Effect of Surface Modification on the Primary Stability of Dental Implants by Plasma Oxidation and Storage Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimen Preparation
2.2. Surface Characterization
2.3. Cell Responses
2.3.1. Cell Attachment Assay
2.3.2. Cell Proliferation Assay
2.3.3. Cell Differentiation Assay
2.4. Animal Experiments
2.5. Statistical Analysis
3. Result
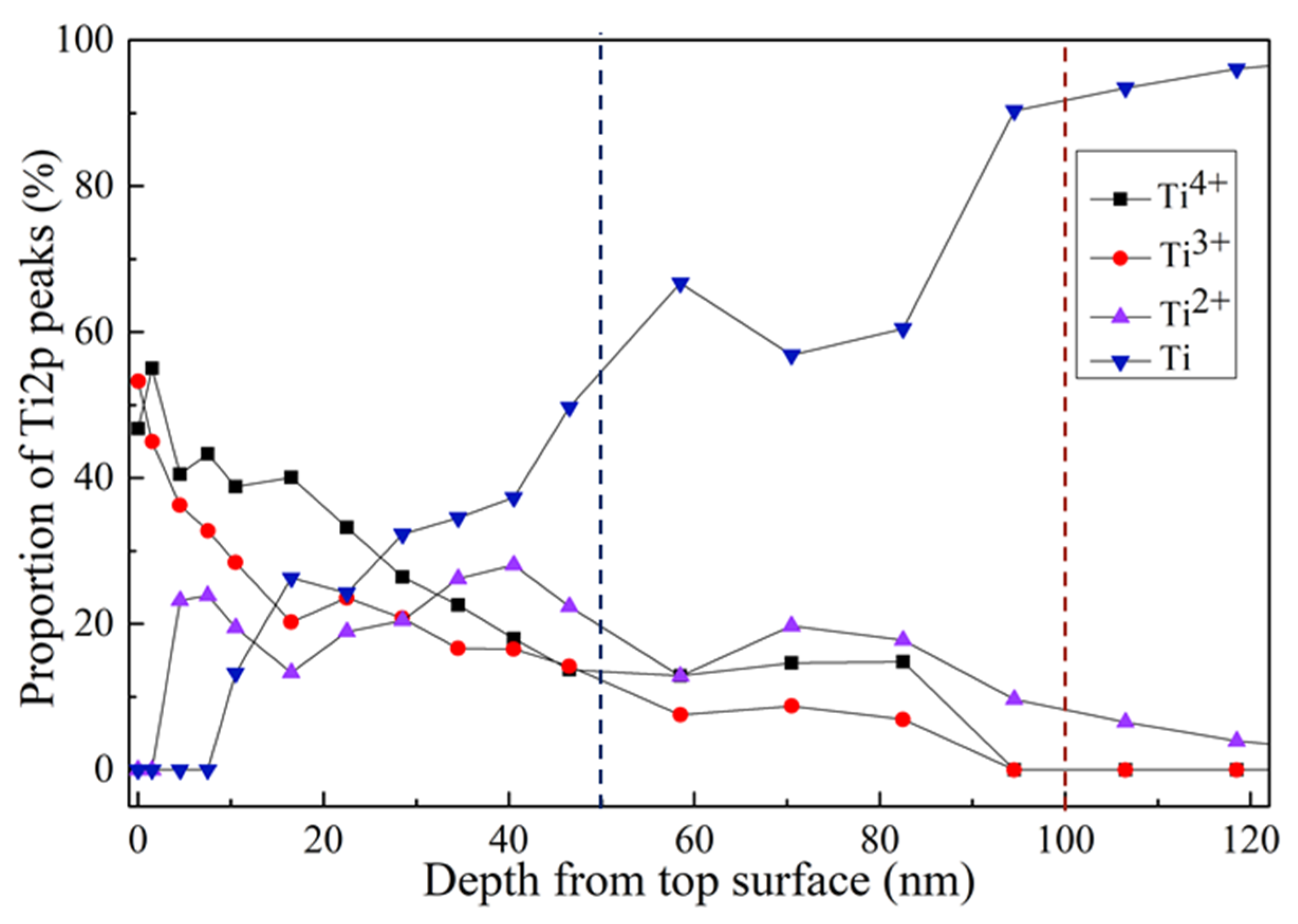
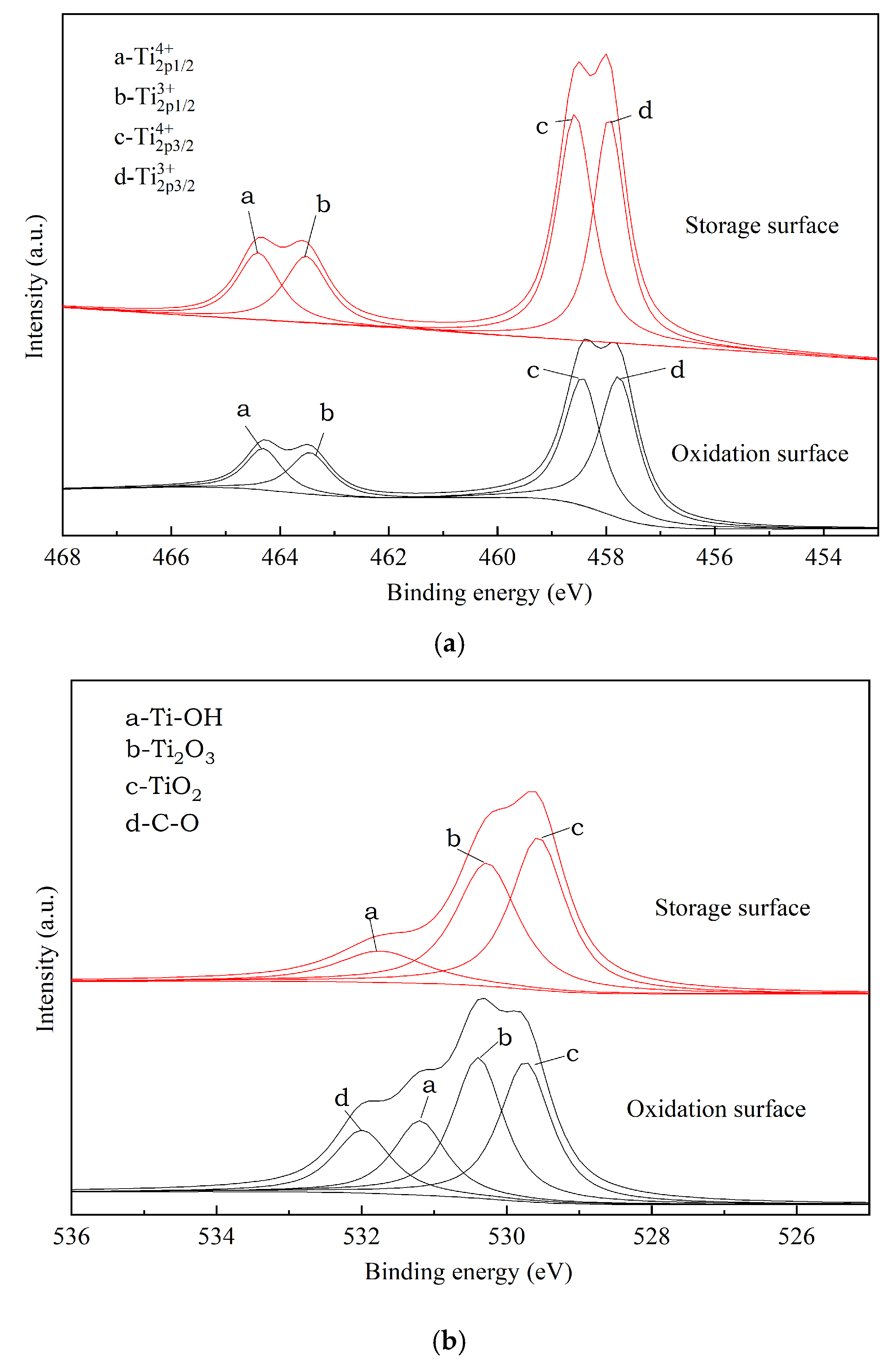
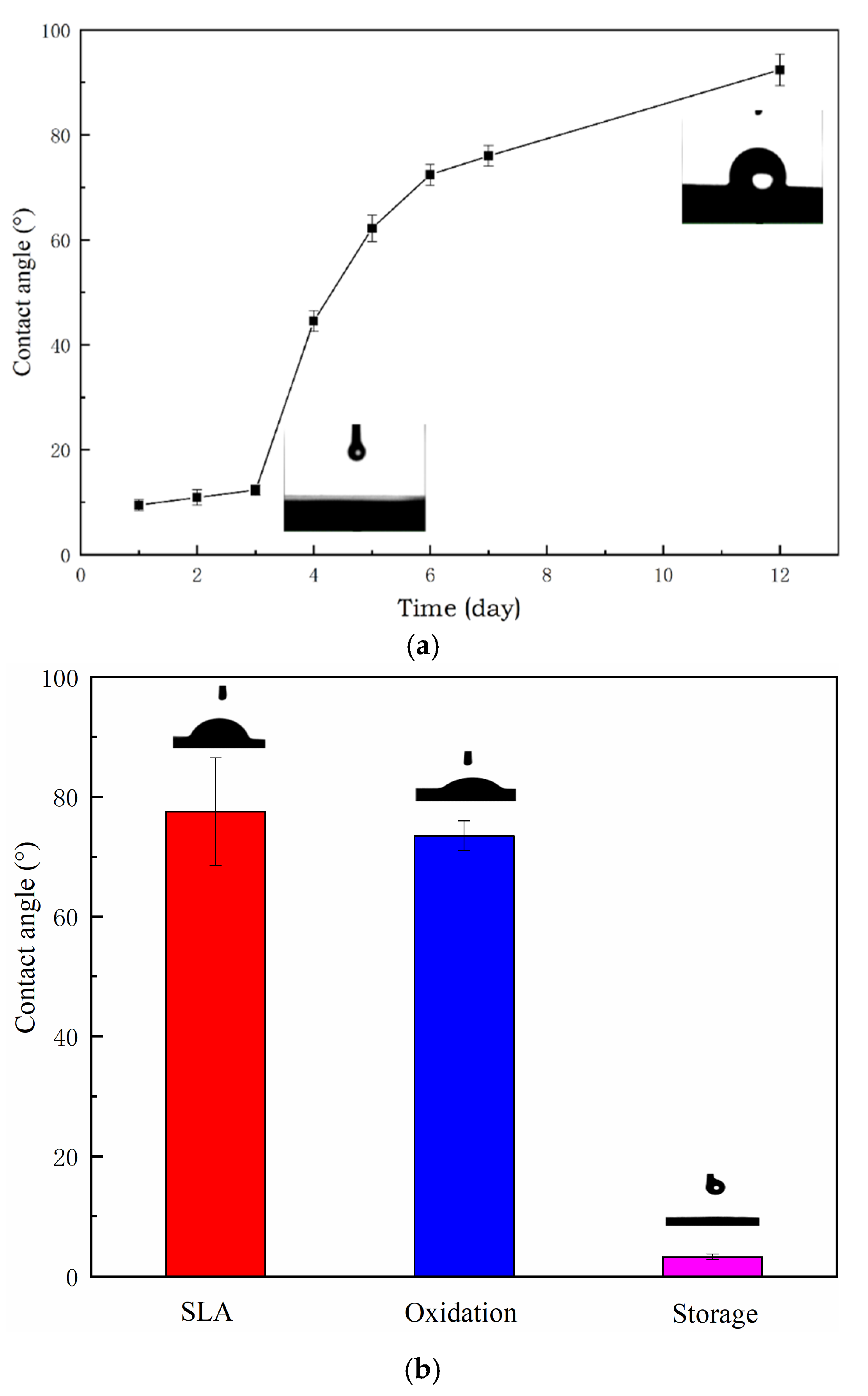
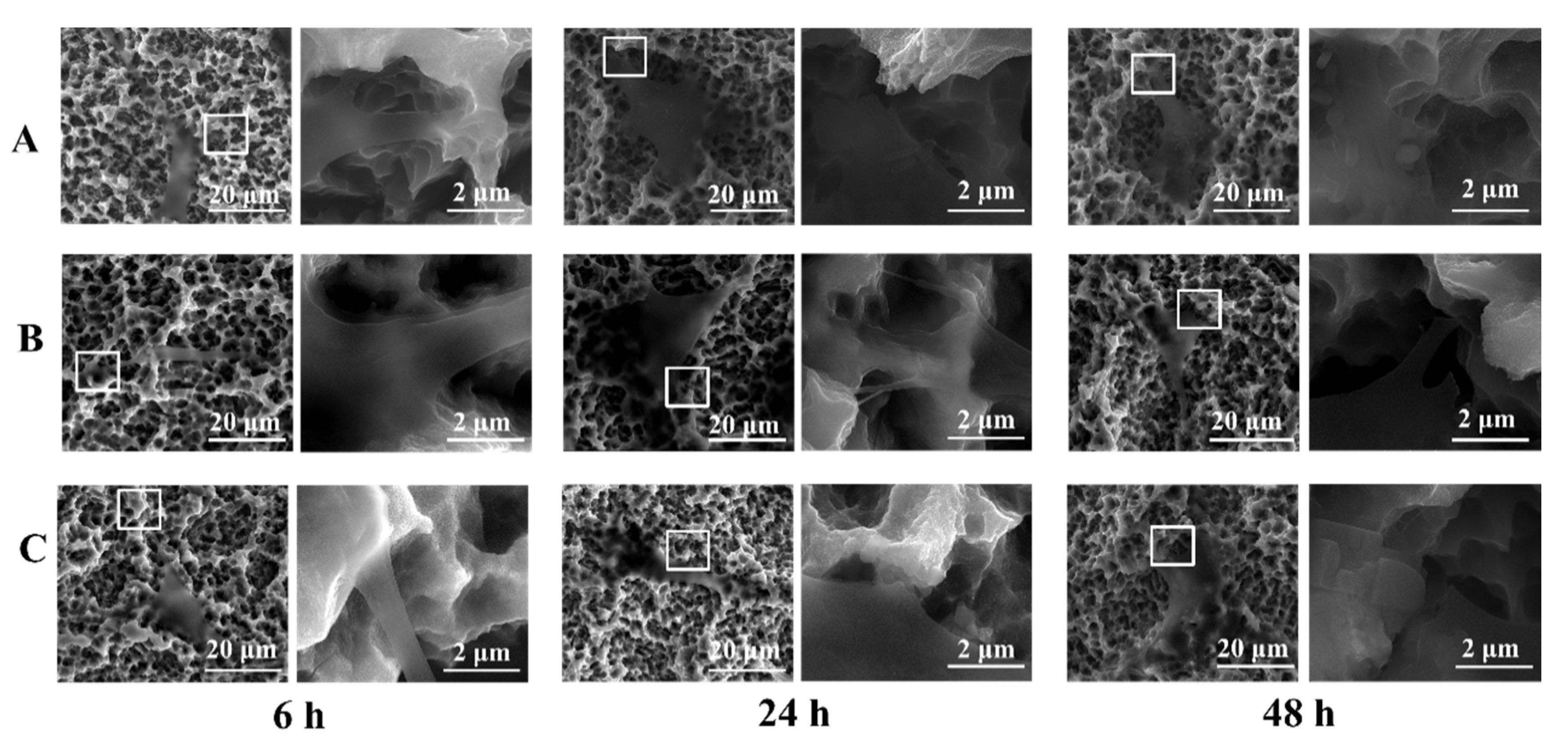
3.1. Surface Characterization
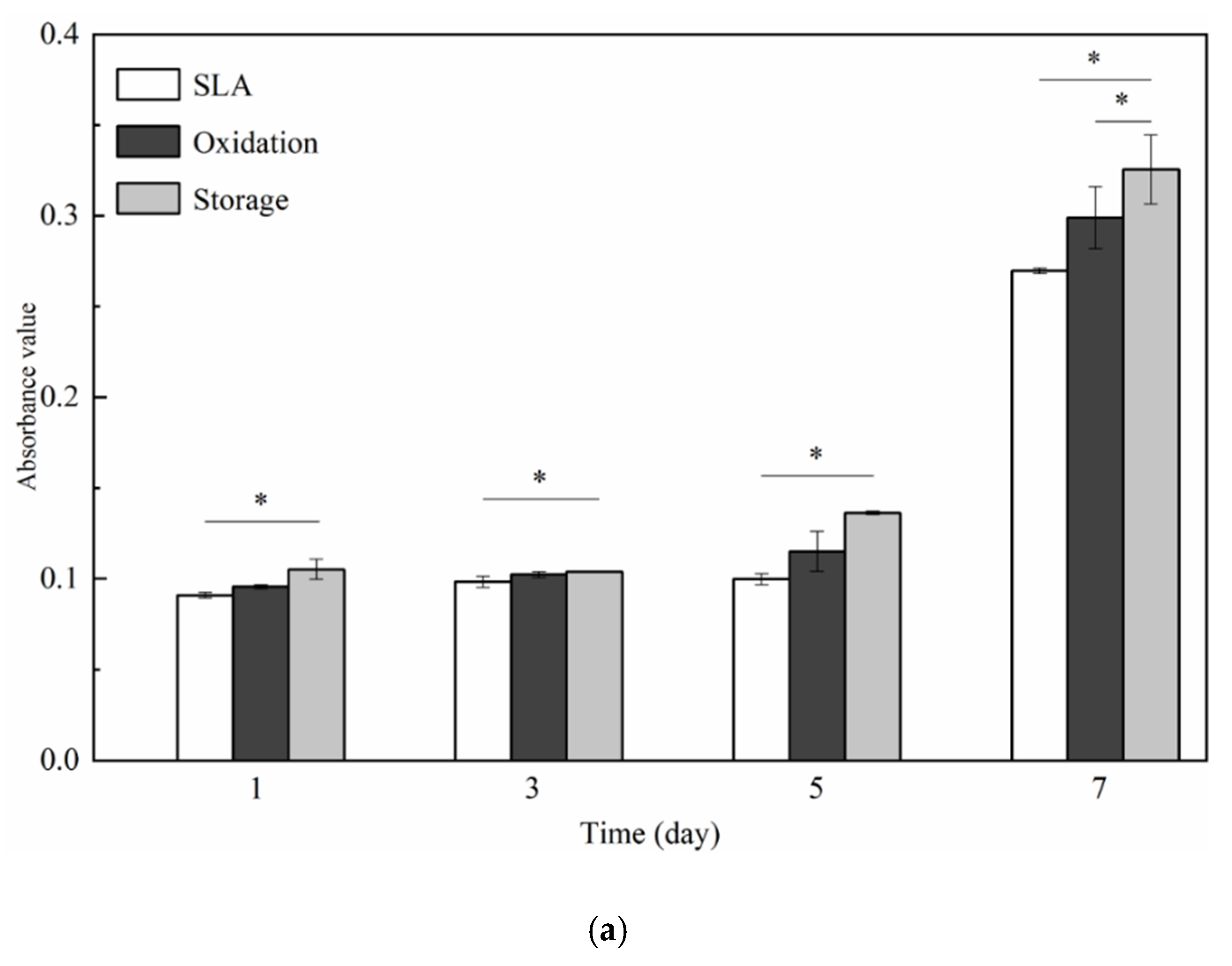
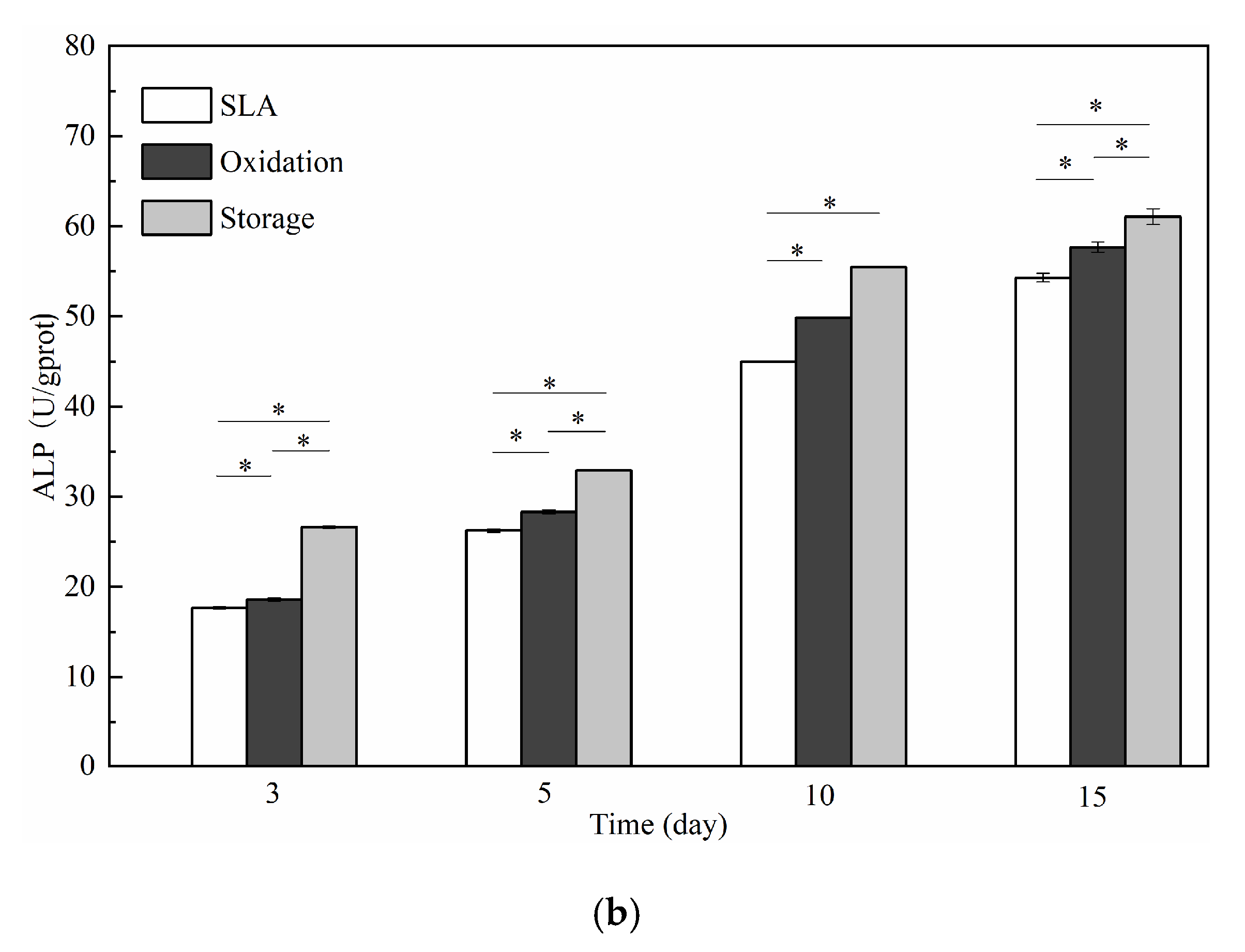
3.2. Cell Responses: Attachment, Proliferation, Differentiation, Morphology, and Morphometry
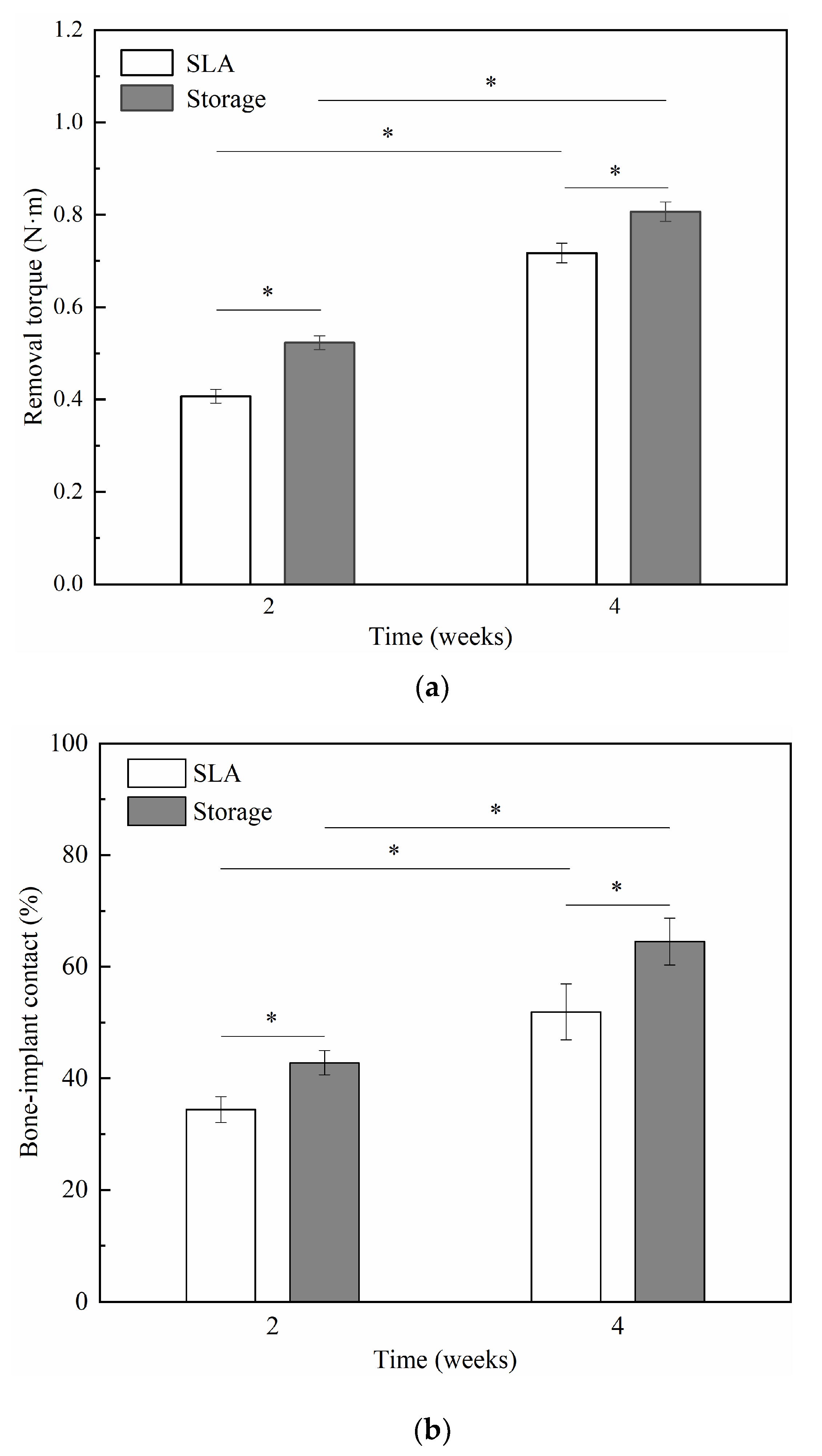
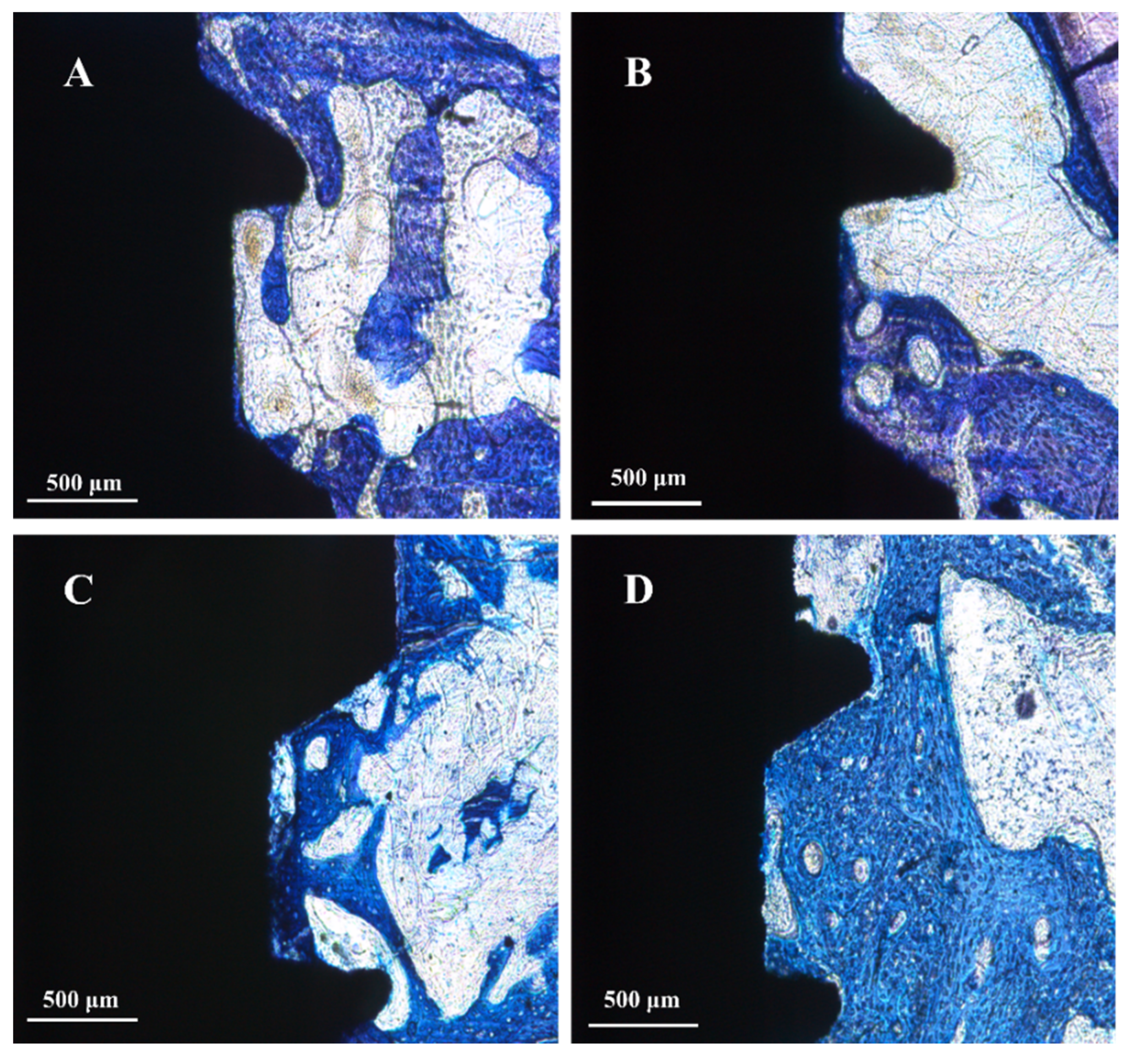
3.3. Animal Experiment
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rautray, T.R.; Narayanan, R.; Kim, K.-H. Ion implantation of titanium based biomaterials. Prog. Mater. Sci. 2011, 56, 1137–1177. [Google Scholar] [CrossRef]
- Webster, T.J.T.; Yao, C. Anodization: A Promising Nano Modification Technique of Titanium-Based Implants for Orthopedic Applications. Surg. Tools Med. Devices 2016, 52, 55–79. [Google Scholar] [CrossRef]
- Abdulgani, A.; Muhamad, A.H.; Georges, D.C. Implant Stability: Methods and Recent Advances. IOSR JDMS 2017, 16, 13–23. [Google Scholar] [CrossRef]
