Investigation of Wettability, Drying and Water Condensation on Polyimide (Kapton) Films Treated by Atmospheric Pressure Air Dielectric Barrier Discharge
Abstract
1. Introduction
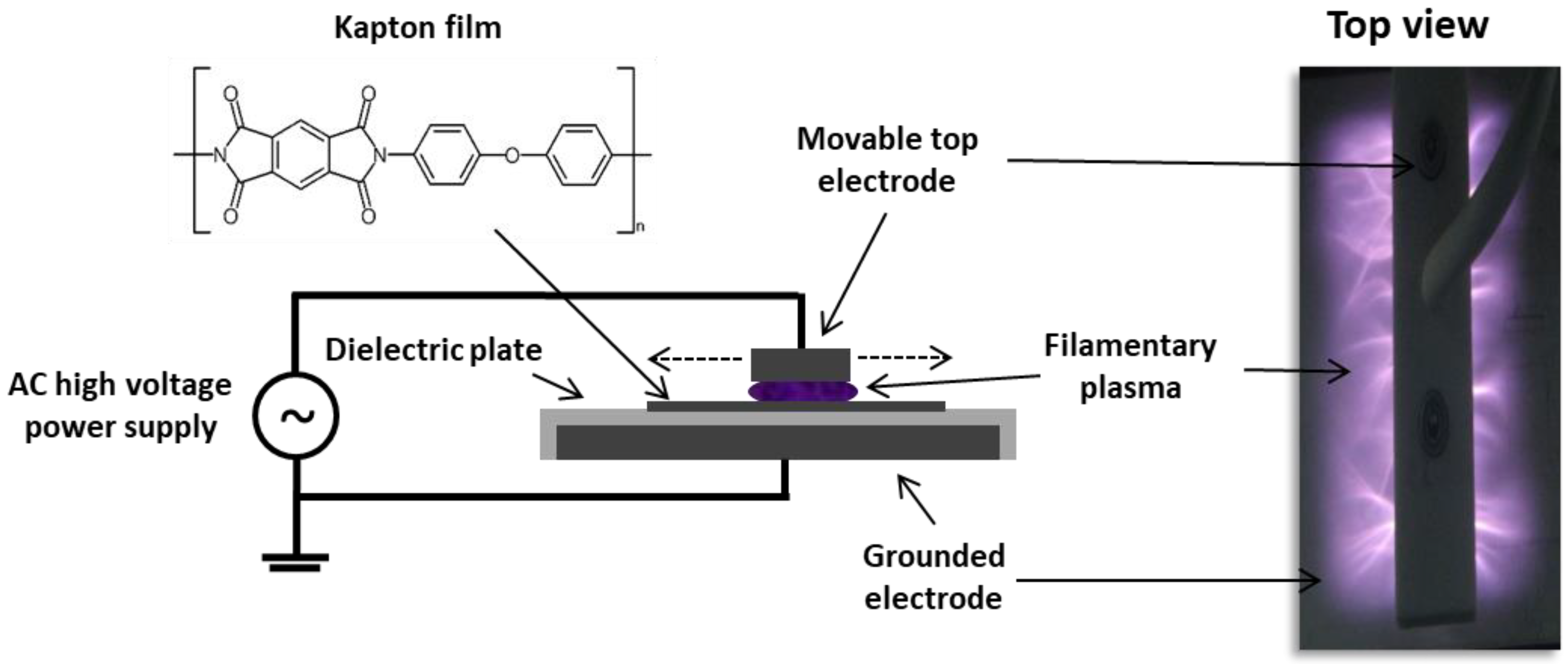
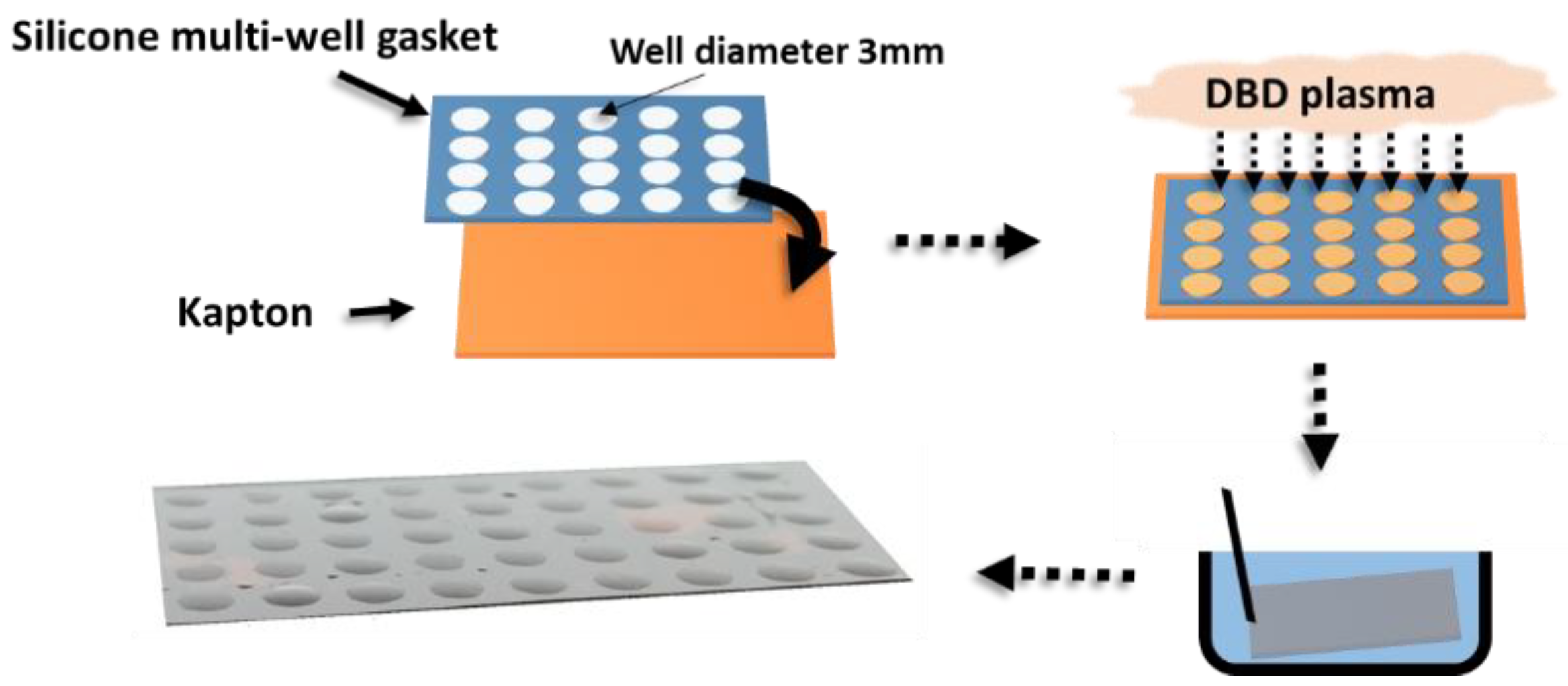
2. Materials and Methods
3. Results and Discussion
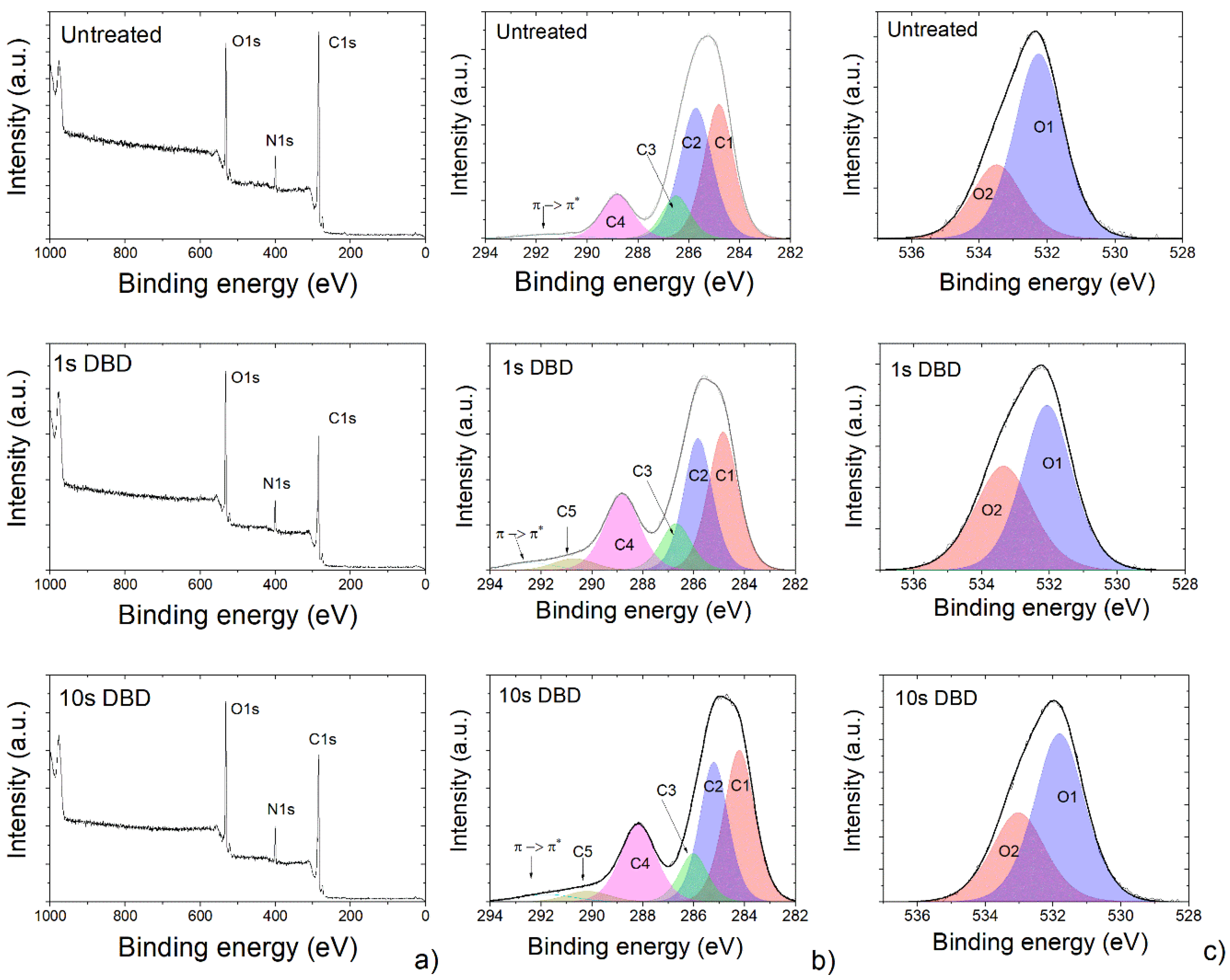
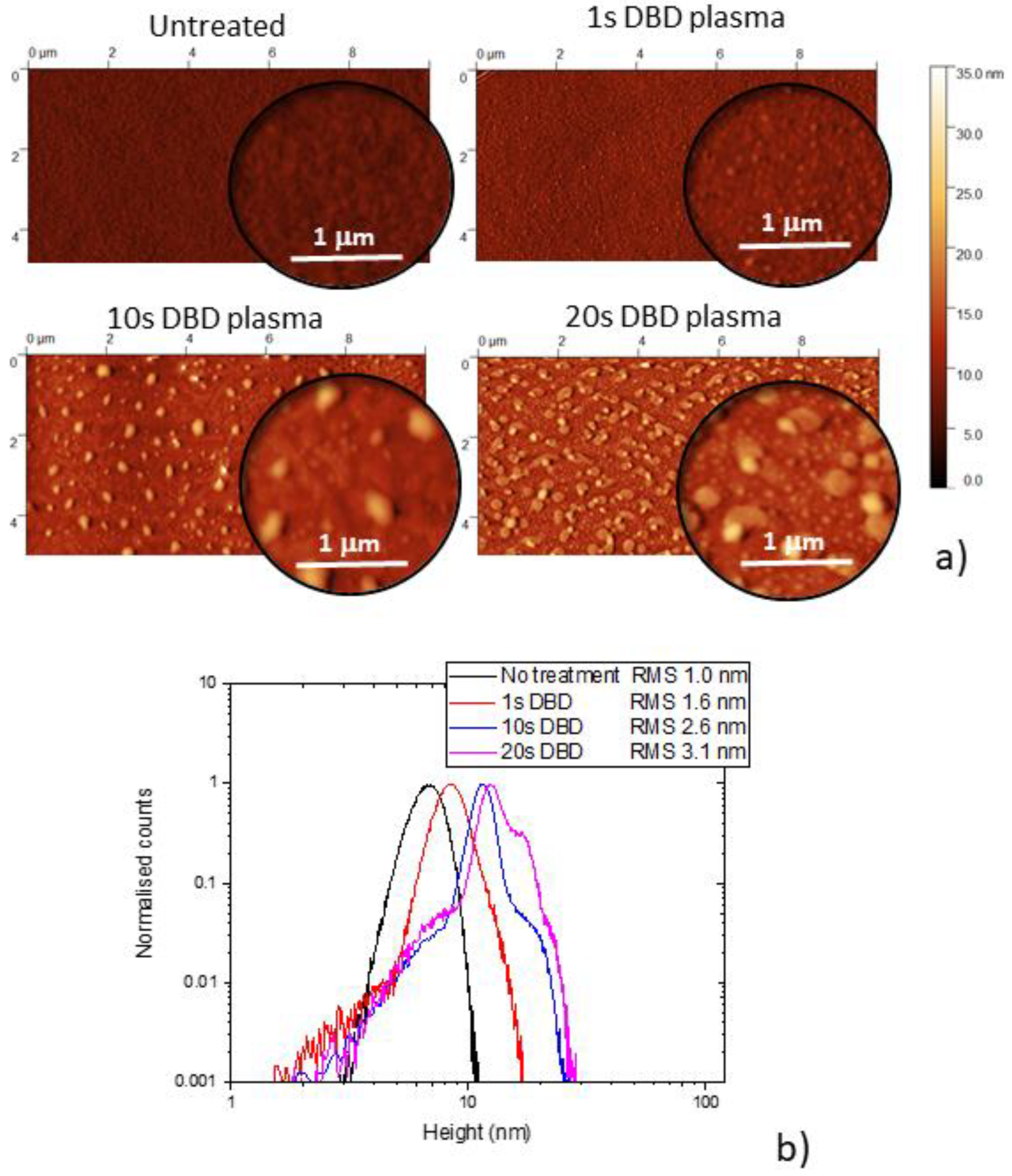
3.1. Morphology and Surface Chemical Composition
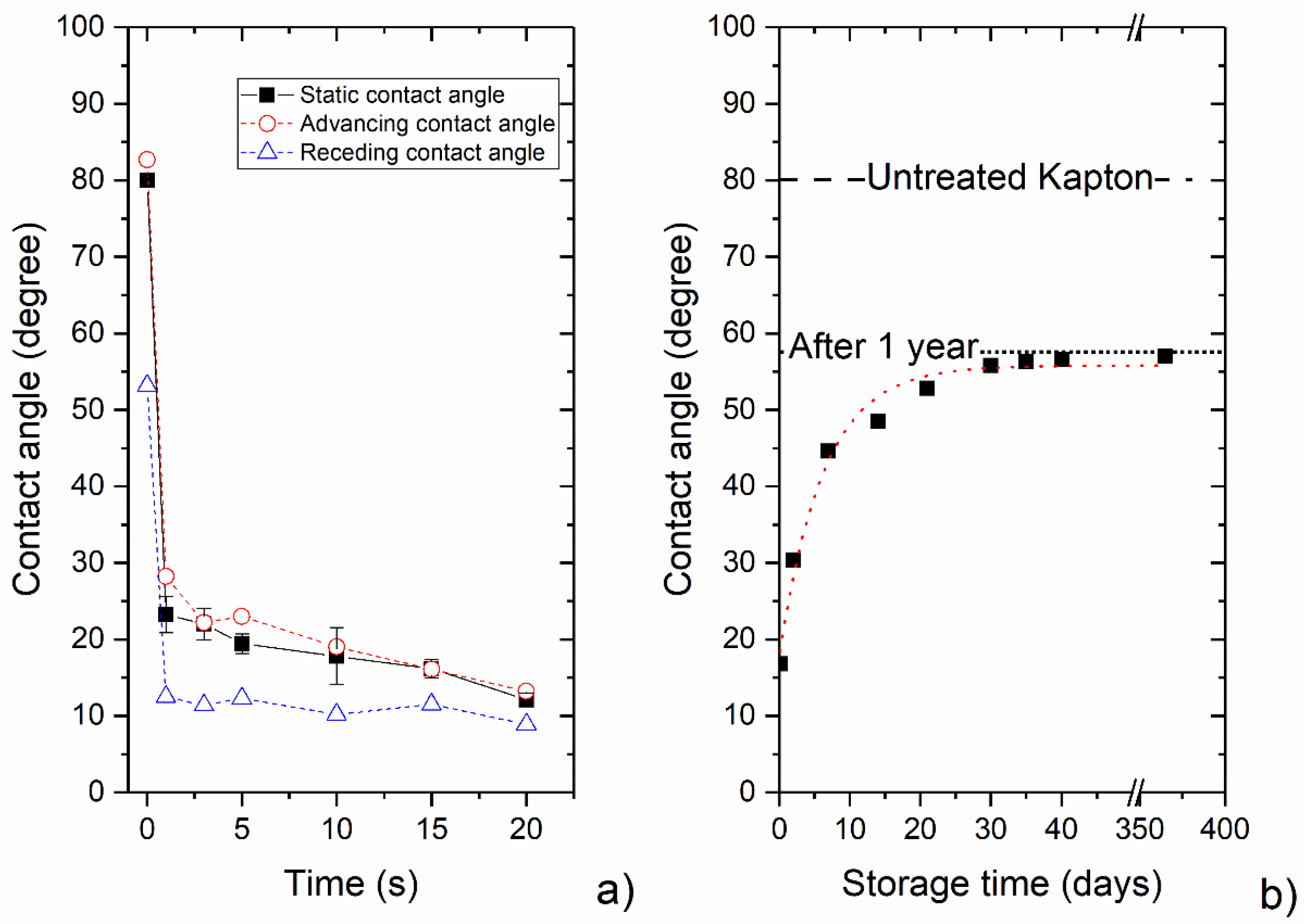
3.2. Wettability
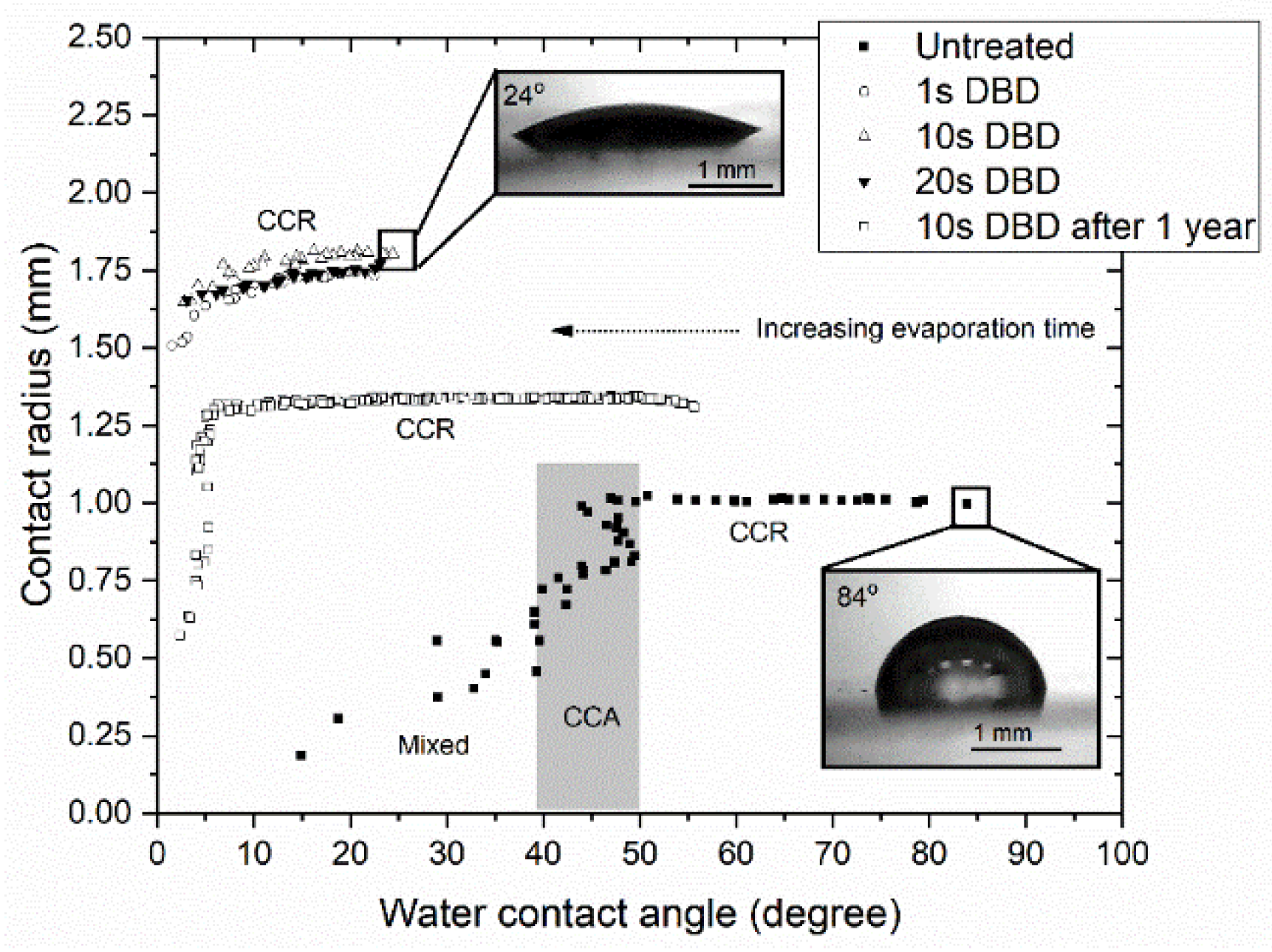
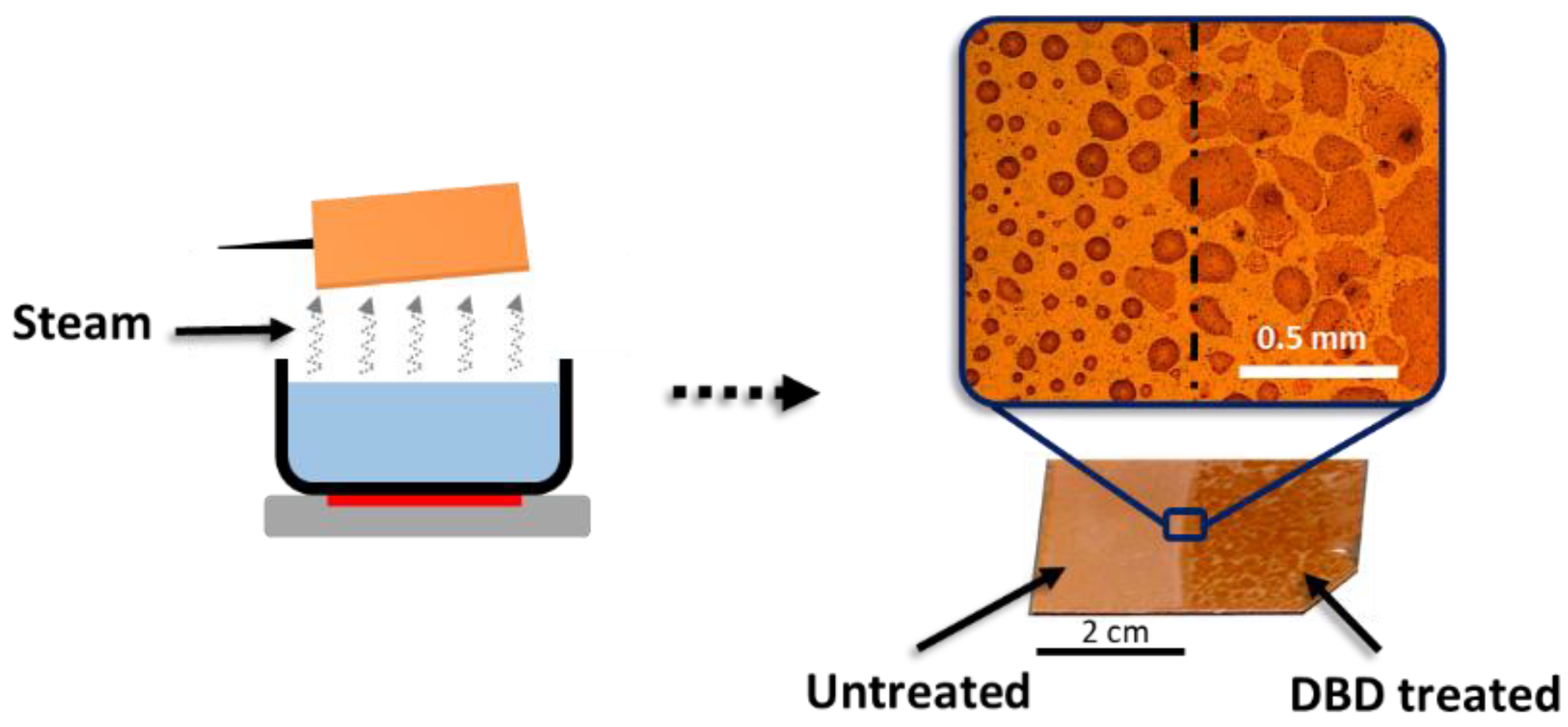
3.3. Droplet Drying and Condensation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pecora, A.; Maiolo, L.; Cuscunà, M.; Simeone, D.; Minotti, A.; Mariucci, L.; Fortunato, G. Low-temperature polysilicon thin film transistors on polyimide substrates for electronics on plastic. Solid State Electron. 2008, 52, 348–352. [Google Scholar] [CrossRef]
- Hosseindokht, Z.; Mohammadpour, R.; Asadian, E.; Paryavi, M.; Rafii-Tabar, H.; Sasanpour, P. Low cost flexible pressure sensor using laser scribed GO/RGO periodic structure for electronic skin applications. Superlattice. Microst. 2020, 140, 106470. [Google Scholar] [CrossRef]
- Han, S.-T.; Peng, H.; Sun, Q.; Venkatesh, S.; Chung, K.-S.; Lau, S.C.; Zhou, Y.; Roy, V.A.L. An Overview of the Development of Flexible Sensors. Adv. Mater. 2017, 29, 1700375. [Google Scholar] [CrossRef]
- Lindner, E.; Cosofret, V.V.; Ufer, S.; Buck, R.P.; Kusy, R.P.; Ash, R.B.; Nagle, H.T. Flexible (Kapton-based) microsensor arrays of high stability for cardiovascular applications. J. Chem. Soc. Faraday Trans. 1993, 89, 361–367. [Google Scholar] [CrossRef]
- Bahramiabarghouei, H.; Porter, E.; Santorelli, A.; Gosselin, B.; Popović, M.; Rusch, L.A. Flexible 16 Antenna Array for Microwave Breast Cancer Detection. IEEE Trans. Biomed. Eng. 2015, 62, 2516–2525. [Google Scholar] [CrossRef]
