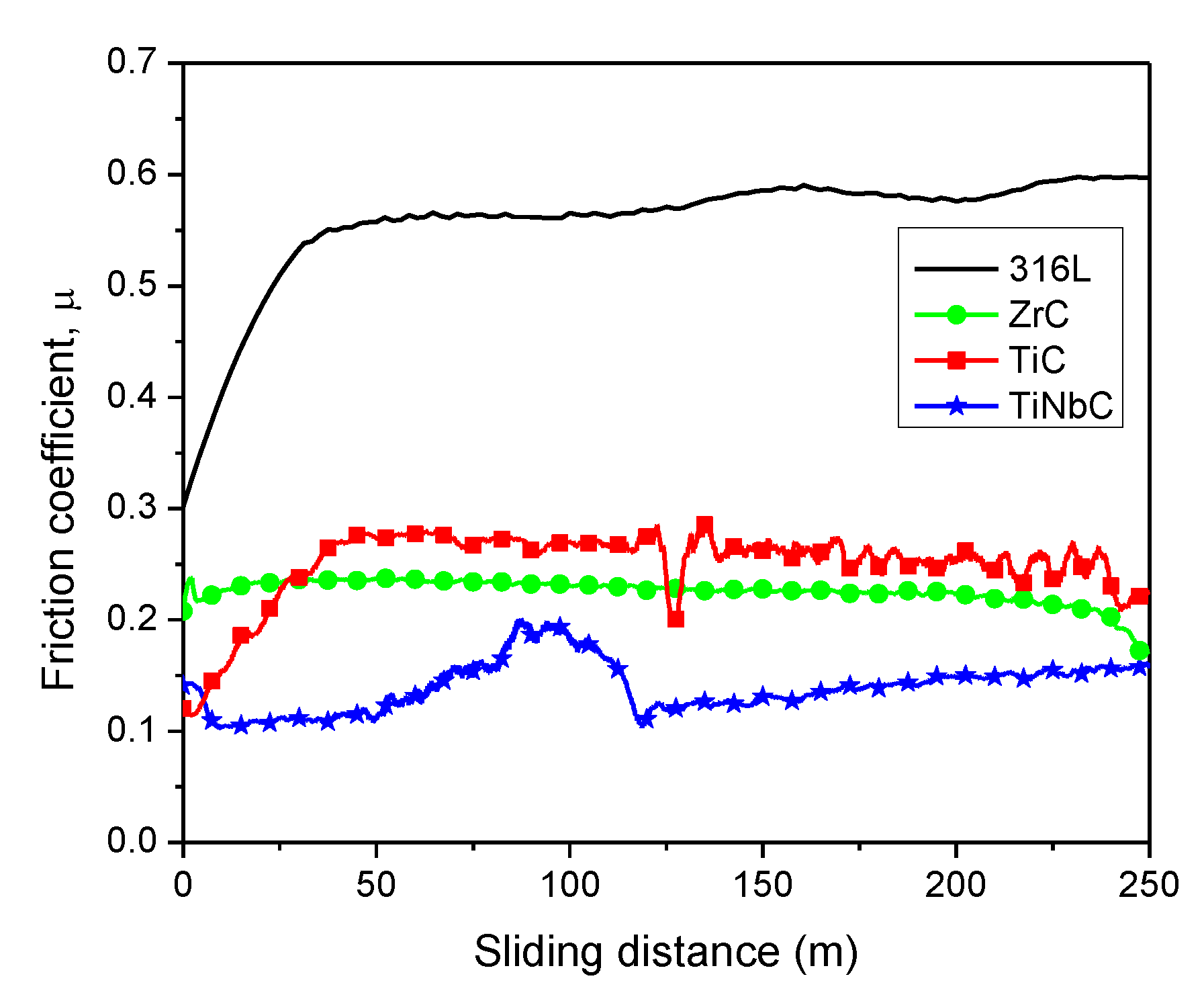
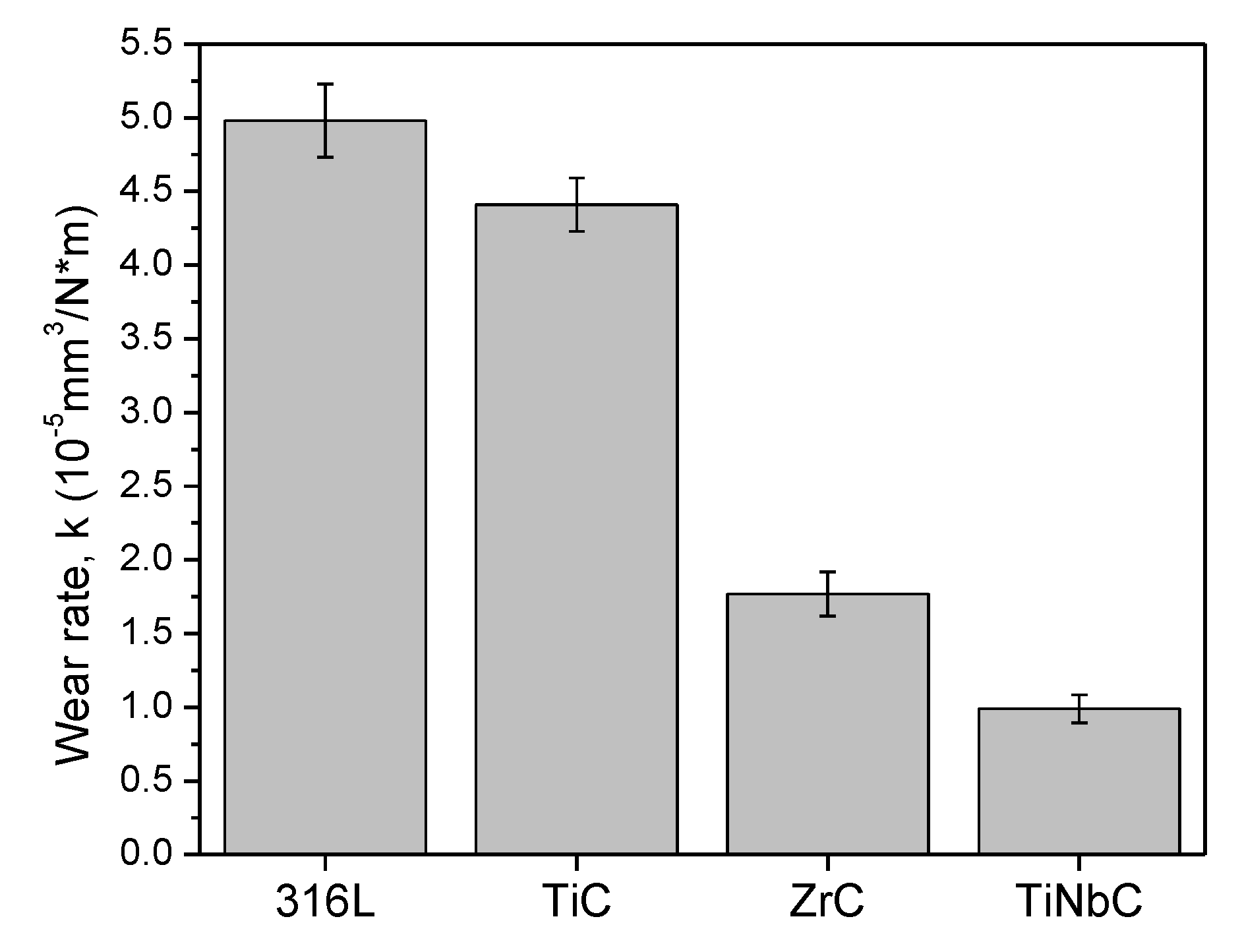
3.5. In Vitro Tribocorrosive Performance
4. Discussion
5. Conclusions
- TiC and ZrC have (111) preferred orientation, while TiNbC has a strong (200) orientation, suggesting that the strain energy was dominant in both binary coatings and indicating that in the re-nucleation process, the strain energy exceeds the surface energy;
- TiNbC exhibited the smallest crystallite size compared to TiC and ZrC coatings;
- TiC was rougher than ZrC and TiNbC; the lowest roughness was found for TiNbC coatings;
- The highest hardness and adhesion were found for TiNbC, followed by TiC and ZrC;
- All coatings improved the corrosion resistance of 316L uncoated stainless steel. TiNbC coating showed the best corrosion behavior (lowest icorr = 0.55 µA/cm2, highest Rp = 81.27 kΩ, highest protective efficiency = 57.7%), followed by ZrC (icorr = 0.59 µA/cm2, Rp = 73.61 kΩ, protective efficiency = 54.6%);
- The TiC coating was more porous than ZrC and TiNbC. There are small differences between the porosity of ZrC and TiNbC;
- TiNbC has the lowest µ (1.6) and k (0.99 × 10−5 mm3·N−1∙m−1) values, indicating the best tribocorrosive performance in 0.9% NaCl at 37 ± 0.5 °C.