- Jeong, S.-J.; Wang, G.; Choi, B.-D.; Hwang, Y.-H.; Kim, B.-H.; Ko, Y.-M.; Jeong, M.-J. Secretory Leukocyte Protease Inhibitor (SLPI) Increases Focal Adhesion in MC3T3 Osteoblast on Titanium Surface. J. Nanosci. Nanotechnol. 2015, 15, 200–204. [Google Scholar] [CrossRef]
- Manara, S.; Paolucci, F.; Palazzo, B.; Marcaccio, M.; Foresti, E.; Tosi, G.; Sabbatini, S.; Sabatino, P.; Altankov, G.; Roveri, N. Electrochemically-assisted deposition of biomimetic hydroxyapatite–collagen coatings on titanium plate. Inorganica Chim. Acta 2008, 361, 1634–1645. [Google Scholar] [CrossRef]
- Jin, G.; Qin, H.; Cao, H.; Qian, S.; Zhao, Y.; Peng, X.; Zhang, X.; Liu, X.; Chu, P.K. Synergistic effects of dual Zn/Ag ion implantation in osteogenic activity and antibacterial ability of titanium. Biomaterials 2014, 35, 7699–7713. [Google Scholar] [CrossRef]
- Mändl, S.; Sader, R.; Thorwarth, G.; Krause, D.; Zeilhofer, H.-F.; Horch, H.; Rauschenbach, B. Biocompatibility of titanium based implants treated with plasma immersion ion implantation. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2003, 206, 517–521. [Google Scholar] [CrossRef]
- Yang, C.-H.; Li, Y.-C.; Tsai, W.-F.; Ai, C.-F.; Huang, H.-H. Oxygen plasma immersion ion implantation treatment enhances the human bone marrow mesenchymal stem cells responses to titanium surface for dental implant application. Clin. Oral Implant. Res. 2013, 26, 166–175. [Google Scholar] [CrossRef]