- Herrero, J.; Guillén, C. Transparent films on polymers for photovoltaic applications. Vacuum 2002, 67, 611–616. [Google Scholar] [CrossRef]
- Bedoya-Pinto, A.; Donolato, M.; Gobbi, M.; Hueso, L.E.; Vavassori, P. Flexible spintronic devices on Kapton. Appl. Phys. Lett. 2014, 104, 062412. [Google Scholar]
- Ennis, C.P.; Kaiser, R.I. Mechanistical studies on the electron-induced degradation of polymethylmethacrylate and Kapton. Phys. Chem. Chem. Phys. 2010, 12, 14902–14915. [Google Scholar] [CrossRef]
- Rahnamoun, A.; Engelhart, S.P.; Humagain, S.; Koerner, H.; Plis, E.; Kennedy, W.J.; Cooper, R.; Greenbaum, S.G.; Hoffmann, R.; van Duin, A.C.T. Chemical dynamics characteristics of Kapton polyimide damaged by electron beam irradiation. Polymer 2019, 176, 135–145. [Google Scholar] [CrossRef]
- Gouzman, I.; Girshevitz, O.; Grossman, E.; Eliaz, N.; Sukenik, C.N. Thin Film Oxide Barrier Layers: Protection of Kapton from Space Environment by Liquid Phase Deposition of Titanium Oxide. ACS Appl. Mater. Interfaces 2010, 2, 1835–1843. [Google Scholar] [CrossRef]
- Gallais, L.; Bergeret, E.; Wang, B.; Guerin, M.; Bènevent, E. Ultrafast laser ablation of metal films on flexible substrates. Appl. Phys. A 2014, 115, 177–188. [Google Scholar] [CrossRef]
- Ghosh, I.; Konar, J.; Bhowmick, A.K. Surface properties of chemically modified polyimide films. J. Adhes. Sci. Technol. 1997, 11, 877–893. [Google Scholar] [CrossRef]
- Bachman, B.J.; Vasile, M.J. Ion bombardment of polyimide films. J. Vac. Sci. Technol. A 1989, 7, 2709–2716. [Google Scholar] [CrossRef]
- Shin, J.; Jeun, J.; Kang, P. Surface modification and characterization of N+ ion implantation on polyimide film. Macromol. Res. 2010, 18, 227–232. [Google Scholar] [CrossRef]