Author Contributions
Funding
Conflicts of Interest
References
- Manivasagam, G.; Dhinasekaran, D.; Rajamanickam, A. Biomedical implants: Corrosion and its prevention—A review. Recent Pat. Corros. Sci. 2010, 2, 40–54. [Google Scholar] [CrossRef]
- Ibrahim, M.Z.; Sarhan, A.A.D.; Yusuf, F.; Hamdi, M. Biomedical materials and techniques to improve the tribological, mechanical and biomedical properties of orthopedic implants—A review article. J. Alloy. Compd. 2017, 714, 636–667. [Google Scholar] [CrossRef]
- Balamurugan, A.; Rajeswari, S.; Balossier, G.; Rebelo, A.H.S.; Ferreira, J.M.F. Corrosion aspects of metallic implants—An overview. Mater. Corros. 2008, 59, 855–869. [Google Scholar] [CrossRef]
- Niinomi, M. Advances in Metallic Biomaterials; Niinomi, M., Narushima, T., Nakai, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; ISBN 978-3-662-46835-7. [Google Scholar]
- Ivanova, E.P.; Bazaka, K.; Crawford, R.J. Metallic biomaterials: Types and advanced applications. In New Functional Biomaterials for Medicine and Healthcare; Woodhead Publishing: Cambridge, UK, 2014; pp. 121–147. [Google Scholar]
- Hussein, M.A.; Mohammed, A.S.; Al-Aqeeli, N. Wear characteristics of metallic biomaterials: A review. Materials (Basel) 2015, 8, 2749–2768. [Google Scholar] [CrossRef]
- Wang, G.; Zreiqat, H. Functional coatings or films for hard-tissue applications. Materials (Basel) 2010, 9, 3994–4050. [Google Scholar] [CrossRef] [PubMed]
- Sargeant, A.; Goswami, T. Hip implants—Paper VI—Ion concentrations. Mater. Des. 2007, 28, 155–171. [Google Scholar] [CrossRef]
- Ege, D.; Duru, İ.; Kamali, A.R.; Boccaccini, A.R. Nitride, zirconia, alumina, and carbide coatings on Ti6Al4V femoral heads: Effect of deposition techniques on mechanical and tribological properties. Adv. Eng. Mater. 2017, 19, 1700177. [Google Scholar] [CrossRef]
- Du, S.; Zhang, K.; Wen, M.; Qin, Y.; Li, R.; Jin, H.; Bao, X.; Ren, P.; Zheng, W. Optimizing the tribological behavior of tantalum carbide coating for the bearing in total hip joint replacement. Vacuum 2018, 150, 222–231. [Google Scholar] [CrossRef]
- Cho, S.M.; Park, J.W.; Han, H.S.; Seok, H.K.; Moon, M.W.; Kim, Y.C. Multifunctional composite coating as a wear-resistant layer for the bearing in total hip joint replacement. ACS Appl. Mater. Interfaces 2013, 5, 395–403. [Google Scholar] [CrossRef]
- Yate, L.; Coy, L.E.; Gregurec, D.; Aperador, W.; Moya, S.E.; Wang, G. Nb-C nanocomposite films with enhanced biocompatibility and mechanical properties for hard-tissue implant applications. ACS Appl. Mater. Interfaces 2015, 25, 6351–6358. [Google Scholar] [CrossRef]
- Zhang, K.; Wen, M.; Meng, Q.N.; Hu, C.Q.; Li, X.; Liu, C.; Zheng, W.T. Effects of substrate bias voltage on the microstructure, mechanical properties and tribological behavior of reactive sputtered niobium carbide films. Surf. Coat. Technol. 2012, 212, 185–191. [Google Scholar] [CrossRef]
- Heimann, R.B. Plasma-Sprayed hydroxylapatite-based coatings: Chemical, mechanical, microstructural, and biomedical properties. J. Therm. Spray Technol. 2016, 25, 827–850. [Google Scholar] [CrossRef]
