A Sinter Visualization Device for Observing the Relationship Between Fillers and Porosity of Precursor-Derived Ceramic Coatings
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Sample
2.2. Visualization Method of the Coating Sintering Process
3. Results and Discussion
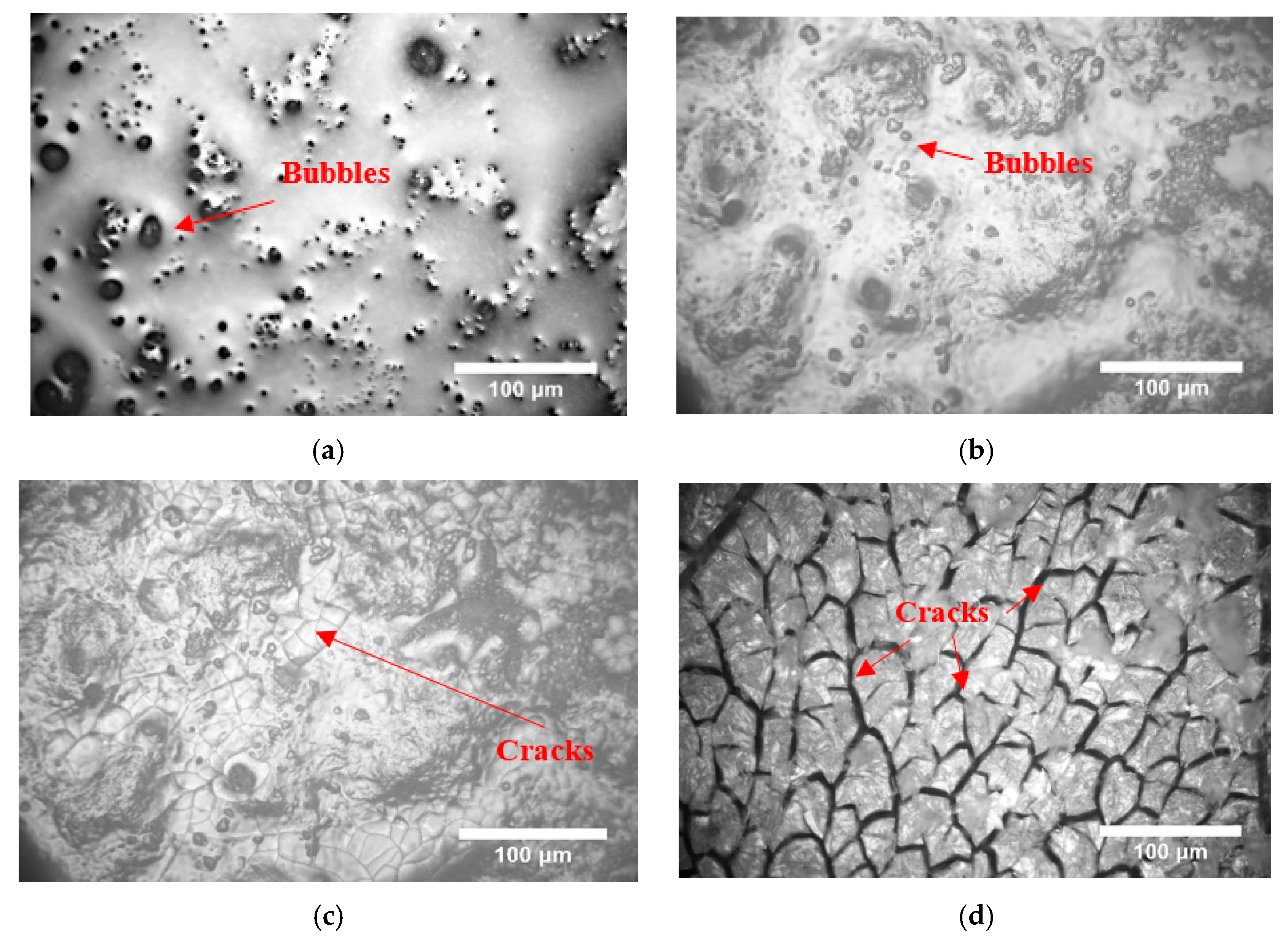
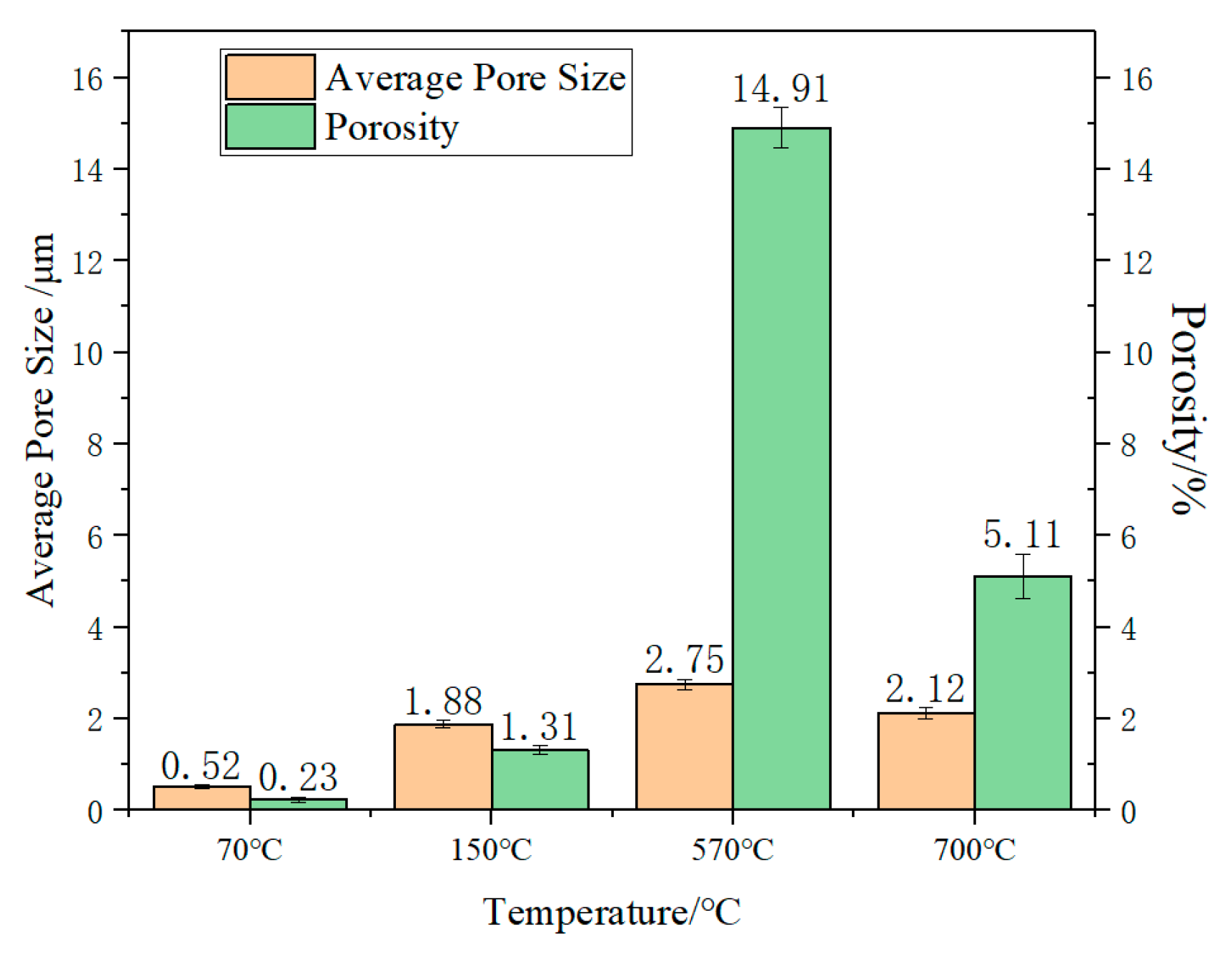
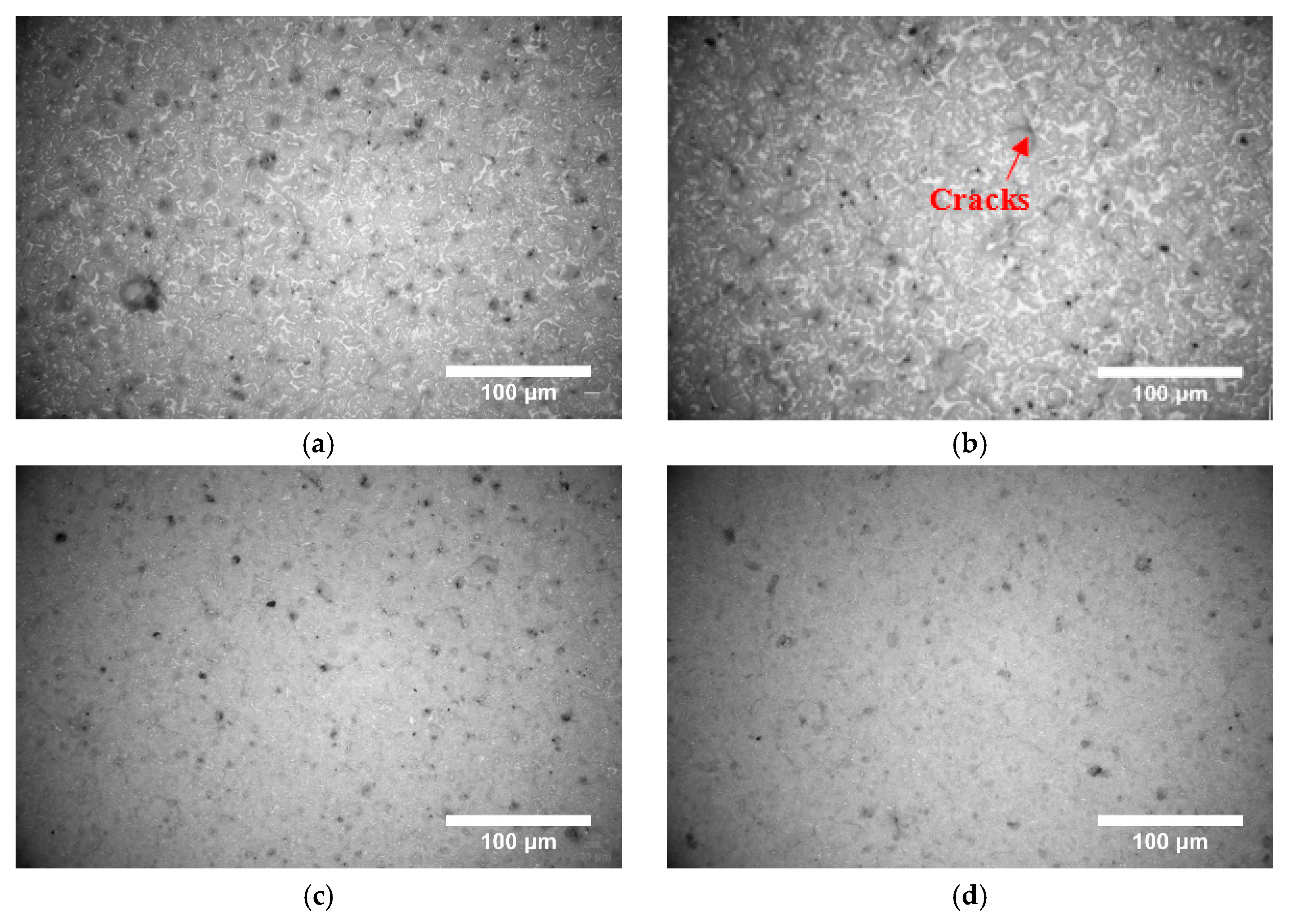