- Ektessabi, A.M.; Hakamata, S. XPS study of ion beam modifiedpolyimide films. Thin Solid Films 2000, 377–378, 621–625. [Google Scholar] [CrossRef]
- Gotoh, K.; Nakata, Y.; Tagawa, M.; Tagawa, M. Wettability of ultraviolet excimer-exposed PE, PI and PTFE films determined by the contact angle measurements. Colloids Surf. A Physicochem. Eng. Asp. 2003, 224, 165–173. [Google Scholar] [CrossRef]
- Kylián, O.; Drábik, M.; Polonskyi, O.; Čechvala, J.; Artemenko, A.; Gordeev, I.; Choukourov, A.; Matolínová, I.; Slavínská, D.; Biederman, H. Deposition of nanostructured fluorocarbon plasma polymer films by RF magnetron sputtering of polytetrafluoroethylene. Thin Solid Films 2011, 519, 6426–6431. [Google Scholar] [CrossRef]
- Kylián, O.; Kuzminova, A.; Hanuš, J.; Slavínská, D.; Biederman, H. Super-hydrophilic SiOx coatings prepared by plasma enhanced chemical vapor deposition combined with gas aggregation source of nanoparticles. Mater. Lett. 2018, 227, 5–8. [Google Scholar] [CrossRef]
- Tsougeni, K.; Vourdas, N.; Tserepi, A.; Gogolides, E.; Cardinaud, C. Mechanisms of oxygen plasma nanotexturing of organic polymer surfaces: From stable super hydrophilic to super hydrophobic surfaces. Langmuir 2009, 25, 11748–117459. [Google Scholar] [CrossRef]
- Rymuszka, D.; Terpiłowski, K.; Borowski, P.; Holysz, L. Time-dependent changes of surface properties of polyether ether ketone caused by air plasma treatment. Polym. Int. 2016, 65, 827–834. [Google Scholar] [CrossRef]
- Vesel, A.; Primc, G.; Zaplotnik, R.; Mozetic, M. Applications of highly non-equilibrium low pressure oxygen plasma for treatment of polymers and polymer composites on an industrial scale. Plasma Phys. Control Fusion 2020, 62, 024008. [Google Scholar] [CrossRef]