- Lin, Z.; Wang, Y.; Wang, D.-N.; Zhao, B.-H.; Li, J.-C. Porous structure preparation and wettability control on titanium implant. Surf. Coatings Technol. 2013, 228, S131–S136. [Google Scholar] [CrossRef]
- Rupp, F.; Scheideler, L.; Olshanska, N.; De Wild, M.; Wieland, M.; Geis-Gerstorfer, J. Enhancing surface free energy and hydrophilicity through chemical modification of microstructured titanium implant surfaces. J. Biomed. Mater. Res. Part A 2005, 76, 323–334. [Google Scholar] [CrossRef]
- Tugulu, S.; Löwe, K.; Scharnweber, D.; Schlottig, F. Preparation of superhydrophilic microrough titanium implant surfaces by alkali treatment. J. Mater. Sci. Mater. Electron. 2010, 21, 2751–2763. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Jin, S.; Geng, S.; Deng, C.; Lin, Z.; Zhao, B. Maintenance and Restoration Effect of the Surface Hydrophilicity of Pure Titanium by Sodium Hydroxide Treatment and its Effect on the Bioactivity of Osteoblasts. Coatings 2019, 9, 222. [Google Scholar] [CrossRef]
- Haibin, L.; Lei, W.; Xueyang, Z.; Mingdeng, R.; Zehong, G.; Lei, Z. Effects of Hydrocarbons Contamination on Initial Responses of Osteoblast-like Cells on Acid-Etched Titanium Surface. Rare Met. Mater. Eng. 2013, 42, 1558–1562. [Google Scholar] [CrossRef]