3.1. Surface Morphology of PSZ During Heating
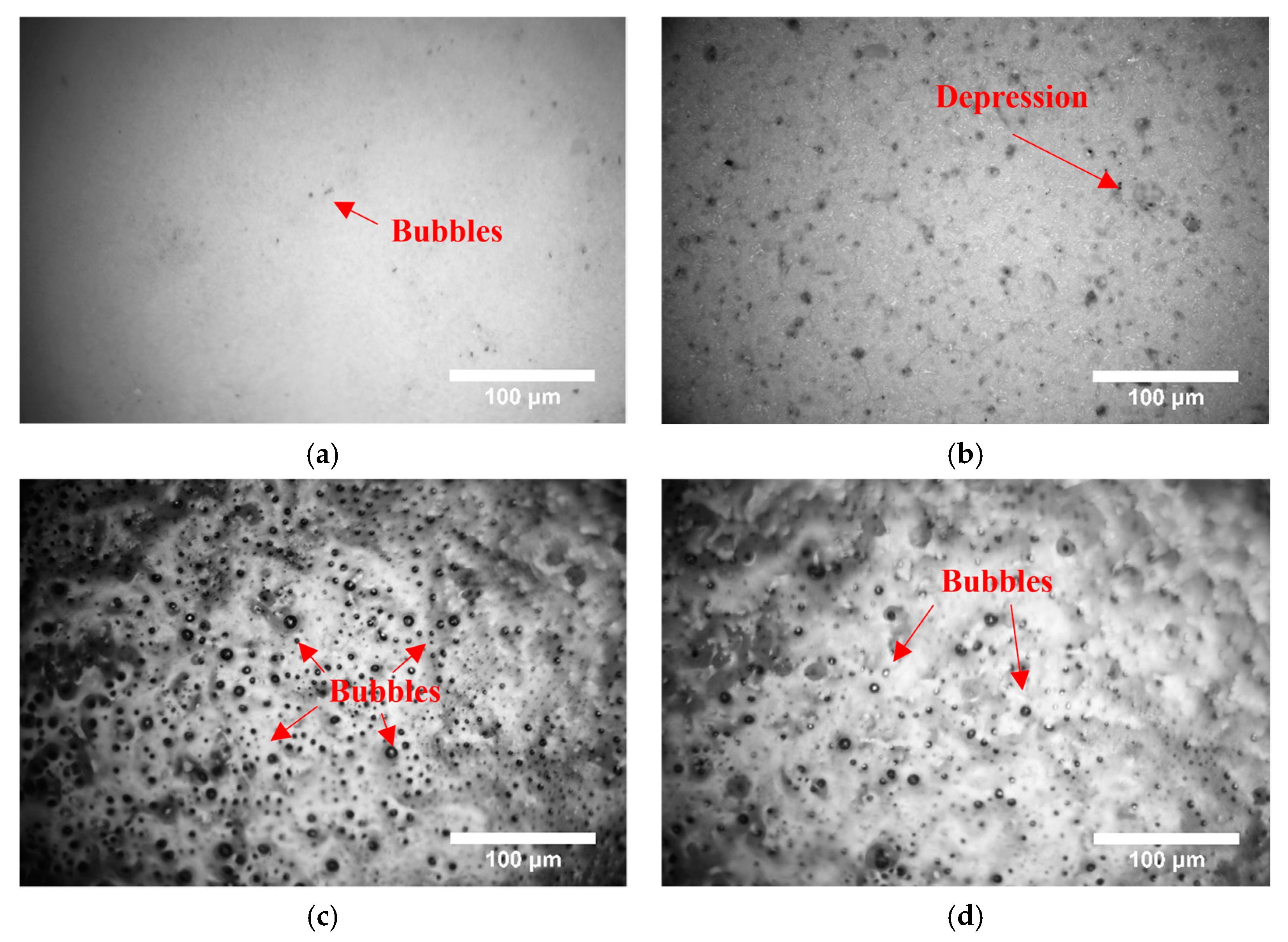
3.2. Surface Morphology after Adding Glass Powder to PSZ During Heating
3.3. Surface Morphology after Adding Glass Powder and ZrO2 to PSZ during Heating
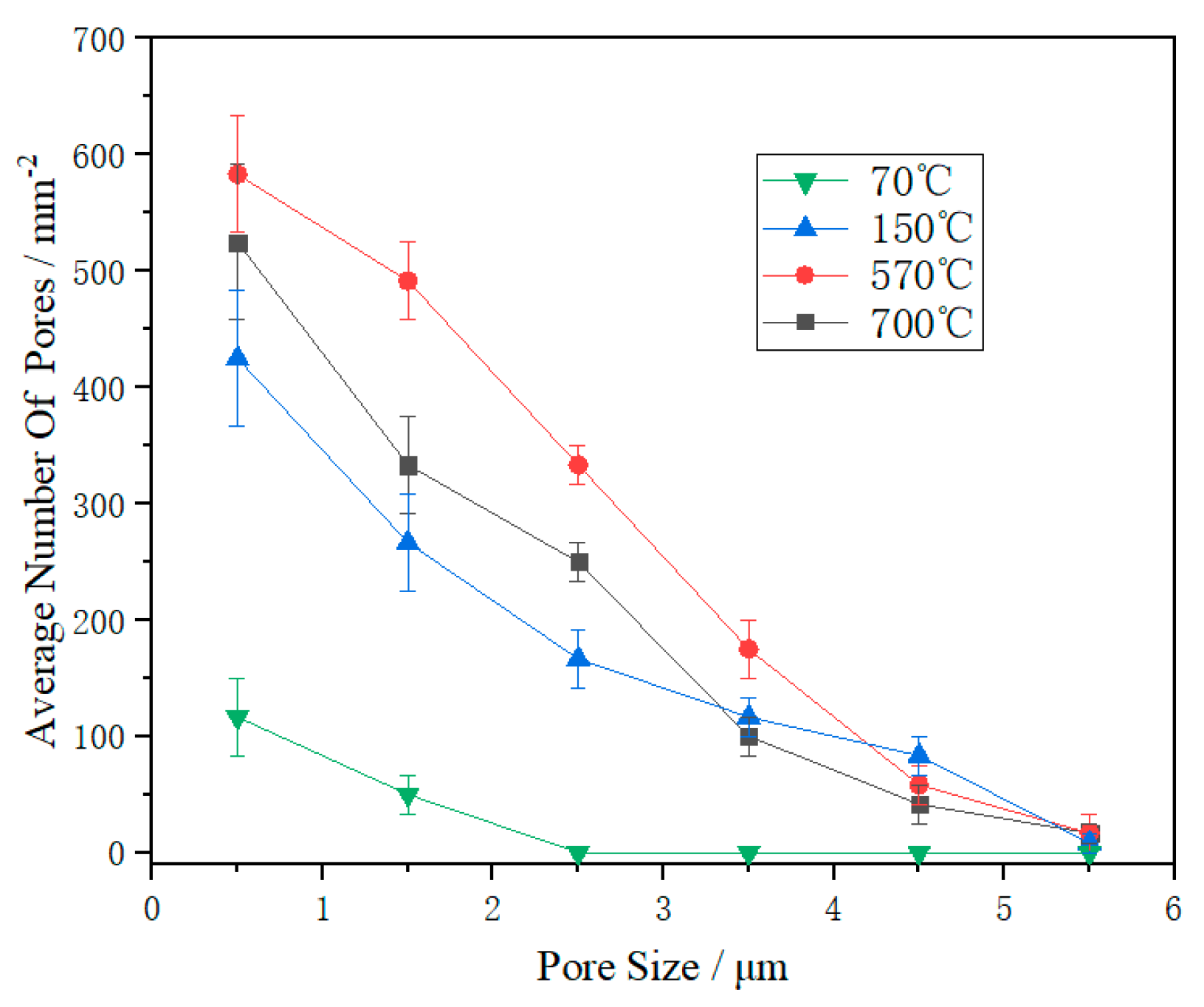
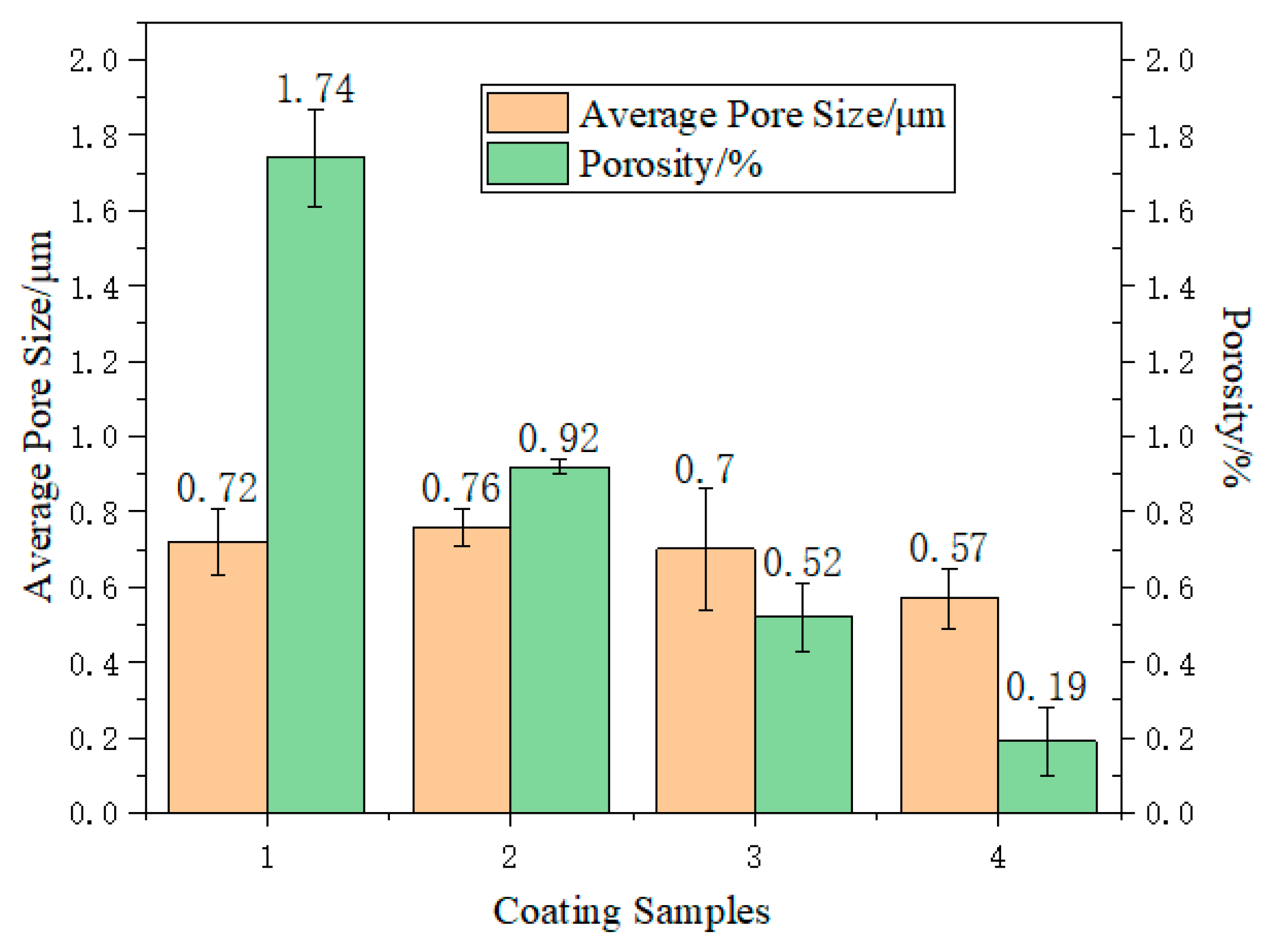
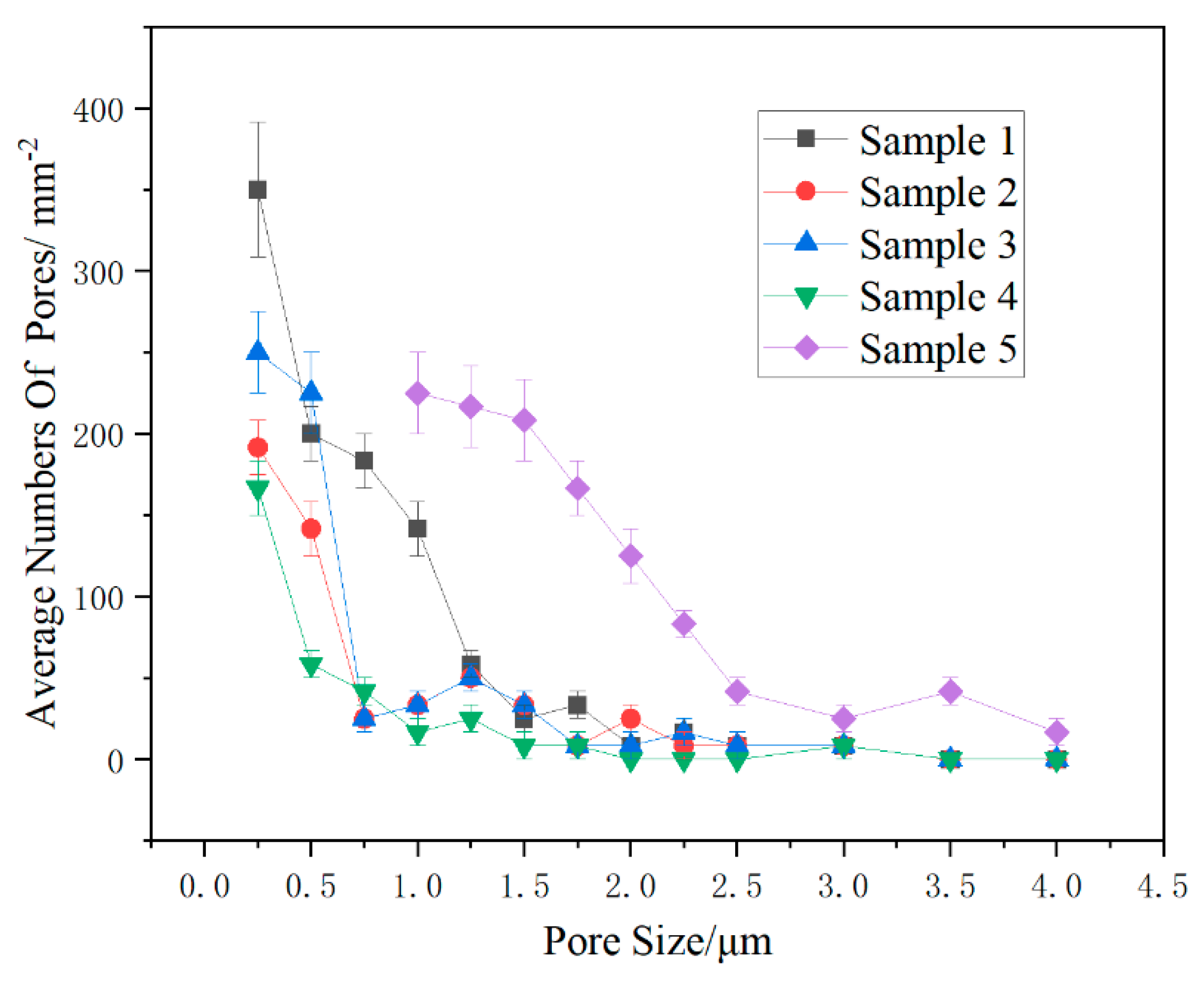
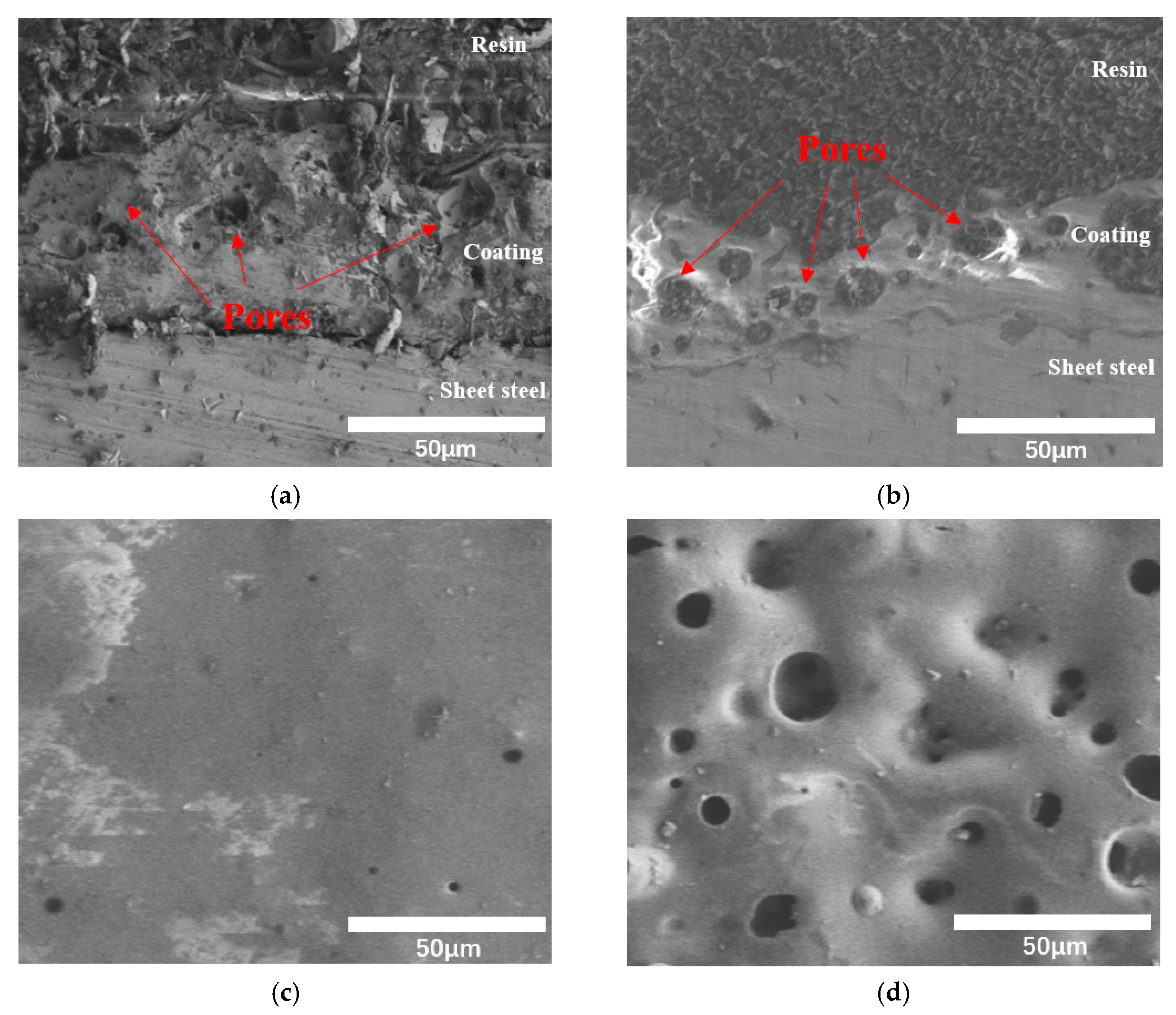