- Cui, N.-Y.; Brown, N.M.D. Modification of the surface properties of a polypropylene (PP) film using an air dielectric barrier discharge plasma. Appl. Surf. Sci. 2002, 189, 31–38. [Google Scholar] [CrossRef]
- Esena, P.; Riccardi, C.; Zanini, S.; Tontini, M.; Poletti, G.; Orsini, F. Surface modification of PET film by a DBD device at atmospheric pressure. Surf. Coatings Technol. 2005, 200, 664–667. [Google Scholar] [CrossRef]
- Borcia, G.; Anderson, C.A.; Brown, N.M.D. The surface oxidation of selected polymers using an atmospheric pressure air dielectric barrier discharge. Part I. Appl. Surf. Sci. 2004, 221, 203–214. [Google Scholar] [CrossRef]
- Borcia, G.; Anderson, C.A.; Brown, N.M.D. The surface oxidation of selected polymers using an atmospheric pressure air dielectric barrier discharge. Part II. Appl. Surf. Sci. 2004, 225, 186–197. [Google Scholar] [CrossRef]
- Homola, T.; Matoušek, J.; Hergelová, B.; Kormunda, M.; Wu, L.Y.L.; Černák, M. Activation of poly(methyl methacrylate) surfaces by atmospheric pressure plasma. Polym. Degrad. Stab. 2012, 97, 886–892. [Google Scholar] [CrossRef]
- Bilek, M.M.M.; Vandrovcová, M.; Shelemin, A.; Kuzminova, A.; Kylián, O.; Biederman, H.; Bačáková, L.; Weiss, A.S. Plasma treatment in air at atmospheric pressure that enables reagent-free covalent immobilization of biomolecules on polytetrafluoroethylene (PTFE). Appl. Surf. Sci. 2020, 518, 146128. [Google Scholar] [CrossRef]
- Kuzminova, A.; Vandrovcová, M.; Shelemin, A.; Kylián, O.; Choukourov, A.; Hanuš, J.; Bačáková, L.; Slavínská, D.; Biederman, H. Treatment of poly (ethylene terephthalate) foils by atmospheric pressure air dielectric barrier discharge and its influence on cell growth. Appl. Surf. Sci. 2015, 357, 689–695. [Google Scholar] [CrossRef]
- Inagaki, H.; Tasaka, S.; Hibi, K. Improved adhesion between plasma-treated polyimide film and evaporated copper. J. Adhes. Sci. Technol. 1994, 8, 395–410. [Google Scholar] [CrossRef]