- Daugaard, H.; Bechtold, J.E.; Soballe, K. HA-coated implant: Bone interface in total joint arthroplasty. In Bone-Implant Interface in Orthopedic Surgery: Basic Science to Clinical Applications; Springer: London, UK, 2014; ISBN 9781447154099. [Google Scholar]
- Li, L.; Bai, W.; Wang, X.; Gu, C.; Jin, G.; Tu, J. Mechanical properties and in vitro and in vivo biocompatibility of a-C/a-C:Ti nanomultilayer films on Ti6Al4V alloy as medical implants. ACS Appl. Mater. Interfaces 2017, 9, 15933–15942. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhao, X.; Ding, M.H.; Zheng, H.; Zhang, H.S.; Zhang, B.; Li, X.Q.; Wu, G.Y. Surface modification of biomedical AISI 316L stainless steel with zirconium carbonitride coatings. Appl. Surf. Sci. 2015, 340, 113–119. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Qin, Y.G.; Qing, Y.A.; Deng, R.P.; Jin, H.; Li, R.Y.; Rehman, J.; Wen, M.; Zhang, K. TiCuN solid solution coating: Excellent wear-resistant biocompatible material to protect artificial joint. Mater. Lett. 2018, 227, 145–148. [Google Scholar] [CrossRef]
- Sánchez-López, J.C.; Martínez-Martínez, D.; López-Cartes, C.; Fernández, A. Tribological behaviour of titanium carbide/amorphous carbon nanocomposite coatings: From macro to the micro-scale. Surf. Coat. Technol. 2008, 202, 4011–4018. [Google Scholar] [CrossRef]
- Kumar, D.D.; Kaliaraj, G.S.; Kirubaharan, A.M.K.; Alagarsamy, K.; Vishwakarma, V.; Baskaran, R. Biocorrosion and biological properties of sputtered ceramic carbide coatings for biomedical applications. Surf. Coat. Technol. 2019, 374, 569–578. [Google Scholar] [CrossRef]
- Finšgar, M.; Uzunalić, A.P.; Stergar, J.; Gradišnik, L.; Maver, U. Novel chitosan/diclofenac coatings on medical grade stainless steel for hip replacement applications. Sci. Rep. 2016, 24, 26653. [Google Scholar] [CrossRef]
- Anders, A. Unfiltered and filtered cathodic arc deposition. In Handbook of Deposition Technologies for Films and Coatings; William Andrew: Norwich, NY, USA, 2010; ISBN 9780815520313. [Google Scholar]
- Zardiackas, L.D.; Kraay, M.J.; Freese, H.L.; International, A. Titanium, Niobium, Zirconium, and Tantalum for Medical and Surgical Applications; ASTM STP 1471; ASTM: West Conshohocken, PA, USA, 2006; ISBN 9780803134973. [Google Scholar]
- Naganawa, T.; Ishihara, Y.; Iwata, T.; Koide, M.; Ohguchi, M.; Ohguchi, Y.; Murase, Y.; Kamei, H.; Sato, N.; Mizuno, M.; et al. In vitro biocompatibility of a new titanium-29niobium-13tantalum-4.6zirconium alloy with osteoblast-like MG63 cells. J. Periodontol. 2004, 75, 1701–1707. [Google Scholar] [CrossRef]
- Kolegar, T.; Matousek, M.; Vilemova, M.; Stary, V. Adhesion of biocompatible TiNb coating. Acta Polytech. CTU Proc. 2017, 8, 5–7. [Google Scholar] [CrossRef]
- Lin, H.; Gao, S.; Dai, C.; Chen, Y.; Shi, J. A two-dimensional biodegradable niobium carbide (MXene) for photothermal tumor eradication in NIR-I and NIR-II biowindows. J. Am. Chem. Soc. 2017. [Google Scholar] [CrossRef] [PubMed]
- Eisenbarth, E.; Velten, D.; Müller, M.; Thull, R.; Breme, J. Biocompatibility of β-stabilizing elements of titanium alloys. Biomaterials 2004, 25, 5705–5713. [Google Scholar] [CrossRef] [PubMed]