3.4. Cross Section and Surface Morphology of Coating
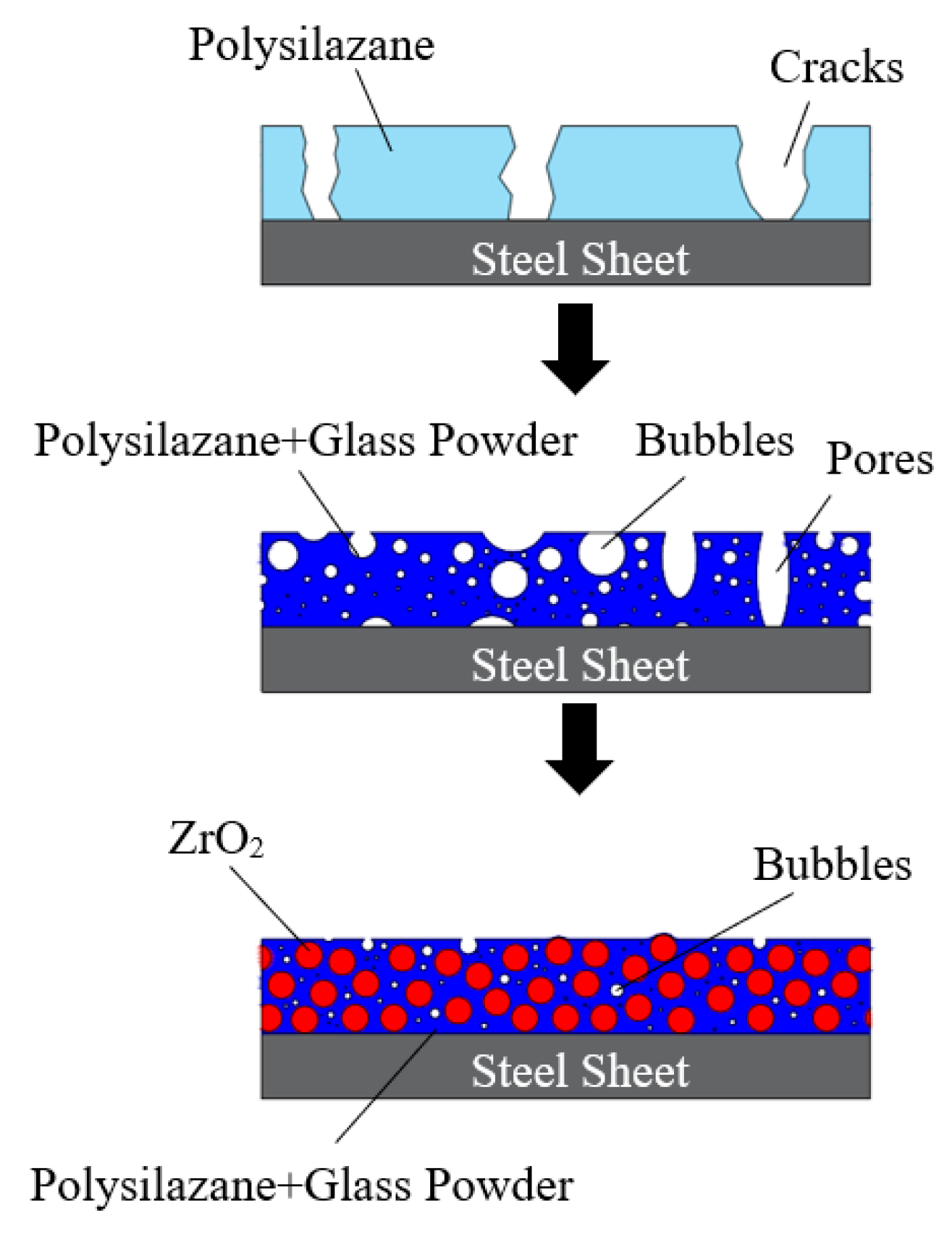
3.5. Mechanism of Pore Inhibition by Adding Fillers to the Coating
4. Conclusions
- When the temperature increased, the PSZ-derived ceramic coating cracked and released gas, and the porosity increased gradually. Additionally, many cracks formed on the coating surface. After adding glass powder, the cracks in the coating disappeared. When the temperature increased, the porosity increased and then decreased. Thus, increasing the sintering temperature and prolonging the sintering time effectively reduced and even eliminated porosity. Further, the addition of ZrO2 effectively prevented the formation of large bubbles and reduced pore size;
- When the ratio of PSZ, glass powder, and ZrO2 was 1:2:1, the porosity was at its lowest. The coating surface was mainly comprised of 1 μm micropores. After sintering, the pore distribution in the coating cross-section showed a decrease in the large pores and thus the coating became denser. However, more research is needed to eliminate macropores;
- Mechanism used for pores inhibition by adding fillers in coating was the healing effect of the glass powder. In addition, ZrO2 played a skeleton role which can adjust the thermal expansion coefficient. After the glass powder melted, its good fluidity facilitated the healing of cracks. ZrO2 formed a skeleton structure to prevent cracks and inhibit the formation of large pores. Moreover, ZrO2 facilitated small bubbles with small flow resistance to overflow from the coating, thus reducing the porosity and the number of large pores.