- Usami, K.; Ishijima, T.; Toyoda, H. Rapid plasma treatment of polyimide for improved adhesive and durable copper film deposition. Thin Solid Films 2012, 521, 22–26. [Google Scholar] [CrossRef]
- Lin, Y.-S.; Liu, H.-M.; Chen, C.-L. Plasma surface modification of polyimide films by air glow discharge for copper metallization on microelectronic flex substrates. Surf. Coat. Technol. 2006, 200, 3775–3785. [Google Scholar] [CrossRef]
- Lee, S.B.; Kim, Y.-K. Adhesion Improvement of Polyimide/Metal Interface by He/O2/NF3 Atmospheric Pressure Plasma. Plasma Process. Polym. 2009, 6, S525–S529. [Google Scholar] [CrossRef]
- Peng, S.; Li, L.; Li, W.; Wang, C.; Guo, Y.; Shi, J.; Zhang, J. Surface Modification of Polyimide Film by Dielectric Barrier Discharge at Atmospheric Pressure. Plasma Sci. Technol. 2016, 18, 337–341. [Google Scholar] [CrossRef]
- Park, W.J.; Yoon, S.G.; Jung, W.S.; Yoon, D.H. Effect of dielectric barrier discharge on surface modification characteristics of polyimide film. Surf. Coat. Technol. 2007, 201, 5017–5020. [Google Scholar] [CrossRef]
- Kostov, K.G.; dos Santos, A.L.R.; Honda, R.Y.; Nascente, P.A.P.; Kayama, M.E.; Algatti, M.A.; Mota, R.P. Treatment of PET and PU polymers by atmospheric pressure plasma generated in dielectric barrier discharge in air. Surf. Coat. Technol. 2010, 204, 3064–3068. [Google Scholar] [CrossRef]
- Ren, C.-S.; Wang, K.; Nie, Q.-Y.; Wang, D.-Z.; Guo, S.-H. Surface modification of PE film by DBD plasma in air. Appl. Surf. Sci. 2008, 255, 3421–3425. [Google Scholar] [CrossRef]
- Kormunda, M.; Homola, T.; Matousek, J.; Kovacik, D.; Cernak, M.; Pavlik, J. Surface analysis of poly(ethylene naphthalate) (PEN) films treated at atmospheric pressure using diffuse coplanar surface barrier discharge in air and in nitrogen. Polym. Degrad. Stab. 2012, 97, 547–553. [Google Scholar] [CrossRef]