- Constantin, L.R.; Parau, A.C.; Balaceanu, M.; Dinu, M.; Vladescu, A. Corrosion and tribological behaviour in a 3.5% NaCl solution of vacuum arc deposited ZrCN and Zr–Cr–Si–C–N coatings. Proc. Inst. Mech. Eng. Part J J. Eng. Tribol. 2019, 233, 158–169. [Google Scholar] [CrossRef]
- Vladescu, A.; Pruna, V.; Kulesza, S.; Braic, V.; Titorencu, I.; Bramowicz, M.; Gozdziejewska, A.; Parau, A.; Cotrut, C.M.; Pana, I.; et al. Influence of Ti, Zr or Nb carbide adhesion layers on the adhesion, corrosion resistance and cell proliferation of titania doped hydroxyapatite to the Ti6Al4V alloy substrate, utilizable for orthopaedic implants. Ceram. Int. 2019, 45, 1710–1723. [Google Scholar] [CrossRef]
- Vladescu, A.; Titorencu, I.; Dekhtyar, Y.; Jinga, V.; Pruna, V.; Balaceanu, M.; Dinu, M.; Pana, I.; Vendina, V.; Braic, M. In vitro biocompatibility of Si alloyed multi-principal element carbide coatings. PLoS ONE 2016, 11. [Google Scholar] [CrossRef] [PubMed]
- Dinu, M.; Pana, I.; Braic, V.; Miculescu, F.; Balaceanu, M.; Vladescu, A.; Braic, M. In vitro corrosion resistance of Si containing multi-principal element carbide coatings. Mater. Corros. 2016, 67. [Google Scholar] [CrossRef]
- Cotrut, C.M.; Vitelaru, C.; Dinu, M.; Petreus, T.; Vladescu, A. Synthesis and characterization of TiSiON biocompatible thin films used in biomedical applications. Sci. Adv. Mater. 2015, 7, 1351–1360. [Google Scholar] [CrossRef]
- Vladescu, A.; Dinu, M.; Braic, M.; Vitelaru, C.; Balaceanu, M.; Tarcolea, M.; Braic, V.; Baciu, F.; Cotrut, C.M. The effect of TiSiN interlayers on the bond strength of ceramic to NiCr and CoCr alloys. Ceram. Int. 2015, 41, 8051–8058. [Google Scholar] [CrossRef]
- Dinu, M.; Braic, V.; Colease, G.; Baciu, F.; Cotrut, C.M.; Braic, M.; Tarcolea, M.; Vitelaru, C.; Vladescu, A.; Vranceanu, D.M. TiSiN coatings for improved bond strength of CoCr alloy to dental ceramic. Key Eng. Mater. 2014, 587, 275–281. [Google Scholar] [CrossRef]
- EN 1071-3. Advanced Technical Ceramics - Methods of Test for Ceramic Coatings - Part 3 : Determination of Adhesion and Other Mechanical Failure Modes by a Scratch Test; The European Committee for Standardization CEN: Brussels, Belgium, 2004. [Google Scholar]
- ASTM G102—89. Standard Practice for Calculation of Corrosion Rates and Related Information from Electrochemical Measurements. In Annual Book of ASTM Standards; ASTM International: West Conshohocken, PA, USA, 2010. [Google Scholar]
- EN 1071-3. Advanced Technical Ceramics. Methods of Test for Ceramic Coatings - Part 13 : Determination of Wear Rate by the Pin-on-Disk Method; The European Committee for Standardization CEN: Brussels, Belgium, 2010. [Google Scholar]
- Kothari, D.C.; Kale, A.N. Recent trends in surface engineering using cathodic arc technique. Surf. Coat. Technol. 2002, 158–159, 174–179. [Google Scholar] [CrossRef]
- Constantin, L.; Braic, M.; Dinu, M.; Balaceanu, M.; Braic, V.; Farcau, C.; Vladescu, A. Effects of Zr, Nb, or Si addition on the microstructural, mechanical, and corrosion resistance of TiCN hard coatings. Mater. Corros. 2016, 67, 929–938. [Google Scholar] [CrossRef]