Author Contributions
Funding
Conflicts of Interest
References
- Fedel, M.; Gómez, F.J.R.; Rossi, S.; Deflorian, F. Characterization of polyorganosilazane-derived hybrid coatings for the corrosion protection of mild steel in chloride solution. Coatings 2019, 9, 680. [Google Scholar] [CrossRef]
- Riedel, R.; Mera, G.; Hauser, R.; Klonczynski, A. Silicon-based polymer-derived ceramics: Synthesis properties and applications—A Review. Ceram. Soc. Jpn. 2006, 114, 425–444. [Google Scholar] [CrossRef]
- Günthner, M.; Kraus, T.; Dierdorf, A.; Decker, D.; Krenkel, W.; Motz, G. Advanced coatings on the basis of Si(C)N precursors for protection of steel against oxidation. J. Eur. Ceram. Soc. 2008, 29, 2061–2068. [Google Scholar] [CrossRef]
- Colombo, P.; Mera, G.; Riedel, R.; Sorarù, G.D. Polymer-derived ceramics: 40 years of research and innovation in advanced ceramics. J. Am. Ceram. Soc. 2010, 93, 1805–1837. [Google Scholar] [CrossRef]
- Justus, T.; Gonçalves, P.; Seifert, M.; Leite, M.L.; Probst, S.M.H.; Binder, C.; Motz, G.; Klein, A.N. Oxidation resistance and microstructure evaluation of a polymer derived ceramic (PDC) composite coating applied onto sintered steel. Materials 2019, 12, 914. [Google Scholar] [CrossRef] [PubMed]
- Vijay, V.; Biju, V.M.; Devasia, R. Active filler controlled polymer pyrolysis—A promising route for the fabrication of advanced ceramics. Cerm. Int. 2016, 42, 15592–15596. [Google Scholar] [CrossRef]
- Yang, D.; Yu, Y.; Zhao, X.; Song, Y.; Lopez-Honorato, E.; Xiao, P.; Lai, D. Fabrication of silicon carbide (SIC) coatings from pyrolysis of polycarbosilane/aluminum. J. Inorg. Organomet. Polym. Mater. 2011, 21, 534–540. [Google Scholar] [CrossRef]
- Seifert, M.; Travitzky, N.; Krenkel, W.; Motz, G. Multiphase ceramic composites derived by reaction of Nb and SiCN precursor. J. Eur. Ceram. Soc. 2014, 34, 1913–1921. [Google Scholar] [CrossRef]
- Günthner, M.; Kraus, T.; Krenkel, W.; Motz, G.; Dierdorf, A.; Decker, D. Particle-filled PHPS silazane-based coatings on steel. Int. J. Appl. Ceram. Technol. 2009, 6, 373–380. [Google Scholar] [CrossRef]
- Konegger, T.; Potzmann, R.; Puchberger, M.; Liersch, A. Matrix–filler interactions in polysilazane-derived ceramics with Al2O3 and ZrO2 fillers. J. Eur. Ceram. Soc. 2011, 31, 3021–3031. [Google Scholar] [CrossRef]
- Farhadian, M.; Raeissi, K.; Golozar, M.A.; Labbaf, S.; Hajilou, T.; Barnoush, A. 3D-Focused ion beam tomography and quantitative porosity evaluation of ZrO2-SiO2 composite coating; amorphous SiO2 as a porosity tailoring agent. Appl. Surf. Sci. 2020, 511, 145567. [Google Scholar] [CrossRef]
- Günthner, M.; Schütz, A.; Glatzel, U.; Wang, K.; Bordia, R.K.; Greißl, O.; Krenkel, W.; Motz, G. High performance environmental barrier coatings, Part I: Passive filler loaded SiCN system for steel. J. Eur. Ceram. Soc. 2011, 31, 3003–3010. [Google Scholar] [CrossRef]
- Schütz, A.; Günthner, M.; Motz, G.; Greißl, O.; Glatzel, U. High temperature (salt melt) corrosion tests with ceramic-coated steel. Mater. Chem. Phys. 2015, 159, 10–18. [Google Scholar] [CrossRef]