- Upadhyay, D.J.; Cui, N.-Y.; Anderson, C.A.; Brown, N.M.D. A comparative study of the surface activation of polyamides using an air dielectric barrier discharge. Colloid. Surf. A 2004, 248, 47–56. [Google Scholar] [CrossRef]
- Kuzminova, A.; Shelemin, A.; Kylián, O.; Choukourov, A.; Valentová, H.; Krakovský, I.; Nedbal, J.; Slavínská, D.; Biederman, H. Study of the effect of atmospheric pressure air dielectric barrier discharge on nylon 6, 6 foils. Polym. Degrad. Stab. 2014, 110, 378–388. [Google Scholar] [CrossRef]
- Kuzminova, A.; Kretková, T.; Kylián, O.; Hanuš, J.; Khalakhan, I.; Prukner, V.; Doležalová, E.; Šimek, M.; Biederman, H. Etching of polymers, proteins and bacterial spores by atmospheric pressure DBD plasma in air. J. Phys. D Appl. Phys. 2017, 50, 135201. [Google Scholar] [CrossRef]
- Fowkes, F.M. Attractive forces at interfaces. Ind. Eng. Chem. 1964, 56, 40–52. [Google Scholar] [CrossRef]
- Beamson, G.; Briggs, D. High Resolution XPS of Organic Polymers; John Wiley & Sons: Chichester, UK, 1992. [Google Scholar]
- Flitsch, R.; Shih, D.-Y. An XPS study of argon ion beam and oxygen RIE modified BPDA-PDA polyimide as related to adhesion. J. Adhes. Sci. Technol. 1996, 10, 1241–1253. [Google Scholar] [CrossRef]
- Shao, T.; Zhang, C.; Long, K.; Zhang, D.; Wang, J.; Yan, P.; Zhou, Y. Surface modification of polyimide films using unipolar nanosecond-pulse DBD in atmospheric air. Appl. Surf. Sci. 2010, 256, 3888–3894. [Google Scholar] [CrossRef]
- Brennan, W.J.; Feast, W.J.; Munro, H.S.; Walker, S.A. Investigation of the ageing of plasma oxidized PEEK. Polymer 1991, 32, 1527–1530. [Google Scholar] [CrossRef]