- Pelleg, J.; Zevin, L.Z.; Lungo, S.; Croitoru, N. Reactive-sputter-deposited TiN films on glass substrates. Thin Solid Films 1991, 197, 117–128. [Google Scholar] [CrossRef]
- Barna, P.B.; Adamik, M. Fundamental structure forming phenomena of polycrystalline films and the structure zone models. Thin Solid Films 1998, 317, 27–33. [Google Scholar] [CrossRef]
- Oh, U.C.; Je, J.H. Effects of strain energy on the preferred orientation of TiN thin films. J. Appl. Phys. 1993, 74, 1692. [Google Scholar] [CrossRef]
- Petrov, I.; Barna, P.B.B.; Hultman, L.; Greene, J.E.E. Microstructural evolution during film growth. J. Vac. Sci. Technol. Vac. Surf. Film. 2003, 21, S117. [Google Scholar] [CrossRef]
- Khorsand Zak, A.; Abd. Majid, W.H.; Abrishami, M.E.; Yousefi, R. X-ray analysis of ZnO nanoparticles by Williamson-Hall and size-strain plot methods. Solid State Sci. 2011, 13, 251–256. [Google Scholar] [CrossRef]
- Ma, Y.; Chang, Y.C.; Yin, J.Z. Evaluation of lattice strain in ZnO thin films based on Williamson-Hall analysis. J. Optoelectron. Adv. Mater. 2019, 21, 702–709. [Google Scholar]
- Münz, W.D.; Smith, I.J.; Lewis, D.B.; Creasey, S. Droplet formation on steel substrates during cathodic steered arc metal ion etching. Vacuum 1997, 48, 473–481. [Google Scholar] [CrossRef]
- Yate, L.; Coy, L.E.; Aperador, W. Robust tribo-mechanical and hot corrosion resistance of ultra-refractory Ta-Hf-C ternary alloy films. Sci. Rep. 2017, 7, 3080. [Google Scholar] [CrossRef]
- Vladescu, A.; Vranceanu, D.M.; Kulesza, S.; Ivanov, A.N.; Bramowicz, M.; Fedonnikov, A.S.; Braic, M.; Norkin, I.A.; Koptyug, A.; Kurtukova, M.O.; et al. Influence of the electrolyte’s pH on the properties of electrochemically deposited hydroxyapatite coating on additively manufactured Ti64 alloy. Sci. Rep. 2017, 7, 16819. [Google Scholar] [CrossRef]
- Dinu, M.; Mouele, E.S.M.; Parau, A.C.; Vladescu, A.; Petrik, L.F.; Braic, M. Enhancement of the corrosion resistance of 304 stainless steel by Cr-N and Cr(N,O) coatings. Coatings 2018, 8, 132. [Google Scholar] [CrossRef]
- Adachi, K.; Hutchings, I.M. Wear-mode mapping for th micro-scale abrasion test. Wear 2003, 255, 23–29. [Google Scholar] [CrossRef]
- Ducheyne, P. Comprehensive Biomaterials, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2017; ISBN 9780081006924. [Google Scholar]
- Mandes, A.; Vladoiu, R.; Prodan, G.; Dinca, V.; Porosnicu, C.; Dinca, P. The properties of binary and Ternary Ti based coatings produced by Thermionic Vacuum Arc (TVA) technology. Coatings 2018, 8, 114. [Google Scholar] [CrossRef]
- Mujawar, S.H.; Inamdar, A.I.; Betty, C.A.; Ganesan, V.; Patil, P.S. Effect of post annealing treatment on electrochromic properties of spray deposited niobium oxide thin films. Electrochim. Acta 2007, 52, 4899–4906. [Google Scholar] [CrossRef]
- ISO BS. Standards 13779-2: 2000:Implants for Surgery—Hydroxyapatite—Part 2: Coatings of Hydroxyapatite; British Standards Institution: London, UK, 2000. [Google Scholar]
- Eliaz, N.; Ritman-Hertz, O.; Aronov, D.; Weinberg, E.; Shenhar, Y.; Rosenman, G.; Weinreb, M.; Ron, E. The effect of surface treatments on the adhesion of electrochemically deposited hydroxyapatite coating to titanium and on its interaction with cells and bacteria. J. Mater. Sci. Mater. Med. 2011, 22, 1741–1752. [Google Scholar] [CrossRef]