- Deshpande, S.; Kulkarni, A.; Sampath, S.; Herman, H. Application of image analysis for characterization of porosity in thermal spray coatings and correlation with small angle neutron scattering. Surf. Coat. Technol. 2004, 187, 6–16. [Google Scholar] [CrossRef]
- Kulkarni, A.; Wang, Z.; Nakamura, T.; Sampath, S.; Goland, G.; Herman, H.; Allen, J.; Ilavsky, J.; Long, G.; Frahm, J. Comprehensive microstructural characterization and predictive property modeling of plasma-sprayed zirconia coatings. Acta Mater. 2003, 51, 2457–2475. [Google Scholar] [CrossRef]
- Fowler, D.B.; Riggs, W.; Russ, J. Inspecting thermally sprayed coatings. Adv. Mater. Process. 1990, 138, 1990–2009. [Google Scholar]
- Kong, J.; Kong, M.; Zhang, X.; Chen, L.; An, L. Magnetoceramics from the bulk pyrolysis of polysilazane cross-linked by polyferrocenylcarbosilanes with hyperbranched topology. ACS Appl. Mater. Interfaces 2013, 5, 10367–10375. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Ju, H.; Kim, J. Pyrolysis behavior of polysilazane and polysilazane-coated-boron nitride for high thermal conductive composite. Compos. Sci. Technol. 2017, 141, 1–7. [Google Scholar] [CrossRef]
- Barroso, G.S.; Krenkel, W.; Motz, G. Low thermal conductivity coating system for application up to 1000 °C by simple PDC processing with active and passive fillers. J. Eur. Ceram. Soc. 2015, 35, 3339–3348. [Google Scholar] [CrossRef]
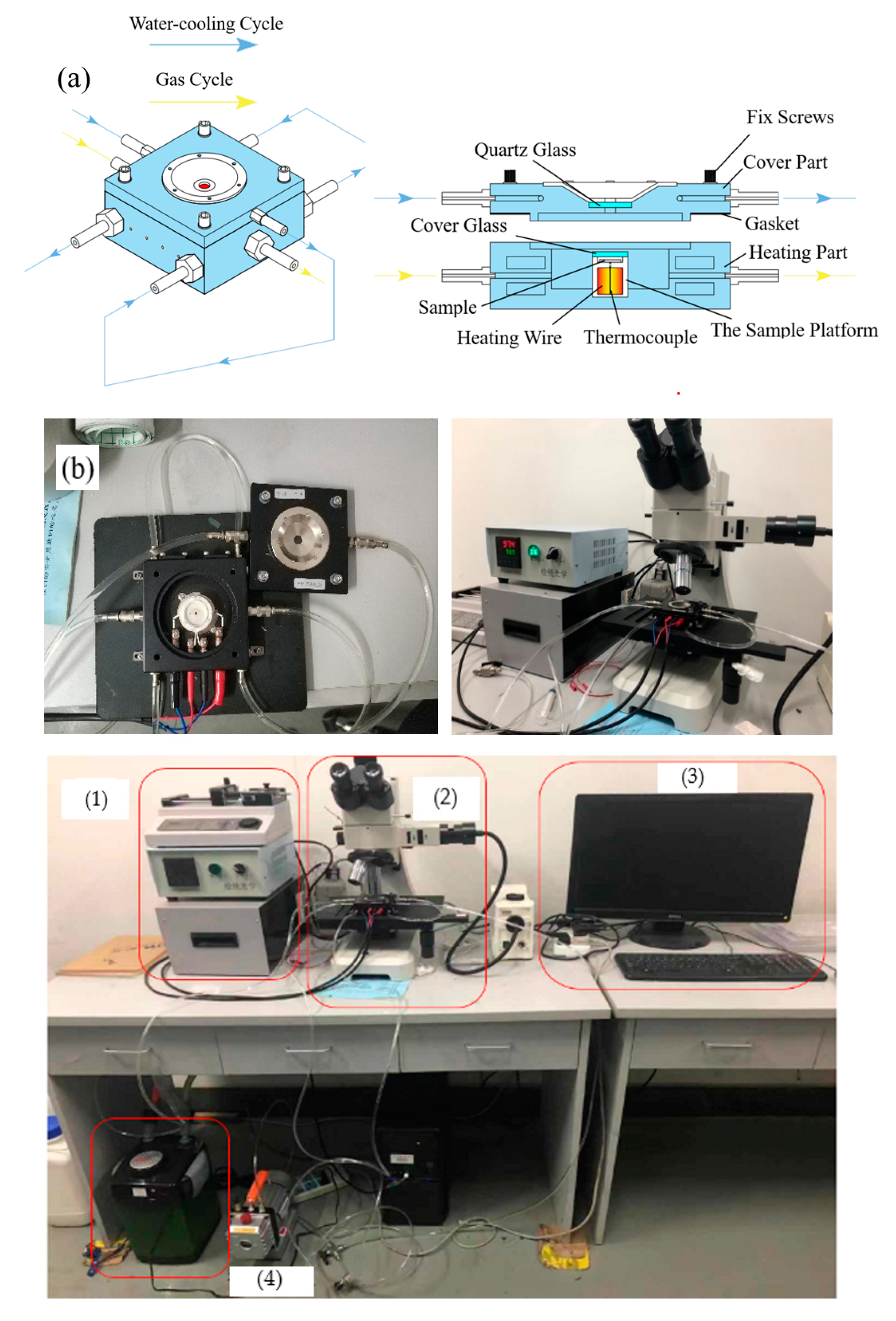
| Material | Type | Density (g/cm3) | Average Diameter d50 (μm) |
|---|---|---|---|
| PSZ | DURAZANE 1800 | 0.900~1.005 | – |
| ZrO2 | BYZ(Y-TZP)-2 | 6.05 | 1 |
| Butyl Acetate | B119685 | 0.88 | – |
| Glass Powder | Glass 8470 | 2.84 | 3.3 |
| Sample | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| PSZ/glass powder/ZrO2 | 1:1:2 | 1:2:2 | 1:1:1 | 1:2:1 | 1:1:0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, G.; Wang, J.; Wang, J.; Chi, Z.; Zhang, G.; Zhou, Z.; Feng, Z.; Xiong, Y. A Sinter Visualization Device for Observing the Relationship Between Fillers and Porosity of Precursor-Derived Ceramic Coatings. Coatings 2020, 10, 552. https://doi.org/10.3390/coatings10060552
Wang G, Wang J, Wang J, Chi Z, Zhang G, Zhou Z, Feng Z, Xiong Y. A Sinter Visualization Device for Observing the Relationship Between Fillers and Porosity of Precursor-Derived Ceramic Coatings. Coatings. 2020; 10(6):552. https://doi.org/10.3390/coatings10060552
Chicago/Turabian StyleWang, Guangxin, Jinqing Wang, Jie Wang, Zuohe Chi, Guangxue Zhang, Zhiyi Zhou, Zhi Feng, and Yunhao Xiong. 2020. "A Sinter Visualization Device for Observing the Relationship Between Fillers and Porosity of Precursor-Derived Ceramic Coatings" Coatings 10, no. 6: 552. https://doi.org/10.3390/coatings10060552
APA StyleWang, G., Wang, J., Wang, J., Chi, Z., Zhang, G., Zhou, Z., Feng, Z., & Xiong, Y. (2020). A Sinter Visualization Device for Observing the Relationship Between Fillers and Porosity of Precursor-Derived Ceramic Coatings. Coatings, 10(6), 552. https://doi.org/10.3390/coatings10060552