- Jacobs, T.; De Geyter, N.; Morent, R.; Van Vlierberghe, S.; Dubruel, P.; Leys, C. Plasma modification of PET foils with different crystallinity. Surf. Coatings. Technol. 2011, 205, S511–S515. [Google Scholar] [CrossRef]
- Borcia, C.; Punga, I.L.; Borcia, G. Surface properties and hydrophobic recovery of polymers treated by atmospheric-pressure plasma. Appl. Surf. Sci. 2014, 317, 103–110. [Google Scholar] [CrossRef]
- Bormashenko, E.; Chaniel, G.; Grynyov, R. Towards understanding hydrophobic recovery of plasma treated polymers: Storing in high polarity liquids suppresses hydrophobic recovery. Appl. Surf. Sci. 2013, 273, 549–553. [Google Scholar] [CrossRef]
- Štefaníková, R.; Kretková, T.; Kuzminova, A.; Hanuš, J.; Vaidulych, M.; Kylián, O.; Biederman, H. Influence of atmospheric pressure dielectric barrier discharge on wettability and drying of poly(ether-ether-ketone) foils. Polym. Degrad. Stab. 2018, 150, 114–121. [Google Scholar] [CrossRef]
- Picknett, R.G.; Bexon, R. The evaporation of sessile or pendant drops in still air. J. Colloid Interface Sci. 1977, 61, 336–350. [Google Scholar] [CrossRef]
- Nguyen, T.A.H.; Nguyen, A.V. Transient volume of evaporating sessile droplets: 2/3, 1/1, or another power law? Langmuir 2014, 30, 6544–6547. [Google Scholar] [CrossRef]
- Hu, H.; Larson, R.G. Evaporation of a sessile droplet on a substrate. J. Phys. Chem. B 2002, 106, 1334–1344. [Google Scholar] [CrossRef]
- Schonfeld, F.; Graf, K.; Hardt, S.; Butt, H.J. Evaporation dynamics of sessile liquid drops in still air with constant contact radius. Int. J. Heat Mass Transfer 2008, 51, 3696–3699. [Google Scholar] [CrossRef]
- Nguyen, T.A.H.; Nguyen, A.V.; Hampton, M.A.; Xu, Z.P.; Huang, L.; Rudolph, V. Theoretical and experimental analysis of droplet evaporation on solid surfaces. Chem. Eng. Sci. 2012, 69, 522–529. [Google Scholar] [CrossRef]