- Shtansky, D.V.; Gloushankova, N.A.; Sheveiko, A.N.; Kharitonova, M.A.; Moizhess, T.G.; Levashov, E.A.; Rossi, F. Design, characterization and testing of Ti-based multicomponent coatings for load-bearing medical applications. Biomaterials 2005, 26, 2909–2924. [Google Scholar] [CrossRef]
- Li, Q.; Lei, Y.; Fu, H. Growth mechanism, distribution characteristics and reinforcing behavior of (Ti, Nb)C particle in laser cladded Fe-based composite coating. Appl. Surf. Sci. 2014, 316, 610–616. [Google Scholar] [CrossRef]



| Deposition Parameters | TiC | ZrC | NbC |
|---|---|---|---|
| Base pressure | 2 × 10−3 Pa | ||
| Working pressure | 1 × 10−1 Pa | 6 × 10−2 Pa | 1 × 10−1 Pa |
| CH4 mass flow rate | 120 sccm | ||
| Arc current on each cathode | 90 A | 130 A | 90 A |
| Substrate bias voltage | −150 V | ||
| Deposition duration | 40–50 min | ||
| Coating | Elemental Composition (at.%) | C/ΣMecoating | T (hkl) | d (nm) | ε | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ti | Zr | Nb | C | Fe | (200) | (111) | (220) | (222) | ||||
| TiC | 48.68 ± 1.2 | - | - | 48.05 ± 1.3 | 3.27 ± 0.5 | 0.99 | 0.409 | 0.437 | - | 0.110 | 16.3 | 0.023 |
| ZrC | - | 49.51 ± 1.3 | - | 48.14 ± 1.2 | 2.35 ± 0.4 | 0.97 | 0.294 | 0.442 | - | 0.173 | 13.1 | 0.014 |
| TiNbC | 32.56 ± 1.2 | - | 12.97 ± 0.7 | 49.00 ± 1.3 | 2.47 ± 0.4 | 1.07 | 0.659 | 0.027 | 0.277 | 0.034 | 8.1 | 0.008 |
| Substrate | Coating | H (GPa) | Lc (N) |
|---|---|---|---|
| 316L | - | 4.3 ± 0.2 | - |
| TiC | 39.4 ± 0.3 | 18 ± 1 ÷ 20 ± 1 | |
| ZrC | 32.2 ± 0.2 | 26 ± 1 ÷ 28 ± 1 | |
| TiNbC | 40.3 ± 0.3 | 28 ± 1 ÷ 30 ± 1 |
| Sample | Ei = 0 (mV) | icorr (µA/cm2) | Rp (kΩ) | P | Pe (%) |
|---|---|---|---|---|---|
| 316L | −336 ± 23 | 1.3 ± 0.3 | 2.54 ± 1.1 | - | - |
| TiC | −268 ± 11 | 0.70 ± 0.2 | 62.75 ± 3.8 | 0.0307 ± 0.0011 | 46.2 ± 0.3 |
| ZrC | −251 ± 18 | 0.59 ± 0.2 | 73.61 ± 4.6 | 0.02449 ± 0.0014 | 54.6 ± 0.2 |
| TiNbC | −273 ± 16 | 0.55 ± 0.2 | 81.27 ± 4.7 | 0.02424 ± 0.0013 | 57.7 ± 0.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pana, I.; Vladescu, A.; Constantin, L.R.; Sandu, I.G.; Dinu, M.; Cotrut, C.M. In Vitro Corrosion and Tribocorrosion Performance of Biocompatible Carbide Coatings. Coatings 2020, 10, 654. https://doi.org/10.3390/coatings10070654
Pana I, Vladescu A, Constantin LR, Sandu IG, Dinu M, Cotrut CM. In Vitro Corrosion and Tribocorrosion Performance of Biocompatible Carbide Coatings. Coatings. 2020; 10(7):654. https://doi.org/10.3390/coatings10070654
Chicago/Turabian StylePana, Iulian, Alina Vladescu, Lidia R. Constantin, Ioan G. Sandu, Mihaela Dinu, and Cosmin M. Cotrut. 2020. "In Vitro Corrosion and Tribocorrosion Performance of Biocompatible Carbide Coatings" Coatings 10, no. 7: 654. https://doi.org/10.3390/coatings10070654
APA StylePana, I., Vladescu, A., Constantin, L. R., Sandu, I. G., Dinu, M., & Cotrut, C. M. (2020). In Vitro Corrosion and Tribocorrosion Performance of Biocompatible Carbide Coatings. Coatings, 10(7), 654. https://doi.org/10.3390/coatings10070654




.jpg)