- Sista, S.; Wen, C.; Hodgson, P.D.; Pande, G. The influence of surface energy of titanium-zirconium alloy on osteoblast cell functions in vitro. J. Biomed. Mater. Res. Part A 2011, 97, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Kopf, B.S.; Ruch, S.; Berner, S.; Spencer, N.D.; Maniura-Weber, K. The role of nanostructures and hydrophilicity in osseointegration: In-vitroprotein-adsorption and blood-interaction studies. J. Biomed. Mater. Res. Part A 2015, 103, 2661–2672. [Google Scholar] [CrossRef]
- Gittens, R.A.; Scheideler, L.; Rupp, F.; Hyzy, S.L.; Geis-Gerstorfer, J.; Schwartz, Z.; Boyan, B.D. A review on the wettability of dental implant surfaces II: Biological and clinical aspects. Acta Biomater. 2014, 10, 2907–2918. [Google Scholar] [CrossRef]
- Preshaw, P. Summary of: Implant surface characteristics and their effect on osseointegration. Br. Dent. J. 2015, 218, 292–293. [Google Scholar] [CrossRef]
- Poon, C.; Hasim, F. Carcinoid tumour metastases to the maxilla. Int. J. Oral Maxillofac. Surg. 2007, 36, 869–870. [Google Scholar] [CrossRef]
- Lang, N.P.; Salvi, G.E.; Huynh-Ba, G.; Ivanovski, S.; Donos, N.; Bosshardt, D. Early osseointegration to hydrophilic and hydrophobic implant surfaces in humans. Clin. Oral Implant. Res. 2011, 22, 349–356. [Google Scholar] [CrossRef]