- Pittoni, P.G.; Chang, C.-C.; Yu, T.-S.; Lin, S.-Y. Evaporation of water drops on polymer surfaces: Pinning, depinning and dynamics of the triple line. Colloids Surf. A 2013, 432, 89–98. [Google Scholar] [CrossRef]
- Sheng, Q.; Sun, J.; Wang, Q.; Wang, W.; Wang, H.S. On the onset of surface condensation: Formation and transition mechanisms of condensation mode. Sci. Rep. 2016, 6, 30764. [Google Scholar] [CrossRef]

| Treatment Time (s) | C (at.%) | O (at.%) | N (at.%) | O/C |
|---|---|---|---|---|
| 0 | 78 | 16 | 6 | 0.21 |
| 1 | 71 | 22.5 | 6.5 | 0.32 |
| 10 | 71 | 21.5 | 7.5 | 0.30 |
| 20 | 71 | 22 | 7 | 0.31 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khomiakova, N.; Hanuš, J.; Kuzminova, A.; Kylián, O. Investigation of Wettability, Drying and Water Condensation on Polyimide (Kapton) Films Treated by Atmospheric Pressure Air Dielectric Barrier Discharge. Coatings 2020, 10, 619. https://doi.org/10.3390/coatings10070619
Khomiakova N, Hanuš J, Kuzminova A, Kylián O. Investigation of Wettability, Drying and Water Condensation on Polyimide (Kapton) Films Treated by Atmospheric Pressure Air Dielectric Barrier Discharge. Coatings. 2020; 10(7):619. https://doi.org/10.3390/coatings10070619
Chicago/Turabian StyleKhomiakova, Natalia, Jan Hanuš, Anna Kuzminova, and Ondřej Kylián. 2020. "Investigation of Wettability, Drying and Water Condensation on Polyimide (Kapton) Films Treated by Atmospheric Pressure Air Dielectric Barrier Discharge" Coatings 10, no. 7: 619. https://doi.org/10.3390/coatings10070619
APA StyleKhomiakova, N., Hanuš, J., Kuzminova, A., & Kylián, O. (2020). Investigation of Wettability, Drying and Water Condensation on Polyimide (Kapton) Films Treated by Atmospheric Pressure Air Dielectric Barrier Discharge. Coatings, 10(7), 619. https://doi.org/10.3390/coatings10070619