- Hsu, H.C.; Wu, S.C.; Hsu, S.K. Surface Modification of Commercially Pure Ti Treated with Aqueous NaOH Treatment and Ethyl Alcohol Aging. J. Med. Biol. Eng. 2013, 33, 331–336. [Google Scholar] [CrossRef]
- Lin, Z.; Lee, G.-H.; Liu, C.-M.; Lee, I.-S. Controls in wettability of TiOx films for biomedical applications. Surf. Coatings Technol. 2010, 205, S391–S397. [Google Scholar] [CrossRef]
- Lin, Z.; Li, S.-J.; Sun, F.; Ba, D.-C.; Li, X.-C. Surface characteristics of a dental implant modified by low energy oxygen ion implantation. Surf. Coatings Technol. 2019, 365, 208–213. [Google Scholar] [CrossRef]
- Wall, I.; Donos, N.; Carlqvist, K.; Jones, F.; Brett, P. Modified titanium surfaces promote accelerated osteogenic differentiation of mesenchymal stromal cells in vitro. Bone 2009, 45, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Hotchkiss, K.M.; Ayad, N.B.; Hyzy, S.L.; Boyan, B.; Olivares-Navarrete, R. Dental implant surface chemistry and energy alter macrophage activation in vitro. Clin. Oral Implant. Res. 2016, 28, 414–423. [Google Scholar] [CrossRef] [PubMed]
- Connor, P.A.; Dobson, K.D.; McQuillan, A.J. Infrared Spectroscopy of the TiO2/Aqueous Solution Interface. Langmuir 1999, 15, 2402–2408. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Liu, X.; Gao, S.; Huang, B.; Dai, Y. Preparation of Ti3+ self-doped TiO2 nanoparticles and their visible light photocatalytic activity. Chin. J. Catal. 2015, 36, 389–399. [Google Scholar] [CrossRef]
- Chou, W.-C.; Wang, R.C.-C.; Huang, C.-L.; Lee, T.-M. The effect of plasma treatment on the osseointegration of rough titanium implant: A histo-morphometric study in rabbits. J. Dent. Sci. 2018, 13, 267–273. [Google Scholar] [CrossRef]
- Raimbault, O.; Benayoun, S.; Anselme, K.; Mauclair, C.; Bourgade, T.; Kietzig, A.-M.; Girard-Lauriault, P.-L.; Valette, S.; Donnet, C. The effects of femtosecond laser-textured Ti-6Al-4V on wettability and cell response. Mater. Sci. Eng. C 2016, 69, 311–320. [Google Scholar] [CrossRef]
- Lu, X.; Wang, Y.; Yang, X.; Zhang, Q.; Zhao, Z.; Weng, L.-T.; Leng, Y. Spectroscopic analysis of titanium surface functional groups under various surface modification and their behaviorsin vitroandin vivo. J. Biomed. Mater. Res. Part A 2007, 84, 523–534. [Google Scholar] [CrossRef]
- Schliephake, H.; Scharnweber, D. Chemical and biological functionalization of titanium for dental implants. J. Mater. Chem. 2008, 18, 2404. [Google Scholar] [CrossRef]
- Bodhak, S.; Bose, S.; Bandyopadhyay, A. Role of surface charge and wettability on early stage mineralization and bone cell–materials interactions of polarized hydroxyapatite. Acta Biomater. 2009, 5, 2178–2188. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.H.; Wang, Y.T.; Tsai, W.F.; Ai, C.F.; Lin, M.C.; Huang, H.H. Effect of oxygen plasma immersion ion implantation treatment on corrosion resistance and cell adhesion of titanium surface. Clin. Oral Implants Res. 2011, 22, 1426–1432. [Google Scholar] [CrossRef] [PubMed]
- Chang, B.; Song, W.; Han, T.; Yan, J.; Li, F.; Zhao, L.; Kou, H.; Zhang, Y. Influence of pore size of porous titanium fabricated by vacuum diffusion bonding of titanium meshes on cell penetration and bone ingrowth. Acta Biomater. 2016, 33, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, G.; Liu, Y.; Zhao, X.; Zou, D.; Zhu, C.; Jin, Y.; Huang, Q.; Sun, J.; Liu, X.; et al. The synergistic effect of hierarchical micro/nano-topography and bioactive ions for enhanced osseointegration. Biomater. 2013, 34, 3184–3195. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Sun, J.-F.; Chu, P.K.; Han, Y.; Zhang, Y.-M. Bone integration capability of a series of strontium-containing hydroxyapatite coatings formed by micro-arc oxidation. J. Biomed. Mater. Res. Part A 2013, 101, 2465–2480. [Google Scholar] [CrossRef]
- Raphel, J.; Karlsson, J.; Galli, S.; Wennerberg, A.; Lindsay, C.; Haugh, M.G.; Pajarinen, J.; Goodman, S.B.; Jimbo, R.; Andersson, M.; et al. Engineered protein coatings to improve the osseointegration of dental and orthopaedic implants. Biomaterials 2016, 83, 269–282. [Google Scholar] [CrossRef]
- Tejero, R.; Anitua, E.; Orive, G. Toward the biomimetic implant surface: Biopolymers on titanium-based implants for bone regeneration. Prog. Polym. Sci. 2014, 39, 1406–1447. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, F.; Li, S.-J.; Li, X.-C.; Wang, L.; Ba, D.-C.; Song, G.-Q.; Sun, C.-S.; Lin, Z. Effect of Surface Modification on the Primary Stability of Dental Implants by Plasma Oxidation and Storage Treatment. Coatings 2020, 10, 622. https://doi.org/10.3390/coatings10070622
Sun F, Li S-J, Li X-C, Wang L, Ba D-C, Song G-Q, Sun C-S, Lin Z. Effect of Surface Modification on the Primary Stability of Dental Implants by Plasma Oxidation and Storage Treatment. Coatings. 2020; 10(7):622. https://doi.org/10.3390/coatings10070622
Chicago/Turabian StyleSun, Fei, Shao-Jie Li, Xin-Chang Li, Lei Wang, De-Chun Ba, Gui-Qiu Song, Chuan-Sheng Sun, and Zeng Lin. 2020. "Effect of Surface Modification on the Primary Stability of Dental Implants by Plasma Oxidation and Storage Treatment" Coatings 10, no. 7: 622. https://doi.org/10.3390/coatings10070622
APA StyleSun, F., Li, S.-J., Li, X.-C., Wang, L., Ba, D.-C., Song, G.-Q., Sun, C.-S., & Lin, Z. (2020). Effect of Surface Modification on the Primary Stability of Dental Implants by Plasma Oxidation and Storage Treatment. Coatings, 10(7), 622. https://doi.org/10.3390/coatings10070622



